Abstract
Listeriosis is an emerging zoonotic infection of humans and ruminants worldwide caused by Listeria monocytogenes (LM). In both host species, CNS disease accounts for the high mortality associated with listeriosis and includes rhombencephalitis, whose neuropathology is strikingly similar in humans and ruminants. This review discusses the current knowledge about listeric encephalitis, and involved host and bacterial factors. There is an urgent need to study the molecular mechanisms of neuropathogenesis, which are poorly understood. Such studies will provide a basis for the development of new therapeutic strategies that aim to prevent LM from invading the brain and spread within the CNS.
1. Introduction
The Gram-positive bacterium Listeria monocytogenes (LM) was first isolated in a human patient with meningitis 1921 and subsequently worldwide from a wide range of mammalian and nonmammalian species, notably farm ruminants [1–4]. However, it was not until the 1980s as a result of several human epidemics that listeriosis was recognized as a serious and frequently fatal foodborne disease and research activity on the disease was substantially intensified [5–7]. Since then the incidence has risen steadily including large outbreaks making listeriosis to a major public health issue [8–12]. Clinical syndromes associated with LM infection are similar in all susceptible hosts and include febrile gastroenteritis, septicemia, abortion, and central nervous system (CNS) infections such as meningitis, meningoencephalitis, and rhombencephalitis [11, 13, 14]. CNS involvement is a characteristic feature and accounts for the high mortality associated with listeriosis [11, 15, 16]. Currently, the agent is one of the best-studied bacterial pathogens for various reasons. Most importantly, it serves as model system for the study of innate and cell-mediated immunity, host-pathogen interactions, and intracellular survival of pathogens [17–25]. More recently, the bacterium has been investigated as a vector of heterologous proteins for vaccination and immunotherapy of cancer and infectious diseases [26–30]. However, although much progress has been made in these various fields of research, the pathogenesis and transmission of the CNS infection in its natural hosts, most notably the pathologically intriguing rhombencephalitis, is largely unknown. Particularly, not much is known about bacterial determinants that are associated with neurovirulence [31–33]. The purpose of this review is to summarize the current knowledge of the CNS form of LM infection in the natural host.
2. Listeria monocytogenes: An Emerging Foodborne Pathogen
LM belongs to the bacterial genus Listeria, which are Gram-positive, nonspore-forming, facultatively anaerobic, and intracellular coccobacilli. The genus comprises currently six species including LM, L. ivanovii, L. welschimeri, L. seeligeri, L. grayi, and L. innocua [6]. Of those, only two species are considered potentially pathogenic: LM and L. ivanovii [6]. LM is the major pathogen of listeriosis and the only species of the genus that poses a serious public health risk. It causes invasive and often fatal disease including CNS infection in numerous animal species including farm ruminants, horses, dogs, pigs, deer, South American camelids, cats, and men. In contrast, L. ivanovii is considered only mildly pathogenic and seems to affect almost exclusively ruminants, causing abortion, still-births, and neonatal septicemia, but not CNS infections [4, 6, 34]. Both LM and L. ivanovii hold a group of virulence genes such as the positive regulatory factor A, internalins, hemolysins, phospholipases, a hexose phosphate transporter and others, which enable them to replicate within and spread between eukaryotic cells [21, 35, 36]. These virulence genes are absent or present in a nonfunctional form in the other four Listeria species that are considered primarily apathogenic saprophytes, although they have been very rarely isolated from humans and animals [37–43]. Accordingly, this review is confined to the discussion of LM.
LM is ubiquitously distributed and grows in a wide variety of environments including soil, water, plant matter, diverse food items, and intestinal tract of mammalian hosts [6]. In addition, the bacterium has well adapted to an intracellular life-cycle that is critical for its pathogenic potential. LM is a biofilm-producer and as compared to most other pathogenic bacteria relatively resistant to hostile environmental conditions including low pH, high salt concentrations and low temperatures [3, 44–46]. These properties render LM remarkably tenacious against numerous food-processing and food-preserving procedures and thus hazardous for the food industry. Hence, the bacterium has emerged as an important foodborne pathogen and is a major cause for large food recalls due to bacterial contamination [47, 48]. Although LM is able to infect a wide range of animal species, it occurs primarily in farm ruminants and humans [4, 14]. In both hosts, the prevalence of listeriosis has risen significantly since the 1980s resulting in intensified surveillance and control of LM in food industry, which contributed to a decrease of human listeriosis cases in the last two decades [49, 50]. However, in various European countries its prevalence has again increased in the last few years [9, 10, 51–53].
3. Listeriosis in Humans
3.1. Incidence
LM has been linked to sporadic episodes as well as large outbreaks of human illness worldwide [7–10, 12, 54–56]. The vast majority of human listeriosis cases occurs following consumption of contaminated food [57]. Although relatively rare (the annual incidence rate ranges from 1 to 10 cases per million), listeriosis has an important impact on public health given that it is responsible for the highest hospitalisation and mortality rates amongst foodborne infections and LM is a common food contaminant [15, 57, 58]. The case fatality rate ranges from 24% to 52% despite adequate antimicrobial treatment [11, 15, 59–65].
3.2. Clinical Aspects
LM has the propensity to cause invasive disease in well-defined risk groups including pregnant women, individuals at the extremes of age (newborns or elderly people), and patients with underlying conditions. The list of such underlying conditions is long and includes malignancies, diabetes mellitus, alcoholism, chronic hepatic and renal diseases, organ transplantation, autoimmune diseases, AIDS, immunosuppressive treatments (e.g., steroids), and treatments reducing the gastric acid secretion [6, 11, 14, 15, 52, 59–62]. However, listeriosis can occur in otherwise healthy individuals [8, 11].
The infection manifests in various syndromes, ranging from mild febrile gastroenteritis to serious invasive disease including septicaemia, abortions, and CNS disease [5, 6, 11]. In addition to these syndromes, listeriosis may present as a local infection including dermatitis, endocarditis, pericarditis, pneumonia, peritonitis, arthritis, hepatitis, and endophthalmitis [66–72]. Infection of nonpregnant adults leads to bacteremia and CNS disease in most cases [11, 13, 65], and listeriosis is nowadays the second to fifth most common etiology of human bacterial meningitis in the Western hemisphere [15, 59–63, 73–79]. The CNS form in humans generally develops as a diffuse meningitis/meningoencephalitis, usually associated with bacteremia. Meningitis prevails in neonates, elderly people and patients with immunosuppressive disorders or other concurrent conditions [11, 59, 60, 62, 74, 75, 80]. Less common CNS manifestations include abscesses in the cerebrum or cerebellum, and in up to 24% of patients encephalitis targeting the brainstem (rhombencephalitis), but the latter is probably under-recognized [13, 80–83]. Rhombencephalitis has been first described in 1957 by Eck as an unusual form of listeriosis [84]. In contrast to meningitis, it appears to occur predominantly in previously healthy patients without any predisposing conditions [13, 16, 82, 85]. The clinical course is usually biphasic, with a prodrome of unspecific symptoms consisting of headache, malaise, nausea, vomiting, and fever in the first phase during between 4 and 10 days, followed by progressive brainstem deficits with asymmetric cranial nerves palsy, cerebellar dysfunctions, hemi- or tetraparesis, sensory deficits, respiratory insufficiency, impairment of consciousness, and sometimes seizures [13, 81, 82, 85]. Blood and spinal fluid cultures are positive in 60% and 40% of patients, respectively [81, 82]. The condition is fatal unless treated early and survivors commonly have significant neurological sequelae [14, 81].
3.3. Neuropathology
In most cases of human listeriosis, the CNS form manifests as a diffuse suppurative meningitis occasionally also extending into the ventricles [86]. Rhombencephalitis involves primarily the medulla oblongata, pons and midbrain with infiltrates targeting frequently nuclei and tracts of cranial nerves [85, 87]. Lesions may extend into the cerebellum and further rostrally into the thalamus and basal nuclei [87]. Cellular infiltrations consist of agglomerates of microglial cells, microabscesses with neutrophils and macrophages, occasionally accompanied by neuronal necrosis and neuronophagia [87].
4. Listeriosis in Ruminants
4.1. Incidence
Listeriosis is of major veterinary importance in the three farm ruminant species cattle, sheep, and goats [4], not only by virtue of significant economical losses in livestock production due to morbidity and high mortality in animals, but also with regard to food safety and public health representing a possible link between the environment and human infection.
In ruminants, the foodborne route of LM infection has been well established long before it was shown in humans [4]. Many studies have indicated that poor-quality silage is commonly contaminated with LM and focused on spoiled silage as source for listeriosis outbreaks [4, 88–105]. In line with these results, fecal shedding of LM in cattle is associated with contamination of silage [106, 107]. The investigation of an epidemiological link between silage feeding and listeriosis in ruminants, however, gave inconsistent results. Whilst some studies could isolate matching LM strains in brains of affected animals and silage samples, others yielded unrelated strains [92, 94, 95, 98, 101, 108, 109]. A recent study detected a higher prevalence of the bacterium in samples collected from the immediate cattle environment (feed bunks, water through and beddings) and in cattle feces than in silage challenging the view that silage is the only source of LM infection [92]. This finding is in line with reports and our own observations of outbreaks unrelated to silage feeding [110–113].
Recent prevalence estimates of listeric encephalitis in cattle, sheep, and goats, based on neuropathological survey studies in Europe, range between 7.5% and 29.4% and a neuropathological survey of fallen stock in Switzerland identified listeriosis as the most important CNS disease of small ruminants [50, 114–117]. With reference to the small ruminant population in Switzerland the prevalence of listeric encephalitis was 216 cases/million sheep and 500 cases/million goats per year and thus exceeded significantly the number of human cases (between 1.4 and 9 cases/million inhabitants per year) [50, 58]. Similar data are not available for bovines. However, in neuropathological surveillance schemes for bovine spongiform encephalopathy in various countries, listeriosis scores as the most frequent neurological disease in cattle [114–117]. The importance of these data is underlined by significant economical losses in life stock industry caused by listeriosis, the likely role of ruminants as reservoir for human pathogenic strains and therefore its impact on food safety [118–121].
4.2. Clinical Aspects
The infection usually occurs in five distinct clinical presentations, of which encephalitis is by far the most common form, followed by abortions, whilst neonatal septicaemia, mastitis, and keratoconjunctivitis/uveitis occur quite rarely [3, 4, 102, 122]. These syndromes seldom overlap within the same animal or the same flock [4, 102, 123–127]. Some authors speculate that encephalitis occurs as a distinct syndrome and more frequently than other clinical syndromes in farm ruminants because immunity acquired through ingestion of contaminated silage protects against septicemia and abortion but is not fully effective in protection against encephalitis [4]. Furthermore, ruminants may commonly be asymptomatic intestinal carriers of the organism [90, 92, 128–130]. In contrast to humans, the classical CNS presentation in ruminants is rhombencephalitis, whereas diffuse meningitis or meningoencephalitis has only exceptionally been reported [131]. Listeric rhombencephalitis was first described in sheep as “circling disease” in New Zealand and since then has been reported in all three ruminant species around the world [4, 50, 98, 102, 105, 108, 111, 114, 125, 131–141]. Cattle appear to be less susceptible to the infection than small ruminants [4]. Occasionally, listeriosis may occur as an outbreak, particularly in sheep and goats [101, 104, 111, 113, 125, 127] (and own observations). The mortality rate is high despite antibiotic treatment [102]. Ruminants and in particular bovines are frequently exposed to relatively high environmental levels of LM cells [92, 120]. As in humans, the disease usually has a low attack rate affecting individual animals within a flock, although it is assumed that all animals are exposed to a similar infectious dose of LM [4, 102, 142, 143]. Therefore, most authors speculate that—similar to the situation in humans—hitherto unidentified underlying predisposing conditions facilitate the development of clinical listeric disease. In agreement with this hypothesis, cattle shed increased numbers of LM in their feces after transport stress [106]. However, although some authors described various concurrent conditions associated with natural listeriosis, the existence of such predisposing factors for listeric encephalitis has not yet been sufficiently proven neither in epidemiological investigations nor in experimental settings [4, 102, 111, 133, 144–155]. Furthermore, routine pathological examinations of ruminants with listeric rhombencephalitis uncommonly reveal significant concurrent disease (own observations). In this context, it is intriguing that the majority of human rhombencephalitis cases occurs in otherwise healthy individuals [82, 85, 156].
For unkown reasons, the incubation period for encephalitis is longer compared to the other conditions (septicaemia, abortion) and varies between 1 and 7 weeks [4, 105, 157–159]. Clinical signs of listeric encephalitis are similar in all three farm ruminants but vary depending on the topography of the CNS lesions. Generally they are characterized by unilateral or bilateral brainstem and cranial nerve (CN) deficits [4, 148, 160–162]. Common manifestations include masticatory problems, failure of jaw closure, hypoalgesia of the head (involvement of CN V), drooping of ears, upper eye lids and lips (involvement of CN VII), deficits of the palpebral and menace reflex (CNs V and VII), problems of swallowing (CNs IX and X), tongue palsy (CN XII), circling, head tilt and leaning to one side (vestibular system), nystagmus (CN VIII), and drooling of saliva (Figures 1 and 2). Other more unspecific signs include fever, dullness, and anorexia. In the terminal stage, animals become recumbent and may show convulsions. Rare cases have been described, in which limb paralysis occurred due to affection of the spinal cord alone (myelitis) without involvement of the brain [134, 163, 164]. The course of infection in small ruminants (sheep and goats) is generally acute and animals die within 1–3 days after clinical signs became apparent. In cattle, the course is more prolonged [4, 158].
Figure 1.
Cow with rhombencephalitis due to Listeria monocytogenes infection. The cow has problems with swallowing (note the feed below her head) and shows increased salivation, facial paralysis with drooping of left ear and upper eyelid, courtesy of Dr. Mireille Meylan, Vetsuisse Faculty, University of Bern.
Figure 2.
Recumbent goat with rhombencephalitis due to Listeria monocytogenes infection. The animal has a head-tilt and pleurothotonus; its right ear is drooping.
4.3. Neuropathology
Gross lesions of the brain are generally absent, but occasionally a greyish-tan discoloration and malacia or simply hyperaemic vessels can be observed in the brainstem. In contrast, histological lesions are pathognomic for the disease and include a combination of suppurative parenchymal lesions (microabscesses) and necrosis with perivascular lymphohistiocytic cuffings and gliosis (Figures 3 and 4). In severe cases, microabscesses may coalesce to large areas of suppuration. These changes are frequently accompanied by a meningitis. Lesions are commonly unilaterally pronounced and centered on the medulla oblongata and pons (Figure 3). However, they consistently spread rostrally into the midbrain, diencephalons, and telencephalon and caudally into the spinal cord [165]. Lesions in the rostral brain show a consistent topography in selective fiber tracts. The inflammatory process involves the ependyma and choroid plexi only in exceptional cases and is then always associated with rhombencephalitis [131, 165, 166].
Figure 3.
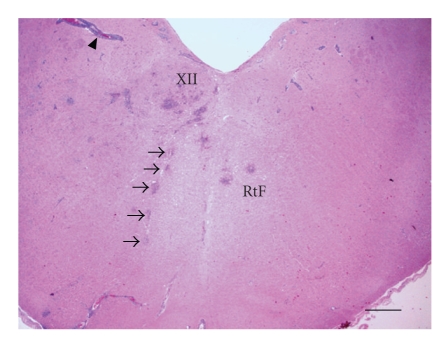
Rhombencephalitis in a sheep: Brainstem at the obex region with multiple microabscesses and perivascular cuffing (arrowhead). Microabscesses involve the hypoglossal nucleus (XII), its intracerebral root (arrows), and the reticular formation (RtF). Hematoxylin & Eosin stain (H&E), bar = 740 μm.
Figure 4.
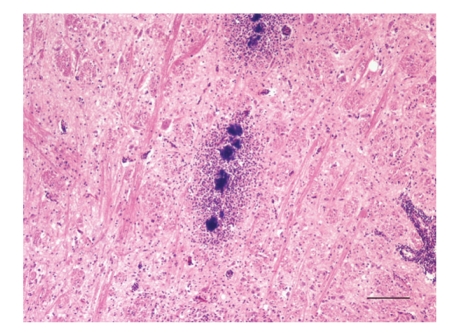
Rhombencephalitis in a sheep: Microabscesses with central bacterial colonies aligned along an axonal tract within the reticular formation (medulla oblongata). H&E, bar = 100 μm.
5. Pathogenesis of Listeriosis and Key Virulence Factors of L. monocytogenes
The pathogenicity of LM depends on its capacity to resist hostile environmental conditions and invade and replicate in both professional phagocytes and nonphagocytic host cells, which is determined by at least 50 genes scattered in the genome [22, 167]. Our knowledge of the pathogenesis at the host and cellular level largely derives from infections in various laboratory animals, notably the mouse, and in vitro models.
5.1. Cellular Interactions
At the cellular level, the infection cycle is regulated by the synchronized operation of various virulence factors. The intracellular life cycle and the intercellular spread of LM has been intensively studied revealing its molecular adaptation to the intracellular microenvironment. The reader is, therefore, referred to various reviews for detailed information [17–19, 21, 168]. The invasion of the host cell is mediated by interaction between internalins, listeric surface ligands, and their respective host cell-receptors. A large number of internalins and internalin-like proteins have been identified by genome sequencing analysis of several LM strains [18, 169–171] and the diversity of internalins within the range of the different Listeria species and LM strains could possibly explain the variation in virulence and pathogenicity [172]. Amongst those, Internalin A and B (InlA, InlB) are the best studied and these have been detected only in LM so far [6, 173, 174]. The former interacts with E-cadherin, which is mostly expressed on epithelial cells in species-specific manner [175]. Notably, E-cadherin is expressed on cells of three host barriers that could determine the clinical syndromes: intestinal barrier, blood-brain barrier, and placental barrier [176–179]. In contrast, InlB promotes the invasion of a wide variety of mammalian cells through interaction with three receptors: Met, globular C1q receptor (gC1qR), and proteoglycans [180–183]. Recently, other internalins, notably internalin J, have been identified as key factors for virulence of LM [184–190]. There is recent evidence that entry of LM into the host cell requires additional factors such as clathrin-mediated endocytosis [23, 191, 192]. Once within the cell, LM is caught in a single-layer membrane phagocytic vacuole and has to transiently resist phagosomal killing [193, 194]. The bacterium escapes from the phagosome and moves into the host cell cytoplasm by employing a pore-forming toxin, listeriolysin-O (LLO), assisted by two phospholipases, phosphatidyl-inositol phospholipase C (PlcA) and phosphatidylcholine phospholipase C (PlcB) [195, 196]. Free in the cytoplasm, LM replicates rapidly [197, 198]. A surface protein of LM, Actin A (ActA), recruits host actin filaments and induces their polymerization to a so-called actin comet tail at one bacterial pole enabling the bacterium to move freely within the cytoplasm and to spread to neighboring cells by formation of cellular membrane protrusions that are engulfed by adjacent cells [17]. The resulting secondary double-membrane vacuole within the neighbor cell is lysed by PlcB and LLO and a new infection cycle starts over again [199]. It is believed that the direct intercellular spread permits the bacterium to multiply and diffuse within tissues protected from host defenses by avoiding the contact with the extracellular compartement.
5.2. Infectious Process In Vivo
Whilst the different steps of the intracellular infection cycle and key virulence factors involved are well known [6, 18, 19, 21, 200], the knowledge of the infectious process in vivo, notably in the natural host, is currently limited. It is generally believed that LM enters the host primarily through the intestine after oral intake of contaminated food. In rare cases, direct skin exposure to LM, for example, through contaminated abortive material, may lead to cutaneous infections [201, 202]. Several virulence factors enable LM to resist the exposure to a highly acidic environment, proteolytic enzymes, and bile salts during its gastroduodenal passage [44, 203–209]. Subsequently, LM crosses the intestinal barrier by actively adhering to and invading enterocytes through interactions between host-cell receptors and internalins, namely, InlA [178]. After intestinal translocation it invades the bloodstream and reaches liver and spleen (primary target organs) hematogeneously. There, resident hepatic and splenic macrophages kill the invading bacteria leading to control of the infection [210]. This initial step is thought to be subclinical and common due to the high prevalence of LM in food. In normal individuals, such exposure to listerial antigens probably contributes to the maintenance of memorial T-cells [211]. The unrestricted replication of LM in the primary target organs as it may occur in immunocompromized individuals may result in hematogenous dissemination to other organs and in overt clinical disease. LM has a predilection for the placenta and CNS (secondary target organs) that determines the main clinical syndromes. This predilection is believed to reflect the inherent ability of LM to cross the blood-brain and the placental barrier, likely by the interaction of bacterial internalins and their host cellular receptors [17, 21]. Recently, it has been shown that both InlA and B are required for the crossing of the placenta [177, 212]. In contrast, such an interaction remains to be shown to mediate the crossing of the blood-brain barrier.
Much of the information reviewed above has been derived from experimental work in laboratory rodents and cell cultures, whereas the role of LM virulence factors in its natural hosts is practically not known. InlA may play a key-role in human virulence as it is indicated by an epidemiological study, which detected a truncated form of InlA in 35% of LM food isolates versus only 4% of clinical isolates [213].
5.3. Genomic Organisation of Virulence Genes
The whole-genome sequences of LM and of the related nonpathogenic Listeria innocua and Listeria welshimeri have been determined, and their comparison has pioneered the identification of virulence factors of LM [169, 170, 214–216]. Two clusters of genes are required for the intracellular life-cycle of LM. The genes that encode the key virulence factors PlcA, LLO, ActA, and PlcB are clustered in a 10 kb virulence locus on the chromosome, the Listeria pathogenicity island 1 (LIPI-1), and are under the control of a transcriptional activator, the positive regulatory factor A (PrfA), [200, 217]. The latter itself is regulated by environmental conditions, namely, the temperature [218–220]. At mammalian host temperature (37°C), PrfA is translated and thus PrfA-dependent virulence genes are transcribed permitting LM to switch from an environmental bacterium into a intracellular pathogen. The second cluster consists of an operon with only two genes, inlA and inlB. Additionally to inlA and inlB, a high number of virulence genes encoding for internalin-like genes are scattered within the genome [171].
6. Neuropathogenesis
The means by which LM invades the brain have been subject of speculation for decades in both human and veterinary medicine [4, 14, 221]. From the pathological point of view, the variation of neuropathological patterns that are associated with CNS infection suggests strongly that the pathogen is able to invade the brain by both hematogenous spread or by migration along axons. However, the pathogenesis of both major manifestations of CNS infection—meningitis and rhombencephalitis—is largely unknown. Notably, the infectious dose required host and pathogen factors involved, particularly the role of LM virulence factors, reasons for the low attack rate, molecular mechanisms of brain invasion, and dynamics of the CNS infection including factors determining the outcome remain challenging.
6.1. Meningitis
The meningeal form with its diffuse distribution—as it occurs frequently in humans—is likely to be a result of hematogenous spread to the brain and crossing of the blood brain barrier. Indeed, in murine models of listeriosis bacteraemia is required for CNS invasion and lesions as well as bacteria are mainly observed in the meninges, choroid plexi and ependyme [222–225]. In vitro and in vivo experiments could show that LM is able to cross the blood-brain barrier by direct invasion of endothelial cells, cell-to-cell spread from infected phagocytes to endothelial cells, or by entry between endothelial cells within infected phagocytes [222, 226–232]. At present, the molecular mechanisms of breaching the blood-brain barrier by LM are still virtually unknown. Given that the endothelium and the choroid plexus epithelium of the blood-brain barrier may express E-cadherin [179, 233–235], some authors suggest that a receptor interaction between the cellular E-cadherin and bacterial internalin A might be the underlying mechanism of blood-brain-barrier crossing, similar to what happens at the intestinal and placental barrier [17, 21, 177, 212]. Epidemiologic data in humans do not indicate that InlA contributes to neuroinvasion from the bloodstream [213]. Two further virulence genes have been proposed to play a role in CNS infection based on investigations of mutant strains in the mouse model, plcB and gtcA, a gene that mediates teichoic acid glycolisation. PlcB is not indispensable, since plcB-negative mutants were able to cause delayed encephalitis in the mouse-model [31]. Mutations involving the gtcA gene that encodes putative cell wall components caused attenuated growth of LM in the brain of mice, and thus the authors speculate that these mutations caused a lower efficiency in the passage of the blood-brain-barrier [32].
6.2. Rhombencephalitis in the Natural Host
In humans, rhombencephalitis occurs in up to 24% of listeriosis patients [13, 80–83]. Both distribution and nature of the lesions are very similar in listeric rhombencephalitis of people and ruminants [87, 165]. However, despite the significant losses in livestock industry due to listeriosis and the growing impact of this zoonosis in ruminants and humans [8, 9, 50], surprisingly few studies have been focused on the pathogenesis of encephalitic disease in its natural hosts in the last decades [33, 165, 236]. This is in contrast to the large number of studies on listeric CNS disease in mice and rats, which are not naturally susceptible to LM infection due to species-specific properties of E-cadherin that functions as a receptor for internalin A [31, 175, 222–225, 237–252]. Because experimental data and observations in natural disease diverge, the pathogenesis of listeric rhombencephalitis and particularly the mechanisms of brainstem predilection are still controversial [131, 135, 136, 166, 222, 253]. However, the neuropathological pattern of the natural disease and the observation of intraaxonal and intraneuronal bacteria (Figures 5 and 6) strongly suggest that foodborne LM cells invade the brainstem by axonal migration along various cranial nerves [87, 131, 136, 165, 254]. Correspondingly, in an outbreak of listeric myelitis in sheep ascending infection via the sensory nerves following dermatitis was suspected and subcutaneous injection of LM in the lumbar and thoracic regions may cause lumbar and thoracic myelitis in mice [163, 255]. These observations indicate that the site of bacterial invasion determines the topography of CNS lesions. Once in the brainstem, LM likely spreads further rostrally to higher brain centers and caudally to the spinal cord along axonal connections [165]. According to this view, isolation of the agent from the cerebrospinal fluid (CSF) in ruminant encephalitis usually fails indicating that it rarely enters the CSF during infection [137]. The intriguing topography of lesions that hit systematically and particularly the rhombencephalon is atypical for a bacterium. As a general rule, bacteria invade the brain hematogenously. During septicemia, bacteria may cross the blood-brain-barrier at the level of the microvasculature causing meningitis, choroiditis, and ependymitis. Alternatively, microorganisms may travel to the brain lodging in septic thromboemboli that get trapped within parenchymal vessels and produce disseminated suppurative lesions within the brain parenchyma that with time develop to abscesses [86]. In this context, it is fascinating that a second ubiquitous and facultatively intracellular bacterium, which is able to spread from cell to cell by actin-polymerization—Burkholderia pseudomallei—causes a brainstem encephalitis in men and animals akin to that of LM [256–261]. Therefore, it is thought that Burkholderia pseudomallei like LM probably moves to the brainstem by centripetal axonal migration. Furthermore, in the vast majority of cases LM is isolated from the brain, but not from other organs [131, 158, 262]. Taken together, these data do not support a hematogenous infection. LM rather enters submucosal nerve endings through mucosal injuries anywhere in the oropharyngeal and nasal cavity, lips, conjunctiva, or gut [135, 165]. It has been attempted to infect sheep using various inoculation routes including intramuscular, intradermal, subcutaneous, intracarotid, intracerebral, intravenous, oral, intraruminal, intravaginal, and conjunctival inoculation [133, 145–147, 152, 262–265]. However, typical lesions of rhombencephalitis could only rarely be reproduced, and clinical responses after the subcutaneous injection of LM in sheep and goats or after oral challenge are generally minimal [3, 133, 263, 265, 266]. In contrast, experimental exposure of injured oral mucosa to LM and intranerval inoculations may cause lesions in the brainstem of mice, sheep and goats reminiscent of the natural disease [135, 157, 159, 237, 241, 267, 268]. Myelitis, and radiculitis in sheep were produced by injecting LM into the left infraorbital nerve [269].
Figure 5.
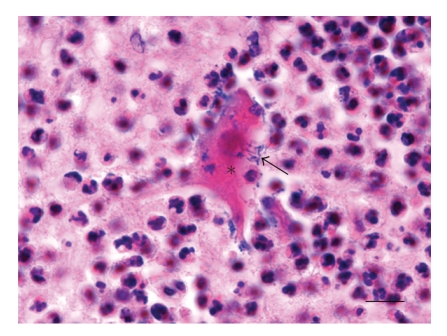
Rhombencephalitis in a sheep: necrotic neuron (asterisk) with intraneuronal Listeria monocytogenes (arrow) in a microabscess of the medulla oblongata. H&E, bar = 13 μm.
Figure 6.
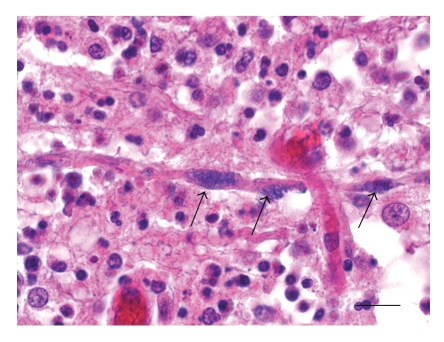
Rhombencephalitis in a sheep: intraaxonal Listeria monocytogenes (arrows) within a microabscess of the medulla oblongata. H&E, bar = 13 μm.
Some groups claim that hematogenous infection with LM leads to rhombencephalitis, and targeting of the brainstem is determined by its high microvascular density [166, 222, 244, 270]. However, lesions were frequently not restricted to rhombencephalitis, but included additional severe and diffuse choroiditis, meningitis and disseminated microabscesses in other brain areas, which is in contrast to the natural disease.
6.3. Rhombencephalitis in Experimental Laboratory Animals
Experimental research on listeric encephalitis, however, yielded contradictory results. The main obstacle for the study of listeric encephalitis is the lack of an animal or in vitro model that reflects the natural infection and consistently reproduces rhombencephalitis. Most experiments in laboratory rodents, notably the mouse, reflect unnatural infection routes by employing intracerebral or intravenous infection, as these species are highly resistant to lethal infection and CNS invasion following oral inoculations of LM, which imitate the natural route of infection, due to an amino acid mutation in E-cadherin, the cellular receptor for InlA [175]. Although an increasing number of studies aim towards natural routes of infection [176, 223], numerous studies still employ parenteral inoculations. This problem may be overcome in future with the use of the genetically engineered E16P knock-in mouse that expresses human E-cadherin in all tissues [271]. Animals inoculated intracerebrally or intravenously suffer a severe and diffuse meningoencephalitis and choroiditis, but not rhombencephalitis [31, 222–225, 245–247, 250, 251]. Differences in neuropathological expression of CNS listeriosis between laboratory animals and the natural host may not only reflect variation in exposure route but also species-specific anatomical and physiological differences of the brain. Furthermore, most experimental studies of encephalitis in laboratory animals and cell cultures have been carried out with the LM EGD strain and mutants, which are serotype 1/2a and may not reflect the virulence mechanisms of the other two clinically important serotypes 1/2b and notably 4b [31, 32, 222, 239, 241, 242, 270, 272]. Therefore, although laboratory animal models have contributed to the understanding of the pathogenesis in listeriosis, the results obtained in these models cannot be automatically extrapolated to humans and ruminants, since small rodents are not naturally susceptible to LM infection.
6.4. Mechanisms of Neural Spread
Although many data strongly indicate a local invasion via centripetal migration along axons, the mechanisms of this process are virtually unknown. The first riddle to solve is how LM is able to pass the mucosal barrier and enter the submucosal nerve endings. At present, it is thought that LM passes the mucosal epithelium of the upper gastrointestinal tract through small mucosal abrasions. There are two potential scenarios that may explain axonal invasion of cranial nerves: it may occur when LM surface proteins interact with a yet unidentified membrane receptor on the axonal surface or by cell-to-cell spread from infected macrophages in the submucosa [240]. The former hypothesis is supported by the apparently selective infection of neuronal populations in vitro [239]. Potential candidates would be InlA and InlB, which have both been shown to be required for the invasion of other cell types [176–178, 180–183, 212]. Interestingly, the cellular receptor of InlA, E-cadherin, is expressed in murine neuronal subpopulations such as sensory neurons of the trigeminal and dorsal root ganglion [273–275]. Most experimental data favor an axonal invasion via cell-to-cell spread from infected macrophages. In contrast to the observation of intraneuronal and intraaxonal LM during natural encephalitis, in vitro data of experimentally infected rat spinal and ovine brain cell cultures indicate that the bacterium rarely infects neurons [253, 276]. The infection rate increases when neurons are cocultivated with infected macrophages, indicating that LM infects neurons rather by cell-to-cell spread than by direct invasion through receptor interaction [276]. Further evidence for such a cell-to-cell spread of LM from macrophages comes from in vivo data in mice indicating that macrophages and dendritic cells facilitate neuroinvasion [272].
The affection of both motor and sensory nerves indicates that LM spreads by antero- and retrograde axonal migration to the neuronal bodies of brainstem and midbrain, likely by employing its actin tail as suggested by experimental data [87, 165, 239, 240]. Transganglionic migration within sensory nerves and further intracerebral spread between functionally connected neuronal cell populations likely occur via cell-to-cell spread. This would be in line with the importance of PlcB, a virulence factor promoting cell-to-cell spread, in the pathogenesis of experimental listeric meningoencephalitis [31].
It is important to note that much of the information reviewed above has been derived from experimental work in laboratory rodents and cell cultures. Thus, it remains to be demonstrated that these findings are applicable to the disease in its natural hosts.
7. Strain Variation in Relation to Neurovirulence in Humans and Ruminants
The species LM encompasses numerous strains and the genetic diversity amongst them is high [277]. Various strains have been implicated in both human and animal disease and it is not clear which LM subtypes in the environment cause illness. Thus, current surveillance schemes for foods are based on the assumption that all LM isolates are potentially pathogenic resulting in costly recalls in food industry. However, epidemiological studies conjoint with strain subtyping by diverse methods (such as serotyping or genomic approaches) suggest that there are, as yet, poorly understood interstrain differences in virulence and transmission [120, 278–283]. Therefore, research in recent years focused on the identification of molecular markers that determine the strain variation in virulence. Although progress has been made, the conundrum is only fragmentarily unraveled and with regard to the neurological disease it is virtually unknown whether neurotropic strains exist and what determines their propensity for neuroinvasion.
7.1. Serotypes
Although at least 13 serotypes are known, more than 95% of clinical isolates from human epidemics or sporadic cases belong to only three serotypes: 1/2a, 1/2b, and notably 4b, which are not the most common strains amongst environmental and food isolates [213, 279, 281, 284–287]. On the other hand, other serotypes are rarely responsible for human disease regardless of their common isolation from food or environmental specimens [277, 282, 288, 289]. However, food strain types and clinical strains partially overlap and key virulence genes are present in all serotypes [118, 290–293]. Food isolates, though, show more genetic diversity than clinical strains, suggesting that only certain food-derived strains may cause human infection [294]. The majority of LM strains that account for large but temporally and geographically unrelated outbreaks of food-borne listeriosis appear to form two epidemic clones in the serotype 4b, independently of the contaminated source involved [15, 279, 286, 295–297]. This serotype is also responsible for the majority of sporadic infections and is apparently overrepresented in pregnancy-associated cases and meningoencephalitis, whilst 1/2b has been primarily associated with nonpregnant individuals with severe underlying illness and HIV infections [279, 285, 298–301]. Taken together, these data suggest that serotype 4b is more virulent than other serotypes of LM. In line with these results, another study revealed a higher mortality rate in patients infected with strains of serogroup 4b as compared to other serotypes [302].
7.2. Genotypes
The application of genomic subtyping methods resulted in two major evolutionary lineages (lineages I and II) and one minor lineage (lineage III) of LM that apparently differ in host specifity and pathogenic potential [282, 283, 293, 297, 303–305]. Thereof lineage I is highly clonal and contains all 4b food-borne-epidemic isolates despite the different countries concerned and the food vehicles involved as well as additional isolates from sporadic cases. In contrast, lineage III contains no human clinical isolates. Lineage II shows a greater genetic diversity and contains clinical isolates but apparently no isolates from food-borne epidemics. One study that employed repetitive element sequence-based PCR could allocate food isolates in another genomic cluster than clinical isolates from human and animals [306].
7.3. Correlation of LM Strains with Pathogenicity
At present, it is not known though whether the observed divergence in subtype distribution between clinical and environmental isolates reflects potential variation in virulence or adaptation to particular ecological niches (e.g., food processing plants) enabling certain serotypes to contaminate food products at infectious levels [118]. One further explanation for the divergence would be a transmission route different than foodborne [289]. However, in either case a molecular basis for variations remains to be discovered. First steps have been done with the discovery of low-virulent strains that account for a significant proportion of environmental isolates [278, 307–312]. Mutations in their key virulence genes including inlA, inlB, plcB, prfA, hly (LLO-encoding gene), or actA have been detected [313–317]. However, the low virulence is mainly determined by point mutations in diverse virulence genes, which are impossible to detect by most subtyping methods [317]. Accordingly, attempts to use key virulence proteins and genes as targets for discrimination of virulent from avirulent LM strains generally failed because both proteins and genes were present in the entire strain population studied independently of their origin [282, 318–320]. An exception is the low virulence of some strains determined by a truncated form of InlA. An epidemiological study could show that the full-length form of InlA was expressed by 96% of clinical LM isolates versus 65% of food isolates [213, 313]. Internalin J (lmo 2821) is another virulence factor claimed to be a putative marker for differentiation between virulent and avirulent strains as it is invariably present in virulent strains [184, 185, 321].
As epidemiological data suggest interstrain variation in virulence, strong efforts have been made to develop in vitro tests and animal models that reflect the variation in virulence between clinical and environmental strains, though with inconsistent results. Although reproducible virulence differences were observed between LM strains in both cell cultures and animal models [278, 308–311, 322–326], not all studies found a correlation of the virulence in the laboratory with the serotype or the source (e.g., clinical isolate, food isolate) [309, 327]. However, several mouse studies observed a higher infectivity of serotypes 1/2a, 1/2b, 1/2c, and 4b strains than other serotypes [184, 328–330] and some in vitro studies observed virulence differences between clinical and food isolates [307, 326, 331], which is in line with the epidemiological observations.
7.4. L. monocytogenes Strains in Farm Animals
Although little is known about the distribution of clinical LM strains in animals, there is some epidemiological evidence that—like in humans—interstrain differences in virulence and organ tropism exist. In farm ruminants, the different clinical forms rarely overlap in the same herd, and visceral and cerebral listeriosis only exceptionally occur simultaneously in the same animal [126, 282, 332]. Furthermore, LM may cause encephalitis in pregnant ruminants without inducing abortion [262, 333] (own observations). However, the significance of divergent LM strains in the pathogenesis of ruminant listeriosis is not well known. As in humans, serovars 1/2a, 1/2b, and 4b appear to be the most commonly isolated LM strains in ruminants and ruminant LM isolates belong to all three identified evolutionary lineages [162, 282].
7.5. Factors of Neurovirulence
Whilst CNS infection is substantially responsible for the high mortality in both human and ruminant listeriosis, the identification of neurovirulence factors has not received much attention. The particular neuropathological pattern of rhombencephalitis and the absence of other organs involvement in previously healthy patients and ruminants suggests that LM strains with neurotropism exist. But with regard to molecular markers that determine strain variation in neuroinvasion and neurovirulence research is in the dark. A major handicap is the lack of an adequate animal or in vitro model to define and measure neurovirulence.
Older publications describe that in natural ruminant encephalitis either serotype 1 or 4b prevails depending on the geographical area [158, 334]. In experimental infection of sheep, one serotype 4 isolate showed high neurovirulence, whilst serotype 1 did not induce rhombencephalitis [157]. Similar results have been reported by other authors [267, 268, 335, 336]. In this context, it is worthy of note that occasionally different subtypes of LM may be isolated from clinically affected animals during an outbreak [101, 110, 334]. Interestingly, a particular phage type of LM was associated with an unusually high incidence of rhombencephalitis during a Swiss outbreak of human listeriosis [156].
PlcB has been proposed as a virulence factor for encephalitis, but PlcB is not indispensable, since PlcB-negative mutants were able to cause delayed encephalitis in the mouse-model [31]. An epidemiological study of human clinical isolates could associate CNS infections with two particular ActA subtypes [287]. Wiedmann identified one particular LM ribotype that was strongly associated with encephalitis in cattle indicating that this ribotype might be a host-associated subtype [282], and a recent study suggests that lineage I strains may have neurotropism in cattle [337]. These authors observed that encephalitic strains in cattle posses a specific internalin profile (lacking inlF and inlG) and a specific actA type (lacking one actA proline-rich repeat) and therefore speculate that gene-loss events and deletions may be associated with virulence and tissue specificity of the different strains. However, the importance of these genes in neurovirulence and neuroinvasion, whether by hematogenous infection or axonal migration, is not known.
8. Are Ruminants a Zoonotic Reservoir for Human Rhombencephalitis Strains?
The link between ruminant and human listeriosis is not completely understood. Listeriosis is defined a zoonosis, but direct transmission between ruminants and humans rarely occurs and is in most cases associated with nonlife-threatening cutaneous infections through contact with infected cattle or after handling of abortive material [201, 202]. However, it appears reasonable to implicate ruminants as an important natural reservoir for strains causing human infections given that one epidemic clone responsible for a significant proportion of human epidemics has been frequently isolated from cases of ruminant listeriosis [118, 121, 282, 297, 338]. Furthermore, dairy farms are frequently contaminated with LM, particularly as compared to other environments, and its subtype populations in the farm environment encompass commonly strains that have been associated with human illness, whether sporadic or epidemic [48, 90, 92, 107, 119, 120, 128, 139, 291, 339–345]. Ruminants, particularly cattle, contribute to amplification and dispersal of LM into the farm environment [120]. The bacteria can be shed in the feces of clinically affected animals, but also healthy carriers [90, 92, 107, 119, 128, 130, 339, 346, 347]. Raw milk might contain LM either as a consequence of bacterial shedding in the milk or due to exogenous contamination from the dairy farm environment [291, 340, 348–355].
Human listeriosis is principally a food-borne infection and most reported outbreaks of listeriosis in men are attributed to the consumption of contaminated products of animal origin [15, 58, 339, 340, 356–362]. Transmission may occur indirectly through food products from infected animals or healthy carriers that are not processed before consumption as well as raw vegetables that are contaminated by LM containing manure [363]. Most foods of animal origin are treated by procedures that effectively kill LM in raw foods. Therefore, a possible means of transmission of LM strains from ruminants to humans is their introduction and establishment in food processing facilities, and their ability to produce biofilms and to adhere to inert surfaces may significantly contribute to the latter [46, 364, 365]. Supporting this hypothesis, one study identified several LM genotypes that contaminated both dairy-processing and farm environments [366].
Although all these results strongly implicate ruminants as a natural reservoir for LM and a source of human infections, at present, there are no data with regard to the extent of strain population overlap between human and ruminant rhombencephalitis. The identical neuropathology of listeric rhombencephalitis in humans and ruminants, however, indicates that neurotropic strains common to both hosts are responsible for the disease.
9. Conclusions
Rhombencephalitis is an apparently uncommon form of listeriosis in humans, but its prevalence is likely underestimated [13, 80–83]. In contrast, in ruminants it is the most common clinical expression of listeriosis and at the same time the most common CNS disorder [50]. The intriguing distribution and the nature of the lesions are very similar in listeric rhombencephalitis of people and ruminants [87, 165]. Furthermore, ruminants may shed high numbers of LM in their feces [90, 92, 107, 119, 128, 130, 339, 346, 347], and dairy farms are frequently contaminated [92, 120]. Until recently, it has been believed that all LM strains are potentially pathogenic. However, epidemiological evidence suggests that there are strain-specific variations in virulence and research has identified strain variation in respect to virulence in animal and cell culture models, although the results frequently do not correlate with epidemiological data. Taken together, these data indicate that neurotropic strains of LM common to both humans and ruminants might cause the rhombencephalitis and that ruminants and their close environment may be their natural reservoir. The identification of virulent strains causing rhombencephalitis and their differentiation from avirulent and low-virulent strains would help to implement effective control and prevention measures against LM. In the context of the reported increase of LM infections in humans [8–10] and the high prevalence of listeric rhombencephalitis in ruminants [50] there is an urgent need to study host and bacterial factors, which contribute to listeric rhombencephalitis, and notably the molecular mechanisms of neuroinvasion, which are poorly understood. Future research might focus on the identification of candidate bacterial proteins and the respective host cell receptors that determine host cell specificity and tissue tropism. The first steps have been done by identifying the key-players in the crossing of the intestinal and placental barrier. Now it is time to search for those that enable the agent to invade the brain.
Acknowledgment
This work was financed by the Swiss Federal Veterinary Office.
References
- 1.Dumont J, Cotoni L. Bacille semblable à celui du rouget du porc rencontré dans le L.C.R. d’un méningitique. Annales de l’Institut Pasteur. 1921;35:625–633. [Google Scholar]
- 2.Murray EGD, Webb AA, Swan MBR. A disease of rabbits characterized by a large mononuclear monocytosis caused by a hitherto undescribed bacillus Bacterium monocytogenes n. sp. Journal of Pathology & Bacteriology. 1926;29:407–439. [Google Scholar]
- 3.Gray ML, Killinger AH. Listeria monocytogenes and listeric infections. Bacteriological Reviews. 1966;30(2):309–382. doi: 10.1128/br.30.2.309-382.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Low JC, Donachie W. A review of Listeria monocytogenes and listeriosis. Veterinary Journal. 1997;153(1):9–29. doi: 10.1016/s1090-0233(97)80005-6. [DOI] [PubMed] [Google Scholar]
- 5.Schlech WF, III, Lavigne PM, Bortolussi RA, et al. Epidemic listeriosis—evidence for transmission by food. New England Journal of Medicine. 1983;308(4):203–206. doi: 10.1056/NEJM198301273080407. [DOI] [PubMed] [Google Scholar]
- 6.Vázquez-Boland JA, Kuhn M, Berche P, et al. Listeria pathogenesis and molecular virulence determinants. Clinical Microbiology Reviews. 2001;14(3):584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linnan MJ, Mascola L, Lou XD, et al. Epidemic listeriosis associated with Mexican-style cheese. New England Journal of Medicine. 1988;319(13):823–828. doi: 10.1056/NEJM198809293191303. [DOI] [PubMed] [Google Scholar]
- 8.de Valk H, Jacquet C, Goulet V, et al. Surveillance of Listeria infections in Europe. Euro Surveillance. 2005;10(10):251–255. [PubMed] [Google Scholar]
- 9.Goulet V, Hedberg C, Le Monnier A, de Valk H. Increasing incidence of listeriosis in France and other European countries. Emerging Infectious Diseases. 2008;14(5):734–740. doi: 10.3201/eid1405.071395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denny J, McLauchlin J. Human Listeria monocytogenes infections in Europe—an opportunity for improved European surveillance. Euro Surveillance. 2008;13(13, article 5):p. 8082. [PubMed] [Google Scholar]
- 11.Siegman-Igra Y, Levin R, Weinberger M, et al. Listeria monocytogenes infection in Israel and review of cases worldwide. Emerging Infectious Diseases. 2002;8(3):305–310. doi: 10.3201/eid0803.010195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Public health agency of Canada. Listeria monocytogenes outbreak. Anonymous, 2009, http://www.phac-aspc.gc.ca/alert-alerte/listeria/listeria_2009-eng.php.
- 13.Bartt R. Listeria and atypical presentations of Listeria in the central nervous system. Seminars in Neurology. 2000;20(3):361–373. doi: 10.1055/s-2000-9398. [DOI] [PubMed] [Google Scholar]
- 14.Drevets DA, Bronze MS. Listeria monocytogenes: epidemiology, human disease, and mechanisms of brain invasion. FEMS Immunology and Medical Microbiology. 2008;53(2):151–165. doi: 10.1111/j.1574-695X.2008.00404.x. [DOI] [PubMed] [Google Scholar]
- 15.Büla CJ, Bille J, Glauser MP. An epidemic of food-borne listeriosis in western Switzerland: description of 57 cases involving adults. Clinical Infectious Diseases. 1995;20(1):66–72. doi: 10.1093/clinids/20.1.66. [DOI] [PubMed] [Google Scholar]
- 16.Malinverni R, Bille J, Perret C, et al. Epidemic listeriosis. Report of 25 cases in 15 months at the Vaud University Hospital Center. Schweizerische Medizinische Wochenschrift. 1985;115(1):2–10. [PubMed] [Google Scholar]
- 17.Pizarro-Cerda J, Cossart P. Subversion of cellular functions by Listeria monocytogenes. Journal of Pathology. 2006;208(2):215–223. doi: 10.1002/path.1888. [DOI] [PubMed] [Google Scholar]
- 18.Hamon M, Bierne H, Cossart P. Listeria monocytogenes: a multifaceted model. Nature Reviews Microbiology. 2006;4(6):423–434. doi: 10.1038/nrmicro1413. [DOI] [PubMed] [Google Scholar]
- 19.Seveau S, Pizarro-Cerda J, Cossart P. Molecular mechanisms exploited by Listeria monocytogenes during host cell invasion. Microbes and Infection. 2007;9(10):1167–1175. doi: 10.1016/j.micinf.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Cossart P. Listeriology (1926–2007): the rise of a model pathogen. Microbes and Infection. 2007;9(10):1143–1146. doi: 10.1016/j.micinf.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Cossart P, Toledo-Arana A. Listeria monocytogenes, a unique model in infection biology: an overview. Microbes and Infection. 2008;10(9):1041–1050. doi: 10.1016/j.micinf.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 22.Toledo-Arana A, Dussurget O, Nikitas G, et al. The Listeria transcriptional landscape from saprophytism to virulence. Nature. 2009;459(7249):950–956. doi: 10.1038/nature08080. [DOI] [PubMed] [Google Scholar]
- 23.Mostowy S, Cossart P. Cytoskeleton rearrangements during Listeria infection: clathrin and septins as new players in the game. Cell Motility and the Cytoskeleton. 2009;66(10):816–823. doi: 10.1002/cm.20353. [DOI] [PubMed] [Google Scholar]
- 24.Flannagan RS, Cosio G, Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nature Reviews Microbiology. 2009;7(5):355–366. doi: 10.1038/nrmicro2128. [DOI] [PubMed] [Google Scholar]
- 25.Pamer EG. Immune responses to Listeria monocytogenes. Nature Reviews Immunology. 2004;4(10):812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 26.Wallecha A, Carroll KD, Maciag PC, Rivera S, Shahabi V, Paterson Y. Multiple effector mechanisms induced by recombinant Listeria monocytogenes anticancer immunotherapeutics. Advances in Applied Microbiology. 2009;66:1–27. doi: 10.1016/S0065-2164(08)00801-0. [DOI] [PubMed] [Google Scholar]
- 27.Jia Q, Lee B-Y, Clemens DL, Bowen RA, Horwitz MA. Recombinant attenuated Listeria monocytogenes vaccine expressing Francisella tularensis IglC induces protection in mice against aerosolized Type A F. tularensis. Vaccine. 2009;27(8):1216–1229. doi: 10.1016/j.vaccine.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brockstedt DG, Dubensky TW., Jr. Promises and challenges for the development of Listeria monocytogenes-based immunotherapies. Expert Review of Vaccines. 2008;7(7):1069–1084. doi: 10.1586/14760584.7.7.1069. [DOI] [PubMed] [Google Scholar]
- 29.Li Z, Zhang M, Zhou C, Zhao X, Iijima N, Frankel FR. Novel vaccination protocol with two live mucosal vectors elicits strong cell-mediated immunity in the vagina and protects against vaginal virus challenge. Journal of Immunology. 2008;180(4):2504–2513. doi: 10.4049/jimmunol.180.4.2504. [DOI] [PubMed] [Google Scholar]
- 30.Orr MT, Orgun NN, Wilson CB, Way SS. Cutting edge: recombinant Listeria monocytogenes expressing a single immune-dominant peptide confers protective immunity to herpes simplex virus-1 infection. Journal of Immunology. 2007;178(8):4731–4735. doi: 10.4049/jimmunol.178.8.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlüter D, Domann E, Buck C, et al. Phosphatidylcholine-specific phospholipase C from Listeria monocytogenes is an important virulence factor in murine cerebral listeriosis. Infection and Immunity. 1998;66(12):5930–5938. doi: 10.1128/iai.66.12.5930-5938.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Autret N, Dubail I, Trieu-Cuot P, Berche P, Charbit A. Identification of new genes involved in the virulence of Listeria monocytogenes by signature-tagged transposon mutagenesis. Infection and Immunity. 2001;69(4):2054–2065. doi: 10.1128/IAI.69.4.2054-2065.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pohl MA, Wiedmann M, Nightingale KK. Associations among Listeria monocytogenes genotypes and distinct clinical manifestations of listeriosis in cattle. American Journal of Veterinary Research. 2006;67(4):616–626. doi: 10.2460/ajvr.67.4.616. [DOI] [PubMed] [Google Scholar]
- 34.McLauchlin J, Jones D. Erysipelothrix and Listeria. In: Borellio SP, Duerden BI, editors. Topley and Wilson’s Microbiology and Microbial Infections. 9th edition. chapter 30. Vol. 2. London, UK: Arnold; 1999. pp. 683–708. [Google Scholar]
- 35.Domínguez-Bernal G, Müller-Altrock S, González-Zorn B, et al. A spontaneous genomic deletion in Listeria ivanovii identifies LIPI-2, a species-specific pathogenicity island encoding sphingomyelinase and numerous internalins. Molecular Microbiology. 2006;59(2):415–432. doi: 10.1111/j.1365-2958.2005.04955.x. [DOI] [PubMed] [Google Scholar]
- 36.Schmid MW, Ng EYW, Lampidis R, et al. Evolutionary history of the genus Listeria and its virulence genes. Systematic and Applied Microbiology. 2005;28(1):1–18. doi: 10.1016/j.syapm.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Vazquez-Boland JA, Dominguez-Bernal G, Gonzalez-Zorn B, Kreft J, Goebel W. Pathogenicity islands and virulence evolution in Listeria. Microbes and Infection. 2001;3(7):571–584. doi: 10.1016/s1286-4579(01)01413-7. [DOI] [PubMed] [Google Scholar]
- 38.Rocourt J, Hof H, Schrettenbrunner A, Malinverni R, Bille J. Acute purulent meningitis due to Listeria seeligeri in an immunocompetent adult. Schweizerische Medizinische Wochenschrift. 1986;116(8):248–251. [PubMed] [Google Scholar]
- 39.Rapose A, Lick SD, Ismail N. Listeria grayi bacteremia in a heart transplant recipient. Transplant Infectious Disease. 2008;10(6):434–436. doi: 10.1111/j.1399-3062.2008.00333.x. [DOI] [PubMed] [Google Scholar]
- 40.Perrin M, Bemer M, Delamare C. Fatal case of Listeria innocua bacteremia. Journal of Clinical Microbiology. 2003;41(11):5308–5309. doi: 10.1128/JCM.41.11.5308-5309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwaiger K, Stierstorfer B, Schmahl W, Lehmann S, Gallien P, Bauer J. Survey on bacterial CNS infections in roe deer (Capreolus capreolus), red deer (Cervus elaphus) and chamois (Rupicapra rupicapra) in Bavaria. Berliner und Münchener Tierarztliche Wochenschrift. 2005;118(1-2):45–51. [PubMed] [Google Scholar]
- 42.Walker JK, Morgan JH, McLauchlin J, Grant KA, Shallcross JA. Listeria innocua isolated from a case of ovine meningoencephalitis. Veterinary Microbiology. 1994;42(2-3):245–253. doi: 10.1016/0378-1135(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 43.Mauder N, Ecke R, Mertins S, et al. Species-specific differences in the activity of PrfA, the key regulator of listerial virulence genes. Journal of Bacteriology. 2006;188(22):7941–7956. doi: 10.1128/JB.00473-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sleator RD, Gahan CGM, Hill C. A postgenomic appraisal of osmotolerance in Listeria monocytogenes. Applied and Environmental Microbiology. 2003;69(1):1–9. doi: 10.1128/AEM.69.1.1-9.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abram F, Starr E, Karatzas KAG, et al. Identification of components of the sigma B regulon in Listeria monocytogenes that contribute to acid and salt tolerance. Applied and Environmental Microbiology. 2008;74(22):6848–6858. doi: 10.1128/AEM.00442-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borucki MK, Peppin JD, White D, Loge F, Call DR. Variation in biofilm formation among strains of Listeria monocytogenes. Applied and Environmental Microbiology. 2003;69(12):7336–7342. doi: 10.1128/AEM.69.12.7336-7342.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rocourt J, Hogue A, Toyofuku H, Jacquet C, Schlundt J. Listeria and listeriosis: risk assessment as a new tool to unravel a multifaceted problem. American Journal of Infection Control. 2001;29(4):225–227. doi: 10.1067/mic.2001.115681. [DOI] [PubMed] [Google Scholar]
- 48.Roberts AJ, Wiedmann M. Pathogen, host and environmental factors contributing to the pathogenesis of listeriosis. Cellular and Molecular Life Sciences. 2003;60(5):904–918. doi: 10.1007/s00018-003-2225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McLauchlin J. Listeria monocytogenes, recent advances in the taxonomy and epidemiology of listeriosis in humans. Journal of Applied Bacteriology. 1987;63(1):1–11. doi: 10.1111/j.1365-2672.1987.tb02411.x. [DOI] [PubMed] [Google Scholar]
- 50.Oevermann A, Botteron C, Seuberlich T, et al. Neuropathological survey of fallen stock: active surveillance reveals high prevalence of encephalitic listeriosis in small ruminants. Veterinary Microbiology. 2008;130(3-4):320–329. doi: 10.1016/j.vetmic.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 51.Gillespie IA, McLauchlin J, Grant KA, et al. Changing pattern of human listeriosis, England and Wales, 2001–2004. Emerging Infectious Diseases. 2006;12(9):1361–1366. doi: 10.3201/eid1209.051657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gillespie IA, McLauchlin J, Little CL, et al. Disease presentation in relation to infection foci for non-pregnancy- associated human listeriosis in England and Wales, 2001 to 2007. Journal of Clinical Microbiology. 2009;47(10):3301–3307. doi: 10.1128/JCM.00969-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.The Community summary report on trends and sources of zoonoses and zoonotic agents in the European Union in 2007. The EFSA Journal. 2009;223:1–215. [Google Scholar]
- 54.Centers for Disease Control and Prevention (CDC) Outbreak of Listeria monocytogenes infections associated with pasteurized milk from a local dairy—Massachusetts, 2007. Morbidity and Mortality Weekly Report. 2008;57(40):1097–1100. [PubMed] [Google Scholar]
- 55.Vit M, Olejnik R, Dlhý J, et al. Outbreak of listeriosis in the Czech Republic, late 2006—preliminary report. Euro Surveillance. 2007;12(2) doi: 10.2807/esw.12.06.03132-en. Article ID E070208. [DOI] [PubMed] [Google Scholar]
- 56.Kathariou S, Graves L, Buchrieser C, Glaser P, Siletzky RM, Swaminathan B. Involvement of closely related strains of a new clonal group of Listeria monocytogenes in the 1998-1999 and 2002 multistate outbreaks of foodborne listeriosis in the United States. Foodborne Pathogens and Disease. 2006;3(3):292–302. doi: 10.1089/fpd.2006.3.292. [DOI] [PubMed] [Google Scholar]
- 57.Mead PS, Slutsker L, Dietz V, et al. Food-related illness and death in the United States. Emerging Infectious Diseases. 1999;5(5):607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bille J, Blanc DS, Schmid H, et al. Outbreak of human listeriosis associated with tomme cheese in northwest Switzerland, 2005. Euro Surveillance. 2006;11(6):91–93. [PubMed] [Google Scholar]
- 59.McLauchlin J. Human listeriosis in Britain, 1967–1985, a summary of 722 cases: 2. Listeriosis in non-pregnant individuals, a changing pattern of infection and seasonal incidence. Epidemiology and Infection. 1990;104(2):191–201. doi: 10.1017/s0950268800059355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paul ML, Dwyer DE, Chow C, et al. Listeriosis—a review of eighty-four cases. Medical Journal of Australia. 1994;160(8):489–493. [PubMed] [Google Scholar]
- 61.Cherubin CE, Appleman MD, Heseltine PNR, Khayr W, Stratton CW. Epidemiological spectrum and current treatment of listeriosis. Reviews of Infectious Diseases. 1991;13(6):1108–1114. doi: 10.1093/clinids/13.6.1108. [DOI] [PubMed] [Google Scholar]
- 62.Skogberg K, Syrjanen J, Jahkola M, et al. Clinical presentation and outcome of listeriosis in patients with and without immunosuppressive therapy. Clinical Infectious Diseases. 1992;14(4):815–821. doi: 10.1093/clinids/14.4.815. [DOI] [PubMed] [Google Scholar]
- 63.Jones EM, McCulloch SY, Reeves DS, MacGowan AP. A 10 year survey of the epidemiology and clinical aspects of listeriosis in a provincial English city. Journal of Infection. 1994;29(1):91–103. doi: 10.1016/s0163-4453(94)95249-3. [DOI] [PubMed] [Google Scholar]
- 64.Nolla-Salas J, Anto JM, Almela M, et al. Incidence of listeriosis in Barcelona, Spain, in 1990. The Collaborative Study Group of Listeriosis of Barcelona. European Journal of Clinical Microbiology and Infectious Diseases. 1993;12(3):157–161. doi: 10.1007/BF01967105. [DOI] [PubMed] [Google Scholar]
- 65.Gellin BG, Broome CV. Listeriosis. Journal of the American Medical Association. 1989;261(9):1313–1320. [PubMed] [Google Scholar]
- 66.Jackson TL, Eykyn SJ, Graham EM, Stanford MR. Endogenous bacterial endophthalmitis: a 17-year prospective series and review of 267 reported cases. Survey of Ophthalmology. 2003;48(4):403–423. doi: 10.1016/s0039-6257(03)00054-7. [DOI] [PubMed] [Google Scholar]
- 67.Kida K, Osada N, Isahaya K, et al. Listeria endocarditis with acute thoracoabdominal aortic dissection. Internal Medicine. 2007;46(15):1209–1212. doi: 10.2169/internalmedicine.46.6247. [DOI] [PubMed] [Google Scholar]
- 68.Jayaraj K, Di Bisceglie AM, Gibson S. Spontaneous bacterial peritonitis caused by infection with Listeria monocytogenes: a case report and review of the literature. American Journal of Gastroenterology. 1998;93(9):1556–1558. doi: 10.1111/j.1572-0241.1998.00482.x. [DOI] [PubMed] [Google Scholar]
- 69.Vargas V, Alemán C, De Torres I, et al. Listeria monocytogenes-associated acute hepatitis in a liver transplant recipient. Liver. 1998;18(3):213–215. doi: 10.1111/j.1600-0676.1998.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 70.Garcia-Montero M, Rodriguez-Garcia JL, Calyo P, et al. Pneumonia caused by Listeria monocytogenes. Respiration. 1995;62(2):107–109. doi: 10.1159/000196402. [DOI] [PubMed] [Google Scholar]
- 71.Mereghetti L, Marquet-van der Mee N, Laudat P, Loulergue J, Jeannou J, Audurier A. Listeria monocytogenes septic arthritis in a natural joint: report of a case and review. Clinical Microbiology and Infection. 1998;4(3):165–168. doi: 10.1111/j.1469-0691.1998.tb00383.x. [DOI] [PubMed] [Google Scholar]
- 72.Revathi G, Suneja A, Talwar V, Aggarwal N. Fatal pericarditis due to Listeria monocytogenes. European Journal of Clinical Microbiology and Infectious Diseases. 1995;14(3):254–255. doi: 10.1007/BF02310368. [DOI] [PubMed] [Google Scholar]
- 73.Durand ML, Calderwood SB, Weber DJ, et al. Acute bacterial meningitis in adults—a review of 493 episodes. New England Journal of Medicine. 1993;328(1):21–28. doi: 10.1056/NEJM199301073280104. [DOI] [PubMed] [Google Scholar]
- 74.Schuchat A, Robinson K, Wenger JD, et al. Bacterial meningitis in the United States in 1995. Active Surveillance Team. New England Journal of Medicine. 1997;337(14):970–976. doi: 10.1056/NEJM199710023371404. [DOI] [PubMed] [Google Scholar]
- 75.Sigurdardottir B, Bjornsson OM, Jonsdottir KE, Erlendsdottir H, Gudmundsson S. Acute bacterial meningitis in adults: a 20-year overview. Archives of Internal Medicine. 1997;157(4):425–430. doi: 10.1001/archinte.1997.00440250077009. [DOI] [PubMed] [Google Scholar]
- 76.Gellin BG, Broome CV, Bibb WF, Weaver RE, Gaventa S, Mascola L. The epidemiology of listeriosis in the United States—1986. Listeriosis Study Group. American Journal of Epidemiology. 1991;133(4):392–401. doi: 10.1093/oxfordjournals.aje.a115893. [DOI] [PubMed] [Google Scholar]
- 77.Goulet V, Marchetti P. Listeriosis in 225 non-pregnant patients in 1992: clinical aspects and outcome in relation to predisposing conditions. Scandinavian Journal of Infectious Diseases. 1996;28(4):367–374. doi: 10.3109/00365549609037921. [DOI] [PubMed] [Google Scholar]
- 78.Hussein AS, Shafran SD. Acute bacterial meningitis in adults: a 12-year review. Medicine. 2000;79(6):360–368. doi: 10.1097/00005792-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 79.Kyaw MH, Christie P, Jones IG, Campbell H. The changing epidemiology of bacterial meningitis and invasive non-meningitic bacterial disease in Scotland during the period 1983–1999. Scandinavian Journal of Infectious Diseases. 2002;34(4):289–298. doi: 10.1080/00365540110080403. [DOI] [PubMed] [Google Scholar]
- 80.Mylonakis E, Hohmann EL, Calderwood SB. Central nervous system infection with Listeria monocytogenes: 33 years’ experience at a general hospital and review of 776 episodes from the literature. Medicine. 1998;77(5):313–336. doi: 10.1097/00005792-199809000-00002. [DOI] [PubMed] [Google Scholar]
- 81.Antal E-A, Dietrichs E, Loberg EM, Melby KK, Maehlen J. Brain stem encephalitis in listeriosis. Scandinavian Journal of Infectious Diseases. 2005;37(3):190–194. doi: 10.1080/00365540410020938. [DOI] [PubMed] [Google Scholar]
- 82.Armstrong RW, Fung PC. Brainstem encephalitis (rhombencephalitis) due to Listeria monocytogenes: case report and review. Clinical Infectious Diseases. 1993;16(5):689–702. doi: 10.1093/clind/16.5.689. [DOI] [PubMed] [Google Scholar]
- 83.Pollock SS, Pollock TM, Harrison MJG. Infection of the central nervous system by Listeria monocytogenes: a review of 54 adult and juvenile cases. Quarterly Journal of Medicine. 1984;53(211):331–340. [PubMed] [Google Scholar]
- 84.Eck H. Encephalomyelitis listeriaca apostematosa. Schweizerische Medizinische Wochenschrift. 1957;87(9):210–214. [PubMed] [Google Scholar]
- 85.Uldry P-A, Kuntzer T, Bogousslavsky J, et al. Early symptoms and outcome of Listeria monocytogenes rhombencephalitis: 14 adult cases. Journal of Neurology. 1993;240(4):235–242. doi: 10.1007/BF00818711. [DOI] [PubMed] [Google Scholar]
- 86.Gray F, Alonso JM. Bacterial infections of the central nervous system. In: Graham DI, Lantos PL, editors. Greenfield’s Neuropathology. 7th edition. London, UK: Arnold; 2002. pp. 151–193. [Google Scholar]
- 87.Antal E-A, Loberg E-M, Dietrichs E, Maehlen J. Neuropathological findings in 9 cases of Listeria monocytogenes brain stem encephalitis. Brain Pathology. 2005;15(3):187–191. doi: 10.1111/j.1750-3639.2005.tb00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Crump JA, Griffin PM, Angulo FJ. Bacterial contamination of animal feed and its relationship to human foodborne illness. Clinical Infectious Diseases. 2002;35(7):859–865. doi: 10.1086/342885. [DOI] [PubMed] [Google Scholar]
- 89.Driehuis F, Oude Elferink SJWH. The impact of the quality of silage on animal health and food safety: a review. Veterinary Quarterly. 2000;22(4):212–216. doi: 10.1080/01652176.2000.9695061. [DOI] [PubMed] [Google Scholar]
- 90.Ueno H, Yokota K, Arai T, et al. The prevalence of Listeria monocytogenes in the environment of dairy farms. Microbiology and Immunology. 1996;40(2):121–124. doi: 10.1111/j.1348-0421.1996.tb03326.x. [DOI] [PubMed] [Google Scholar]
- 91.Yoshida T, Kato Y, Sato M, Hirai K. Sources and routes of contamination of raw milk with Listeria monocytogenes and its control. Journal of Veterinary Medical Science. 1998;60(10):1165–1168. doi: 10.1292/jvms.60.1165. [DOI] [PubMed] [Google Scholar]
- 92.Mohammed HO, Stipetic K, McDonough PL, Gonzalez RN, Nydam DV, Atwill ER. Identification of potential on-farm sources of Listeria monocytogenes in herds of dairy cattle. American Journal of Veterinary Research. 2009;70(3):383–388. doi: 10.2460/ajvr.70.3.383. [DOI] [PubMed] [Google Scholar]
- 93.Sanaa M, Poutrel B, Menard JL, Serieys F. Risk factors associated with contamination of raw milk by Listeria monocytogenes in dairy farms. Journal of Dairy Science. 1993;76(10):2891–2898. doi: 10.3168/jds.S0022-0302(93)77628-6. [DOI] [PubMed] [Google Scholar]
- 94.Gray ML. Isolation of Listeria monocytogenes from oat silage. Science. 1960;132(3441):1767–1768. doi: 10.1126/science.132.3441.1767. [DOI] [PubMed] [Google Scholar]
- 95.Gray ML. A possible link in the relationship between silage feeding and listeriosis. Journal of the American Veterinary Medical Association. 1960;136(5):205–208. [PubMed] [Google Scholar]
- 96.Fenlon DR. Wild birds and silage as reservoirs of Listeria in the agricultural environment. Journal of Applied Bacteriology. 1985;59(6):537–543. doi: 10.1111/j.1365-2672.1985.tb03357.x. [DOI] [PubMed] [Google Scholar]
- 97.Donald AS, Fenlon DR, Seddon B. The relationship between ecophysiology, indigenous microflora and growth of Listeria monocytogenes in grass silage. Journal of Applied Bacteriology. 1995;79(2):141–148. doi: 10.1111/j.1365-2672.1995.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 98.Vázquez-Boland JA, Dominguez L, Blanco M, et al. Epidemiologic investigation of a silage-associated epizootic of ovine listeric encephalitis, using a new Listeria-selective enumeration medium and phage typing. American Journal of Veterinary Research. 1992;53(3):368–371. [PubMed] [Google Scholar]
- 99.Ryser ET, Arimi SM, Donnelly CW. Effects of pH on distribution of Listeria ribotypes in corn, hay, and grass silage. Applied and Environmental Microbiology. 1997;63(9):3695–3697. doi: 10.1128/aem.63.9.3695-3697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vela AI, Fernandez-Garayzabal JF, Vazquez JA, et al. Molecular typing by pulsed-field gel electrophoresis of Spanish animal and human Listeria monocytogenes isolates. Applied and Environmental Microbiology. 2001;67(12):5840–5843. doi: 10.1128/AEM.67.12.5840-5843.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wiedmann M, Czajka J, Bsat N, et al. Diagnosis and epidemiological association of Listeria monocytogenes strains in two outbreaks of listerial encephalitis in small ruminants. Journal of Clinical Microbiology. 1994;32(4):991–996. doi: 10.1128/jcm.32.4.991-996.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wilesmith JW, Gitter M. Epidemiology of ovine listeriosis in Great Britain. Veterinary Record. 1986;119(19):467–470. doi: 10.1136/vr.119.19.467. [DOI] [PubMed] [Google Scholar]
- 103.Gronstol H. Listeriosis in sheep. Isolation of Listeria monocytogenes from grass silage. Acta Veterinaria Scandinavica. 1979;20(4):492–497. doi: 10.1186/BF03546576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wiedmann M, Arvik T, Bruce JL, et al. Investigation of a listeriosis epizootic in sheep in New York state. American Journal of Veterinary Research. 1997;58(7):733–737. [PubMed] [Google Scholar]
- 105.Low JC, Renton CP. Septicaemia, encephalitis and abortions in a housed flock of sheep caused by Listeria monocytogenes type 1/2. Veterinary Record. 1985;116(6):147–150. doi: 10.1136/vr.116.6.147. [DOI] [PubMed] [Google Scholar]
- 106.Fenlon DR, Wilson J, Donachie W. The incidence and level of Listeria monocytogenes contamination of food sources at primary production and initial processing. Journal of Applied Bacteriology. 1996;81(6):641–650. doi: 10.1111/j.1365-2672.1996.tb03559.x. [DOI] [PubMed] [Google Scholar]
- 107.Ho AJ, Ivanek R, Grohn YT, Nightingale KK, Wiedmann M. Listeria monocytogenes fecal shedding in dairy cattle shows high levels of day-to-day variation and includes outbreaks and sporadic cases of shedding of specific L. monocytogenes subtypes. Preventive Veterinary Medicine. 2007;80(4):287–305. doi: 10.1016/j.prevetmed.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 108.Fensterbank R, Audurier A, Godu J, Guerrault P, Malo N. Listeria strains isolated from sick animals and consumed silage. Annales de Recherches Veterinaires. 1984;15(1):113–118. [PubMed] [Google Scholar]
- 109.Baxter F, Wright F, Chalmers RM, Low JC, Donachie W. Characterization by multilocus enzyme electrophoresis of Listeria monocytogenes isolates involved in ovine listeriosis outbreaks in Scotland from 1989 to 1991. Applied and Environmental Microbiology. 1993;59(9):3126–3129. doi: 10.1128/aem.59.9.3126-3129.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wiedmann M, Mobini S, Cole JR, Jr., Watson CK, Jeffers GT, Boor KJ. Molecular investigation of a listeriosis outbreak in goats caused by an unusual strain of Listeria monocytogenes. Journal of the American Veterinary Medical Association. 1999;215(3):369–371. [PubMed] [Google Scholar]
- 111.Vandegraaff R, Borland NA, Browning JW. An outbreak of listerial meningo-encephalitis in sheep. Australian Veterinary Journal. 1981;57(2):94–96. doi: 10.1111/j.1751-0813.1981.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 112.Belchev L. Listeria enzootic in newborn lambs. Veterinarno-Meditsinski Nauki. 1979;16(3):57–62. [PubMed] [Google Scholar]
- 113.Kumar H, Singh BB, Bal MS, et al. Pathological and epidemiological investigations into listerial encephalitis in sheep. Small Ruminant Research. 2007;71(1–3):293–297. [Google Scholar]
- 114.Heim D, Fatzer R, Hornlimann B, Vandevelde M. Frequency of neurological diseases in cattle. Schweizer Archiv für Tierheilkunde. 1997;139(8):354–362. [PubMed] [Google Scholar]
- 115.Jeffrey M. A neuropathological survey of brains submitted under the Bovine Spongiform Encephalopathy Orders in Scotland. Veterinary Record. 1992;131(15):332–337. doi: 10.1136/vr.131.15.332. [DOI] [PubMed] [Google Scholar]
- 116.McGill IS, Wells GAH. Neuropathological findings in cattle with clinically suspect but histologically unconfirmed bovine spongiform encephalopathy (BSE) Journal of Comparative Pathology. 1993;108(3):241–260. doi: 10.1016/s0021-9975(08)80288-5. [DOI] [PubMed] [Google Scholar]
- 117.Miyashita M, Stierstorfer B, Schmahl W. Neuropathological findings in brains of Bavarian cattle clinically suspected of bovine spongiform encephalopathy. Journal of Veterinary Medicine Series B. 2004;51(5):209–215. doi: 10.1111/j.1439-0450.2004.00755.x. [DOI] [PubMed] [Google Scholar]
- 118.Boerlin P, Piffaretti J-C. Typing of human, animal, food, and environmental isolates of Listeria monocytogenes by multilocus enzyme electrophoresis. Applied and Environmental Microbiology. 1991;57(6):1624–1629. doi: 10.1128/aem.57.6.1624-1629.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Borucki MK, Reynolds J, Gay CC, et al. Dairy farm reservoir of Listeria monocytogenes sporadic and epidemic strains. Journal of Food Protection. 2004;67(11):2496–2499. doi: 10.4315/0362-028x-67.11.2496. [DOI] [PubMed] [Google Scholar]
- 120.Nightingale KK, Schukken YH, Nightingale CR, et al. Ecology and transmission of Listena monocytogenes infecting ruminants and in the farm environment. Applied and Environmental Microbiology. 2004;70(8):4458–4467. doi: 10.1128/AEM.70.8.4458-4467.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Okwumabua O, O’Connor M, Shull E, et al. Characterization of Listeria monocytogenes isolates from food animal clinical cases: PFGE pattern similarity to strains from human listeriosis cases. FEMS Microbiology Letters. 2005;249(2):275–281. doi: 10.1016/j.femsle.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 122.Gitter M. Listeriosis in farm animals in Great Britain. In: Collins CH, Grange JM, editors. Isolation and Identification of Microorganisms of Medical and Veterinary Importance. New York, NY, USA: Academic Press; 1985. pp. 191–200. [Google Scholar]
- 123.McDonald JW. An outbreak of abortion due to Listeria monocytogenes in an experimental flock of sheep. Australian Veterinary Journal. 1967;43(12):564–567. doi: 10.1111/j.1751-0813.1967.tb04803.x. [DOI] [PubMed] [Google Scholar]
- 124.Ladds PW, Dennis SM, Cooper RF. Sequential studies of experimentally induced ovine listerial abortion: clinical changes and bacteriologic examinations. American Journal of Veterinary Research. 1974;35(2):155–160. [PubMed] [Google Scholar]
- 125.Wardrope DD, MacLeod NS. Outbreak of Listeria meningoencephalitis in young lambs. Veterinary Record. 1983;113(10):213–214. doi: 10.1136/vr.113.10.213. [DOI] [PubMed] [Google Scholar]
- 126.Low JC, Wright F, McLauchlin J, Donachie W. Serotyping and distribution of Listeria isolates from cases of ovine listeriosis. Veterinary Record. 1993;133(7):165–166. doi: 10.1136/vr.133.7.165. [DOI] [PubMed] [Google Scholar]
- 127.Wagner M, Melzner D, Bagò Z, et al. Outbreak of clinical listeriosis in sheep: evaluation from possible contamination routes from feed to raw produce and humans. Journal of Veterinary Medicine Series B. 2005;52(6):278–283. doi: 10.1111/j.1439-0450.2005.00866.x. [DOI] [PubMed] [Google Scholar]
- 128.Husu JR. Epidemiological studies on the occurrence of Listeria monocytogenes in the feces of dairy cattle. Zentralblatt für Veterinarmedizin B. 1990;37(4):276–282. doi: 10.1111/j.1439-0450.1990.tb01059.x. [DOI] [PubMed] [Google Scholar]
- 129.Pell AN. Manure and microbes: public and animal health problem? Journal of Dairy Science. 1997;80(10):2673–2681. doi: 10.3168/jds.S0022-0302(97)76227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Skovgaard N, Morgen C-A. Detection of Listeria spp. in faeces from animals, in feeds, and in raw foods of animal origin. International Journal of Food Microbiology. 1988;6(3):229–242. doi: 10.1016/0168-1605(88)90015-3. [DOI] [PubMed] [Google Scholar]
- 131.Charlton KM, Garcia MM. Spontaneous listeric encephalitis and neuritis in sheep. Light microscopic studies. Veterinary Pathology. 1977;14(4):297–313. doi: 10.1177/030098587701400401. [DOI] [PubMed] [Google Scholar]
- 132.Gill DA. “Circling” disease of sheep in New Zealand. Veterinary Journal. 1931;87:60–74. [Google Scholar]
- 133.Gill DA. “Circling” disease: a meningoencephalitis of sheep in New Zealand. Veterinary Journal. 1933;89:258–270. [Google Scholar]
- 134.Gates GA, Blenden DC, Kintner LD. Listeric myelitis in sheep. Journal of the American Veterinary Medical Association. 1967;150(2):200–204. [PubMed] [Google Scholar]
- 135.Barlow RM, McGorum B. Ovine listerial encephalitis: analysis, hypothesis and synthesis. Veterinary Record. 1985;116(9):233–236. doi: 10.1136/vr.116.9.233. [DOI] [PubMed] [Google Scholar]
- 136.Otter A, Blakemore WF. Observation on the presence of Listeria monocytogenes in axons. Acta Microbiologica Hungarica. 1989;36(2-3):125–131. [PubMed] [Google Scholar]
- 137.Peters M, Pohlenz J, Jaton K, Ninet B, Bille J. Studies of the detection of Listeria monocytogenes by culture and PCR in cerebrospinal fluid samples from ruminants with listeric encephalitis. Zentralblatt für Veterinarmedizin B. 1995;42(2):84–88. doi: 10.1111/j.1439-0450.1995.tb00686.x. [DOI] [PubMed] [Google Scholar]
- 138.Johnson GC, Maddox CW, Fales WH, et al. Epidemiologic evaluation of encephalitic listeriosis in goats. Journal of the American Veterinary Medical Association. 1996;208(10):1695–1699. [PubMed] [Google Scholar]
- 139.Erdogan HM, Cetinkaya B, Green LE, Cripps PJ, Morgan KL. Prevalence, incidence, signs and treatment of clinical listeriosis in dairy cattle in England. Veterinary Record. 2001;149(10):289–293. doi: 10.1136/vr.149.10.289. [DOI] [PubMed] [Google Scholar]
- 140.Boerlin P, Boerlin-Petzold F, Jemmi T. Use of listeriolysin O and internalin A in a seroepidemiological study of listeriosis in Swiss dairy cows. Journal of Clinical Microbiology. 2003;41(3):1055–1061. doi: 10.1128/JCM.41.3.1055-1061.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gitter M, Terlecki S, Turnbull PA. An outbreak of visceral and cerebral listeriosis in a flock of sheep in south east England. The Veterinary Record. 1965;77(1):11–15. [PubMed] [Google Scholar]
- 142.Pallaske G. Über das Vorkommen einer seuchenhaften Encephalomyelitis purulenta bei Schafen in Deutschland (Listerella-Infektion) Berliner und Münchener Tierarztliche Wochenschrift. 1940;37:441–445. [Google Scholar]
- 143.Krüger W. Das Vorkommen von Listeria monocytogenes in den verschiedenen Silagen und dessen ätiologische Bedeutung. Archiv für Experimentelle Veterinärmedizin. 1963;17(1):181–203. [Google Scholar]
- 144.Gill DA. Ovine bacterial encephalitis (Circling disease) and the genus Listerella. Australian Veterinary Journal. 1937;13(2):46–56. [Google Scholar]
- 145.Biester HE, Schwartz LH. Studies on Listerella infection in sheep. The Journal of Infectious Diseases. 1939;64:135–144. [Google Scholar]
- 146.Olafson P. Listerella encephalitis (circling disease) of sheep, cattle and goats. Cornell Veterinarian. 1940;30(2):141–150. [Google Scholar]
- 147.Graham R, Dunlap GL, Brandly CA. Ovine and bovine listerellosis in Illinois. Science. 1938;88(2277):171–172. doi: 10.1126/science.88.2277.171. [DOI] [PubMed] [Google Scholar]
- 148.Brugère-Picoux J. Ovine listeriosis. Small Ruminant Research. 2008;76(1-2):12–20. [Google Scholar]
- 149.Gronstol H. Listeriosis in sheep. Experimental listeric infection in sheep treated with various immunosuppressiva. Acta Veterinaria Scandinavica. 1980;21(3):415–427. doi: 10.1186/BF03546874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gronstol H, Overas J. Listeriosis in sheep. Eperythrozoon ovis infection used as a model to study predisposing factors. Acta Veterinaria Scandinavica. 1980;21(4):523–532. doi: 10.1186/BF03546840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gronstol H, Overas J. Listeriosis in sheep. Tick-borne fever used as a model to study predisposing factors. Acta Veterinaria Scandinavica. 1980;21(4):533–545. doi: 10.1186/BF03546841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jakob W. Further experimental studies on the pathogenesis of cerebral listeriosis of the sheep. II. The course of the experimental infection with Listeria monocytogenes under various stress conditions. Archiv für Experimentelle Veterinarmedizin. 1967;21(3):675–684. [PubMed] [Google Scholar]
- 153.Jakob W. Untersuchungen zum Infektionsmodus und zur Pathogenese der Listeriose des Schafes. Archiv für Experimentelle Veterinärmedizin. 1963;17(5):1081–1128. [Google Scholar]
- 154.Olson C, Segre D. An agent enhancing listeriosis of sheep. American Journal of Veterinary Research. 1956;17(63):235–242. [PubMed] [Google Scholar]
- 155.C. Olson C, Grace OD, Segre D, Blore IC. Enhancement of listeriosis in sheep with material from bovine mucosal disease. American Journal of Veterinary Research. 1957;18:303–309. [PubMed] [Google Scholar]
- 156.Malinverni R, Glauser MP, Bille J, Rocourt J. Unusual clinical features of an epidemic of listeriosis associated with a particular phage type. European Journal of Clinical Microbiology. 1986;5(2):169–171. doi: 10.1007/BF02013979. [DOI] [PubMed] [Google Scholar]
- 157.Jakob W. Further experimental studies on the pathogenesis of cerebral listeriosis in sheep. 3. The course of the experimental infection with L. monocytogenes in sheep with high protein and low protein nutrition. Archiv für Experimentelle Veterinarmedizin. 1967;21(4):1061–1072. [PubMed] [Google Scholar]
- 158.Wuilleret A, Despres P, Monteiro L, Bouzakoura C, Wildi E. Bacteriological and histological diagnosis of listeriosis during an ovine enzootic. Schweizer Archiv für Tierheilkunde. 1969;111(11):622–641. [PubMed] [Google Scholar]
- 159.Akiyama Y, Asahi O, Hosoda T. Studies on the mechanism of infection of the brain with Listeria monocytogenes. American Journal of Veterinary Research. 1957;18(66):147–157. [PubMed] [Google Scholar]
- 160.Braun U, Stehle C, Ehrensperger F. Clinical findings and treatment of listeriosis in 67 sheep and goats. Veterinary Record. 2002;150(2):38–42. doi: 10.1136/vr.150.2.38. [DOI] [PubMed] [Google Scholar]
- 161.Schweizer G, Ehrensperger F, Torgerson PR, Braun U. Clinical findings and treatment of 94 cattle presumptively diagnosed with listeriosis. Veterinary Record. 2006;158(17):588–592. doi: 10.1136/vr.158.17.588. [DOI] [PubMed] [Google Scholar]
- 162.Low JC, Wright F, McLauchlin J, Donachie W. Serotyping and distribution of Listeria isolates from cases of ovine listeriosis. Veterinary Record. 1993;133(7):165–166. doi: 10.1136/vr.133.7.165. [DOI] [PubMed] [Google Scholar]
- 163.Seaman JT, Carter GI, Carrigan MJ, Cockram FA. An outbreak of listerial myelitis in sheep. Australian Veterinary Journal. 1990;67(4):142–143. doi: 10.1111/j.1751-0813.1990.tb07733.x. [DOI] [PubMed] [Google Scholar]
- 164.Schweizer G, Fuhrer B, Braun U. Signs of spinal cord disease in two heifers caused by Listeria monocytogenes. Veterinary Record. 2004;154(2):54–55. doi: 10.1136/vr.154.2.54. [DOI] [PubMed] [Google Scholar]
- 165.Oevermann A, Di PS, Doherr MG, et al. Neuropathogenesis of naturally occurring encephalitis caused by Listeria monocytogenes in ruminants. Brain Pathology. 2010;20(2):378–390. doi: 10.1111/j.1750-3639.2009.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cordy DR, Osebold JW. The neuropathogenesis of listeria encephalomyelitis in sheep and mice. The Journal of Infectious Diseases. 1959;104(2):164–173. doi: 10.1093/infdis/104.2.164. [DOI] [PubMed] [Google Scholar]
- 167.Camejo A, Buchrieser C, Couvé E, et al. In vivo transcriptional profiling of Listeria monocytogenes and mutagenesis identify new virulence factors involved in infection. PLoS Pathogens. 2009;5(5) doi: 10.1371/journal.ppat.1000449. Article ID e1000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cossart P, Sansonetti PJ. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science. 2004;304(5668):242–248. doi: 10.1126/science.1090124. [DOI] [PubMed] [Google Scholar]
- 169.Glaser P, Frangeul L, Buchrieser C, et al. Comparative genomics of Listeria species. Science. 2001;294(5543):849–852. doi: 10.1126/science.1063447. [DOI] [PubMed] [Google Scholar]
- 170.Nelson KE, Fouts DE, Mongodin EF, et al. Whole genome comparisons of serotype 4b and 1/2a strains of the food-borne pathogen Listeria monocytogenes reveal new insights into the core genome components of this species. Nucleic Acids Research. 2004;32(8):2386–2395. doi: 10.1093/nar/gkh562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bierne H, Sabet C, Personnic N, Cossart P. Internalins: a complex family of leucine-rich repeat-containing proteins in Listeria monocytogenes. Microbes and Infection. 2007;9(10):1156–1166. doi: 10.1016/j.micinf.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 172.Cabanes D, Dehoux P, Dussurget O, Frangeul L, Cossart P. Surface proteins and the pathogenic potential of Listeria monocytogenes. Trends in Microbiology. 2002;10(5):238–245. doi: 10.1016/s0966-842x(02)02342-9. [DOI] [PubMed] [Google Scholar]
- 173.Gaillard J-L, Berche P, Frehel C, Gouin E, Cossart P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell. 1991;65(7):1127–1141. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- 174.Dramsi S, Biswas I, Maguin E, Braun L, Mastroeni P, Cossart P. Entry of Listeria monocytogenes into hepatocytes requires expression of inIB, a surface protein of the internalin multigene family. Molecular Microbiology. 1995;16(2):251–261. doi: 10.1111/j.1365-2958.1995.tb02297.x. [DOI] [PubMed] [Google Scholar]
- 175.Lecuit M, Dramsi S, Gottardi C, Fedor-Chaiken M, Gumbiner B, Cossart P. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO Journal. 1999;18(14):3956–3963. doi: 10.1093/emboj/18.14.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lecuit M, Vandormael-Pournin S, Lefort J, et al. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science. 2001;292(5522):1722–1725. doi: 10.1126/science.1059852. [DOI] [PubMed] [Google Scholar]
- 177.Lecuit M, Nelson DM, Smith SD, et al. Targeting and crossing of the human maternofetal barrier by Listeria monocytogenes: role of internalin interaction with trophoblast E-cadherin. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(16):6152–6157. doi: 10.1073/pnas.0401434101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lecuit M. Understanding how Listeria monocytogenes targets and crosses host barriers. Clinical Microbiology and Infection. 2005;11(6):430–436. doi: 10.1111/j.1469-0691.2005.01146.x. [DOI] [PubMed] [Google Scholar]
- 179.Rubin LL, Hall DE, Porter S, et al. A cell culture model of the blood-brain barrier. Journal of Cell Biology. 1991;115(6):1725–1735. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bierne H, Cossart P. InIB, a surface protein of Listeria monocytogenes that behaves as an invasin and a growth factor. Journal of Cell Science. 2002;115(17):3357–3367. doi: 10.1242/jcs.115.17.3357. [DOI] [PubMed] [Google Scholar]
- 181.Jonquieres R, Pizarro-Cerda J, Cossart P. Synergy between the N- and C-terminal domains of InlB for efficient invasion of non-phagocytic cells by Listeria monocytogenes. Molecular Microbiology. 2001;42(4):955–965. doi: 10.1046/j.1365-2958.2001.02704.x. [DOI] [PubMed] [Google Scholar]
- 182.Braun L, Ghebrehiwet B, Cossart P. GC1q-R/p32, a C1q-binding protein, is a receptor for the InlB invasion protein of Listeria monocytogenes. EMBO Journal. 2000;19(7):1458–1466. doi: 10.1093/emboj/19.7.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shen Y, Naujokas M, Park M, Ireton K. InIB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell. 2000;103(3):501–510. doi: 10.1016/s0092-8674(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 184.Liu D, Ainsworth AJ, Austin FW, Lawrence ML. Characterization of virulent and avirulent Listeria monocytogenes strains by PCR amplification of putative transcriptional regulator and internalin genes. Journal of Medical Microbiology. 2003;52(12):1065–1070. doi: 10.1099/jmm.0.05358-0. [DOI] [PubMed] [Google Scholar]
- 185.Liu D, Lawrence ML, Austin FW, Ainsworth AJ. A multiplex PCR for species- and virulence-specific determination of Listeria monocytogenes. Journal of Microbiological Methods. 2007;71(2):133–140. doi: 10.1016/j.mimet.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 186.Sabet C, Toledo-Arana A, Personnic N, et al. The Listeria monocytogenes virulence factor InlJ is specifically expressed in vivo and behaves as an adhesin. Infection and Immunity. 2008;76(4):1368–1378. doi: 10.1128/IAI.01519-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sabet C, Lecuit M, Cabanes D, Cossart P, Bierne H. LPXTG protein InlJ, a newly identified internalin involved in Listeria monocytogenes virulence. Infection and Immunity. 2005;73(10):6912–6922. doi: 10.1128/IAI.73.10.6912-6922.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Engelbrecht F, Chun SK, Ochs C, et al. A new PrfA-regulated gene of Listeria monocytogenes encoding at small, secreted protein which belongs to the family of internalins. Molecular Microbiology. 1996;21(4):823–837. doi: 10.1046/j.1365-2958.1996.541414.x. [DOI] [PubMed] [Google Scholar]
- 189.Bergmann B, Raffelsbauer D, Kuhn M, et al. InlA- but not InlB-mediated internalization of Listeria monocytogenes by non-phagocytic mammalian cells needs the support of other internalins. Molecular Microbiology. 2002;43(3):557–570. doi: 10.1046/j.1365-2958.2002.02767.x. [DOI] [PubMed] [Google Scholar]
- 190.Raffelsbauer D, Bubert A, Engelbrecht F, et al. The gene cluster inlC2DE of Listeria monocytogenes contains additional new internalin genes and is important for virulence in mice. Molecular and General Genetics. 1998;260(2-3):144–158. doi: 10.1007/s004380050880. [DOI] [PubMed] [Google Scholar]
- 191.Veiga E, Cossart P. Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nature Cell Biology. 2005;7(9):894–900. doi: 10.1038/ncb1292. [DOI] [PubMed] [Google Scholar]
- 192.Veiga E, Guttman JA, Bonazzi M, et al. Invasive and adherent bacterial pathogens co-Opt host clathrin for infection. Cell Host and Microbe. 2007;2(5):340–351. doi: 10.1016/j.chom.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Archambaud C, Nahori MA, Pizarro-Cerda J, Cossart P, Dussurget O. Control of Listeria superoxide dismutase by phosphorylation. Journal of Biological Chemistry. 2006;281(42):31812–31822. doi: 10.1074/jbc.M606249200. [DOI] [PubMed] [Google Scholar]
- 194.Boneca IG, Dussurget O, Cabanes D, et al. A critical role for peptidoglycan N-deacetylation in Listeria evasion from the host innate immune system. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(3):997–1002. doi: 10.1073/pnas.0609672104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Portnoy DA, Chakraborty T, Goebel W, Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infection and Immunity. 1992;60(4):1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Schnupf P, Portnoy DA. Listeriolysin O: a phagosome-specific lysin. Microbes and Infection. 2007;9(10):1176–1187. doi: 10.1016/j.micinf.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 197.Chico-Calero I, Suárez M, González-Zorn B, et al. Hpt, a bacterial homolog of the microsomal glucose-6-phosphate translocase, mediates rapid intracellular proliferation in Listeria. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(1):431–436. doi: 10.1073/pnas.012363899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.O’Riordan M, Moors MA, Portnoy DA. Listeria intracellular growth and virulence require host-derived lipoic acid. Science. 2003;302(5644):462–464. doi: 10.1126/science.1088170. [DOI] [PubMed] [Google Scholar]
- 199.Vázquez-Boland JA, Kuhn M, Berche P, et al. Listeria pathogenesis and molecular virulence determinants. Clinical Microbiology Reviews. 2001;14(3):584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dussurget O, Pizarro-Cerda J, Cossart P. Molecular determinants of Listeria monocytogenes virulence. Annual Review of Microbiology. 2004;58:587–610. doi: 10.1146/annurev.micro.57.030502.090934. [DOI] [PubMed] [Google Scholar]
- 201.McLauchlin J, Low JC. Primary cutaneous listeriosis in adults: an occupational disease of veterinarians and farmers. Veterinary Record. 1994;135(26):615–617. [PubMed] [Google Scholar]
- 202.Regan EJ, Harrison GAJ, Butler S, et al. Primary cutaneous listeriosis in a veterinarian. Veterinary Record. 2005;157(7):p. 207. doi: 10.1136/vr.157.7.207. [DOI] [PubMed] [Google Scholar]
- 203.Cotter PD, Gahan CGM, Hill C. Analysis of the role of the Listeria monocytogenes F0F1-ATPase operon in the acid tolerance response. International Journal of Food Microbiology. 2000;60(2-3):137–146. doi: 10.1016/s0168-1605(00)00305-6. [DOI] [PubMed] [Google Scholar]
- 204.Cotter PD, Gahan CGM, Hill C. A glutamate decarboxylase system protects Listeria monocytogenes in gastric fluid. Molecular Microbiology. 2001;40(2):465–475. doi: 10.1046/j.1365-2958.2001.02398.x. [DOI] [PubMed] [Google Scholar]
- 205.Begley M, Sleator RD, Gahan CGM, Hill C. Contribution of three bile-associated loci, bsh, pva, and btlB, to gastrointestinal persistence and bile tolerance of Listeria monocytogenes. Infection and Immunity. 2005;73(2):894–904. doi: 10.1128/IAI.73.2.894-904.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Dussurget O, Cabanes D, Dehoux P, et al. Listeria monocytogenes bile salt hydrolase is a PrfA-regulated virulence factor involved in the intestinal and hepatic phases of listeriosis. Molecular Microbiology. 2002;45(4):1095–1106. doi: 10.1046/j.1365-2958.2002.03080.x. [DOI] [PubMed] [Google Scholar]
- 207.Sleator RD, Wemekamp-Kamphuis HH, Gahan CGM, Abee T, Hill C. A PrfA-regulated bile exclusion system (BilE) is a novel virulence factor in Listeria monocytogenes. Molecular Microbiology. 2005;55(4):1183–1195. doi: 10.1111/j.1365-2958.2004.04454.x. [DOI] [PubMed] [Google Scholar]
- 208.Hardy J, Francis KP, DeBoer M, et al. Extracellular replication of Listeria monocytogenes in the murine gall bladder. Science. 2004;303(5659):851–853. doi: 10.1126/science.1092712. [DOI] [PubMed] [Google Scholar]
- 209.Dussurget O. New insights into determinants of Listeria monocytogenes virulence. International Review of Cell and Molecular Biology. 2008;270:1–38. doi: 10.1016/S1937-6448(08)01401-9. [DOI] [PubMed] [Google Scholar]
- 210.Mackaness GB. Cellular resistance to infection. The Journal of Experimental Medicine. 1962;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Munk ME, Kaufmann HE. Listeria monocytogenes reactive T lymphocytes in healthy individuals. Microbial Pathogenesis. 1988;5(1):49–54. doi: 10.1016/0882-4010(88)90080-0. [DOI] [PubMed] [Google Scholar]
- 212.Disson O, Grayo S, Huillet E, et al. Conjugated action of two species-specific invasion proteins for fetoplacental listeriosis. Nature. 2008;455(7216):1114–1118. doi: 10.1038/nature07303. [DOI] [PubMed] [Google Scholar]
- 213.Jacquet C, Doumith M, Gordon JI, et al. A molecular marker for evaluating the pathogenic potential of foodborne Listeria monocytogenes. Journal of Infectious Diseases. 2004;189(11):2094–2100. doi: 10.1086/420853. [DOI] [PubMed] [Google Scholar]
- 214.Buchrieser C, Rusniok C, Kunst F, Cossart P, Glaser P. Comparison of the genome sequences of Listeria monocytogenes and Listeria innocua: clues for evolution and pathogenicity. FEMS Immunology and Medical Microbiology. 2003;35(3):207–213. doi: 10.1016/S0928-8244(02)00448-0. [DOI] [PubMed] [Google Scholar]
- 215.Buchrieser C. Biodiversity of the species Listeria monocytogenes and the genus Listeria. Microbes and Infection. 2007;9(10):1147–1155. doi: 10.1016/j.micinf.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 216.Hain T, Steinweg C, Kuenne CT, et al. Whole-genome sequence of Listeria welshimeri reveals common steps in genome reduction with Listeria innocua as compared to Listeria monocytogenes. Journal of Bacteriology. 2006;188(21):7405–7415. doi: 10.1128/JB.00758-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gouin E, Mengaud J, Cossart P. The virulence gene cluster of Listeria monocytogenes is also present in Listeria ivanovii, an animal pathogen, and Listeria seeligeri, a nonpathogenic species. Infection and Immunity. 1994;62(8):3550–3553. doi: 10.1128/iai.62.8.3550-3553.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Leimeister-Wachter M, Domann E, Chakraborty T. The expression of virulence genes in Listeria monocytogenes is thermoregulated. Journal of Bacteriology. 1992;174(3):947–952. doi: 10.1128/jb.174.3.947-952.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Johansson J, Mandin P, Renzoni A, et al. An RNA thermosensor controls expression of virulence genes in Listeria monocytogenes. Cell. 2002;110(5):551–561. doi: 10.1016/s0092-8674(02)00905-4. [DOI] [PubMed] [Google Scholar]
- 220.Renzoni A, Klarsfeld A, Dramsi S, Cossart P. Evidence that PrfA, the pleiotropic activator of virulence genes in Listeria monocytogenes, can be present but inactive. Infection and Immunity. 1997;65(4):1515–1518. doi: 10.1128/iai.65.4.1515-1518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Drevets DA, Leenen PJM, Greenfield RA. Invasion of the central nervous system by intracellular bacteria. Clinical Microbiology Reviews. 2004;17(2):323–347. doi: 10.1128/CMR.17.2.323-347.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Berche P. Bacteremia is required for invasion of the murine central nervous system by Listeria monocytogenes. Microbial Pathogenesis. 1995;18(5):323–336. doi: 10.1006/mpat.1995.0029. [DOI] [PubMed] [Google Scholar]
- 223.López S, Marco AJ, Prats N, Czuprynski CJ. Critical role of neutrophils in eliminating Listeria monocytogenes from the central nervous system during experimental murine listeriosis. Infection and Immunity. 2000;68(8):4789–4791. doi: 10.1128/iai.68.8.4789-4791.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.López S, Prats N, Marco AJ. Expression of E-selectin, P-selectin, and intercellular adhesion molecule-1 during experimental murine listeriosis. American Journal of Pathology. 1999;155(4):1391–1397. doi: 10.1016/S0002-9440(10)65241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Prats N, Briones V, Blanco MM, et al. Choroiditis and meningitis in experimental murine infection with Listeria monocytogenes. European Journal of Clinical Microbiology and Infectious Diseases. 1992;11(8):744–747. doi: 10.1007/BF01989983. [DOI] [PubMed] [Google Scholar]
- 226.Sibelius U, Chakraborty T, Krögel B, et al. The listerial exotoxins listeriolysin and phosphatidylinositol-specific phospholipase C synergize to elicit endothelial cell phosphoinositide metabolism. Journal of Immunology. 1996;157(9):4055–4060. [PubMed] [Google Scholar]
- 227.Sibelius U, Rose F, Chakraborty T, et al. Listeriolysin is a potent inducer of the phosphatidylinositol response and lipid mediator generation in human endothelial cells. Infection and Immunity. 1996;64(2):674–676. doi: 10.1128/iai.64.2.674-676.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Drevets DA, Sawyer RT, Potter TA, Campbell PA. Listeria monocytogenes infects human endothelial cells by two distinct mechanisms. Infection and Immunity. 1995;63(11):4268–4276. doi: 10.1128/iai.63.11.4268-4276.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Drevets DA. Dissemination of Listeria monocytogenes by infected phagocytes. Infection and Immunity. 1999;67(7):3512–3517. doi: 10.1128/iai.67.7.3512-3517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wilson SL, Drevets DA. Listeria monocytogenes infection and activation of human brain microvascular endothelial cells. Journal of Infectious Diseases. 1998;178(6):1658–1666. doi: 10.1086/314490. [DOI] [PubMed] [Google Scholar]
- 231.Drevets DA, Jelinek TA, Freitag NE. Listeria monocytogenes-infected phagocytes can initiate central nervous system infection in mice. Infection and Immunity. 2001;69(3):1344–1350. doi: 10.1128/IAI.69.3.1344-1350.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Greiffenberg L, Goebel W, Kim KS, et al. Interaction of Listeria monocytogenes with human brain microvascular endothelial cells: InlB-dependent invasion, long-term intracellular growth, and spread from macrophages to endothelial cells. Infection and Immunity. 1998;66(11):5260–5267. doi: 10.1128/iai.66.11.5260-5267.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Abbruscato TJ, Davis TP. Protein expression of brain endothelial cell E-cadherin after hypoxia/aglycemia: influence of astrocyte contact. Brain Research. 1999;842(2):277–286. doi: 10.1016/s0006-8993(99)01778-3. [DOI] [PubMed] [Google Scholar]
- 234.Pal D, Audus KL, Siahaan TJ. Modulation of cellular adhesion in bovine brain microvessel endothelial cells by a decapeptide. Brain Research. 1997;747(1):103–113. doi: 10.1016/s0006-8993(96)01223-1. [DOI] [PubMed] [Google Scholar]
- 235.Szmydynger-Chodobska J, Pascale CL, Pfeffer AN, Coulter C, Chodobski A. Expression of junctional proteins in choroid plexus epithelial cell lines: a comparative study. Cerebrospinal Fluid Research. 2007;4, article 11 doi: 10.1186/1743-8454-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Krueger N, Low C, Donachie W. Phenotypic characterization of the cells of the inflammatory response in ovine encephalitic listeriosis. Journal of Comparative Pathology. 1995;113(3):263–275. doi: 10.1016/s0021-9975(05)80041-6. [DOI] [PubMed] [Google Scholar]
- 237.Antal EA, Løberg EM, Bracht P, Melby KK, Mæhlen J. Evidence for intraaxonal spread of Listeria monocytogenes from the periphery to the central nervous system. Brain Pathology. 2001;11(4):432–438. doi: 10.1111/j.1750-3639.2001.tb00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Domann E, Deckert M, Schlüter D, Chakraborty T. Listeria monocytogenes: a model system to study invasion and spread of bacteria in the central nervous system. Current Topics in Microbiology and Immunology. 2002;265:213–226. doi: 10.1007/978-3-662-09525-6_11. [DOI] [PubMed] [Google Scholar]
- 239.Dons L, Weclewicz K, Jin Y, Bindseil E, Olsen JE, Kristensson K. Rat dorsal root ganglia neurons as a model for Listeria monocytogenes infections in culture. Medical Microbiology and Immunology. 1999;188(1):15–21. doi: 10.1007/s004300050100. [DOI] [PubMed] [Google Scholar]
- 240.Dons L, Jin Y, Kristensson K, Rottenberg ME. Axonal transport of Listeria monocytogenes and nerve-cell-induced bacterial killing. Journal of Neuroscience Research. 2007;85(12):2529–2537. doi: 10.1002/jnr.21256. [DOI] [PubMed] [Google Scholar]
- 241.Jin Y, Dons L, Kristensson K, Rottenberg ME. Neural route of cerebral Listeria monocytogenes murine infection: role of immune response mechanisms in controling bacterial neuroinvasion. Infection and Immunity. 2001;69(2):1093–1100. doi: 10.1128/IAI.69.2.1093-1100.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Jin Y, Lundkvist G, Dons L, Kristensson K, Rottenberg ME. Interferon-γ mediates neuronal killing of intracellular bacteria. Scandinavian Journal of Immunology. 2004;60(5):437–448. doi: 10.1111/j.0300-9475.2004.01500.x. [DOI] [PubMed] [Google Scholar]
- 243.Join-Lambert OF, Ezine S, Le Monnier A, et al. Listeria monocytogenes-infected bone marrow myeloid cells promote bacterial invasion of the central nervous system. Cellular Microbiology. 2005;7(2):167–180. doi: 10.1111/j.1462-5822.2004.00444.x. [DOI] [PubMed] [Google Scholar]
- 244.Altimira J, Prats N, López S, et al. Repeated oral dosing with Listeria monocytogenes in mice as a model of central nervous system listeriosis in man. Journal of Comparative Pathology. 1999;121(2):117–125. doi: 10.1053/jcpa.1999.0303. [DOI] [PubMed] [Google Scholar]
- 245.Schlüter D, Oprisiu SB, Chahoud S, et al. Systemic immunization induces protective CD4+ and CD8+ T cell-mediated immune responses in murine Listeria monocytogenes meningoencephalitis. European Journal of Immunology. 1995;25(8):2384–2391. doi: 10.1002/eji.1830250839. [DOI] [PubMed] [Google Scholar]
- 246.Schlüter D, Chahoud S, Lassmann H, Schumann A, Hof H, Deckert-Schlüter M. Intracerebral targets and immunomodulation of murine Listeria monocytogenes meningoencephalitis. Journal of Neuropathology and Experimental Neurology. 1996;55(1):14–24. doi: 10.1097/00005072-199601000-00002. [DOI] [PubMed] [Google Scholar]
- 247.Schlüter D, Buck C, Reiter S, et al. Immune reactions to Listeria monocytogenes in the brain. Immunobiology. 1999;201(2):188–195. doi: 10.1016/S0171-2985(99)80058-8. [DOI] [PubMed] [Google Scholar]
- 248.Deckert M, Soltek S, Geginat G, et al. Endogenous interleukin-10 is required for prevention of a hyperinflammatory intracerebral immune response in Listeria monocytogenes meningoencephalitis. Infection and Immunity. 2001;69(7):4561–4571. doi: 10.1128/IAI.69.7.4561-4571.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kwok L-Y, Miletic H, Lütjen S, et al. Protective immunosurveillance of the central nervous system by Listeria-specific CD4 and CD8 T cells in systemic listeriosis in the absence of intracerebral Listeria. Journal of Immunology. 2002;169(4):2010–2019. doi: 10.4049/jimmunol.169.4.2010. [DOI] [PubMed] [Google Scholar]
- 250.Virna S, Deckert M, Lütjen S, et al. TNF is important for pathogen control and limits brain damage in murine cerebral listeriosis. Journal of Immunology. 2006;177(6):3972–3982. doi: 10.4049/jimmunol.177.6.3972. [DOI] [PubMed] [Google Scholar]
- 251.Deckert M, Virna S, Sakowicz-Burkiewicz M, et al. Interleukin-1 receptor type 1 is essential for control of cerebral but not systemic listeriosis. American Journal of Pathology. 2007;170(3):990–1002. doi: 10.2353/ajpath.2007.060507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Montgomery DL, Storts RW. Listeria monocytogenes meningoencephalitis: murine models vs. the naturally occurring disease in ruminants. Journal of Neuropathology and Experimental Neurology. 1996;55(5):p. 603. [PubMed] [Google Scholar]
- 253.Peters M, Hewicker-Trautwein M. Studies on the cell tropism of Listeria monocytogenes in ovine fetal brain cell cultures. Veterinary Microbiology. 1996;49(3-4):169–179. doi: 10.1016/0378-1135(95)00185-9. [DOI] [PubMed] [Google Scholar]
- 254.Urbaneck D. Zur Neuropathogenese der Listeriose. Histologische Untersuchungen am N. trigeminus bei spontan an zerebraler Form der Listeriose erkrankten Schafen. Archiv für Experimentelle Veterinärmedizin. 1962;16:61–80. [Google Scholar]
- 255.Asahi O, Hosoda T, Akiyama Y. Studies on the relationship between neuropenetrability of L. monocytogenes and the mechanisms of immunity acquired by subcutaneous infection with its live culture. The Journal of Veterinary Medical Science. 1955;17(supplement):p. 17. [Google Scholar]
- 256.Currie BJ, Fisher DA, Howard DM, Burrow JNC. Neurological melioidosis. Acta Tropica. 2000;74(2-3):145–151. doi: 10.1016/s0001-706x(99)00064-9. [DOI] [PubMed] [Google Scholar]
- 257.Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clinical Microbiology Reviews. 2005;18(2):383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ladds PW, Thomas AD, Pott B. Melioidosis with acute meningoencephalomyelitis in a horse. Australian Veterinary Journal. 1981;57(1):36–38. doi: 10.1111/j.1751-0813.1981.tb07082.x. [DOI] [PubMed] [Google Scholar]
- 259.Wong KT, Puthucheary SD, Vadivelu J. The histopathology of human melioidosis. Histopathology. 1995;26(1):51–55. doi: 10.1111/j.1365-2559.1995.tb00620.x. [DOI] [PubMed] [Google Scholar]
- 260.Stevens JM, Galyov EE, Stevens MP. Actin-dependent movement of bacterial pathogens. Nature Reviews Microbiology. 2006;4(2):91–101. doi: 10.1038/nrmicro1320. [DOI] [PubMed] [Google Scholar]
- 261.Wiersinga WJ, van der Poll T, White NJ, Day NP, Peacock SJ. Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nature Reviews Microbiology. 2006;4(4):272–282. doi: 10.1038/nrmicro1385. [DOI] [PubMed] [Google Scholar]
- 262.Olson C, Rollins CL, Bagdonas V, Blore IC, Segre D. Distribution of Listeria monocytogenes in listeriosis of sheep. The Journal of Infectious Diseases. 1953;93(3):247–256. doi: 10.1093/infdis/93.3.247. [DOI] [PubMed] [Google Scholar]
- 263.Osebold JW, Inouye T. Pathogenesis of Listeria monocytogenes infections in natural hosts. II. Sheep studies. The Journal of Infectious Diseases. 1954;95(1):67–78. doi: 10.1093/infdis/95.1.67. [DOI] [PubMed] [Google Scholar]
- 264.Jakob W. Weitere experimentelle untersuchungen zur pathogenese der zerebralen Listeriose des Schafes. I. Mitteilung: pathologisch-anatomische Befunde nach experimenteller Infektion mit frisch isolierten Listerienstämmen. Archiv für Experimentelle Veterinarmedizin. 1966;20(2):367–381. [PubMed] [Google Scholar]
- 265.Low JC, Donachie W. Clinical and serum antibody responses of lambs to infection by Listeria monocytogenes. Research in Veterinary Science. 1991;51(2):185–192. doi: 10.1016/0034-5288(91)90012-d. [DOI] [PubMed] [Google Scholar]
- 266.Miettinen A, Husu J, Tuomi J. Serum antibody response to Listeria monocytogenes, listerial excretion, and clinical characteristics in experimentally infected goats. Journal of Clinical Microbiology. 1990;28(2):340–343. doi: 10.1128/jcm.28.2.340-343.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Schleicher J, Urbaneck D. Ein beitrag zur experimentellen Listeriose bei Haus- und versuchstieren. X. Mitteilung: experimentell erzeugte zerebrale Listeriose beim schaf durch neurale und paraneurale Injektionen mit geringen kulturdosen an zweigen des nervus trigeminus. Archiv für Experimentelle Veterinarmedizin. 1966;20(1):23–48. [PubMed] [Google Scholar]
- 268.Urbaneck D. Ein beitrag zur experimentellen Listeriose bei Haus- und versuchstieren. III. Mitteilung: versuche zur Klärung der neuropathogenese der zentralnervösen Listerioseform bei schafen durch applikation von listerien am N. trigeminus. Archiv für Experimentelle Veterinarmedizin. 1962;16:641–677. [Google Scholar]
- 269.Borman G, Olson C, Segre D. The trigeminal and facial nerves as pathways for infection of sheep with Listeria monocytogenes. American Journal of Veterinary Research. 1960;21:993–1000. [Google Scholar]
- 270.Blanot S, Joly MM, Vilde F, et al. A gerbil model for rhombencephalitis due to Listeria monocytogenes. Microbial Pathogenesis. 1997;23(1):39–48. doi: 10.1006/mpat.1997.0131. [DOI] [PubMed] [Google Scholar]
- 271.Disson O, Nikitas G, Grayo S, et al. Modeling human listeriosis in natural and genetically engineered animals. Nature Protocols. 2009;4(6):799–810. doi: 10.1038/nprot.2009.66. [DOI] [PubMed] [Google Scholar]
- 272.Jin Y, Dons L, Kristensson K, Rottenberg ME. Colony-stimulating factor 1-dependent cells protect against systemic infection with Listeria monocytogenes but facilitate neuroinvasion. Infection and Immunity. 2002;70(8):4682–4686. doi: 10.1128/IAI.70.8.4682-4686.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Takeichi M, Inuzuka H, Shimamura K, Matsunaga M, Nose A. Cadherin-mediated cell-cell adhesion and neurogenesis. Neuroscience Research. 1990;13(supplement):S92–S96. doi: 10.1016/0921-8696(90)90036-3. [DOI] [PubMed] [Google Scholar]
- 274.Shimamura K, Takeichi M. Local and transient expression of E-cadherin involved in mouse embryonic brain morphogenesis. Development. 1992;116(4):1011–1019. doi: 10.1242/dev.116.4.1011. [DOI] [PubMed] [Google Scholar]
- 275.Shimamura K, Takahashi T, Takeichi M. E-cadherin expression in a particular subset of sensory neurons. Developmental Biology. 1992;152(2):242–254. doi: 10.1016/0012-1606(92)90132-z. [DOI] [PubMed] [Google Scholar]
- 276.Dramsi S, Lévi S, Triller A, Cossart P. Entry of Listeria monocytogenes into neurons occurs by cell-to-cell spread: an in vitro study. Infection and Immunity. 1998;66(9):4461–4468. doi: 10.1128/iai.66.9.4461-4468.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Doumith M, Cazalet C, Simoes N, et al. New aspects regarding evolution and virulence of Listeria monocytogenes revealed by comparative genomics and DNA arrays. Infection and Immunity. 2004;72(2):1072–1083. doi: 10.1128/IAI.72.2.1072-1083.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Conner DE, Scott VN, Summer SS, Bernard DT. Pathogenicity of food-borne, environmental and clinical isolates of Listeria monocytogenes in mice. Journal of Food Protection. 1989;54:1553–1556. [Google Scholar]
- 279.McLauchlin J. Distribution of serovars of Listeria monocytogenes isolated from different categories of patients with listeriosis. European Journal of Clinical Microbiology and Infectious Diseases. 1990;9(3):210–213. doi: 10.1007/BF01963840. [DOI] [PubMed] [Google Scholar]
- 280.Brosch R, Catimel B, Milton G, et al. Virulence heterogeneity of Listeria monocytogenes strains from various sources (human food animal) in immunocompetent mice and its associated typing characteristics. Journal of Food Protection. 1993;56:296–301. doi: 10.4315/0362-028X-56.4.297. [DOI] [PubMed] [Google Scholar]
- 281.McLauchlin J. The pathogenicity of Listeria monocytogenes: a public health perspective. Reviews in Medical Microbiology. 1997;8(1):1–14. [Google Scholar]
- 282.Wiedmann M, Bruce JL, Keating C, Johnson AE, McDonough PL, Batt CA. Ribotypes and virulence gene polymorphisms suggest three distinct Listeria monocytogenes lineages with differences in pathogenic potential. Infection and Immunity. 1997;65(7):2707–2716. doi: 10.1128/iai.65.7.2707-2716.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Wiedmann M. Molecular subtyping methods for Listeria monocytogenes. Journal of AOAC International. 2002;85(2):524–531. [PubMed] [Google Scholar]
- 284.Davies JW, Ewan EP, Varughese P, Acres SE. Listeria monocytogenes infections in Canada. Clinical and Investigative Medicine. 1984;7(4):315–320. [PubMed] [Google Scholar]
- 285.Art D, André P. Clinical and epidemiological aspects of Listeriosis in Belgium, 1985–1990. Zentralblatt für Bakteriologie. 1991;275(4):549–556. doi: 10.1016/s0934-8840(11)80177-5. [DOI] [PubMed] [Google Scholar]
- 286.Schuchat A, Swaminathan B, Broome CV. Epidemiology of human listeriosis. Clinical Microbiology Reviews. 1991;4(2):169–183. doi: 10.1128/cmr.4.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Jacquet C, Gouin E, Jeannel D, Cossart P, Rocourt J. Expression of ActA, Ami, InlB, and listeriolysin O in Listeria monocytogenes of human and food origin. Applied and Environmental Microbiology. 2002;68(2):616–622. doi: 10.1128/AEM.68.2.616-622.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Gracieux P, Roche SM, Pardon P, Velge P. Hypovirulent Listeria monocytogenes strains are less frequently recovered than virulent strains on PALCAM and Rapid’ L. mono media. International Journal of Food Microbiology. 2003;83(2):133–145. doi: 10.1016/s0168-1605(02)00321-5. [DOI] [PubMed] [Google Scholar]
- 289.Kathariou S. Listeria monocytogenes virulence and pathogenicity, a food safety perspective. Journal of Food Protection. 2002;65(11):1811–1829. doi: 10.4315/0362-028x-65.11.1811. [DOI] [PubMed] [Google Scholar]
- 290.Fenlon DR, Stewart T, Donachie W. The incidence, numbers and types of Listeria monocytogenes isolated from farm bulk tank milks. Letters in Applied Microbiology. 1995;20(1):57–60. doi: 10.1111/j.1472-765x.1995.tb00407.x. [DOI] [PubMed] [Google Scholar]
- 291.Jensen NE, Aarestrup FM, Jensen J, Wegener HC. Listeria monocytogenes in bovine mastitis. Possible implication for human health. International Journal of Food Microbiology. 1996;32(1-2):209–216. doi: 10.1016/0168-1605(96)01105-1. [DOI] [PubMed] [Google Scholar]
- 292.Ryser ET, Arimi SM, Bunduki MM, Donnelly CW. Recovery of different Listeria ribotypes from naturally contaminated, raw refrigerated meat and poultry products with two primary enrichment media. Applied and Environmental Microbiology. 1996;62(5):1781–1787. doi: 10.1128/aem.62.5.1781-1787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Trott DJ, Robertson ID, Hampson DJ. Genetic characterisation of isolates of Listeria monocytogenes from man, animals and food. Journal of Medical Microbiology. 1993;38(2):122–128. doi: 10.1099/00222615-38-2-122. [DOI] [PubMed] [Google Scholar]
- 294.Bibb WF, Gellin BG, Weaver R, et al. Analysis of clinical and food-borne isolates of Listeria monocytogenes in the United States by multilocus enzyme electrophoresis and application of the method to epidemiologic investigations. Applied and Environmental Microbiology. 1990;56(7):2133–2141. doi: 10.1128/aem.56.7.2133-2141.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Buchrieser C, Brosch R, Catimel B, Rocourt J. Pulsed-field gel electrophoresis applied for comparing Listeria monocytogenes strains involved in outbreaks. Canadian Journal of Microbiology. 1993;39(4):395–401. doi: 10.1139/m93-058. [DOI] [PubMed] [Google Scholar]
- 296.Jacquet C, Catimel B, Brosch R, et al. Investigations related to the epidemic strain involved in the French listeriosis outbreak in 1992. Applied and Environmental Microbiology. 1995;61(6):2242–2246. doi: 10.1128/aem.61.6.2242-2246.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Piffaretti J-C, Kressebuch H, Aeschbacher M, et al. Genetic characterization of clones of the bacterium Listeria monocytogenes causing epidemic disease. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(10):3818–3822. doi: 10.1073/pnas.86.10.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Ewert DP, Lieb L, Hayes PS, Reeves MW, Mascola L. Listeria monocytogenes infection and serotype distribution among HIV- infected persons in Los Angeles County, 1985–1992. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 1995;8(5):461–465. doi: 10.1097/00042560-199504120-00005. [DOI] [PubMed] [Google Scholar]
- 299.Nolla-Salas J, Bosch J, Gasser I, et al. Perinatal listeriosis: a population based multicenter study in Barcelona, Spain (1990–1996) American Journal of Perinatology. 1998;15(8):461–467. doi: 10.1055/s-2007-994067. [DOI] [PubMed] [Google Scholar]
- 300.Swaminathan B, Gerner-Smidt P. The epidemiology of human listeriosis. Microbes and Infection. 2007;9(10):1236–1243. doi: 10.1016/j.micinf.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 301.Larsson S. Epidemiology of listeriosis in Sweden 1958–1974. Scandinavian Journal of Infectious Diseases. 1979;11(1):47–54. doi: 10.3109/inf.1979.11.issue-1.07. [DOI] [PubMed] [Google Scholar]
- 302.Gerner-Smidt P, Ethelberg S, Schiellerup P, et al. Invasive listeriosis in Denmark 1994–2003: a review of 299 cases with special emphasis on risk factors for mortality. Clinical Microbiology and Infection. 2005;11(8):618–624. doi: 10.1111/j.1469-0691.2005.01171.x. [DOI] [PubMed] [Google Scholar]
- 303.Rasmussen OF, Skouboe P, Dons L, Rossen L, Olsen JE. Listeria monocytogenes exists in at least three evolutionary lines: evidence from flagellin, invasive associated protein and listeriolysin O genes. Microbiology. 1995;141(9):2053–2061. doi: 10.1099/13500872-141-9-2053. [DOI] [PubMed] [Google Scholar]
- 304.Jeffers GT, Bruce JL, McDonough PL, et al. Comparative genetic characterization of Listeria monocytogenes isolates from human and animal listeriosis cases. Microbiology. 2001;147(5):1095–1104. doi: 10.1099/00221287-147-5-1095. [DOI] [PubMed] [Google Scholar]
- 305.Nightingale KK, Windham K, Wiedmann M. Evolution and molecular phylogeny of Listeria monocytogenes isolated from human and animal listeriosis cases and foods. Journal of Bacteriology. 2005;187(16):5537–5551. doi: 10.1128/JB.187.16.5537-5551.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Jeršek B, Gilot P, Gubina M, et al. Typing of Listeria monocytogenes strains by repetitive element sequence-based PCR. Journal of Clinical Microbiology. 1999;37(1):103–109. doi: 10.1128/jcm.37.1.103-109.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Nørrung B, Andersen JK. Variations in virulence between different electrophoretic types of Listeria monocytogenes. Letters in Applied Microbiology. 2000;30(3):228–232. doi: 10.1046/j.1472-765x.2000.00704.x. [DOI] [PubMed] [Google Scholar]
- 308.Roche SM, Velge P, Bottreau E, et al. Assessment of the virulence of Listeria monocytogenes: agreement between a plaque-forming assay with HT-29 cells and infection of immunocompetent mice. International Journal of Food Microbiology. 2001;68(1-2):33–44. doi: 10.1016/s0168-1605(01)00460-3. [DOI] [PubMed] [Google Scholar]
- 309.Pine L, Kathariou S, Quinn F, et al. Cytopathogenic effects in enterocytelike Caco-2 cells differentiate virulent from avirulent Listeria strains. Journal of Clinical Microbiology. 1991;29(5):990–996. doi: 10.1128/jcm.29.5.990-996.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Tabouret M, De Rycke J, Audurier A, Poutrel B. Pathogenicity of Listeria monocytogenes isolates in immunocompromised mice in relation to listeriolysin production. Journal of Medical Microbiology. 1991;34(1):13–18. doi: 10.1099/00222615-34-1-13. [DOI] [PubMed] [Google Scholar]
- 311.Van Langendonck N, Bottreau E, Bailly S, et al. Tissue culture assays using Caco-2 cell line differentiate virulent from non-virulent Listeria monocytogenes strains. Journal of Applied Microbiology. 1998;85(2):337–346. doi: 10.1046/j.1365-2672.1998.00515.x. [DOI] [PubMed] [Google Scholar]
- 312.Nightingale KK, Ivy RA, Ho AJ, et al. InlA premature stop codons are common among Listeria monocytogenes isolates from foods and yield virulence-attenuated strains that confer protection against fully virulent strains. Applied and Environmental Microbiology. 2008;74(21):6570–6583. doi: 10.1128/AEM.00997-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Jonquieres R, Bierne H, Mengaud J, Cossart P. The inlA gene of Listeria monocytogenes LO28 harbors a nonsense mutation resulting in release of internalin. Infection and Immunity. 1998;66(7):3420–3422. doi: 10.1128/iai.66.7.3420-3422.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Roberts A, Chan Y, Wiedmann M. Definition of genetically distinct attenuation mechanisms in naturally virulence-attenuated Listeria monocytogenes by comparative cell culture and molecular characterization. Applied and Environmental Microbiology. 2005;71(7):3900–3910. doi: 10.1128/AEM.71.7.3900-3910.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Roche SM, Gracieux P, Milohanic E, et al. Investigation of specific substitutions in virulence genes characterizing phenotypic groups of low-virulence field strains of Listeria monocytogenes. Applied and Environmental Microbiology. 2005;71(10):6039–6048. doi: 10.1128/AEM.71.10.6039-6048.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Velge P, Herler M, Johansson J, et al. A naturally occurring mutation K220T in the pleiotropic activator PrfA of Listeria monocytogenes results in a loss of virulence due to decreasing DNA-binding affinity. Microbiology. 2007;153, part 4:995–1005. doi: 10.1099/mic.0.2006/002238-0. [DOI] [PubMed] [Google Scholar]
- 317.Témoin S, Roche SM, Grépinet O, Fardini Y, Velge P. Multiple point mutations in virulence genes explain the low virulence of Listeria monocytogenes field strains. Microbiology. 2008;154(3):939–948. doi: 10.1099/mic.0.2007/011106-0. [DOI] [PubMed] [Google Scholar]
- 318.Nishibori T, Cooray K, Xiong H, et al. Correlation between the presence of virulence-associated genes as determined by PCR and actual virulence to mice in various strains of Listeria spp. Microbiology and Immunology. 1995;39(5):345–349. doi: 10.1111/j.1348-0421.1995.tb02211.x. [DOI] [PubMed] [Google Scholar]
- 319.Jaradat ZW, Schutze GE, Bhunia AK. Genetic homogeneity among Listeria monocytogenes strains from infected patients and meat products from two geographic locations determined by phenotyping, ribotyping and PCR analysis of virulence genes. International Journal of Food Microbiology. 2002;76(1-2):1–10. doi: 10.1016/s0168-1605(02)00050-8. [DOI] [PubMed] [Google Scholar]
- 320.Chiu S, Vanderlinde PB, Dykes GA. A comparison of selected methods for measuring the virulence properties of Listeria spp. Canadian Journal of Microbiology. 2006;52(4):301–307. doi: 10.1139/w05-129. [DOI] [PubMed] [Google Scholar]
- 321.Liu D, Lawrence ML, Gorski L, et al. Listeria monocytogenes serotype 4b strains belonging to lineages I and III possess distinct molecular features. Journal of Clinical Microbiology. 2006;44(1):214–217. doi: 10.1128/JCM.44.1.214-217.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Barbour AH, Rampling A, Hormaeche CE. Variation in the infectivity of Listeria monocytogenes isolates following intragastric inoculation of mice. Infection and Immunity. 2001;69(7):4657–4660. doi: 10.1128/IAI.69.7.4657-4660.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Lammerding AM, Glass KA, Gendron-Fitzpatrick A, Doyle MP. Determination of virulence of different strains of Listeria monocytogenes and Listeria innocua by oral inoculation of pregnant mice. Applied and Environmental Microbiology. 1992;58(12):3991–4000. doi: 10.1128/aem.58.12.3991-4000.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Roche SM, Gracieux P, Albert I, et al. Experimental validation of low virulence in field strains of Listeria monocytogenes. Infection and Immunity. 2003;71(6):3429–3436. doi: 10.1128/IAI.71.6.3429-3436.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Roche SM, Kerouanton A, Minet J, et al. Prevalence of low-virulence Listeria monocytogenes strains from different foods and environments. International Journal of Food Microbiology. 2009;130(2):151–155. doi: 10.1016/j.ijfoodmicro.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 326.Jensen A, Thomsen LE, Jørgensen RL, et al. Processing plant persistent strains of Listeria monocytogenes appear to have a lower virulence potential than clinical strains in selected virulence models. International Journal of Food Microbiology. 2008;123(3):254–261. doi: 10.1016/j.ijfoodmicro.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 327.Larsen CN, Nørrung B, Sommer HM, Jakobsen M. In vitro and in vivo invasiveness of different pulsed-field gel electrophoresis types of Listeria monocytogenes. Applied and Environmental Microbiology. 2002;68(11):5698–5703. doi: 10.1128/AEM.68.11.5698-5703.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Kim SH, Bakko MK, Knowles D, Borucki MK. Oral inoculation of A/J mice for detection of invasiveness differences between Listeria monocytogenes epidemic and environmental strains. Infection and Immunity. 2004;72(7):4318–4321. doi: 10.1128/IAI.72.7.4318-4321.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Liu D, Lawrence ML, Wiedmann M, et al. Listeria monocytogenes subgroups IIIA, IIIB, and IIIC delineate genetically distinct populations with varied pathogenic potential. Journal of Clinical Microbiology. 2006;44(11):4229–4233. doi: 10.1128/JCM.01032-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Liu D, Lawrence ML, Ainsworth AJ, Austin FW. Toward an improved laboratory definition of Listeria monocytogenes virulence. International Journal of Food Microbiology. 2007;118(2):101–115. doi: 10.1016/j.ijfoodmicro.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 331.Gudmundsdóttir S, Roche SM, Kristinsson KG, Kristjánsson M. Virulence of Listeria monocytogenes isolates from humans and smoked salmon, peeled shrimp, and their processing environments. Journal of Food Protection. 2006;69(9):2157–2160. doi: 10.4315/0362-028x-69.9.2157. [DOI] [PubMed] [Google Scholar]
- 332.Kidd AR, Terlecki S. Visceral and cerebral listeriosis in a lamb. Veterinary Record. 1966;78(13):453–454. doi: 10.1136/vr.78.13.453. [DOI] [PubMed] [Google Scholar]
- 333.Killinger AH, Mansfield ME. Epizootiology of listeric infection in sheep. Journal of the American Veterinary Medical Association. 1970;157(10):1318–1324. [PubMed] [Google Scholar]
- 334.Behrens H, Krüger A. Differentiation of listeria strains isolated in brain listeriosis of sheep. Berliner und Münchener Tierarztliche Wochenschrift. 1969;82(1):4–6. [PubMed] [Google Scholar]
- 335.Urbaneck D. Ein beitrag zur experimentellen listeriose bei Haus- und l aboratoriumstieren. IV. Mitteilung: versuche zur Klärung der zentralnervösen listerioseform bei schafen durch applikation von listerien im bereich der äusseren haut und verschiedener schleimhäute des Kopfes (paraneurale Infektion) Archiv für Experimentelle Veterinärmedizin. 1963;17:35–53. [Google Scholar]
- 336.Trautwein G, Behrens H. Histopathologie der Meningoencephalitis listeriosa des Schafes. Deutsche Tierarztliche Wochenschrift. 1962;69:149–155. [Google Scholar]
- 337.Pohl MA, Wiedmann M, Nightingale KK. Associations among Listeria monocytogenes genotypes and distinct clinical manifestations of listeriosis in cattle. American Journal of Veterinary Research. 2006;67(4):616–626. doi: 10.2460/ajvr.67.4.616. [DOI] [PubMed] [Google Scholar]
- 338.Nappi R, Bozzetta E, Serra R, et al. Molecular characterization of Listeria monocytogenes strains associated with outbreaks of listeriosis in humans and ruminants and food products by serotyping and automated ribotyping. Veterinary Research Communications. 2005;29(supplement 2):249–252. doi: 10.1007/s11259-005-0054-9. [DOI] [PubMed] [Google Scholar]
- 339.Unnerstad H, Romell A, Ericsson H, Danielsson-Tham ML, Tham W. Listeria monocytogenes in faeces from clinically healthy dairy cows in Sweden. Acta Veterinaria Scandinavica. 2000;41(2):167–171. doi: 10.1186/BF03549648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Van Kessel JS, Karns JS, Gorski L, McCluskey BJ, Perdue ML. Prevalence of Salmonellae, Listeria monocytogenes, and fecal coliforms in bulk tank milk on US dairies. Journal of Dairy Science. 2004;87(9):2822–2830. doi: 10.3168/jds.S0022-0302(04)73410-4. [DOI] [PubMed] [Google Scholar]
- 341.Husu JR, Seppanen JT, Sivela SK, Rauramaa AL. Contamination of raw milk by Listeria monocytogenes on dairy farms. Zentralblatt für Veterinarmedizin. Reihe B. 1990;37(4):268–275. doi: 10.1111/j.1439-0450.1990.tb01058.x. [DOI] [PubMed] [Google Scholar]
- 342.Borucki MK, Gay CC, Reynolds J, et al. Genetic diversity of Listeria monocytogenes strains from a high-prevalence dairy farm. Applied and Environmental Microbiology. 2005;71(10):5893–5899. doi: 10.1128/AEM.71.10.5893-5899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Hassan L, Mohammed HO, McDonough PL, Gonzalez RN. A cross-sectional study on the prevalence of Listeria monocytogenes and Salmonella in New York dairy herds. Journal of Dairy Science. 2000;83(11):2441–2447. doi: 10.3168/jds.S0022-0302(00)75135-6. [DOI] [PubMed] [Google Scholar]
- 344.Ho AJ, Lappi VR, Wiedmann M. Longitudinal monitoring of Listeria monocytogenes contamination patterns in a farmstead dairy processing facility. Journal of Dairy Science. 2007;90(5):2517–2524. doi: 10.3168/jds.2006-392. [DOI] [PubMed] [Google Scholar]
- 345.Garcia E, de Paz M, Rodriguez JL, et al. Exogenous sources of Listeria contamination in raw ewe’s milk. Journal of Food Protection. 1996;59(9):950–954. doi: 10.4315/0362-028X-59.9.950. [DOI] [PubMed] [Google Scholar]
- 346.Esteban JI, Oporto B, Aduriz G, Juste RA, Hurtado A. Faecal shedding and strain diversity of Listeria monocytogenes in healthy ruminants and swine in Northern Spain. BMC Veterinary Research. 2009;5, article 2 doi: 10.1186/1746-6148-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Zundel E, Bernard S. Listeria monocytogenes translocates throughout the digestive tract in asymptomatic sheep. Journal of Medical Microbiology. 2006;55(12):1717–1723. doi: 10.1099/jmm.0.46709-0. [DOI] [PubMed] [Google Scholar]
- 348.Muraoka W, Gay C, Knowles D, Borucki M. Prevalence of Listeria monocytogenes subtypes in bulk milk of the Pacific Northwest. Journal of Food Protection. 2003;66(8):1413–1419. doi: 10.4315/0362-028x-66.8.1413. [DOI] [PubMed] [Google Scholar]
- 349.Yoshida T, Sato M, Hirai K. Prevalence of Listeria species in raw milk from farm bulk tanks in Nagano prefecture. Journal of Veterinary Medical Science. 1998;60(3):311–314. doi: 10.1292/jvms.60.311. [DOI] [PubMed] [Google Scholar]
- 350.Waak E, Tham W, Danielsson-Tham ML. Prevalence and fingerprinting of Listeria monocytogenes strains isolated from raw whole milk in farm bulk tanks and in dairy plant receiving tanks. Applied and Environmental Microbiology. 2002;68(7):3366–3370. doi: 10.1128/AEM.68.7.3366-3370.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Jayarao BM, Donaldson SC, Straley BA, et al. A survey of foodborne pathogens in bulk tank milk and raw milk consumption among farm families in Pennsylvania. Journal of Dairy Science. 2006;89(7):2451–2458. doi: 10.3168/jds.S0022-0302(06)72318-9. [DOI] [PubMed] [Google Scholar]
- 352.Schoder D, Winter P, Kareem A, Baumgartner W, Wagner M. A case of sporadic ovine mastitis caused by Listeria monocytogenes and its effect on contamination of raw milk and raw-milk cheeses produced in the on-farm dairy. Journal of Dairy Research. 2003;70(4):395–401. doi: 10.1017/s0022029903006277. [DOI] [PubMed] [Google Scholar]
- 353.Eilertz I, Danielsson-Tham ML, Hammarberg KE, et al. Isolation of Listeria monocytogenes from goat cheese associated with a case of listeriosis in goat. Acta Veterinaria Scandinavica. 1993;34(2):145–149. doi: 10.1186/BF03548203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Winter P, Schilcher F, Bagò Z, et al. Clinical and histopathological aspects of naturally occurring mastitis caused by Listeria monocytogenes in cattle and ewes. Journal of Veterinary Medicine Series B. 2004;51(4):176–179. doi: 10.1111/j.1439-0450.2004.00751.x. [DOI] [PubMed] [Google Scholar]
- 355.Pintado CMBS, Grant KA, Halford-Maw R, et al. Association between a case study of asymptomatic ovine listerial mastitis and the contamination of soft cheese and cheese processing environment with Listeria monocytogenes in Portugal. Foodborne Pathogens and Disease. 2009;6(5):569–575. doi: 10.1089/fpd.2008.0246. [DOI] [PubMed] [Google Scholar]
- 356.Graves LM, Hunter SB, Ong AR, et al. Microbiological aspects of the investigation that traced the 1998 outbreak of listeriosis in the United States to contaminated hot dogs and establishment of molecular subtyping-based surveillance for Listeria monocytogenes in the PulseNet network. Journal of Clinical Microbiology. 2005;43(5):2350–2355. doi: 10.1128/JCM.43.5.2350-2355.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Olsen SJ, Patrick M, Hunter SB, et al. Multistate outbreak of Listeria monocytogenes infection linked to delicatessen turkey meat. Clinical Infectious Diseases. 2005;40(7):962–967. doi: 10.1086/428575. [DOI] [PubMed] [Google Scholar]
- 358.Mead PS, Dunne EF, Graves L, et al. Nationwide outbreak of listeriosis due to contaminated meat. Epidemiology and Infection. 2006;134(4):744–751. doi: 10.1017/S0950268805005376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Ericsson H, Eklöw A, Danielsson-Tham M-L, et al. An outbreak of listeriosis suspected to have been caused by rainbow trout. Journal of Clinical Microbiology. 1997;35(11):2904–2907. doi: 10.1128/jcm.35.11.2904-2907.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Makino S-I, Kawamoto K, Takeshi K, et al. An outbreak of food-borne listeriosis due to cheese in Japan, during 2001. International Journal of Food Microbiology. 2005;104(2):189–196. doi: 10.1016/j.ijfoodmicro.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 361.Carrique-Mas JJ, Hökeberg I, Andersson Y, et al. Febrile gastroenteritis after eating on-farm manufactured fresh cheese—an outbreak of listeriosis? Epidemiology and Infection. 2003;130(1):79–86. doi: 10.1017/s0950268802008014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Danielsson-Tham ML, Eriksson E, Helmersson S, et al. Causes behind a human cheese-borne outbreak of gastrointestinal listeriosis. Foodborne Pathogens and Disease. 2004;1(3):153–159. doi: 10.1089/fpd.2004.1.153. [DOI] [PubMed] [Google Scholar]
- 363.MacDonald PDM, Whitwam RE, Boggs JD, et al. Outbreak of listeriosis among Mexican immigrants as a result of consumption of illicitly produced Mexican-style cheese. Clinical Infectious Diseases. 2005;40(5):677–682. doi: 10.1086/427803. [DOI] [PubMed] [Google Scholar]
- 364.Tresse O, Shannon K, Pinon A, et al. Variable adhesion of Listeria monocytogenes isolates from food-processing facilities and clinical cases to inert surfaces. Journal of Food Protection. 2007;70(7):1569–1578. doi: 10.4315/0362-028x-70.7.1569. [DOI] [PubMed] [Google Scholar]
- 365.Lunden JM, Miettinen MK, Autio TJ, Korkeala HJ. Persistent Listeria monocytogenes strains show enhanced adherence to food contact surface after short contact times. Journal of Food Protection. 2000;63(9):1204–1207. doi: 10.4315/0362-028x-63.9.1204. [DOI] [PubMed] [Google Scholar]
- 366.Arimi SM, Pritchard TJ, Donnelly CW. Diversity of Listeria ribotypes recovered from dairy cattle, silage, and dairy processing environments. Journal of Food Protection. 1997;60(7):811–816. doi: 10.4315/0362-028X-60.7.811. [DOI] [PubMed] [Google Scholar]