Abstract
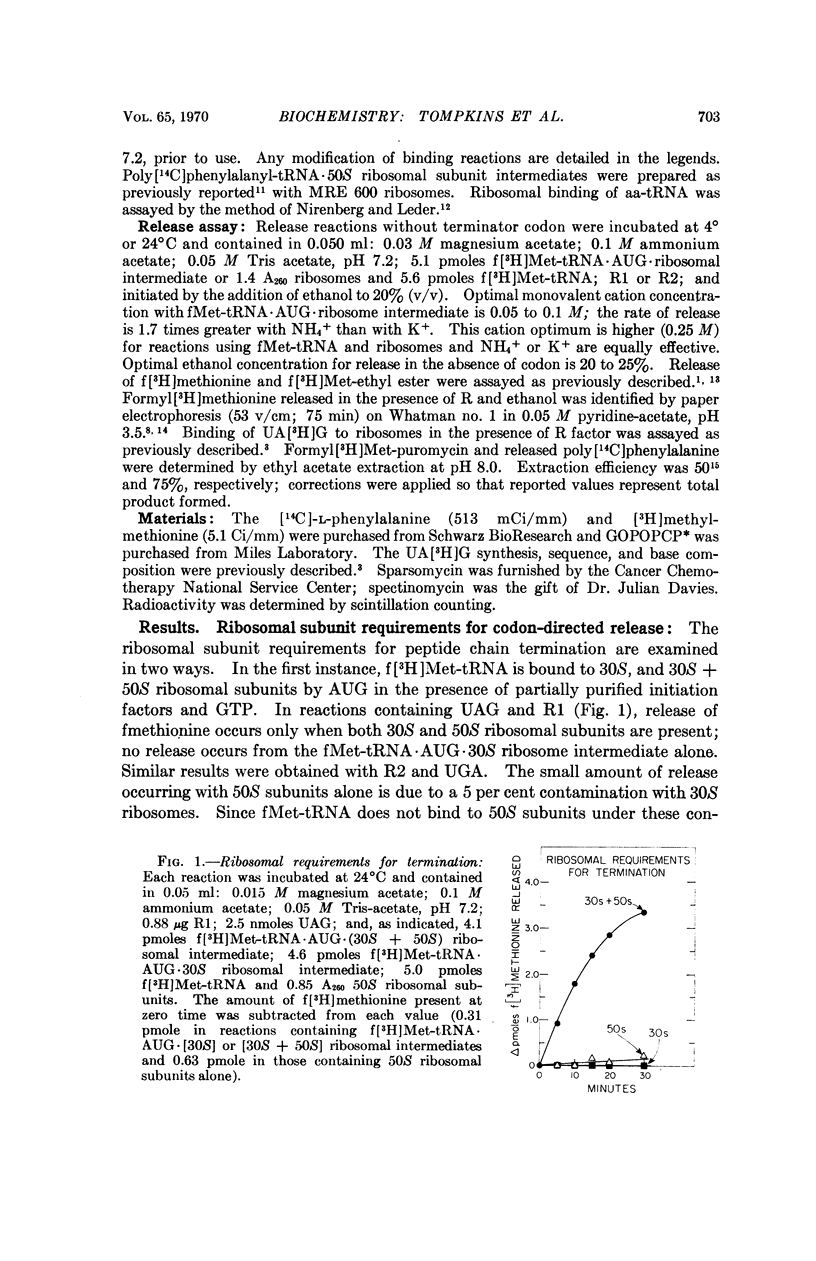
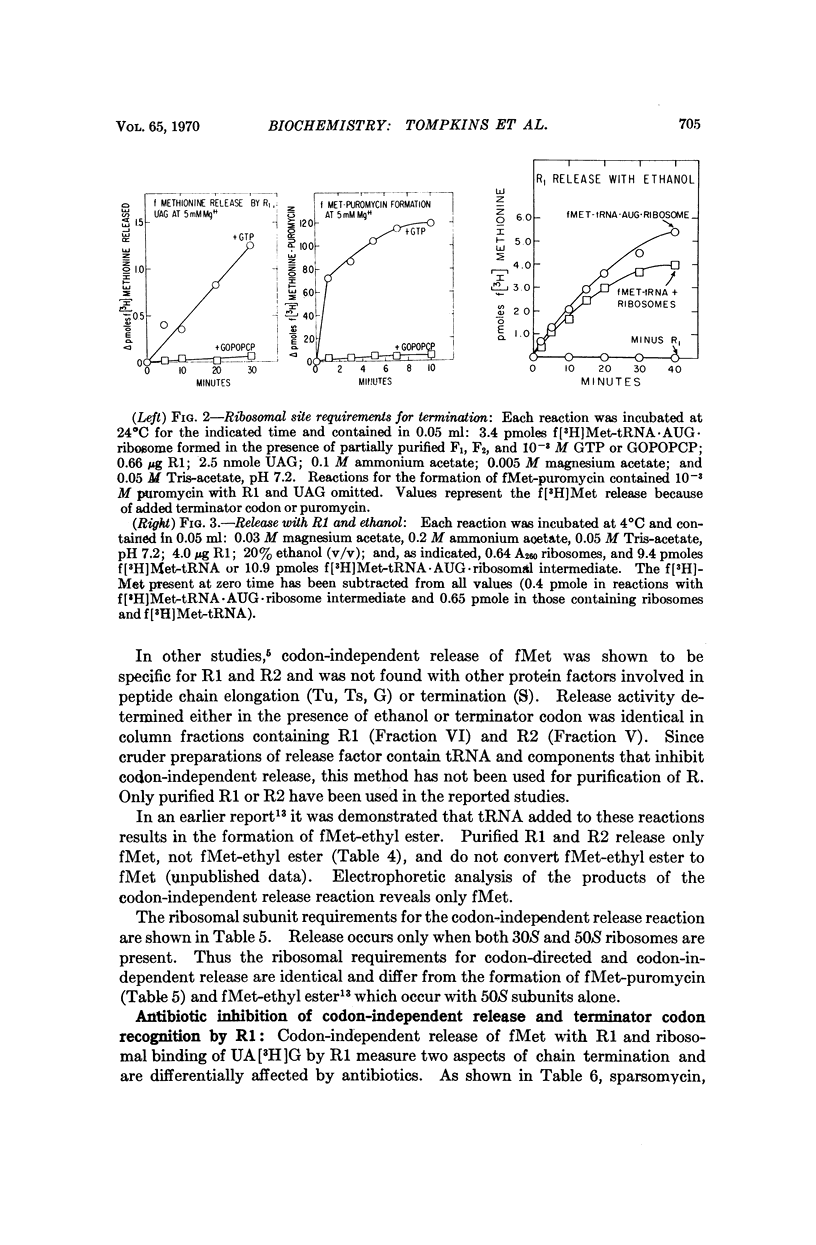
Release factors participate in release of fMet from fMet-tRNA · AUG · ribosome intermediates upon binding to ribosomes. This release requires R factor and occurs in the absence of terminator codon in reactions containing 20 per cent ethanol. Release occurs only when both 30S and 50S ribosomal subunits are present and when fMet-tRNA is located in the ribosomal P site. Release factor-dependent deacylation of fMet-tRNA is inhibited by sparsomycin, amicetin, lincocin, and chloramphenicol, antibiotics which have little effect on binding of R factor to ribosomes. The possible role of peptidyl transferase in the release reaction is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caskey C. T., Tompkins R., Scolnick E., Caryk T., Nirenberg M. Sequential translation of trinucleotide codons for the initiation and termination of protein synthesis. Science. 1968 Oct 4;162(3849):135–138. doi: 10.1126/science.162.3849.135. [DOI] [PubMed] [Google Scholar]
- Erbe R. W., Leder P. Initiation and protein synthesis: translation of di- and tri-codon messengers. Biochem Biophys Res Commun. 1968 Jun 10;31(5):798–803. doi: 10.1016/0006-291x(68)90633-5. [DOI] [PubMed] [Google Scholar]
- Erbe R. W., Nau M. M., Leder P. Translation and translocation of defined RNA messengers. J Mol Biol. 1969 Feb 14;39(3):441–460. doi: 10.1016/0022-2836(69)90137-5. [DOI] [PubMed] [Google Scholar]
- Goldstein J., Milman G., Scolnick E., Caskey T. Peptide chain termination. VI. Purification and site of action of S. Proc Natl Acad Sci U S A. 1970 Feb;65(2):430–437. doi: 10.1073/pnas.65.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey J. W., Thach R. E. Role of guanosine 5'-triphosphate in the initiation of Peptide synthesis, I. Synthesis of formylmethionyl-puromycin. Proc Natl Acad Sci U S A. 1967 Mar;57(3):759–766. doi: 10.1073/pnas.57.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Bursztyn H. Initiation of protein synthesis II. A convenient assay for the ribosome-dependent synthesis of N-formyl-C14-methionylpuromycin. Biochem Biophys Res Commun. 1966 Oct 20;25(2):233–238. doi: 10.1016/0006-291x(66)90586-9. [DOI] [PubMed] [Google Scholar]
- Lucas-Lenard J., Lipmann F. Separation of three microbial amino acid polymerization factors. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1562–1566. doi: 10.1073/pnas.55.6.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman G., Goldstein J., Scolnick E., Caskey T. Peptide chain termination. 3. Stimulation of in vitro termination. Proc Natl Acad Sci U S A. 1969 May;63(1):183–190. doi: 10.1073/pnas.63.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monro R. E. Catalysis of peptide bond formation by 50 S ribosomal subunits from Escherichia coli. J Mol Biol. 1967 May 28;26(1):147–151. doi: 10.1016/0022-2836(67)90271-9. [DOI] [PubMed] [Google Scholar]
- Monro R. E., Vazquez D. Ribosome-catalysed peptidyl transfer: effects of some inhibitors of protein synthesis. J Mol Biol. 1967 Aug 28;28(1):161–165. doi: 10.1016/s0022-2836(67)80085-8. [DOI] [PubMed] [Google Scholar]
- NIRENBERG M., LEDER P. RNA CODEWORDS AND PROTEIN SYNTHESIS. THE EFFECT OF TRINUCLEOTIDES UPON THE BINDING OF SRNA TO RIBOSOMES. Science. 1964 Sep 25;145(3639):1399–1407. doi: 10.1126/science.145.3639.1399. [DOI] [PubMed] [Google Scholar]
- Pestka S., Nirenberg M. Regulatory mechanisms and protein synthesis. X. Codon recognition on 30 S ribosomes. J Mol Biol. 1966 Oct 28;21(1):145–171. doi: 10.1016/0022-2836(66)90085-4. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Caskey C. T. Peptide chain termination. V. The role of release factors in mRNA terminator codon recognition. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1235–1241. doi: 10.1073/pnas.64.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E., Milman G., Rosman M., Caskey T. Transesterification by peptidyl transferase. Nature. 1970 Jan 10;225(5228):152–154. doi: 10.1038/225152a0. [DOI] [PubMed] [Google Scholar]
- Scolnick E., Tompkins R., Caskey T., Nirenberg M. Release factors differing in specificity for terminator codons. Proc Natl Acad Sci U S A. 1968 Oct;61(2):768–774. doi: 10.1073/pnas.61.2.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel Z., Zamir A., Elson D. On the specificity and stability of an enzyme that hydrolyzes N-substituted aminoacyl-transfer RNA's. Proc Natl Acad Sci U S A. 1968 Oct;61(2):701–707. doi: 10.1073/pnas.61.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel Z., Zamir A., Elson D. The possible involvement of peptidyl transferase in the termination step of protein biosynthesis. Biochemistry. 1969 Dec;8(12):5161–5168. doi: 10.1021/bi00840a070. [DOI] [PubMed] [Google Scholar]
- Weisblum B., Davies J. Antibiotic inhibitors of the bacterial ribosome. Bacteriol Rev. 1968 Dec;32(4 Pt 2):493–528. [PMC free article] [PubMed] [Google Scholar]



