Abstract
In humans the circulating concentrations of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) decrease markedly during aging, and have been implicated in age-associated cognitive decline. This has led to the hypothesis that DHEA supplementation during aging may improve memory. In rodents, a cognitive anti-aging effect of DHEA and DHEAS has been observed but it is unclear whether this effect is mediated indirectly through conversion of these steroids to estradiol. Moreover, despite the demonstration of correlations between endogenous DHEA concentrations and cognitive ability in certain human patient populations, such correlations have yet to be convincingly demonstrated during normal human aging. This review highlights important differences between rodents and primates in terms of their circulating DHEA and DHEAS concentrations, and suggests that age-related changes within the human DHEA metabolic pathway may contribute to the relative inefficacy of DHEA replacement therapies in humans. The review also highlights the value of using nonhuman primates as a pragmatic animal model for testing the therapeutic potential of DHEA for age-associate cognitive decline in humans.
Keywords: Dehydroepiandrosterone, Cognitive decline, Intracrinology, Neurosteroidogenesis
Introduction
Dehydroepiandrosterone (DHEA) and its ester, DHEA sulfate (DHEAS; together, referred to hereon as DHEA/S), are together the most abundant circulating hormones in young adult humans and nonhuman primates. Although their exact physiological function is still unclear, they represent a major source of active androgens and estrogens when metabolized in central nervous system (CNS) and peripheral tissues. A number of observations, including a unique age-related profile of production and neuroprotective and pro-cognitive effects on cultured tissue and behaving rodents, have led many researchers to investigate DHEA/S’s role in the aging process and possible therapeutic actions in learning and memory. Despite a wealth of evidence suggesting DHEA/S supplementation can improve memory in rodent models, similar actions in healthy elderly humans has yet to be demonstrated. Nevertheless, it is plausible that hormonal replacement therapies (HRTs) comprising DHEA/S, rather than more conventional sex-steroid HRT, could provide an alternative and possibly safer approach in the treatment of aging-associated human pathologies. This paper provides a brief review of the evidence, from both rodent and human studies, arguing for and against the benefits of DHEA supplementation in the treatment of age-associated cognitive decline, and also offers possible explanations for the inconsistencies in the published literature.
Observations of a DHEA/S–cognition relationship in the elderly
DHEA/S is a prohormone secreted by the zona reticularis of the adrenal glands in a highly age-specific manner. While other adrenal hormones, such as cortisol, show a relatively steady level of secretion throughout aging, DHEA/S synthesis peaks in young adulthood and declines by up to 80% in old age (Orentreich et al. 1992; Labrie et al. 1997). Indeed, it has been suggested that this decline in the DHEA:cortisol ratio underlies some of the cognitive decline associated with aging, as DHEA/S can attenuate the deleterious effects of cortisol (van Niekerk et al. 2001; Karishma and Herbert 2002). Additionally, lower levels of DHEA and DHEAS have been associated with cognitive disorders with a higher prevalence in the elderly, such as Alzheimer’s disease (Weill-Engerer et al. 2002) and depression (Micheal et al. 2000). In men (van Niekerk et al. 2001) and healthy postmenopausal women (Davis et al. 2008), endogenous DHEAS levels are associated with better cognitive ability; however, the only similar study to date in nonhuman primates failed to find such an association (Herndon et al. 1999) and studies of the frail elderly reveal an inverse relationship between DHEAS and cognitive ability (Morrison et al. 1998, 2000). As the previous studies did not simultaneously measure cortisol levels, which are significantly higher in frail versus healthy elderly humans (Varadhan et al. 2008), such findings may be due to a concurrent rise in cortisol resulting in a decreased DHEA:cortisol ratio. While the immediate effects of DHEA/S have not yet been attributed to a specific receptor, some of its protective effects may result from its conversion to sex steroids. For example, it has been estimated that 30–50% of active sex steroids in men and 75% (100% after menopause) of active sex steroids in women are derived peripherally from DHEA/S (Labrie 1991). Thus, an 80% decline in DHEA from the adrenals may be greatly enhancing cognitive deficits due to the decline in sex steroid production from the gonads.
Healthy aging is often accompanied by a decline in cognitive ability that does not meet the criteria for dementia, termed age-associated mental impairment, or AAMI (Larrabee and Crook 1994). Included in this decline are deficits in working, spatial, and episodic memory (Verhaeghen and Salthouse 1997), which, in part, is maintained by the prefrontal cortex and hippocampus. As the age-related cellular changes in these areas can be reduced by estrogen (Hao et al. 2007; Saravia et al. 2007), the age-related loss of DHEA/S may further exacerbate the age-related loss of sex steroids from the gonads, thereby potentiating the deficit in these memory domains seen in old age. Some evidence suggests that HRT involving estrogen may attenuate cognitive decline (Sherwin 2007a; Løkkegaard et al. 2002), particularly in women at risk for developing Alzheimer’s disease (Hu et al. 2006; Yue et al. 2007). These results remain controversial, however, as other HRT replacement studies, including that of the Women’s Health Initiative, have shown null effects or negative influences of HRT on cognitive decline (Craig et al. 2005; Lethaby et al. 2008). Many postulate that these discrepancies may be due to the age of HRT initiation relative to menopause, as in both humans and rodents this variable seems to determine the direction of estrogen’s effect on cognition (Kang et al. 2004; Daniel et al. 2006; Sherwin 2007b; Bohacek et al. 2008). Regardless, estrogen therapy carries with it an increased risk for breast malignancy (Rohan et al. 2008). In contrast, DHEA/S does not exhibit the same proliferative effects on breast cancer tissue as estrogen (Labrie et al. 1998), yet can be converted into estrogen in some peripheral tissues. Consequently, it is plausible that supplementation with DHEA/S may offer some of the same benefits as estrogen replacement, but with reduced risk.
Effects of DHEA/S and neurosteroidogenesis in rodents
While a receptor specific to DHEA or DHEAS has not been isolated, rodent studies have observed a number of effects of the steroid that, on a cellular level, may improve memory. DHEA/S has been shown to antagonize the androgen receptor and agonize estrogen receptor β; thus, it may exert some of the same actions as estradiol (Chen et al. 2005). Also, DHEA/S may affect synaptic plasticity in the hippocampus through antagonism of the GABAA receptor (Majewska 1992), facilitating long-term potentiation via NMDA agonism (Mellon and Griffin 2002; Chen et al. 2006), and enhancing glutamate release during learning (Lhullier et al. 2004). As mentioned earlier, DHEA/S significantly protects the CNS against the effects of cortisol by attenuating its suppression of neurogenesis (Karishma and Herbert 2002). Additionally, DHEA/S has been observed to be neuroprotective against oxygen-glucose deprivation (Kaasik et al. 2001), oxidative stress (Bastianetto et al. 1999; Kumar et al. 2008), and excitotoxicity (Kimonides et al. 1998; Mao and Barger 1998), as well as effective at enhancing cell survival, proliferation, and neurogenesis (Karishma and Herbert 2002; Suzuki et al. 2004). In line with this evidence, in vivo studies have shown an anti-amnestic effect of DHEAS (Flood et al. 1988) as well as an anti-aging effect on cognition (Flood and Roberts 1988) in rodents.
Although DHEA/S supplementation studies have been performed both in vitro, using cell culture, and in vivo, using rodents, in many cases endogenous neurosteroidogenesis was not blocked. Therefore, the observed effects may have been mediated by endogenous conversion of DHEA/S to estradiol. This is certainly plausible given that estradiol supplementation can produce many similar results (DeNicola et al. 2008). The proteins and enzymes necessary for the conversion of DHEA/S to estradiol, as well as for the synthesis of DHEA/S from cholesterol (Fig. 1), are present in a region-dependent manner in the rodent brain (Zwain and Yen 1999; Hojo et al. 2003; Kohchi et al. 1998; Gottfried-Blackmore et al. 2008), suggesting that this may be a valid mechanism of action. Interestingly, adrenalectomy combined with gonadectomy has no effect on the central levels of DHEA/S in rodents (Robel et al. 1987) and suppression of adrenal activity with dexamethasone has no effect on DHEA/S levels in the nonhuman primate brain (Corpechot et al. 1981), suggesting that the hormone is indeed synthesized de novo from cholesterol in the brain. Indeed, locally produced estradiol has been shown to have significant impacts on hippocampal synaptic plasticity in rodent models (Kretz et al. 2004; Rune and Frotscher 2005; Mukai et al. 2006). It is important to note, however, that while similar neurosteroidogenesis has been suggested in primates and humans (Robel et al. 1987), this pathway has yet to be investigated in detail.
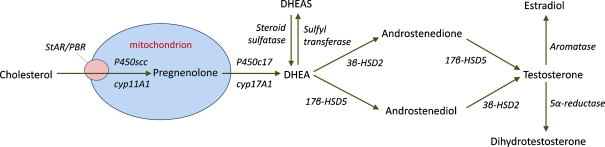
Fig. 1.
Schematic representing sex steroid synthesis from cholesterol and DHEA. Cholesterol is transported into the mitochondrion by steroidogenic acute regulatory protein (StAR) and the peripheral benzodiazepine receptor (PBR), where it is converted into pregnenolone by the cytochrome p450 side-chain cleavage enzyme (p450scc). After diffusing into the cytoplasm, pregnenolone is converted to DHEA by a 17,20-desmolase enzyme, p450c17. DHEA can then be converted to its ester via sulfyl transferase (and back to DHEA by steroid sulfatase), or can be converted to testosterone by 3β- and 17β-hydroxysteroid dehydrogenase (HSD). Aromatization of testosterone then results in the formation of estradiol, or 5α-reduction forms dihydrotestosterone (Stoffel-Wagner 2003). Enzymes are depicted in italics
Clinical studies of DHEA/S supplementation
Because of the strong pro-cognitive and anti-aging effects of DHEA/S previously observed in rodents, attempts have been made to examine the efficacy of DHEA/S supplementation in elderly humans. There is general agreement that DHEA supplementation can exert beneficial effects on mood and well-being in populations with adrenal insufficiency (Arlt et al. 1999; Bloch et al. 1999; Hunt et al. 2000) and depression (Wolkowitz et al. 1999; Schmidt et al. 2005), and that it shows some benefit in a primate model of Parkinson’s disease (Bélanger et al. 2006); this suggests that DHEA supplementation might also enhance cognitive functions in the elderly. Additionally, low circulating DHEA/S levels are thought to play a role in the development of Alzheimer’s disease (Weill-Engerer et al. 2002) and in some domains of memory impairment (van Niekerk et al. 2001; Davis et al. 2008), again, supporting the hypothesis that DHEA/S supplementation may improve cognition in the elderly. So far, however, clinical studies of DHEA/S supplementation have failed to provide convincing evidence in support of this hypothesis. No studies of DHEA replacement on healthy elderly populations, either acute administration or chronic (up to 12 months) supplementation, have shown a benefit in memory with treatment (Wolf et al. 1997, 1998; Wolf and Kirschbaum 1999; Arlt et al. 2001; Grimley Evans et al. 2006; Kritz-Silverstein et al. 2008), and some have even observed a negative effect on memory (Wolf et al. 1998; Parsons et al. 2006). DHEA supplementation has also shown no benefit in the treatment of Alzheimer’s disease (Wolkowitz et al. 2003).
Sources of discrepancies and future directions
With the substantial evidence pointing to a cognitive benefit of DHEA/S supplementation in rodents, why are similar causal effects of DHEA/S not seen in human studies? One possible explanation may lie in the method of supplementation. DHEA is marketed as a dietary supplement in the United States, most often in doses of 25–50 mg per day, and thus this is the form and dose most often chosen for clinical studies, although doses as high as 450 mg per day have also been used (Bloch et al. 1999). In rodent studies, however, the sulfated form of DHEA (DHEAS) has been commonly used. DHEA has a half-life of only 30 min while the half-life of DHEAS can be up to 12 h (Wolf and Kirschbaum 1999), thus, rapid clearance of DHEA may explain the lack of observed effects. A difference in the adrenal glands of rodents and humans may be another cause of discrepancy: while humans synthesize a large portion of DHEA/S from the adrenal glands, rodent adrenal glands do not, and it has been proposed that most, if not all, active DHEA/S is produced locally. Similarly, rodents lack the observed age-related decrease in DHEA/S production, and thus DHEA/S “replacement” may not be as physiologically relevant in a rodent model as in a primate or human model (Wolf and Kirschbaum 1999). It should also be emphasized that the human clinical studies were performed in elderly subjects, who already had attenuated circulating DHEA/S concentrations. Therefore, the studies specifically tested whether DHEA supplementation could reverse cognitive decline. They did not, however, examine whether maintenance of “youthful” circulating DHEA/S concentrations can prevent or delay age-associated cognitive decline. It is generally assumed that the beneficial effects of estrogen on cognitive function are dependent upon when the HRT is initiated relative to the onset of menopause, and so the lack of an obvious beneficial effects of DHEA supplementation are not surprising.
To fully understand the potential for DHEA/S supplementation to yield cognitive benefits in humans it might be necessary to perform studies in nonhuman primate animal models, rather than rodents. Rhesus macaques, for example are long-lived primates that show many similar aging-associated changes in cognitive function to those seen in humans. Furthermore, like humans rhesus macaques show similar adrenal gland structure and endocrine function (Conley et al. 2004; Nguyen and Conley 2008; Abbott and Bird 2008), and show a marked age-related decline in circulating DHEAS concentrations (Urbanski et al. 2004; Downs et al. 2008). Also, like women, female rhesus macaques undergo menopause (Downs and Urbanski 2006). One possible target of investigation includes the neurosteroidogenic pathway (see Fig. 1). An age-related decline in expression of the enzymes necessary to form estradiol from DHEA/S may explain the negative results reported by clinical studies. In particular, the enzyme responsible for the age-related decline in DHEA/S production in the adrenal glands, 17,20-desmolase (Liu et al. 1990; encoded by the p450c17 gene and necessary in forming DHEA from pregnenolone), may also decline in central tissues, resulting in reduced central production of DHEA. Other genes responsible for the conversion of DHEA itself to estradiol may decline in expression as well. Targeting these enzymes instead of, or in addition to, DHEA/S supplementation may provide another route of cognitive therapy in healthy older adults. Because rhesus macaques can be maintained under tightly controlled environmental conditions (including temperature, photoperiod, diet, and medication), and subjected to long-term in vivo studies, they may represent an ideal animal model in which to comprehensively examine the therapeutic potential of DHEA in elderly humans. Moreover, because rhesus macaques can yield high quality mRNA from post-mortem tissues, in vitro studies could help to elucidate the underlying mechanisms by which DHEA/S exert its beneficial effects within the CNS (Racchi et al. 2003).
Acknowledgements
This work was supported by National Institute of Health grants: AG-019914, AG-026472, AG-029612 HD-29186, and RR-00163.
References
- Abbott DH, Bird IM (2008) Nonhuman primates as models for human adrenal androgen production: function and dysfunction Rev Endocr Metab Disord doi:10.1007/s11154-008-9099-8 [DOI] [PMC free article] [PubMed]
- Arlt W, Callies F, Vlijmen JC, et al. Dehydroepiandrosterone replacement in women with adrenal insufficiency. N Engl J Med. 1999;341:1013–1020. doi: 10.1056/NEJM199909303411401. [DOI] [PubMed] [Google Scholar]
- Arlt W, Callies F, Koehler I, et al. Dehydroepiandrosterone supplementation in healthy men with and age-related decline of dehydroepiandrosterone secretion. J Clin Endocrinol Metab. 2001;86:4684–4692. doi: 10.1210/jc.86.10.4686. [DOI] [PubMed] [Google Scholar]
- Bastianetto S, Ramassamy C, Poirer J, et al. Dehydroepiandrosterone (DHEA) protects hippocampal cells from oxidative stress-induced damage. Mol Brain Res. 1999;66:35–41. doi: 10.1016/S0169-328X(99)00002-9. [DOI] [PubMed] [Google Scholar]
- Bélanger N, Grégoire L, Bédard PJ, et al. DHEA improves symptomatic treatment of moderately and severely impaired MPTP monkeys. Neurobiol Aging. 2006;27:1684–1693. doi: 10.1016/j.neurobiolaging.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau MA, et al. Dehydroepiandrosterone treatment of midlife dysthymia. Biol Psychiatry. 1999;45:1533–1541. doi: 10.1016/S0006-3223(99)00066-9. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Bearl AM, Daniel JM. Long-term ovarian hormone deprivation alters the ability of sebsewuent oestradiol replacement to regulate choline acetyltransferase protein levels in the hippocampus and prefrontal cortex of middle-aged rats. J Neuroendocrinol. 2008;20:1023–1027. doi: 10.1111/j.1365-2826.2008.01752.x. [DOI] [PubMed] [Google Scholar]
- Chen F, Knecht K, Birzin E, et al. Direct agonist/antagonist functions of DHEA. Endocrinology. 2005;146:4568–4576. doi: 10.1210/en.2005-0368. [DOI] [PubMed] [Google Scholar]
- Chen L, Miyamoto Y, Furuya K, et al. Chronic DHEAS administration facilitates hippocampal long-term potentiation via an amplification of Src-dependent MDA receptor signaling. Neuropharmacology. 2006;51:659–670. doi: 10.1016/j.neuropharm.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Conley AJ, Pattison JC, Bird IM. Variations in adrenal androgen production among (nonhuman) primates. Sem Reprod Med. 2004;22:311–326. doi: 10.1055/s-2004-861548. [DOI] [PubMed] [Google Scholar]
- Corpechot C, Robel P, Axelson M, et al. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc Natl Acad Sci USA. 1981;78:4704–4707. doi: 10.1073/pnas.78.8.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig MC, Maki PM, Murphy DGM. The Women’s Health Initiative Memory Study: findings and implications for treatment. Lancet Neurol. 2005;4:190–194. doi: 10.1016/S1474-4422(05)01016-1. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Julst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Davis SR, Shah SM, McKEnzie DP, et al. Dehydroepiandrosterone sulfate levels are associated with more favorable cognitive function in women. J Clin Endocrinol Metab. 2008;93:801–808. doi: 10.1210/jc.2007-2128. [DOI] [PubMed] [Google Scholar]
- DeNicola AF, Pietranera L, Beauquis J, et al. Steroid protection in aging and age-associated diseases. Exp Gerontol. 2008;44:34–40. doi: 10.1016/j.exger.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Downs JL, Urbanski HF. Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macacaca mulatta) Biol Reprod. 2006;75:539–546. doi: 10.1095/biolreprod.106.051839. [DOI] [PubMed] [Google Scholar]
- Downs JL, Mattison JA, Ingram DK, et al. Effect of age and caloric restriction on circadian adrenal steroid rhythms in rhesus macaques. Neurobiol Aging. 2008;29:1412–1422. doi: 10.1016/j.neurobiolaging.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood JF, Roberts E. Dehydroepiandrosterone improves memory in aging mice. Brain Res. 1988;448:178–181. doi: 10.1016/0006-8993(88)91116-X. [DOI] [PubMed] [Google Scholar]
- Flood JF, Smith GE, Roberts E. Dehydroepiandrosterone and its sulfate enhance memory retention in mice. Brain Res. 1988;447:269–278. doi: 10.1016/0006-8993(88)91129-8. [DOI] [PubMed] [Google Scholar]
- Gottfried-Blackmore A, Sierra A, Jellinck PH, et al. Brain microglia express steroid-converting enzymes in the mouse. J Steroid Biochem Mol Biol. 2008;109:96–107. doi: 10.1016/j.jsbmb.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimley Evans J, Malouf R, Huppert F et al. (2006) Dehydroepiandrosterone (DHEA) supplementation for cognitive function in healthy elderly people. Cochrane Database of Syst Rev CD006221 [DOI] [PMC free article] [PubMed]
- Hao J, Rapp PR, Janssen WGM, et al. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc Natl Acad Sci USA. 2007;104:11465–11470. doi: 10.1073/pnas.0704757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon JG, Lacreuse A, Ladinsky E, et al. Age-related decline in DHEAS is not related to cognitive impairement in aged monkeys. NeuroReport. 1999;10:3507–3511. doi: 10.1097/00001756-199911260-00008. [DOI] [PubMed] [Google Scholar]
- Hojo Y, Hattori T, Enami T, et al. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P450c17α and P450 aromatase localized in neurons. Proc Natl Acad Sci USA. 2003;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Yue Y, Zuo PP, et al. Evaluation of neuroprotective effects of long-term low dose hormone replacement therapy on postmenopausal women brain hippocampus using magnetic resonance scanner. Chin Med Sci J. 2006;21:214–218. [PubMed] [Google Scholar]
- Hunt PJ, Gurnell EM, Huppert FA, et al. Improvement in mood and fatigue after dehydroepiandrosterone replacement in Addison's disease in a randomized, double blind trial. J Clin Endocrinol Metab. 2000;85:4650–4656. doi: 10.1210/jc.85.12.4650. [DOI] [PubMed] [Google Scholar]
- Kaasik A, Kalda A, Jaako K, et al. Dehydroepiandrosterone sulphate prevents oxygen-glucose deprivation-induced injury in cerebellar granule cell culture. Neuroscience. 2001;102:427–432. doi: 10.1016/S0306-4522(00)00489-9. [DOI] [PubMed] [Google Scholar]
- Kang JH, Weuve J, Grodstein F. Postmenopausal hormone therapy and risk of cognitive decline in community-dwelling aging women. Neurology. 2004;63:101–107. doi: 10.1212/01.wnl.0000132522.13574.67. [DOI] [PubMed] [Google Scholar]
- Karishma KK, Herbert J. DHEA stimulates neurogenesis in the hippocampus of the rat, promotes survival of newly formed neurons, and prevents corticosterone-induced suppression. Eur J NeuroSci. 2002;16:445–453. doi: 10.1046/j.1460-9568.2002.02099.x. [DOI] [PubMed] [Google Scholar]
- Kimonides VG, Khatibi NH, Svendsen CN, et al. Dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEAS) protect hippocampal neurons against excitatory amino acid-induced neurotoxicity. Proc Natl Acad Sci USA. 1998;95:1852–1857. doi: 10.1073/pnas.95.4.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohchi C, Ukena K, Tsutsui K. Age- and region-specific expressions of the mRNAs encoding for steroidogenic enzymes P450scc, P450c17, and 3bhsd in the postnatal rat brain. Brain Res. 1998;801:233–238. doi: 10.1016/S0006-8993(98)00585-X. [DOI] [PubMed] [Google Scholar]
- Kretz O, Fester L, Wehrenberg U, et al. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004;24:5913–5921. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritz-Silverstein D, Muhlen D, Laughlin GA, et al. Effects of dehydroepiandrosterone supplementation on cognitive function and quality of life: the DHEA and wellness (DAWN) trial. J Am Geriatr Soc. 2008;56:1292–1298. doi: 10.1111/j.1532-5415.2008.01768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Taha A, Sharma D, et al. Effect of DHEA on MAO activity, lipid peroxidation, and lipofuscin accumulation in aging rat brain regions. Biogerontology. 2008;9:235–246. doi: 10.1007/s10522-008-9133-y. [DOI] [PubMed] [Google Scholar]
- Labrie F. Intracrinology. Mol Cell Endocrinol. 1991;78:C113–C118. doi: 10.1016/0303-7207(91)90116-A. [DOI] [PubMed] [Google Scholar]
- Labrie F, Bélanger A, Cusan L, et al. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab. 1997;82:2396–2402. doi: 10.1210/jc.82.8.2396. [DOI] [PubMed] [Google Scholar]
- Labrie F, Bélanger A, Luu-The V, et al. DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: its role during aging. Steroids. 1998;63:322–328. doi: 10.1016/S0039-128X(98)00007-5. [DOI] [PubMed] [Google Scholar]
- Larrabee GJ, Crook TH. Estimated prevalence of age-associated memory impairment derived from standardized tests of memory function. Int Psychogeriatr. 1994;6:95–104. doi: 10.1017/S1041610294001663. [DOI] [PubMed] [Google Scholar]
- Lethaby A, Hovervorst E, Richards M et al. (2008) Hormone replacement therapy for cognitive function in postmenopausal women. Chochrane Database of Syst Rev CD003122 [DOI] [PMC free article] [PubMed]
- Lhullier FLR, Nicolaidis R, Riera NG, et al. Dehydroepiandrosterone increases synaptosomal glutamate release and improves the performance in inhibitory avoidance task. Pharmacol Biochem Behav. 2004;77:601–606. doi: 10.1016/j.pbb.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Liu CH, Laughlin GA, Fischer UG, et al. Marked attenuation of ultradian and circadian rhythms of dehydroepiandrosterone in postmenopausal women: evidence for a reduced 17, 20-desmolase activity. J Clin Endocrinol Metab. 1990;71:900–906. doi: 10.1210/jcem-71-4-900. [DOI] [PubMed] [Google Scholar]
- Løkkegaard E, Pederson AT, Laursen P, et al. The influence of hormone replacement therapy on the aging-related change in cognitive performance. Analysis based on a Danish cohort study. Maturitas. 2002;42:209–218. doi: 10.1016/S0378-5122(02)00076-2. [DOI] [PubMed] [Google Scholar]
- Majewska MD. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobiol. 1992;38:379–395. doi: 10.1016/0301-0082(92)90025-A. [DOI] [PubMed] [Google Scholar]
- Mao X, Barger SW. Neuroprotection by dehydroepiandrosterone-sulfate: role of an NFκB-like factor. NeuroReport. 1998;9:759–763. doi: 10.1097/00001756-199803090-00036. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol Metab. 2002;13:35–42. doi: 10.1016/S1043-2760(01)00503-3. [DOI] [PubMed] [Google Scholar]
- Micheal A, Jenaway A, Paykel ES, et al. Altered salivary dehydroepiandrosterone levels in major depression in adults. Biol Psychiatry. 2000;48:989–995. doi: 10.1016/S0006-3223(00)00955-0. [DOI] [PubMed] [Google Scholar]
- Morrison MF, Katz IR, Parmelee P, et al. Dehydroepiandrosterone sulfate (DHEA-S) and psychiatric and laboratory measures of frailty in a residential care population. Am J Geriatr Psychiatry. 1998;6:277–284. [PubMed] [Google Scholar]
- Morrison MG, Redei E, TenHave T, et al. Dehydroepiandrosterone sulfate and psychiatric measures in a frail, elderly residential care population. Biol Psychiatry. 2000;47:144–150. doi: 10.1016/S0006-3223(99)00099-2. [DOI] [PubMed] [Google Scholar]
- Mukai H, Tsuruqizawa T, Ogiue-Ikeda M, et al. Local neurosteroid production in the hippocampus: influence on synaptic plasticity of memory. Neuroendocrinology. 2006;84:255–263. doi: 10.1159/000097747. [DOI] [PubMed] [Google Scholar]
- Nguyen AD, Conley AJ (2008) Adrenal androgens in humans and nonhuman primates: production, zonation and regulation. In: Flück CE, Miller WL (eds) Disorders of the human adrenal cortex. Endocr Dev Karger Basel 13:33–54 [DOI] [PubMed]
- Orentreich N, Brind JL, Vogelman JH, et al. Long-term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. J Clin Endocrinol Metab. 1992;75:1002–1004. doi: 10.1210/jc.75.4.1002. [DOI] [PubMed] [Google Scholar]
- Parsons TD, Kratz KM, Thompson E, et al. DHEA supplementation and cognition in postmenopausal women. Int J Neurosci. 2006;116:141–155. doi: 10.1080/00207450500341506. [DOI] [PubMed] [Google Scholar]
- Racchi M, Balduzzi C, Corsini E. Dehydroeiandrosterone (DHEA) and the aging brain: flipping a coin in the “fountain of youth”. CNS Drug Rev. 2003;9:21–40. doi: 10.1111/j.1527-3458.2003.tb00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robel P, Bourreau E, Corpechot C, et al. Neuro-steroids: 3β-hydroxy-Δ5-derivatives in rat and monkey brain. J Steroid Biochem. 1987;27:649–655. doi: 10.1016/0022-4731(87)90133-6. [DOI] [PubMed] [Google Scholar]
- Rohan TE, Negassa A, Chlebowski RT, et al. Conjugated equine estrogen and risk of benign proliferative breast disease: a randomized controlled trial. J Natl Cancer Inst. 2008;100:563–571. doi: 10.1093/jnci/djn075. [DOI] [PubMed] [Google Scholar]
- Rune GM, Frotscher M. Neurosteroid synthesis in the hippocampus: role in synaptic plasticity. Neurosci. 2005;136:833–842. doi: 10.1016/j.neuroscience.2005.03.056. [DOI] [PubMed] [Google Scholar]
- Saravia F, Beauquis J, Pietranera, et al. Neuroprotective effects of estradiol in hippocampal neurons and glia of middle age mice. Psychoneuroendocrinology. 2007;32:480–492. doi: 10.1016/j.psyneuen.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Daly RC, Bloch M, et al. Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Arch Gen Psychiatry. 2005;62:154–162. doi: 10.1001/archpsyc.62.2.154. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. The clinical relevance of the relationship between estrogen and cognition in women. J Steroid Biochem Mol Biol. 2007;106:151–156. doi: 10.1016/j.jsbmb.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. The critical period hypothesis: can it explain discrepancies in the oestrogen-cognition literature? J Neuroendocrinol. 2007;19:77–81. doi: 10.1111/j.1365-2826.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- Stoffel-Wagner B. Neurosteroid biosynthesis in the human brain and its clinical implications. Ann N Y Acad Sci. 2003;1007:64–78. doi: 10.1196/annals.1286.007. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Wright LS, Marwah P, et al. Mitotic and neurogenic effects of DHEA on human neural stem cell cultures derived from the fetal cortex. Proc Natl Acad Sci USA. 2004;101:3202–3207. doi: 10.1073/pnas.0307325101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski HF, Downs JL, Garyfallou VT, et al. Effect of caloric restriction on the 24-hour plasma DHEAS and cortisol profiles of young and old male rhesus macaques. Ann N Y Acad Sci. 2004;1019:443–447. doi: 10.1196/annals.1297.081. [DOI] [PubMed] [Google Scholar]
- Niekerk JK, Huppert JA, Herbert J. Salivary cortisol and DHEA: association with measures of cognition and well-being in normal older men, and effects of three months of DHEA supplementation. Psychoneuroendocrinology. 2001;26:591–612. doi: 10.1016/S0306-4530(01)00014-2. [DOI] [PubMed] [Google Scholar]
- Varadhan R, Walston J, Cappola AR, et al. Higher levels and blunted diurnal variation of cortisol in frail older women. J Gerontol A Biol Sci Med Sci. 2008;63:190–195. doi: 10.1093/gerona/63.2.190. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Salthouse TA. Meta-analyses of age-cognition relations in adulthood: estimates of linear and nonlinear age effects and structural models. Psychol Bull. 1997;122:231–249. doi: 10.1037/0033-2909.122.3.231. [DOI] [PubMed] [Google Scholar]
- Weill-Engerer S, David JP, Sazdovitch V, et al. Neurosteroid quantification in human brain regions: comparison between Alzheimer’s and non-demented patients. J Clin Endocrinol Metab. 2002;87:5238–5142. doi: 10.1210/jc.2002-020878. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Kirschbaum C. Actions of dehydroepiandrosterone and its sulfate in the central nervous system: effects on cognition and emotion in animals and humans. Brain Res Rev. 1999;30:264–288. doi: 10.1016/S0165-0173(99)00021-1. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Neumann O, Hellhammer DH, et al. Effects of a two-week physiological dehydroepiandrosterone substitution on cognitive performance and well-being in healthy elderly women and men. J Clin Endocrinol Metab. 1997;82:2363–2367. doi: 10.1210/jc.82.7.2363. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Kudielka BM, Hellhammer DH, et al. Opposing effects of DHEA replacement in elderly subjects on declarative memory and attention after exposure to a laboratory stressor. Psychoneuroendocrinology. 1998;23:617–629. doi: 10.1016/S0306-4530(98)00032-8. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Kramer JH, Reus VI, et al. Double-blind treatment of major depression with dehydroepiandrosterone. Am J Psychiatry. 1999;156:646–649. doi: 10.1176/ajp.156.4.646. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Kramer JH, Reus VI, et al. DHEA treatment of Alzheimer’s disease: a randomized, double-blind, placebo-controlled study. Neurology. 2003;60:1071–1076. doi: 10.1212/01.wnl.0000052994.54660.58. [DOI] [PubMed] [Google Scholar]
- Yue Y, Hu L, Tian QJ, et al. Effects of long-term, low-dose sex hormone replacement therapy on hippocampus and cognition of postmenopausal women of different apoE genotypes. Acta Pharmacol Sin. 2007;28:1129–1135. doi: 10.1111/j.1745-7254.2007.00618.x. [DOI] [PubMed] [Google Scholar]
- Zwain IH, Yen SSC. DHEA: biosynthesis and metabolism in the brain. Endocrinology. 1999;140:880–887. doi: 10.1210/en.140.2.880. [DOI] [PubMed] [Google Scholar]