Abstract
Calorie restriction (CR) slows aging and is thought to improve insulin sensitivity in laboratory animals. In contrast, decreased insulin signaling and/or mild insulin resistance paradoxically extends maximal lifespan in various genetic animal models of longevity. Nothing is known regarding the long-term effects of CR on glucose tolerance and insulin action in lean healthy humans. In this study we evaluated body composition, glucose, and insulin responses to an oral glucose tolerance test and serum adipokines levels in 28 volunteers, who had been eating a CR diet for an average of 6.9 ± 5.5 years, (mean age 53.0 ± 11 years), in 28 age-, sex-, and body fat-matched endurance runners (EX), and 28 age- and sex-matched sedentary controls eating Western diets (WD). We found that the CR and EX volunteers were significantly leaner than the WD volunteers. Insulin sensitivity, determined according to the HOMA-IR and the Matsuda and DeFronzo insulin sensitivity indexes, was significantly higher in the CR and EX groups than in the WD group (P = 0.001). Nonetheless, despite high serum adiponectin and low inflammation, ∼40% of CR individuals exhibited an exaggerated hyperglycemic response to a glucose load. This impaired glucose tolerance is associated with lower circulating levels of IGF-1, total testosterone, and triiodothyronine, which are typical adaptations to life-extending CR in rodents.
Keywords: Calorie restriction, Endurance exercise, Glucose tolerance, Insulin action, Adipokines, Glycation
Introduction
Long-term calorie restriction (CR) without malnutrition, the most robust intervention to extend average and maximal lifespan in rodents (Fontana & Klein 2007), improves insulin sensitivity, reduces fasting glucose, and insulin concentration in rodents and monkeys (Masoro et al. 1992; Kemnitz et al. 1994; Lane et al. 1995; Cefalu et al. 1997; Barzilai et al. 1998; Gresl et al. 2001), and in some (Lane et al. 1995; Masoro et al. 1992), but not all cases (Kalant et al. 1988; Bodkin et al. 1995; Cefalu et al. 1997; Barzilai et al. 1998), improves glucose disposal compared with ad libitum fed animals. It has been proposed that improved insulin sensitivity, and reduced plasma glucose with less glycation of macromolecules, play a major role in the life-extending effect of CR (Cerami 1985). However, recent evidence suggests that localized (e.g. adipose tissue and brain) or systemic insulin resistance is associated with increased life span in various genetically altered mice. Fat-specific insulin-receptor knockout (FIRKO) mice, which lack the insulin receptor in adipose tissue, brain insulin receptor substrate 2 null mice, insulin receptor substrate 1 null mice, and Klotho mice are long-lived, despite reduced insulin signaling and/or mild lifelong insulin resistance (Bluher et al. 2003; Taguchi et al. 2007; Selman et al. 2008; Kurosu et al. 2005). Moreover, maximum lifespan is not increased in rats that maintain a low body fat mass by performing regular exercise, whereas maximal lifespan is increased in sedentary paired weight rats that are food-restricted, even though the adipocytes of the exercising animals are more insulin sensitive than the adipocytes of the CR animals (Craig et al. 1987; Holloszy 1997). Finally, transgenic mice overexpressing GLUT4 protein in heart, skeletal muscle, and adipose tissue have lower average daily values of plasma glucose over the lifespan than wild-type mice but do not live longer, whereas maximal lifespan is increased in GLUT-4 transgenic and non-transgenic mice on CR (McCarter et al. 2007).
We are not aware of any studies that have evaluated the effects of long-term CR on glucose homeostasis in humans. Therefore, the purpose of the present study was to determine the effect of long-term CR on glucose tolerance, insulin action, and metabolic variables that affect glucose homeostasis. Circulating levels of glucoregulatory variables (e.g. adiponectin, resistin, IL-6, TNF-alpha receptors, and free fatty acids), glucose, and insulin before and after a glucose load were determined in lean men and women who had been practicing severe CR for years, in age-, sex-, and total body fat-matched endurance runners (EX) consuming a high-calorie diet, and in age- and sex-matched non-obese sedentary subjects consuming Western diets (WD).
Materials and methods
Study subjects Twenty-eight individuals who had been practicing severe CR with adequate nutrition for an average of ∼7 years (range 3–20 years) were recruited through the Calorie Restriction Society; four were from the St. Louis area and the others came to the Washington University Medical Center from other cities in the USA and Canada. Twenty-eight individuals, who are long-term endurance runners, matched with the CR group in terms of age, sex, height, and body fat were used as a lean comparison group. The endurance runners ran an average 48 miles/week (range 20 to 90 miles/week), and had been training regularly for an average of 21 years (range 5–35 years). Twenty-eight sedentary (regular exercise <1 h per week) individuals eating typical Western diets matched with the CR and EX groups in terms of age, sex, and height served as a sedentary comparison group. The characteristics of the study participants are shown in Table 1. None of the participants had evidence of chronic disease, including cardiovascular, lung, gastrointestinal, and autoimmune diseases, or cancer. In particular, none of the CR volunteers reported a diagnosis of type 2 diabetes, impaired glucose tolerance, or impaired fasting glucose before or after starting CR. Only one CR participant reported a positive family history of type 2 diabetes, defined as having at least one diabetic first degree relative, he was in the CR-NGT group. None of the participants were smokers. In addition, none of the participants were taking medications that could have affected the outcome variables. All of the study participants were weight stable, i.e. less than 2 kg weight change in the preceding 6 months. This study was approved by the Human Studies Committee of Washington University School of Medicine, and all participants gave informed consent.
Table 1.
Physical characteristics of the study subjects
| CR group (n = 28) | EX group (n = 28) | WD group (n = 28) | Among group P | |
|---|---|---|---|---|
| Age (years) | 53.0 ± 11 | 54.0 ± 11 | 53.0 ± 10 | ns |
| Sex (M/F) | 24/4 | 24/4 | 24/4 | |
| Height (m) | 1.73 ± 0.1 | 1.75 ± 0.1 | 1.76 ± 0.1 | ns |
| Weight (kg) | 58.1 ± 6.0*,** | 68.0 ± 7.6* | 81.1 ± 14.5 | 0.0001 |
| BMI (kg/m2) | 19.5 ± 1.7*,** | 22.2 ± 2.1* | 26.0 ± 3.0 | 0.0001 |
| Total body fat (%) | ||||
| Men | 9.7 ± 4.6* | 10.9 ± 4.5* | 23.2 ± 6.2 | 0.0001 |
| Women | 20.5 ± 9.9 | 20.1 ± 1.7 | 32.0 ± 7.8 | 0.085 |
| Trunk fat (%) | ||||
| Men | 7.0 ± 5.0* | 8.4 ± 6.0* | 25.2 ± 8.4 | 0.0001 |
| Women | 14.1 ± 8.8 | 13.2 ± 2.6 | 27.5 ± 10.4 | 0.056 |
| Lean mass (kg) | ||||
| Men | 51.7 ± 4.8*,** | 59.2 ± 5.0 | 59.9 ± 8.8 | 0.0001 |
| Women | 38.9 ± 5.3 | 40.3 ± 3.0 | 35.6 ± 2.0 | ns |
Values are means ± SD
*P ≤ 0.0001, significantly different from Western diet group; **P ≤ 0.001, significantly different from EX group
Anthropometrics and body composition Height was measured without shoes to the nearest 0.1 cm. Body weight was obtained on a balance scale in the morning after a 12-h fast. Body mass index (BMI) was calculated by dividing body weight (in kilograms) by the square of height (in meters). Total body fat mass and fat free mass were determined by dual-energy X-ray absorptiometry (QDR 1000/w, Hologic, Waltham, MA, USA).
Dietary assessment Participants were instructed by a research dietician to record all food and beverages consumed, preparation methods, and portion sizes for seven consecutive days. To assist with portion size determinations, measuring spoon and cup sets were provided to the participants, and the food diaries had a ruler imprinted on the back cover. Food records were analyzed by using the NDS-R program (version 4.03_31), which is the Nutrition Data System for research from the Nutrition Coordinating Center at the University of Minnesota.
Fasting blood draw and OGTT All the subjects tested were on a stable diet, at a stable weight, with a stable level of exercise, and without acute illness or recent hospitalization. All of the study participants were eating more than 150 g of carbohydrate per day. All the CR volunteers were admitted to the Washington University GCRC the day before the oral glucose tolerance test (OGTT) and were given a meal containing at least 150 g of carbohydrate between 6 and 8 pm. A venous blood sample was taken to determine glucose, insulin, C-peptide, fructosamine, soluble receptor for advanced glycation end-products (sRAGE), and several circulating glucoregulatory factors concentrations after an overnight fast. Whole blood was allowed to clot and then centrifuged. The serum was stored at −80°C. Commercial ELISA kits were used to measure serum adiponectin (B-Bridge International, Sunnyvale, CA, USA), IL-6, resistin, TNF-α, TNF R-I and TNF R-II, sRAGE (Quantakine High Sensitive, R&D Systems, Minneapolis, MN, USA), and high sensitive C-reactive protein (ALPCO Diagnostics, Windham, NH, USA). Commercially available radioimmunoassay kits were used to measure insulin-like growth factor 1 (IGF-1) and total testosterone, (Diagnostic Systems Laboratories Inc, Webster, TX, USA) and leptin (Linco Research Inc, St. Charles, MO, USA). Serum T3 concentrations were determined by using microparticle enzyme immunoassay (Abbott Laboratories, North Chicago, IL, USA). Fructosamine was assayed with an automatic analyzer Modular Hitachi (Roche, Mannheim, Germany). Plasma free fatty acids were determined using the NEFAC test kit (Wako Chemicals USA, Inc., Richmond, VA, USA). Glycerol-blanked triglycerides were measured by automated enzymatic commercial kits (Miles-Technicon, Tarrytown, NY, USA). High-density lipoprotein cholesterol (HDL-C) was measured in plasma after precipitation of apolipoprotein B-containing lipoproteins by dextran sulfate (50,000 MW) and magnesium.Two-hour, 75-g OGTTs were started between 7 and 9 am. The participants were instructed to refrain from exercise for at least 48 h prior to the OGTT. Plasma glucose was measured by the glucose oxidase method (YSI Stat Plus, Yellow Springs, OH, USA), insulin and C-peptide by double antibody radioimmunoassay (Linco Research). Total areas under the curve (AUCs) were calculated for the OGTT plasma glucose and insulin responses using the trapezoidal rule (Allison et al. 1995). Insulin resistance was calculated using homeostasis model assessment of insulin resistance {HOMA-IR = [fasting glucose (mmol/l) × fasting insulin (mIU/L)]/22.5} (Matthews et al. 1985). An index of insulin sensitivity (ISI) was calculated according to the method of Matsuda and Defronzo (Matsuda & DeFronzo 1999).
VO2max measurement VO2max was determined by indirect calorimetry during an incremental exercise test to exhaustion (Kohrt et al. 1991). Participants walked on a level treadmill at a pace that elicited 60–70% of age-predicted maximal heart rate for a 5-min warm-up. The speed was then set at the fastest comfortable pace, and the grade was increased 1–2% every 1–2 min until volitional exhaustion, electrocardiographic changes, or other abnormalities that rendered it unsafe to continue.
Statistical analysis One-way analysis of variance (ANOVA) was used to compare group variables followed by Tukey post hoc testing where indicated. One-way ANOVA by ranks was performed for non-normally distributed data. Unpaired Student t test (normally distributed variables with approximately equal standard deviations) and Wilcoxon two-samples test (variables not normally distributed, or with unequal standard deviations) were used to compare group variables in the CR-NGT and CR-IGT subgroups. Pearson correlation was used to assess associations between continuous variables. Statistical significance was set at P < 0.05 for all tests. All data were analyzed by using SPSS FOR WINDOWS software, version 12.0 (SPSS Inc, Chicago). All values are expressed as means ± SD.
Results
Body weight and composition
Mean values for body weight and BMI were significantly different between the three groups (Table 1). Total body fat and trunk fat were similar in the CR and EX groups, and lower than in the WD group (Table 1). In men, lean mass was lower in the CR group than in the EX and WD group (Table 1).
Nutrient intake
Nutrient intakes differed significantly between the diet groups. The CR subjects designed their diets in order to consume a balance of foods that supply more than 100% of the recommended daily intake (RDI) for all the essential nutrients, while minimizing energy content (1,112–2,415 kcal/day). They eat a wide variety of vegetables, fruits, nuts, low-fat dairy products, egg whites, wheat and soy proteins, fish, and meat (∼23% calories from protein, ∼28% from fat, ∼48% from complex carbohydrates, and 1% from alcohol). All of the CR groups strictly avoid refined and processed foods containing trans-fatty acids and high glycemic foods (e.g. refined carbohydrates, potato, white rice, and soft drinks). The comparison group ate typical US diets containing nearly twice as many calories as the CR group (1,976–3,537 kcal/day; ∼16% calories from protein, ∼32% from fat, ∼48% from carbohydrates, and 4% from alcohol). As expected the EX group ate a typical American diet but with a higher energy intake to compensate for the energy expended due to the high volume of daily training (2,651–4,459 kcal/day; ∼16% calories from protein, ∼32% from fat, ∼49% from carbohydrates, and 3% from alcohol).
Circulating adipokines, cytokines, and markers of glycation
Serum adiponectin concentration was significantly higher in the CR group than in the EX or WD groups (Table 2). Serum resistin concentration was significantly lower in the CR group than in the WD group (Table 2). Serum IL-6 concentration was significantly and similarly lower in the CR and EX groups than in the WD group (Table 2). Serum soluble TNF R-I and TNF R-II concentrations were significantly and similarly lower in the CR and EX groups than in the WD group (Table 2). Serum fructosamine concentration was significantly higher in the CR group, and tended to be higher in the WD group, than in the EX group (Table 2). Serum sRAGE concentration was significantly higher in the EX group than in the WD group, and tended to be higher than in the CR group (Table 2). Fasting serum free fatty acids were significantly higher in the CR group than in the WD group (Table 2).
Table 2.
Circulating adipokines, cytokines and markers of glycation
| CR group (n = 28) | EX group (n = 28) | WD group (n = 28) | Among group P | |
|---|---|---|---|---|
| Adiponectin (μg/mL) | 15.7 ± 8.2*,** | 11.1 ± 5.5 | 9.5 ± 4.3 | 0.001 |
| Resistin (pg/mL) | 7.0 ± 2.2*** | 8.1 ± 1.7 | 8.7 ± 2.3 | 0.015 |
| IL-6 (pg/ml) | 0.73 ± 0.3* | 0.71 ± 0.3* | 1.21 ± 0.8 | 0.001 |
| s-TNF R-I (ng/mL) | 1.05 ± 0.33*** | 0.95 ± 0.28* | 1.30 ± 0.27 | 0.0001 |
| s-TNF R-II (ng/mL) | 2.77 ± 0.83*** | 2.81 ± 0.69*** | 3.40 ± 0.84 | 0.008 |
| Fructosamine (μmol/L) | 269 ± 40** | 241 ± 17 | 262 ± 34 | 0.005 |
| sRAGE (μg/mL) | 1.27 ± 0.66 | 1.63 ± 0.53*** | 1.11 ± 0.69 | 0.01 |
| Free fatty acids (mEq/L) | 0.72 ± 0.35*** | 0.59 ± 0.18 | 0.51 ± 0.20 | 0.015 |
All values are means ± SD
*P ≤ 0.003, significantly different from Western diet group; **P ≤ 0.05, significantly different from EX group; ***P ≤ 0.05, significantly different from Western diet group
Glucose tolerance and insulin action
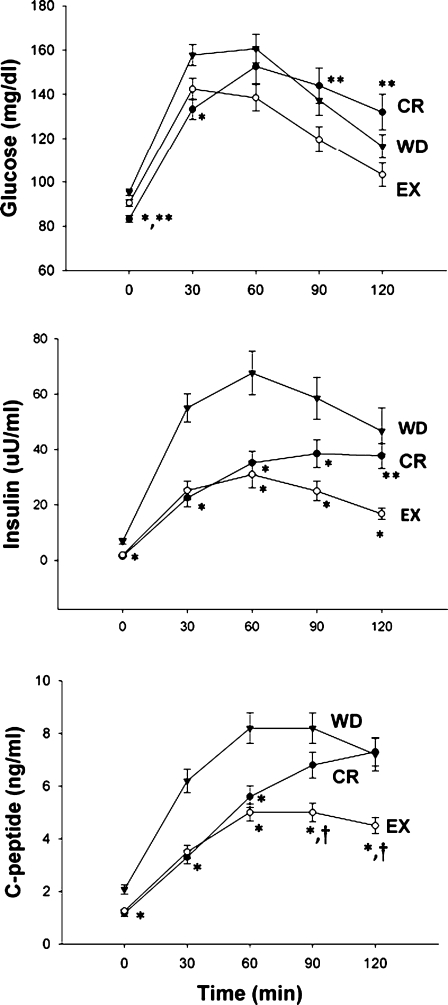
Insulin sensitivity, assessed by HOMA-IR and ISI by Matsuda and De Fronzo were significantly higher in the CR and EX groups than in the WD group (P = 0.001; Table 3). Nonetheless, the 120-min plasma glucose level during the oral glucose tolerance test was higher in the CR group, despite a fasting plasma glucose concentration that was significantly lower in the CR group than in the EX and WD groups (Table 3, Fig. 1). Plasma 30-min glucose concentration was significantly lower in the CR group than in the WD group, whereas plasma 90- and 120-min glucose concentration was significantly higher in the CR group than in the EX group (Table 3, Fig. 1). Plasma insulin concentrations measured at 0, 30, 60, and 90 min after the glucose load were significantly lower in the CR and EX groups than in the WD group (Table 3, Fig. 1). Plasma C-peptide concentrations measured at 0, 30, and 60 min after the glucose load were significantly lower in the CR and EX groups than in the WD group (Table 3, Fig. 1). Plasma C-peptide concentrations measured at 90 min after the glucose load were significantly lower in the EX group than in the CR and WD group (Fig. 1). Plasma 2-h insulin and C-peptide concentrations were significantly lower in the EX group than in the CR and WD groups (Table 3, Fig. 1).
Table 3.
Indices of glucose tolerance and insulin action
| CR group (n = 28) | EX group (n = 28) | WD group (n = 28) | Among group P | |
|---|---|---|---|---|
| HOMA-IR | 0.29 ± 0.1* | 0.44 ± 0.3* | 1.6 ± 1.3 | 0.0001 |
| ISI | 18.5 ± 6.7* | 20.4 ± 9.2* | 7.0 ± 3.6 | 0.0001 |
| Fasting glucose (mg/dL) | 83 ± 8*,** | 91 ± 8 | 95 ± 8 | 0.0001 |
| Fasting insulin (µU/mL) | 1.4 ± 0.7* | 2.0 ± 1.3* | 6.9 ± 5.6 | 0.0001 |
| Fasting C-peptide (ng/mL) | 1.17 ± 0.6* | 1.26 ± 0.35* | 2.1 ± 0.9 | 0.0001 |
| 2-h glucose (mg/dL) | 132 ± 42** | 103 ± 28 | 116 ± 28 | 0.008 |
| 2-h insulin (µU/mL) | 37.7 ± 24*** | 16.8 ± 11**** | 46.7 ± 43.9 | 0.001 |
| 2-h C-peptide (ng/mL) | 7.3 ± 2.9** | 4.5 ± 1.6**** | 7.2 ± 3.3 | 0.0001 |
| Glucose AUC × 103 (mg min/dL) | 16.1 ± 3.2 | 14.9 ± 2.6 | 16.8 ± 3.0 | 0.054 |
| Insulin AUC × 103 (µU min/mL) | 3.5 ± 1.7* | 2.7 ± 1.8* | 6.2 ± 3.6 | 0.0001 |
| C-peptide AUC × 103 (ng min/mL) | 0.59 ± 0.19**** | 0.49 ± 0.15* | 0.82 ± 0.29 | 0.0001 |
| C-peptide AUC/glucose AUC | 0.038 ± 0.012**** | 0.033 ± 0.010* | 0.048 ± 0.013 | 0.0001 |
Values are means ± SD
HOMA-IR homeostasis model assessment of insulin resistance, ISI insulin sensitivity index determined according to Matsuda and DeFronzo (Matsuda & DeFronzo 1999), AUC total area under the curve.
*P = 0.0001, significantly different from Western diet group; **P ≤ 0.006, significantly different from EX group; ***P ≤ 0.05, significantly different from EX group; ****P ≤ 0.05, significantly different from Western diet group
Fig. 1.
Long-term effects of CR and EX on glucose tolerance and insulin action. Mean (±SE) plasma glucose, insulin, and C-peptide concentrations before and after an oral glucose tolerance test for the calorie-restricted (CR) group (black circle), the exercise (EX) group (open circle), and the Western diet (WD) group (black triangle). Baseline values were significantly different between groups for glucose, insulin, and C-peptide (ANOVA). *P ≤ 0.05, significantly different from WD group (Tukey’s tests). **P ≤ 0.05, significantly different from EX group (Tukey’s tests). †P ≤ 0.05, significantly different from CR group (Tukey’s tests)
Body composition, glucose tolerance, insulin action, hormones, and growth factors in two subgroups of CR volunteers.
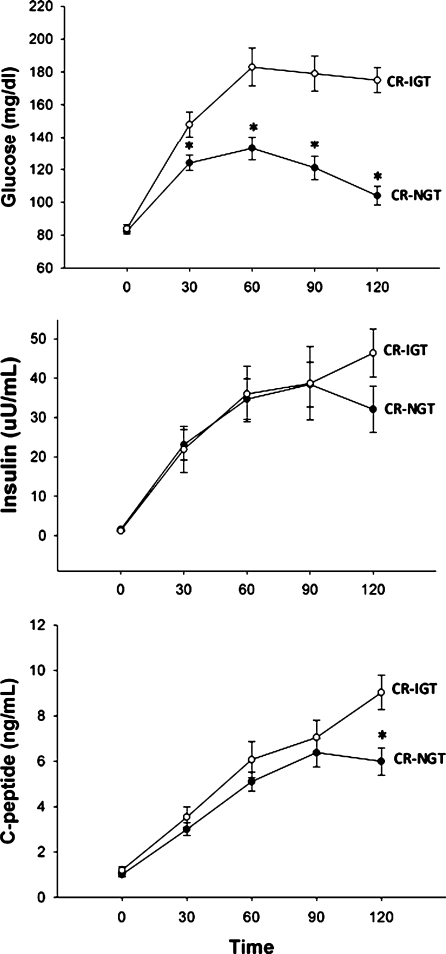
It was evident on inspection of the data that the CR participants fell into two groups, those with normal glucose tolerance (CR-NGT; n = 17) and those with impaired glucose tolerance, i.e., a 2-h glucose level above 140 mg/dl (CR-IGT; n = 11). To try to obtain some insight regarding the mechanism responsible for this difference, we did a post hoc evaluation of the data. There were no significant differences between the CR-NGT and CR-IGT groups in either the HOMA-IR (0.32 ± 0.20 versus 0.24 ± 0.10) or the ISI (19.6 ± 7.6 versus 16.8 ± 4.7). Fasting plasma glucose, insulin, and C-peptide concentrations were similarly low in the two CR subgroups (Fig. 2). Plasma 30-, 60-, 90-, and 120-min glucose concentrations were significantly higher in the CR-IGT subgroup than in the CR-NGT subgroup (Fig. 2). Glucose AUC was significantly higher in the CR-IGT group 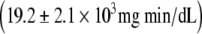 than in the CR-NGT subgroup (
than in the CR-NGT subgroup (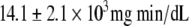 ; P = 0.0001). Plasma insulin and C-peptide concentrations after the glucose load were not significantly different between the two CR subgroups except for the 120-min C-peptide value, which was higher in the CR-IGT groups (Fig. 2). Insulin AUC and C-peptide AUC were not significantly different between the CR-IGT group (
; P = 0.0001). Plasma insulin and C-peptide concentrations after the glucose load were not significantly different between the two CR subgroups except for the 120-min C-peptide value, which was higher in the CR-IGT groups (Fig. 2). Insulin AUC and C-peptide AUC were not significantly different between the CR-IGT group (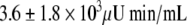 and
and 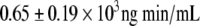 ) and the NGT-CR group (
) and the NGT-CR group (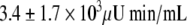 and
and 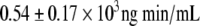 , respectively).
, respectively).
Fig. 2.
Glucose tolerance and insulin action in glucose-intolerant and glucose-tolerant CR individuals. Mean (±SE) plasma glucose, insulin, and C-peptide concentrations before and after an oral glucose tolerance test for the calorie restricted normal glucose tolerance (CR-NGT) subgroup (black circle) and the calorie restricted impaired glucose tolerance (CR-IGT) subgroup (open circle). *P ≤ 0.05, significantly different from CR-IGT subgroup (Student t test)
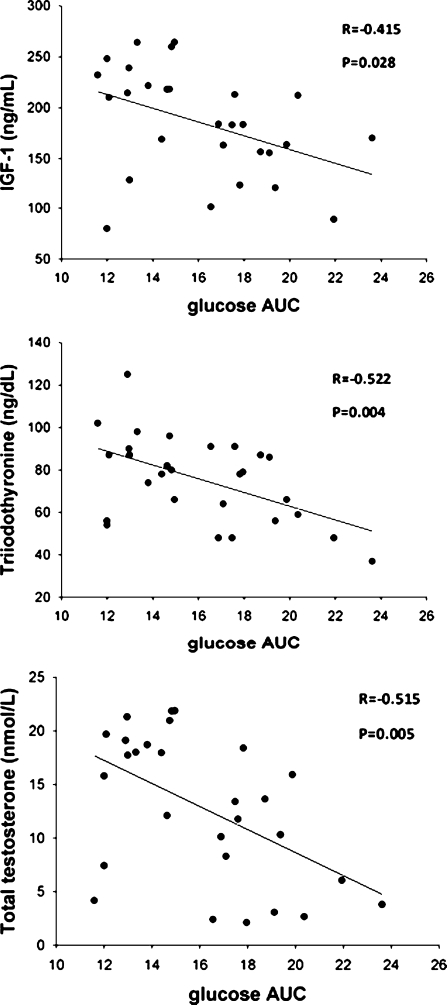
Mean values for age, total body fat, and lean mass were not different between the two CR subgroups, whereas BMI was significantly lower in the CR-IGT subgroup than in the CR-NGT subgroup (Table 4). Total energy intake was not different between the two CR subgroups, but fiber intake was significantly higher in the CR-IGT group (Table 4). VO2max was markedly lower in the CR-IGT subgroup than in the CR-NGT subgroup (Table 4). Serum concentrations of metabolic and inflammatory markers (i.e., HDL-cholesterol, triglycerides, free fatty acids, fructosamine, and C-reactive protein) and adipokines (i.e., adiponectin, leptin) were also not significantly different between the two CR subgroups (Table 4). In contrast, serum concentration of total testosterone and IGF-1 were significantly lower, and serum triiodothyronine concentration tended to be lower, in the CR-IGT group than in the CR-NGT group (Table 4). In the CR group glucose AUC was inversely correlated with serum IGF-1, total testosterone concentrations, and triiodothyronine (Fig. 3).
Table 4.
Body composition, hormones, and growth factors in two subgroups of CR volunteers
| CR-EGT (n = 17) | CR-IGT (n = 11) | P value | |
|---|---|---|---|
| Age (years) | 52.6 ± 11 | 53.6 ± 12 | ns |
| Energy intake (kcal/day) | 1,729 ± 375 | 1,858 ± 350 | ns |
| Total fiber (g/day) | 44 ± 16 | 69 ± 24 | 0.008 |
| VO2max (ml kg−1 min−1) | 44.2 ± 6.8 | 34.5 ± 7.0 | 0.001 |
| BMI (kg/m2) | 20.0 ± 1.8 | 18.6 ± 0.9 | 0.034 |
| Body fat (%) | 11.1 ± 6.7 | 11.6 ± 7.0 | ns |
| Lean mass (kg) | 51.2 ± 6.8 | 47.9 ± 6.0 | ns |
| HDL-c (mg/dL) | 60.0 ± 16 | 63.5 ± 24 | ns |
| Triglycerides (mg/dL) | 55.8 ± 18 | 56.4 ± 20 | ns |
| Free fatty acids (mEq/L) | 0.70 ± 0.32 | 0.73 ± 0.39 | ns |
| Fructosamine (μmol/L) | 260 ± 38 | 284 ± 41 | ns |
| hsCRP (mg/L) | 0.29 ± 0.35 | 0.18 ± 0.13 | ns |
| Adiponectin (μg/mL) | 13.9 ± 5.1 | 18.5 ± 11.2 | ns |
| Leptin (ng/mL) | 1.9 ± 1.5 | 1.8 ± 1.0 | ns |
| IGF-1 (ng/mL) | 205 ± 50 | 154 ± 41 | 0.01 |
| Total testosterone (nmol/L) | 15.5 ± 6 | 8.7 ± 6 | 0.007 |
| Triiodothyronine (ng/dL) | 79.8 ± 20 | 68.7 ± 18 | ns |
Values are means ± SD
Fig. 3.
Relationship between glucose AUC and serum IGF-1 concentration (upper panel), serum triiodothyronine concentration (middle panel), and serum total testosterone concentration (lower panel) in the CR group. Pearson correlation was used to assess associations between continuous variables
Discussion
In this study, we compared glucose tolerance, insulin action, and adipokine production in healthy weight-stable lean men and women, who were consuming a self-imposed CR diet, containing more than 100% of the RDI for all essential nutrients, for 3 to 20 years, with age-, sex-, and body fat- matched endurance runners, and age- and sex-matched sedentary individuals, who were consuming Western diets. Our data show that insulin sensitivity, determined by HOMA-IR index and the Matsuda and DeFronzo ISI, was significantly higher in the CR and EX groups than in the WD group. However, although the average values for the HOMA-IR and the Matsuda ISI indicate that the CR group was more insulin sensitive than the WD group, ∼40% of the CR individuals exhibited impaired glucose tolerance in response to a glucose load, which suggests that HOMA-IR is not a good surrogate marker of insulin sensitivity in people practicing CR. Interestingly, serum fructosamine concentration, a marker of glycation, was higher in the CR and WD groups than in the EX group.
Aging and many age-associated diseases in both humans and rodents are associated with progressive increase in fasting insulin concentration and insulin resistance (Meigs et al. 2003; Basu et al. 2003; Reaven 1995). However, the relationship between aging/aging-related diseases and insulin resistance is confounded by associated factors such as excessive abdominal adiposity, decreased physical activity, hyperinsulinemia, dyslipedemia, inflammation, and other metabolic and hormonal components of the metabolic syndrome (Barzilai et al. 1998; Reaven 1995). It is intriguing that based on the 2-h OGTT plasma glucose values, 11 of our CR subjects met diagnostic criteria for glucose intolerance (Genuth et al. 2003), and six of the CR-NGT subjects had high–normal 2-h glucose values, even though all CR subjects were extremely lean and had very low fasting plasma concentrations of glucose and insulin, and an outstanding metabolic profile (very low triglyceride, high HDL-cholesterol, high adiponectin, and extremely low C-reactive protein concentrations; Fontana et al. 2006). The elevated glucose levels after the glucose load, in the CR-IGT group, compared to the CR-NGT group, cannot reflect acute CR, because all the volunteers were instructed to maintain their usual diet and weight stability. Carbohydrate restriction seems also unlikely as a possible cause for the glucose intolerance in the CR-IGT group, both because 150 g of carbohydrate appears to be sufficient to prevent impairment of glucose tolerance due to carbohydrate deficiency (Wilkerson et al. 1960; American Diabetes Association 2009) and because the CR-NGT group carbohydrate intake was similar to that of the CR-IGT group. Finally, the elevated glucose levels after the glucose load, in the CR-IGT group, compared to the CR-NGT group, cannot be explained by a reduced insulin response as their insulin levels were similar during the OGT. Therefore, our data suggest that severe chronic CR, in some individuals, may be associated with a relative peripheral insulin resistance mainly due to a low muscle mass with decreased capacity to take up glucose. This will have to be confirmed by more definitive studies that can directly assess insulin secretion and sensitivity.
One possible explanation for the reduced glucose disposal following a glucose load in the CR practitioners may be a protective physiological adaptation to prevent hypoglycemia, which is considered part of the adaptive response to fasting (Jensen et al. 1987). This hypothesis is supported by the finding that impaired glucose tolerance in the CR-IGT group was associated with lower circulating levels of IGF-1, total testosterone, leptin, and triiodothyronine, which are key metabolic/hormonal adaptations to CR in rodents (Fontana & Klein 2007). The combination of decreased fasting levels of serum triiodothyronine, leptin, and anabolic hormones (i.e., IGF-1, testosterone, and insulin), and increased levels of adiponectin is a clear indication that these individuals are in a state of “sensing” severe energy restriction.
During periods of severe food deprivation, increased insulin sensitivity and glucose disposal are clearly detrimental to survival of the organism, because of severe hypoglycemia risk. In contrast, decreased insulin sensitivity and glucose disposal could enhance survival, by preventing hypoglycemia and maintaining circulating glucose for organs that require glucose as a fuel (Cahill 2006). This hypothesis is supported by the finding that chronic CR or prolonged starvation in normal mice induces impairment of insulin signaling at a receptor and post-receptor level (Rao 1995; Du et al. 2003). For example, prolonged starvation causes an elevation of the insulin signaling (Akt) inhibitor TRB3 in the liver, despite the very low levels of circulating insulin (Du et al. 2003). Moreover, prolonged fasting in insulin-resistant hypoinsulinemic rats causes a reduction in insulin-induced phosphorylation of the protein Shc (an insulin receptor substrate) in the liver and adipose tissue, whereas a significant increase in Shc activation was observed in adipose tissue, skeletal muscle, and liver of insulin-resistant hyperinsulinemic old rats (Páez-Espinosa et al. 1999). Thus, from an evolutionary point of view, a CR-mediated reduction in insulin signaling could be a protective metabolic response against hypoglycemia when food is scarce.
Interestingly, recent evidence suggests that decreased insulin signaling, and not enhanced insulin sensitivity, is implicated in the delayed aging phenotype of some of the animal models of increased longevity, such as klotho transgenic mice, insulin receptor substrate 1 null mice, brain insulin receptor substrate 2 null mice, and FIRKO mice (Bluher et al. 2003; Taguchi et al. 2007; Selman et al. 2008; Kurosu et al. 2005). It is also intriguing that glucose tolerance in response to a glucose load was reduced, and insulin sensitivity in response to an intravenous insulin tolerance test was enhanced in long-lived Ames dwarf and growth hormone receptor KO mice, that have extremely low circulating levels of fasting IGF-1 and insulin, and hypothyroidism (Dominici et al. 2002; Coschigano et al. 1999). Therefore, it may be possible that severe CR decreases signaling through the insulin pathway in some tissues and this may play a role in mediating expression of anti-aging genes and negatively regulating the expression of pro-aging genes by reducing AKT/protein kinase B (PKB) and class O of forkhead box transcription factors (FoxO) activities (Dominici et al. 2003; Hsieh and Papaconstantinou 2004; Salih and Brunet 2008).
While the finding that various strains of long-lived mice are insulin resistant is interesting relative to our finding of IGT in the CR-IGT group, it must be kept in mind that there is currently no evidence that CR slows primary aging in humans. Also, it is not known which of the biological effects of CR in rodents are responsible for the increase in maximal life span. So, the idea that the “insulin resistance” in the CR-IGT group might have the effect of slowing aging, based on the finding that a number of insulin-resistant strains of mice are long-lived is just a hypothesis/speculation at this point.
The mechanism responsible for the higher glucose tolerance and insulin action, and lower glycation of plasma proteins in our endurance athletes is likely related to exercise itself, rather than changes in body composition caused by exercise. Even though relative fat mass was low and similar in the EX and CR groups, 2-h glucose and 2-h insulin concentrations were ∼22% and ∼55% lower in the EX group than in the CR group. Serum fructosamine concentration, a marker of nonenzymatic glycation of serum proteins that integrates plasma glucose fluctuations over the preceding 2–3 weeks (Tahara and Shima 1995), was higher in the CR group than in the EX group. In addition, soluble RAGE, a decoy receptor that antagonizes the toxic effects of RAGE-mediated signaling, was higher in the EX group than in the CR and WD groups (Koyama et al. 2007). It is well known that endurance exercise training has positive effects on glucose tolerance, insulin sensitivity, and responsiveness in addition to those that result from a low adiposity (Holloszy 2005). Physical training causes multiple adaptations in skeletal muscle that contribute to increased insulin action, including up-regulation of muscle GLUT4 protein, and increased enzyme capacities, that disappears after few days of detraining (Ebeling et al. 1993; Yu et al. 2001; Hughes et al. 1993). While exercise induces an acute, insulin-independent activation of glucose transport in muscle, this effect wears off within 3 to 4 h and is replaced by increases in insulin sensitivity and responsiveness (Holloszy 2005).
This study has potential limitations. One is the fact that the CR volunteers are a heterogeneous group, and therefore differences in macro- or micronutrient content of their diets as well as in fitness (as evidenced by the lower VO2 max in the IGT-CR group) may be responsible, at least in part, for the differences in glucose tolerance. Another limitation was the lack of use of a glucose clamp to calculate insulin sensitivity. On the other hand, a limitation of the hyperinsulinemic euglycemic clamp is that utilizes steady-state insulin levels that may be supraphysiological, resulting in a reversal of the normal portal to peripheral insulin gradient.
In conclusion, the results of this study demonstrate that endurance exercise training is associated with better glucose tolerance, insulin action, and protein glycation, than severe CR, independent of body fat mass and basal adipokine production. The results of this study provide the new information that long-term severe CR is associated with impaired glucose tolerance in some individuals, presumably because of decreased insulin-mediated glucose disposal. This reduced glucose disposal is associated with lower circulating levels of IGF-1, total testosterone, and triiodothyronine, which are typical adaptations to CR in rodents.
Acknowledgements
We are grateful to the study participants for their cooperation and to the staff of the Applied Physiology Laboratory and nurses of the General Clinical Research Center at WUMS for their skilled assistance. The study design was developed by LF and JOH; data collection was performed and supervised by LF; data analyses and interpretation were performed by LF, SK, and JOH; writing was performed by LF, SK, and JOH. LF had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All the authors declare that they participated in the study as mentioned above and that they reviewed and approved the manuscript in its final version.
Financial disclosures The author had no conflicts of interest.
Funding/support This study was supported by Grant Number UL1 RR024992 from the National Center for Research Resources (a component of the National Institutes of Health and NIH Roadmap for Medical Research), by Istituto Superiore di Sanità/National Institutes of Health Collaboration Program Grant, a grant from the Longer Life Foundation (an RGA/Washington University Partnership), and a donation from the Scott and Annie Appleby Charitable Trust.
Role of the sponsor The funding agencies had no role in the analysis or interpretation of the data or in the decision to submit the report for publication.
References
- Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes Care. 1995;18:245–250. doi: 10.2337/diacare.18.2.245. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(Suppl 1):S62–S67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Banerjee S, Hawkins M, Chen W, Rossetti L. Caloric restriction reverses hepatic insulin resistance in aging rats by decreasing visceral fat. J Clin Invest. 1998;101:1353–1361. doi: 10.1172/JCI485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Breda E, Oberg AL, Powell CC, Man C, Basu A, Vittone JL, Klee GG, Arora P, Jensen MD, Toffolo G, Cobelli C, Rizza RA. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003;52:1738–1748. doi: 10.2337/diabetes.52.7.1738. [DOI] [PubMed] [Google Scholar]
- Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- Bodkin NL, Ortmeyer HK, Hansen BC. Long-term dietary restriction in older-aged rhesus monkeys: effects on insulin resistance. J Gerontol A Biol Sci Med Sci. 1995;50:B142–B147. doi: 10.1093/gerona/50a.3.b142. [DOI] [PubMed] [Google Scholar]
- Cahill GF., Jr Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258. [DOI] [PubMed] [Google Scholar]
- Cefalu WT, Wagner JD, Wang ZQ, Bell-Farrow AD, Collins J, Haskell D, Bechtold R, Morgan T. A study of caloric restriction and cardiovascular aging in cynomolgus monkeys (Macaca fascicularis): a potential model for aging research. J Gerontol A Biol Sci Med Sci. 1997;52:B10–B19. doi: 10.1093/gerona/52a.1.b10. [DOI] [PubMed] [Google Scholar]
- Cerami A. Hypothesis: glucose as a mediator of aging. J Am Geriatr Soc. 1985;33:626–634. doi: 10.1111/j.1532-5415.1985.tb06319.x. [DOI] [PubMed] [Google Scholar]
- Coschigano KT, Riders ME, Bellush LL, Kopchick JJ (1999) Glucose metabolism in growth hormone receptor/binding protein gene disrupted mice. Annual Meeting, The Endocrine Society. Abstract P3, p. 553
- Craig BW, Garthwaite SM, Holloszy JO. Adipocyte insulin resistance: effects of aging, obesity, exercise, and food restriction. J Appl Physiol. 1987;62:95–100. doi: 10.1063/1.339112. [DOI] [PubMed] [Google Scholar]
- Dominici FP, Hauck S, Argentino DP, Bartke A, Turyn D. Increased insulin sensitivity and upregulation of insulin receptor, insulin receptor substrate (IRS)-1 and IRS-2 in liver of Ames dwarf mice. J Endocrinol. 2002;173:81–94. doi: 10.1677/joe.0.1730081. [DOI] [PubMed] [Google Scholar]
- Dominici FP, Argentino DP, Bartke A, Turyn D. The dwarf mutation decreases high dose insulin responses in skeletal muscle, the opposite of effects in liver. Mech Ageing Dev. 2003;124:819–827. doi: 10.1016/S0047-6374(03)00136-2. [DOI] [PubMed] [Google Scholar]
- Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003;300:1574–1577. doi: 10.1126/science.1079817. [DOI] [PubMed] [Google Scholar]
- Ebeling P, Bourey R, Koranyi L, Tuominen JA, Groop LC, Henriksson J, Mueckler M, Sovijärvi A, Koivisto VA. Mechanism of enhanced insulin sensitivity in athletes. Increased blood flow, muscle glucose transport protein (GLUT-4) concentration, and glycogen synthase activity. J Clin Invest. 1993;92:1623–1631. doi: 10.1172/JCI116747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Klein S. Aging, adiposity, and calorie restriction. JAMA. 2007;297:986–994. doi: 10.1001/jama.297.9.986. [DOI] [PubMed] [Google Scholar]
- Fontana L, Klein S, Holloszy JO, Premachandra BN. Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J Clin Endocrinol Metab. 2006;91:3232–3235. doi: 10.1210/jc.2006-0328. [DOI] [PubMed] [Google Scholar]
- Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P, Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.12.3331. [DOI] [PubMed] [Google Scholar]
- Gresl TA, Colman RJ, Roecker EB, Havighurst TC, Huang Z, Allison DB, Bergman RN, Kemnitz JW. Dietary restriction and glucose regulation in aging rhesus monkeys: a follow-up report at 8.5 yr. Am J Physiol Endocrinol Metab. 2001;281:E757–E765. doi: 10.1152/ajpendo.2001.281.4.E757. [DOI] [PubMed] [Google Scholar]
- Holloszy JO. Mortality rate and longevity of food-restricted exercising male rats: a reevaluation. J Appl Physiol. 1997;82:399–403. doi: 10.1152/jappl.1997.82.2.399. [DOI] [PubMed] [Google Scholar]
- Holloszy JO. Exercise-induced increase in muscle insulin sensitivity. J Appl Physiol. 2005;99:338–343. doi: 10.1152/japplphysiol.00123.2005. [DOI] [PubMed] [Google Scholar]
- Hsieh CC, Papaconstantinou J. Akt/PKB and p38 MAPK signaling, translational initiation and longevity in Snell dwarf mouse livers. Mech Ageing Dev. 2004;125:785–798. doi: 10.1016/j.mad.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Hughes VA, Fiatarone MA, Fielding RA, Kahn BB, Ferrara CM, Shepherd P, Fisher EC, Wolfe RR, Elahi D, Evans WJ. Exercise increases muscle GLUT-4 levels and insulin action in subjects with impaired glucose tolerance. Am J Physiol. 1993;264:E855–E862. doi: 10.1152/ajpendo.1993.264.6.E855. [DOI] [PubMed] [Google Scholar]
- Jensen MD, Haymond MW, Gerich JE, Cryer PE, Miles JM. Lipolysis during fasting. Decreased suppression by insulin and increased stimulation by epinephrine. J Clin Invest. 1987;79:207–213. doi: 10.1172/JCI112785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalant N, Stewart J, Kaplan R. Effect of diet restriction on glucose metabolism and insulin responsiveness in aging rats. Mech Ageing Dev. 1988;46:89–104. doi: 10.1016/0047-6374(88)90117-0. [DOI] [PubMed] [Google Scholar]
- Kemnitz JW, Roecker EB, Weindruch R, Elson DF, Baum ST, Bergmann RN. Dietary restriction increases insulin sensitivity and lowers blood glucose in rhesus monkeys. Am J Physiol. 1994;266:E540–E547. doi: 10.1152/ajpendo.1994.266.4.E540. [DOI] [PubMed] [Google Scholar]
- Kohrt WM, Malley MT, Coggan AR, Spina RJ, Ogawa T, Ehsani AA, Bourey RE, Martin WH, 3rd, Holloszy JO. Effects of gender, age, and fitness level on the response of VO2max to training in 60- to 71-year olds. J Appl Physiol. 1991;71:2004–2011. doi: 10.1152/jappl.1991.71.5.2004. [DOI] [PubMed] [Google Scholar]
- Koyama H, Yamamoto H, Nishizawa Y. RAGE and soluble RAGE: potential therapeutic targets for cardiovascular diseases. Mol Med. 2007;13:625–635. doi: 10.2119/2007-00087.Koyama. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Ball SS, Ingram DK, Cutler RG, Engel J, Reed V, Roth GS. Diet restriction in rhesus monkeys lowers fasting and glucose-stimulated glucoregulatory end points. Am J Physiol. 1995;268:E941–E948. doi: 10.1152/ajpendo.1995.268.5.E941. [DOI] [PubMed] [Google Scholar]
- Masoro EJ, McCarter RJM, Katz MS, McMahan CA. Dietary restriction alters the characteristics of glucose fuel use. . J Gerontol Biol Sci. 1992;47:B202–B208. doi: 10.1093/geronj/47.6.b202. [DOI] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rodenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- McCarter R, Mejia W, Ikeno Y, Monnier V, Kewitt K, Gibbs M, McMahan A, Strong R. Plasma glucose and the action of calorie restriction on aging. J Gerontol A Biol Sci Med Sci. 2007;62:1059–1070. doi: 10.1093/gerona/62.10.1059. [DOI] [PubMed] [Google Scholar]
- Meigs JB, Muller DC, Nathan DM, Blake DR, Andres R, Baltimore Longitudinal Study of Aging The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes. 2003;52:1475–1484. doi: 10.2337/diabetes.52.6.1475. [DOI] [PubMed] [Google Scholar]
- Páez-Espinosa EV, Rocha EM, Velloso LA, Boschero AC, Saad MJ. Insulin-induced tyrosine phosphorylation of Shc in liver, muscle and adipose tissue of insulin resistant rats. Mol Cell Endocrinol. 1999;156:121–129. doi: 10.1016/S0303-7207(99)00137-9. [DOI] [PubMed] [Google Scholar]
- Reaven GM. Pathophysiology of insulin resistance in human disease. Physiol Rev. 1995;75:473–486. doi: 10.1152/physrev.1995.75.3.473. [DOI] [PubMed] [Google Scholar]
- Rao RH. Adaptations in glucose homeostasis during chronic nutritional deprivation in rats: hepatic resistance to both insulin and glucagon. Metabolism. 1995;44:817–824. doi: 10.1016/0026-0495(95)90199-X. [DOI] [PubMed] [Google Scholar]
- Salih DA, Brunet A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol. 2008;20:126–136. doi: 10.1016/j.ceb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C, Lingard S, Choudhury AI, Batterham RL, Claret M, Clements M, Ramadani F, Okkenhaug K, Schuster E, Blanc E, Piper MD, Al-Qassab H, Speakman JR, Carmignac D, Robinson IC, Thornton JM, Gems D, Partridge L, Withers DJ. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J. 2008;22:807–818. doi: 10.1096/fj.07-9261com. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- Tahara Y, Shima K. Kinetics of HbA1c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care. 1995;18:440–447. doi: 10.2337/diacare.18.4.440. [DOI] [PubMed] [Google Scholar]
- Yu M, Blomstrand E, Chibalin AV, Wallberg-Henriksson H, Zierath JR, Krook A. Exercise-associated differences in an array of proteins involved in signal transduction and glucose transport. J Appl Physiol. 2001;90:29–34. doi: 10.1152/jappl.2001.90.1.29. [DOI] [PubMed] [Google Scholar]
- Wilkerson HL, Hyman H, Kaufman M, McCuistion AC, Francis JO. Diagnostic evaluation of oral glucose tolerance tests in nondiabetic subjects after various levels of carbohydrate intake. N Engl J Med. 1960;262:1047–1053. doi: 10.1056/NEJM196005262622101. [DOI] [PubMed] [Google Scholar]