Abstract
A postural vertical (PV) tilted backward has been put forward as a reason explaining the backward disequilibrium often observed in elderly fallers. This raises the question of a possible ageing process of the PV involving a backward tilt of verticality perception increasing with age. We have explored this hypothesis by measuring PV in pitch using the wheel paradigm in 87 healthy subjects aged from 20 to 97 years. The possibility that this physiological ageing accelerated in the second part of life was also analysed. Two indices were calculated: the mean orientation (PV-orient) and the dispersion (PV-uncert). The correlation between age and PV-orient was r = −0.2 (p < 0.05). Added to the fact that PV was twice as shifted backward in the 38 seniors over 50 years (−1.15° ± 1.40°) as in the 49 young adults under 50 years (−0.45° ± 0.97°; t = 2.75, p < 0.01), this indicates the existence of a physiological ageing process on the direction perceived as vertical by the whole body, with a slight backward shift of PV throughout the life span. The correlation between age and PV-uncert was r = 0.35 (p < 0.001) in all subjects and r = 0.59 (p < 0.001) in seniors. This indicates that subjects get less and less accurate in their perception of the postural vertical with age, especially very old subjects who show great uncertainty in determining with their body the direction of the vertical. Taken together, these findings indicate that the internal model of verticality is less robust in elderly people. This may play a part in their postural decline.
Keywords: Verticality, Postural control, Subjective vertical, Postural vertical, Ageing
Introduction
The perception of the vertical (subjective vertical) is perfectly aligned with the gravitational vertical in normal subjects. Certain diseases may alter the perception of the vertical, in the frontal and/or the sagittal plane. In the frontal plane, a tilted perception of the vertical and/or an uncertainty in the perception of the vertical is frequently observed in patients with a hemisphere stroke (Bonan et al. 2006; Brandt et al. 1994; Perennou et al. 1998; Perennou et al. 2000; Perennou et al. 2008; Saj et al. 2005; Yelnik et al. 2002), or a peripheral or central vestibular disease (Aoki et al. 1999; Bisdorff et al. 1996; Bronstein et al. 2003; Dieterich and Brandt 1992). In the sagittal plane, a tilted perception of the vertical has recently been found in elderly fallers (Manckoundia et al. 2007). Some recent papers have clearly established that lateropulsion and retropulsion may be the consequences of a tilted internal model of verticality, in the frontal and the sagittal plane, respectively (Manckoundia et al. 2007; Perennou et al. 2008). Among the different modalities to evaluate the subjective vertical, i.e. the visual vertical, the haptic vertical and the postural vertical (PV), the latter is the most relevant in explaining balance disorders (Manckoundia et al. 2007; Perennou et al. 1998; Perennou et al. 2008). For research purposes, the measurement of PV is often performed by means of a motorised driven machine (Bisdorff et al. 1996; Ito and Gresty 1996; Van Beuzekom and Van Gisbergen 2000), little suited to disabled patients in terms of accessibility into the machine and also in terms of noise and ill-secured surroundings. A non-motorised paradigm, namely the wheel paradigm, has been recently designed to measure the PV in a clinical context, both in the frontal plane (Mazibrada et al. 2008; Perennou 2006) and in the sagittal plane (Manckoundia et al. 2007). Although this paradigm has been found to be relevant in a clinical context, its normative values and clinimetric properties remain to be investigated in a wide number of subjects of different ages, especially in measurements concerning the sagittal plane. In light of two recent studies in which PV was measured in the sagittal plane using the wheel paradigm in young and older healthy people (Barbieri et al. 2008; Manckoundia et al. 2007), a question arose about a possible ageing process involving the perception of PV, characterised by a slight backward tilt in the normal elderly. Whether this possible physiological ageing shows acceleration in the second part of life was also open to question. These were the issues of this study.
Materials and methods
Subjects
The PV in the pitch plane was measured in 87 able-bodied subjects (38 females and 49 males) aged from 20 to 97 years. Fifty years being the value closest to the median and mean age (45 and 48 years, respectively) and divisible by ten, was used as the cut-off point to divide this cohort into two age groups: young adults <50 years (n = 49), senior >50 years (n = 38). Subjects were recruited among the students, paramedical and medical staff working in our laboratory and rehabilitation centre, and among their relatives for the oldest participants. All gave their informed consent to participate in this study according to the recommendations of the local ethics committee. None of them reported having any known neurological or motor disorder and their score on the backward disequilibrium test (Manckoundia et al. 2007) was 0, which means that they were perfectly upright whilst seated, standing with and without vision and performing sit to stand or stand to sit movements. All had a normal gait.
Ten young adults repeated the measurement procedures a second time with another experimenter in order to test the inter-experimenter reproducibility.
Measurement of PV
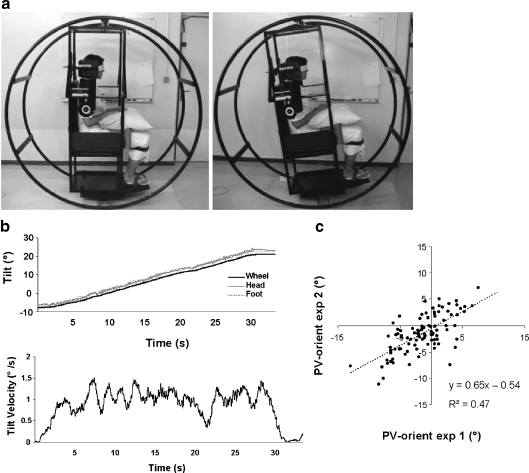
The experimental setting (Fig. 1a) used was that designed by Manckoundia et al. (2007) to measure the backward tilt of PV in the elderly and derived from that previously elaborated by Bisdorff et al. (1996) to evaluate the PV in the frontal and sagittal planes. The subjects were strapped in a sitting position to a framework inside a 180-cm diameter × 80-cm wide drum made of welded steel tubes, with head, trunk, thighs and legs restrained by webbing and pads and aligned upright, and the feet strapped on a plane support. The experimental setting used was designed to prevent subjects from moving. Before each measurement of PV, the experimenters visually ensured that no movement was possible. The assessment started only after this check. Subjects’ eyes were closed and entirely masked.
Fig. 1.
a Experimental setting to measure PV. Left the subject is oriented upright; right the subject is inclined −10° backward. b Wheel rotation induces the same body rotation, symbolised by no movement of the head and the feet (upper part). The mean angular velocity was around 1–1.5° per second, except when the movement started/stopped for minor positional adjustments (lower part). c Scatter plot of the PV orientations measured for each angle by the two experimenters with ten subjects (n = 100). The equation and the coefficient of determination (r²) of the linear regression are noted
Measurement of the PV was carried out according to a random order after several practise trials to familiarise the subject with the procedure. No feedback was given to subjects about their performance before the whole assessment was completed. In total darkness, the subjects were randomly tilted to a given position (backward or forward at 10°, 15°, 20°, 25° or 30°), then the wheel was manually rolled in the opposite direction, as steadily and smoothly as possible at an approximate velocity of 1.5° per second which is the threshold of stimulation of the semicircular vestibular organs (Benson et al. 1989), until subjects reported reaching an upright position. Small adjustments around this position were then performed if needed until the subjects were satisfied that they were perfectly vertical. To control rolling velocity, only two well-trained experimenters, capable of manually ensuring rolling velocity at less than 1.5° per second, carried out the assessments.
Ten trials were performed in a pseudo-random sequence, five from front to back (starting position 10°, 15°, 20°, 25° or 30°) and five from back to front (starting position −10°, −15°, −20°, −25° or −30°) alternatively. Trials were performed according to an unpredictable experimental plan with an initial wheel position which was neither constant nor specific in order to prevent subjects from using time representation to perform the task. The orientation of the PV was measured by means of an inclinometer indicating 0° for the gravitational vertical, negative values for backward orientation and positive values for forward orientation. The PV orientation was obtained by averaging first the five trials from the front (PV-front), then the five trials from the back (PV-back) and finally the ten trials (PV-orient). The total PV uncertainty (PV-uncert) was obtained by averaging the standard deviation of the five trials from front to back (PV-front SD) and that of the five trials from back to front (PV-back SD).
Pilot measurements
Kinematic analysis during PV measurement
In order to check that subjects were passively tilted with the wheel without significant differential displacements of body segments and that the tilt velocity was close to 1.5° per second, pilot measurements were associated in one subject to kinematic analysis with the SMART-e movement analysis system (sample rate, 120 Hz). Measures were taken with reflective targets (15 mm diameter) arranged all around the wheel and at the head and feet of the subject. SMART acquisition started and stopped with a 1- or 2-s delay before and after the wheel tilt.
Inter-experimenter reproducibility
PV measurements in ten subjects were taken two times by two different experimenters (exp 1 and exp 2) well trained to manipulate the set-up. Half of the subjects completed their first session with exp1 and the other half with exp2 to avoid a session effect. Between each session, the wheel was manually replaced in the vertical position and the subject remained strapped to the seat with eyes open for 5 min. The second experimenter was not informed of the results obtained in the first session.
Statistics
Statistical analysis was performed using the programme SPSS (version 14.0 for Windows). The normality of PV-orient and PV-uncert distributions was tested using the Kolmogorov–Smirnov test. PV-orient and PV-uncert were normal in the 87 subjects. PV-orient and PV-uncert were also normal in the young adults, whereas in the seniors, only PV-orient was normal. Correlation analyses (for the entire group or the two age groups) between age and PV-orient or PV-uncert were carried out using the Pearson test if the distribution considered was normal or the Spearman test if the distribution considered was not normal. Regarding the PV-orient in all subjects and in the two specific age groups, normal PV-orient and differences from the vertical (zero) were tested using the sample T test. Comparisons between the young adults and the seniors were carried out by means of the Kolmogorov–Smirnov test. Groups were also compared with regard to the positive/negative ratio of PV using the χ² test. Differences for age, gender and procedure (starting direction and starting position) were tested using ANOVAs (with Tukey tests as post hoc analyses). Regarding the PV-uncert, comparisons between the young adults and the senior adults were carried out using a Mann–Whitney test and a Kolmogorov–Smirnov test. Regarding the inter-experimenter reproducibility, the error in PV-orient was calculated as the difference between the two experimenters and compared from the vertical (zero) using a sample T test. In addition, the correlation between measurements of each trial was calculated using the Pearson correlation. Data are given in the text as mean ± standard deviation (SD).
Results
Pilot measurements
Kinematic analysis during PV measurement
During the manual rotation of the wheel, no unwanted movements of the head or feet appeared, meaning that the body passively followed the wheel orientation (Fig. 1b—upper part). The derivative of the wheel position signal (velocity) showed that the mean angular velocity was situated between 1° and 1.5° per second, except during the start/stop movements for minor positional adjustments (Fig. 1b—lower part).
Inter-experimenter reproducibility
The inter-experimenter reproducibility was accurate with a mean absolute error of 0.8° and no systematic error between the two experimenters or difference from the reference 0° (0.05° ± 1.18°, t = −0.16, p > 0.05). There was no difference between the two experimenters, whatever the starting position (F = 0.01, p > 0.05). The correlation between the PV-orient as measured by exp 1 and exp 2 was r = 0.7, p < 0.001 (Fig. 1c).
Age influence on verticality perception
Correlation analysis
PV data according to the demographic characteristics of subjects are given in Table 1. Tested in all subjects, the distribution of PV-orient and PV-uncert were Gaussian. The correlation between age and PV-orient was r = −0.2 (p < 0.05), meaning that as age increased PV magnitude increased with a negative polarity. The correlation between age and PV-uncert was r = 0.35 (p < 0.001), meaning that uncertainty increased with age. No gender effect was found on PV-orient [F(1,86) = 1; p > 0.05] or on PV-uncert [F(1,86) = 2.66; p > 0.05].
Table 1.
Comparative analysis of PV-orient and PV-uncert for each group and gender, presented in the form mean ± standard deviation
| Variable | Group | Total | Female | Male | |||
|---|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | ||
| PV-orient | Young | 49 | −0.45 ± 0.97 | 23 | −0.45 ± 1.00 | 26 | −0.50 ± 0.96 |
| Senior | 38 | −1.15 ± 1.40 | 15 | −0.86 ± 1.08 | 23 | −1.34 ± 1.56 | |
| Total | 87 | −0.76 ± 1.22 | 38 | −0.61 ± 1.04 | 49 | −0.87 ± 1.34 | |
| PV-uncert | Young | 49 | 1.83 ± 0.62 | 23 | 1.73 ± 0.57 | 26 | 1.91 ± 0.66 |
| Senior | 38 | 2.21 ± 1.21 | 15 | 1.94 ± 0.55 | 23 | 2.39 ± 1.48 | |
| Total | 87 | 1.99 ± 0.94 | 38 | 1.81 ± 0.57 | 49 | 2.14 ± 1.13 | |
Young vs. senior adults
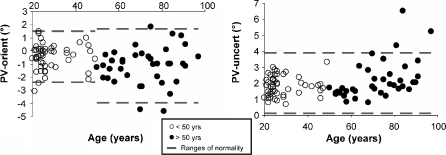
Since PV-orient distributions for both groups were Gaussian, groups were compared using a parametric T test. PV was more shifted backward in seniors (−1.15° ± 1.40°) than in young adults (−0.45° ± 0.97°; t = 2.75, p < 0.01), PV being significantly different from the gravitational vertical both in seniors (t = −5.07, p < 0.001) and in young adults (t = −3.28, p < 0.01). The ranges of normality per group, defined as mean ± 2SD, ranged from −2.39° to 1.5° and from −3.95° to 1.65° in young adults and seniors, respectively (Fig. 2a). The symmetry of these ranges of normality was then analysed by adding up the PV-front and the PV-back and by comparing the sum to 0° with a sample T test. As distributions of the two age groups were normal, a sample T test was performed for each group. For each group, the value found was different from zero, meaning that normative values were not symmetrical around the physical vertical (young adults = −0.91° ± 1.93°, t = −3.28, p < 0.01; senior adults = −2.3° ± 2.79°, t = −5.07, p < 0.001). In addition, PV values were more frequently negative in seniors than in young adults (32 negative/six positive vs. 32 negative/17 positive; χ² = 3.93, p < 0.05). PV data was also more scattered in seniors than in young adults (Z = 1.56, p < 0.05).
Fig. 2.
Age-related distribution of a the mean PV orientation and b the mean PV uncertainty. The two age groups are represented. For each group, the ranges of normality are represented by the values contained between the upper and lower dotted lines
Since distribution of PV-uncert data was Gaussian in subjects under 50 years but not over, groups were compared using a Mann–Whitney test. Surprisingly, no significant difference was found (ZU = −0.94, p > 0.05). The apparent discrepancy between the existence of a significant ageing process on the 87 subjects and the lack of difference between groups was further analysed by testing non parametric correlations between age and PV-uncert in each group. No correlation was found in young adults (r = 0.1, p > 0.05) whereas PV-uncert strongly correlated with age in seniors (r = 0.59, p < 0.001). Taken all together, these results indicate that the ageing process bearing on the uncertainty was not progressive throughout the life span but subject to acceleration in the second part of life. In addition, dispersions of PV-uncert data were similar in both groups (Z = 0.88, p > 0.05). Finally, ranges of normality calculated in the 87 subjects as mean ± 2SD were from 0.11° to 3.87° (Fig. 2b).
Influence of the rotation direction and starting position on PV
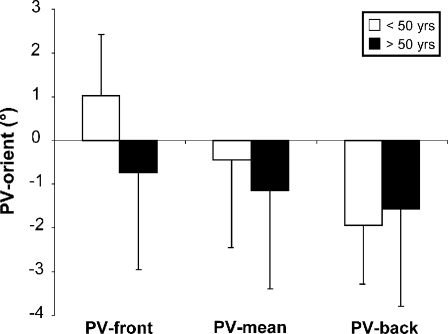
These statistical analyses were only performed on PV-orient. Figure 3 deals with the influence of the rotation direction of the wheel on PV. A two-factor ANOVA (two groups/two starting directions) showed an age effect [F(1,174) = 6.51; p < 0.05], an influence of the rotation direction of the wheel [F(1,174) = 48.73; p < 0.001], with an interaction between the two factors [F(1,174) = 15.38; p < 0.001]. This interaction was further analysed by means of a one-way ANOVA for each group. In young adults, a strong direction effect was revealed [F(1,97) = 116.88; p < 0.001]. Until age 50, the perception of the vertical was attracted towards the direction of the starting tilt of the wheel, i.e. forward (1.04°) when the wheel was rotated from front to back (PV-front) and backward (−1.94°) when rotated back to front (PV-back). In contrast, no direction effect was found in the elderly (>50 years) [F(1,75) = 2.69; p > 0.05] who always perceived the vertical slightly backward, irrespective of the starting position of the wheel (Fig. 3).
Fig. 3.
Influence of the starting direction on the mean PV orientation in subjects of less (white box) and more (black box) than 50 years of age. The means and the standard deviations are represented for the five trials from front to back (PV-front), for the ten trials (PV-mean) and for the five trials from back to front (PV-back)
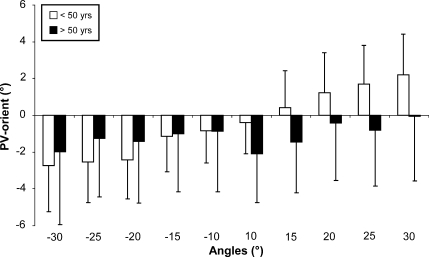
The influence of the various starting positions (±10°, 15°, 20°, 25° and 30°) was then tested by means of a two-factor ANOVA (group/starting position). This analysis showed significant differences between the groups [F(1,869) = 14.55; p < 0.001] and between the starting positions [F(9,869) = 16.07; p < 0.001] with an interaction between the two factors [F(9,869) = 6.52; p < 0.001]. In subjects under 50 years of age, the farther the starting position from the vertical, the more the PV was tilted in the same direction (Fig. 4, white box). In senior subjects, the PV was oriented backward whatever the starting position (Fig. 4, black box).
Fig. 4.
Influence of the starting angle on the mean PV orientation, in subjects younger than 50 (white box) and older than 50 (black box). The means and the standard deviations are represented for each angle
Discussion
The objectives of this study concerned the pitch plane: to determine the metrological properties of the wheel paradigm, to determine normative values of PV and to test the existence of a possible physiological ageing of PV.
The present study is the first to investigate PV in pitch in a large group of normal people of various ages. Few studies have investigated PV in healthy subjects (most involving a small number of subjects) serving either as controls for patients with a disease (Anastasopoulos et al. 1997a; Bisdorff et al. 1996; Clark and Graybiel 1963; Ito and Gresty 1996; Manckoundia et al. 2007) and/or tested in various experimental conditions (Barbieri et al. 2008; Bringoux et al. 2003; Ceyte et al. 2007). They provided a first but limited indication of the expected normal values of PV in pitch. By assessing numerous healthy subjects covering all age groups, the present study is a further step in the definition of normative values of PV in pitch, confirming that in young healthy adults the perception of the PV is accurate and close to the gravitational vertical (Anastasopoulos et al. 1997b; Bisdorff et al. 1996).
The existence of a backward tilted perception of PV in aged individuals has recently been reported by Manckoundia et al. (2007) who investigated an older population of fallers, with balance disorders and severe retropulsion. Only eight old normal subjects serving as controls were investigated, in whom PV was found to be significantly negative, leading the authors to hypothesise the existence of a physiological ageing process on PV. The present study confirms this hypothesis and reveals that senior adults over 50 years feel themselves upright when tilted backward at about 1° (this figure represents twice the corresponding backward tilt in young subjects), with the magnitude of this tilt linearly increasing with age. This finding seems robust, supported by an investigation of 87 healthy adults from 20 to 97 years old. In addition, the present study shows that, with age, healthy subjects are less and less accurate in aligning their whole body to the direction of the gravitational vertical, a finding previously reported by Bisdorff et al. (1996), but not analysed in Manckoundia’s study (2007). Major PV uncertainty associated with normal PV orientation was also observed in peripheral/central vestibular disorders (Bisdorff et al. 1996) and in deafferented patients (Mazibrada et al. 2008). In our study, the elderly subjects reported the absence of any known neurological or motor disorders, they did not present a backward disequilibrium nor did they report falling. However, it is well known that all the major sensory and motor systems important for balance and mobility decline with age (Lord and Ward 1994). Physiological ageing is associated with reduced functioning in vestibular, visual and somaesthetic systems (Lord et al. 1996) which are all implied in the building up and the updating of a central representation of verticality (Brandt et al. 1994; Mittelstaedt 1998; Perennou et al. 1998). In particular, the PV is mainly governed by somatosensory graviceptive information, provided by visceral graviceptors in the trunk and by tactile afferents and proprioception (Barbieri et al. 2008; Bronstein 1999; Clark and Graybiel 1963; Mittelstaedt 1998; Perennou et al. 1998). It is thus probable that the greater PV uncertainty observed in the elderly can be attributed to the age-related decline of one of these systems, which was not specifically assessed in this study. Beyond sensory systems, the findings of the present study indicate that the internal model of verticality is less robust in elderly people and that this may play a part in their postural decline.
Regarding the experimental procedure and its influence on PV orientation, two points have to be discussed. In the younger population (<50 years), the PV tended to be oriented in the direction of the initial wheel position, slightly forward when rotating the wheel from the front to the back and conversely. Furthermore, the greater the angle of the starting position relative to the vertical, the more the PV was biassed. These effects corroborate previous studies (Bisdorff et al. 1996; Clark and Graybiel 1963; Perennou et al. 2002) and may be assimilated to an Aubert effect (i.e. errors in judgement of visual orientations) when the subject is tilted. The construction of the reference of verticality is based on multi-sensory integration and a correct perception of the PV is linked to an intact somatosensory contribution (Manckoundia et al. 2007; Mittelstaedt 1998). Its recalibration in case of wheel tilt is probably due to over-weighting of the somatosensory contribution to gravity perception, while the contribution of the otoliths remains constant. This may explain why the PV was oriented in the direction of the initial wheel position: the internal referential of verticality is attracted towards the initial tilt, provided that correct somatosensory integration is obtained (Mittelstaedt 1998). These effects were nonetheless counterbalanced by the fact that the experimental procedure was symmetrically randomised. Strikingly, the elderly seemed to be less sensitive to the direction and the amplitude of the wheel rotation. They were systematically oriented slightly backward, whatever the wheel rotation, with a mean PV orientation comparable to that observed in the younger population. On the basis of a recent study showing that an alteration of the proprioception input led to the same phenomenon (Barbieri et al. 2008), the latter finding seems to argue in favour of a decline in somatosensory information with age.
Could our results be due to a methodological bias? First, we raise the question of whether the backward inclination in the normal range is due to an internal referential which is backwardly inclined or due to the device. The normal range (−2.39° to 1.5° under 50 years and −3.95° to 1.65° over 50 years) was more backward than the gravitational reference (i.e. 0°). Contrary to the normative values determined in the frontal plane (−2.5° to 2.5°) (Mazibrada et al. 2008; Perennou et al. 2008), the normative values obtained here are not symmetrical around the physical vertical. A methodological bias cannot be excluded. Due to the importance of somaesthetic input and tactile afferent in the perception of PV (Bronstein 1999; Mittelstaedt 1998; Perennou et al. 1998), coupled with the fact that the device used imposes more contact surface on the back of the body, PV as measured in our conditions may be attracted slightly backward. Alternatively this means that our PV is slightly and naturally tilted backward, at least in the sitting position used in the wheel paradigm. Secondly, the choice of using different starting positions may be discussed. An initial wheel position which was neither constant nor specific allowed us to prevent subjects from using time representation to perform the task. This procedure, which precludes fixing a single starting angle, is usual in the literature for measuring PV (Bisdorff et al. 1996; Bronstein et al. 2003; Ito and Gresty 1996; Perennou et al. 2008; Van Beuzekom and Van Gisbergen 2000). Furthermore, Fig. 4 shows a clear influence of the starting position, varying with age, which confirmed the relevance of our procedure. Finally, the validity of our non-motorised device may also be questioned, especially in terms of the relative motion between the subject and the wheel, the rolling velocity and measurement reproducibility. Pilot measurements showed that it is possible to passively tilt subjects with the drum without significant differential displacements of body segments. These pilot measurements were performed both in the frontal (Perennou et al. 2008) and in the sagittal plane (present paper). Performed in several subjects (only one trial in one subject is displayed in Fig. 1), they prove that it is possible to perfectly pad and strap subjects in the device, and thus avoid any undesirable motion. Particular attention was paid to the setting up of each subject included in the present study, and the experiment started only once the operator had checked that the subject was firmly maintained in the device. It was easy to see whether or not this condition was respected. Regarding the rolling velocity, only two well-trained experimenters took the measurements, which reduced variations in the rolling velocity. In addition, the balanced design of the drum with a large radius and high inertia (weight = 120 kg) ensured that the rotation was silent and smooth at an average velocity determined by the experimenter. Although satisfactory, the inter-operator reproducibility was not perfect if one considers the correlation coefficient between their measurements (0.7). In fact, this coefficient correlation underestimates the actual reproducibility because of the very low PV values. The mean absolute error between both operators was quite low (0.8°) and the mean error not significantly different from 0°. These results show that PV measurement using the wheel paradigm is accurate and reproducible. However, this reproducibility will have to be further investigated in more subjects including subjects showing a bias in PV. Taken together, these results confirm those recently obtained using the same paradigm in the frontal plane (Perennou et al. 2008), which argues in favour of the validity of the wheel paradigm and of the procedure used. This mechanical paradigm also boasts two other advantages: it is silent and thus free of the possibly disturbing noise of some motorised machines and the transfer of frail and/or disabled people into the “machine” remains relatively easy.
Zago et al. (2009) suggested that an internal model is built up by experience. Our study argues for a deterioration of the internal model of verticality with ageing. This is not contradictory with the fact that experience could contribute to a more robust internal model of verticality. Ageing is associated with decreased cerebral ability that could deteriorate the updating of internal models.
In conclusion, our study argues for age-related alterations of the internal model of verticality, which could play a role in the normal postural decline of elderly people. The possible link with postural behaviours such as a mild backward disequilibrium frequently observed in the elderly (Manckoundia et al. 2008) remains to be investigated. If we assume that the net backward disequilibrium often observed in elderly people is partly due to a net backward tilt in the PV (Manckoundia et al. 2007), our findings indicate that this net backward disequilibrium is not caused by a low magnitude physiological ageing of PV. It is rather due to a pathological mechanism which remains to be investigated.
Footnotes
Guillaume Barbieri and Anne-Sophie Gissot have contributed equally to the paper
References
- Anastasopoulos D, Bhatia K, Bisdorff A, Bronstein AM, Gresty MA, Marsden CD. Perception of spatial orientation in spasmodic torticollis. Part I: the postural vertical. Mov Disord. 1997;12:561–569. doi: 10.1002/mds.870120413. [DOI] [PubMed] [Google Scholar]
- Anastasopoulos D, Haslwanter T, Bronstein A, Fetter M, Dichgans J. Dissociation between the perception of body verticality and the visual vertical in acute peripheral vestibular disorder in humans. Neurosci Lett. 1997;233:151–153. doi: 10.1016/S0304-3940(97)00639-3. [DOI] [PubMed] [Google Scholar]
- Aoki M, Ito Y, Burchill P, Brookes GB, Gresty MA. Tilted perception of the subjective ‘upright’ in unilateral loss of vestibular function. Am J Otol. 1999;20:741–747. [PubMed] [Google Scholar]
- Barbieri G, Gissot AS, Fouque F, Casillas JM, Pozzo T, Perennou D. Does proprioception contribute to the sense of verticality? Exp Brain Res. 2008;185:545–552. doi: 10.1007/s00221-007-1177-8. [DOI] [PubMed] [Google Scholar]
- Benson AJ, Hutt EC, Brown SF. Thresholds for the perception of whole body angular movement about a vertical axis. Aviat Space Environ Med. 1989;60:205–213. [PubMed] [Google Scholar]
- Bisdorff AR, Wolsley CJ, Anastasopoulos D, Bronstein AM, Gresty MA. The perception of body verticality (subjective postural vertical) in peripheral and central vestibular disorders. Brain. 1996;119(Pt 5):1523–1534. doi: 10.1093/brain/119.5.1523. [DOI] [PubMed] [Google Scholar]
- Bonan IV, Guettard E, Leman MC, Colle FM, Yelnik AP. Subjective visual vertical perception relates to balance in acute stroke. Arch Phys Med Rehabil. 2006;87:642–646. doi: 10.1016/j.apmr.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Brandt T, Dieterich M, Danek A. Vestibular cortex lesions affect the perception of verticality. Ann Neurol. 1994;35:403–412. doi: 10.1002/ana.410350406. [DOI] [PubMed] [Google Scholar]
- Bringoux L, Nougier V, Barraud PA, Marin L, Raphel C. Contribution of somesthetic information to the perception of body orientation in the pitch dimension. Q J Exp Psychol A. 2003;56:909–923. doi: 10.1080/02724980245000016. [DOI] [PubMed] [Google Scholar]
- Bronstein AM. The interaction of otolith and proprioceptive information in the perception of verticality. The effects of labyrinthine and CNS disease. Ann N Y Acad Sci. 1999;871:324–333. doi: 10.1111/j.1749-6632.1999.tb09195.x. [DOI] [PubMed] [Google Scholar]
- Bronstein AM, Perennou DA, Guerraz M, Playford D, Rudge P. Dissociation of visual and haptic vertical in two patients with vestibular nuclear lesions. Neurology. 2003;61:1260–1262. doi: 10.1212/01.wnl.0000086815.22816.dc. [DOI] [PubMed] [Google Scholar]
- Ceyte H, Cian C, Zory R, Barraud PA, Roux A, Guerraz M. Effect of Achilles tendon vibration on postural orientation. Neurosci Lett. 2007;416:71–75. doi: 10.1016/j.neulet.2007.01.044. [DOI] [PubMed] [Google Scholar]
- Clark B, Graybiel A. Perception of the postural vertical in normals and subjects with labyrinthine defects. J Exp Psychol. 1963;65:490–494. doi: 10.1037/h0045606. [DOI] [PubMed] [Google Scholar]
- Dieterich M, Brandt T. Wallenberg's syndrome: lateropulsion, cyclorotation, and subjective visual vertical in thirty-six patients. Ann Neurol. 1992;31:399–408. doi: 10.1002/ana.410310409. [DOI] [PubMed] [Google Scholar]
- Ito Y, Gresty MA. Shift of subjective reference and visual orientation during slow pitch tilt for the seated human subject. Brain Res Bull. 1996;40:417–421. doi: 10.1016/0361-9230(96)00136-0. [DOI] [PubMed] [Google Scholar]
- Lord SR, Lloyd DG, Li SK. Sensori-motor function, gait patterns and falls in community-dwelling women. Age Ageing. 1996;25:292–299. doi: 10.1093/ageing/25.4.292. [DOI] [PubMed] [Google Scholar]
- Lord SR, Ward JA. Age-associated differences in sensori-motor function and balance in community dwelling women. Age Ageing. 1994;23:452–460. doi: 10.1093/ageing/23.6.452. [DOI] [PubMed] [Google Scholar]
- Manckoundia P, Mourey F, Perennou D, Pfitzenmeyer P. Backward disequilibrium in elderly subjects. Clin Interv Aging. 2008;3:667–672. doi: 10.2147/cia.s3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manckoundia P, Mourey F, Pfitzenmeyer P, Hoecke JV, Perennou D. Is backward disequilibrium in the elderly caused by an abnormal perception of verticality? A pilot study. Clin Neurophysiol. 2007;118:786–793. doi: 10.1016/j.clinph.2006.11.274. [DOI] [PubMed] [Google Scholar]
- Mazibrada G, Tariq S, Perennou D, Gresty M, Greenwood R, Bronstein AM. The peripheral nervous system and the perception of verticality. Gait Posture. 2008;27:202–208. doi: 10.1016/j.gaitpost.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Mittelstaedt H. Origin and processing of postural information. Neurosci Biobehav Rev. 1998;22:473–478. doi: 10.1016/S0149-7634(97)00032-8. [DOI] [PubMed] [Google Scholar]
- Perennou D. Postural disorders and spatial neglect in stroke patients: a strong association. Restor Neurol Neurosci. 2006;24:319–334. [PubMed] [Google Scholar]
- Perennou DA, Amblard B, Laassel el M, Benaim C, Herisson C, Pelissier J. Understanding the pusher behavior of some stroke patients with spatial deficits: a pilot study. Arch Phys Med Rehabil. 2002;83:570–575. doi: 10.1053/apmr.2002.31198. [DOI] [PubMed] [Google Scholar]
- Perennou DA, Amblard B, Leblond C, Pelissier J. Biased postural vertical in humans with hemispheric cerebral lesions. Neurosci Lett. 1998;252:75–78. doi: 10.1016/S0304-3940(98)00501-1. [DOI] [PubMed] [Google Scholar]
- Perennou DA, Leblond C, Amblard B, Micallef JP, Rouget E, Pelissier J. The polymodal sensory cortex is crucial for controlling lateral postural stability: evidence from stroke patients. Brain Res Bull. 2000;53:359–365. doi: 10.1016/S0361-9230(00)00360-9. [DOI] [PubMed] [Google Scholar]
- Perennou DA, Mazibrada G, Chauvineau V, Greenwood R, Rothwell J, Gresty MA, Bronstein AM. Lateropulsion, pushing and verticality perception in hemisphere stroke: a causal relationship? Brain. 2008;131:2401–2413. doi: 10.1093/brain/awn170. [DOI] [PubMed] [Google Scholar]
- Saj A, Honore J, Bernati T, Coello Y, Rousseaux M. Subjective visual vertical in pitch and roll in right hemispheric stroke. Stroke. 2005;36:588–591. doi: 10.1161/01.STR.0000155740.44599.48. [DOI] [PubMed] [Google Scholar]
- Beuzekom AD, Gisbergen JA. Properties of the internal representation of gravity inferred from spatial-direction and body-tilt estimates. J Neurophysiol. 2000;84:11–27. doi: 10.1152/jn.2000.84.1.11/F. [DOI] [PubMed] [Google Scholar]
- Yelnik AP, Lebreton FO, Bonan IV, Colle FM, Meurin FA, Guichard JP, Vicaut E. Perception of verticality after recent cerebral hemispheric stroke. Stroke. 2002;33:2247–2253. doi: 10.1161/01.STR.0000027212.26686.48. [DOI] [PubMed] [Google Scholar]
- Zago M, McIntyre J, Senot P, Lacquaniti F. Visuo-motor coordination and internal models for object interception. Exp Brain Res. 2009;192:571–604. doi: 10.1007/s00221-008-1691-3. [DOI] [PubMed] [Google Scholar]