Abstract
Apolipoprotein D (apo D) is a lipocalin present in the nervous system that may be related to processes of reinnervation, regeneration and neuronal cell protection. On the other hand, apo D expression has been correlated, in some brain regions, with normal ageing and neurodegenerative diseases. To elucidate the regional and cellular expression of apo D in normal human brain during ageing, we performed a detailed and extensive study in samples of post-mortem human cerebral cortices. To achieve this study, slot-blot techniques, for protein and mRNA, as well as immunohistochemistry and hybridohistochemistry methods, were used. A positive correlation for apo D expression with ageing was found; furthermore, mRNA levels, as well as the protein ones, were higher in the white than in the grey matter. Immunohistochemistry and non-isotopic in situ hybridization showed that apo D is synthesised in both neurons and glial cells. Apo D expression is notorious in oligodendrocytes, but with ageing, the number of neurons that synthesise apo D is increased. Our results indicate that apo D could play a fundamental role in central nervous system ageing and in the reduction of products derived from lipid peroxidation. The increment in the expression of apo D with ageing can be included in a global mechanism of cellular protection to prevent the deleterious effects caused by ageing.
Keywords: Apolipoprotein D, Ageing, Human, Frontal cortex, In situ hybridization, Immunohistochemistry
Introduction
Human apolipoprotein D (apo D), first isolated by McConathy and Alaupovic (1973, 1976) in plasma high-density lipoproteins, was classically considered a member of the apolipoproteins. However, further studies revealed that apo D belongs to two-microglobulin super family or lipocalin family (Drayna et al. 1987), a group of small extra-cellular proteins involved in the transport of specific hydrophobic ligands (Flower 1996). This lipocalin is associated with lecithin-cholesterol acyltransferase in plasma, probably stabilising the activity of the enzyme and helping in the efflux of cholesterol and its esters (Steyrer and Kostner 1988). Therefore, it has been hypothesised that the function of this protein could be cholesterol transport from peripheral tissues to liver for catabolism (Drayna et al. 1986). It has also been showed that apo D binds several hydrophobic ligands, including cholesterol, progesterone, pregnenolone, arachidonic acid, bilirubin and pheromones (Goessling and Zucker 2000; Morais-Cabral et al. 1995; Pearlman et al. 1973; Rassart et al. 2000; Zeng et al. 1996). In addition, apo D is expressed in a wide variety of tissues in mammals, like pancreas, kidney, placenta, adrenal gland, spleen and brain (Boyles et al. 1990b; Provost et al. 1991; Seguin et al. 1995; Smith et al. 1990). The interstitial and connective fibroblasts near blood vessels are the main cell type that accumulates apo D. A different local role for apo D depending on the tissue and the associated ligand has been proposed (Provost et al. 1991).
In the peripheral nervous system (PNS), apo D is synthesised and accumulated, as well as other apolipoproteins, by fibroblasts during regeneration following a lesion or axotomy (Boyles et al. 1990a; Del Signore et al. 2006; Spreyer et al. 1990) where it is thought to be implicated in the distribution of lipids during regeneration processes. Within central nervous system (CNS), apo D expression has been showed in pia matter cells, perivascular cells, pericytes, astrocytes, oligodendrocytes and some scattered neurons (Navarro et al. 1998; Provost et al. 1991; Seguin et al. 1995; Smith et al. 1990). A difference in staining patterns between glia and neurons has been observed in human brain regions (Navarro et al. 1998). Northern blot analysis of total mRNA extract from grey and white matters of human and rabbit brains showed that the white matter is the main site of apo D gene expression, which proves the hypothesis of apo D synthesis in fibrous astrocytes and oligodendrocytes (Provost et al. 1991). Furthermore, some studies have reported apo D mRNA in neuroglia and perivascular cells by in situ hybridization (ISH) (Provost et al. 1991; Sánchez et al. 2002; Smith et al. 1990). It has been shown, in vivo, that astrocytes and fibroblast synthesise and constitutively secrete apo D under some particular conditions (Do Carmo et al. 2002; Patel et al. 1995). These authors suggest that apo D has a physiological role in cholesterol metabolism in the nervous system. Neuronal synthesis of apo D has been reported as a suspicion but has never been confirmed (Provost et al. 1991; Smith et al. 1990).
A specific induction of apo D expression is observed during the repair following brain experimental injury in animals (Franz et al. 1999; Montpied et al. 1999; Ong et al. 1997; Rickhag et al. 2008; Terrisse et al. 1999; Trieu and Uckun 2000). The increase in apo D was mainly observed in astrocytes and neurons and recently in oligodendrocytes in injured regions (Rickhag et al. 2008). However, it is yet unknown whether these observations are due to an increased biosynthesis and/or uptake of apo D by these cells. The studies suggest that apo D could be a good marker of degeneration, being its expression induced as soon as any type of injury occurs.
In humans, increased levels of apo D in the brain have been demonstrated in several neuropathologies, such as Alzheimer’s disease (AD), scrapie and schizophrenia (Dandoy-Dron et al. 1998; Terrisse et al. 1998; Thomas et al. 2001), as well as in normal ageing (Belloir et al. 2001; Kalman et al. 2000). In AD, increased levels of apo D have been reported in cerebrospinal fluid (CSF), hippocampus and cortex (Belloir et al. 2001; Kalman et al. 2000; Terrisse et al. 1998). Furthermore, not only neurons and reactive astrocytes but also some characteristic hallmarks of AD as senile plaques and amyloid vessel deposits show apo D immunoreactivity (Desai et al. 2005; Del Valle et al. 2003; Navarro et al. 2001, 2003). In the same way, increased apo D expression has been demonstrated in pre-frontal cortex and caudate nucleus of subjects suffering from schizophrenia and bipolar disorders (Thomas et al. 2001). These findings suggest the hypothesis that apo D is a marker for brain regions that undergo some types of neuropathology (Thomas et al. 2001). Moreover, it could be part of a defence system acting in situations of unbalanced oxidative stress, which has been suggested, as an underlying mechanism in some neurodegenerative diseases (Muffat et al. 2008; Ordóñez et al. 2006; Sánchez et al. 2006). Like what occurs in the PNS, the increased apo D expression in the CNS in these conditions allows to speculate a role in repair or remodelling processes secondary to neurodegeneration (Boyles et al. 1990a). Apo D may serve as a lipid carrier to and from cells and/or it may be a scavenger to deleterious molecules or free radicals like lipid peroxidation products (Ganfornina et al. 2008).
Although recent studies reported that apo D expression is upregulated in ageing and AD (Belloir et al. 2001; Kalman et al. 2000; Kang et al. 2003; Lee et al. 2000), detailed and extensive study in the course of adult human life has not been previously achieved. In this study, we have measured the levels of apo D protein and mRNA along human adult life in the grey as well as in the white matter of pre-frontal cortex. In addition, protein and mRNA localization and distribution is described by hybrido- and immunohistochemistry methods.
Methods
Subjects
Human tissues were provided by the Department of Pathologic Anatomy of the Central Hospital of Asturias. Twenty-six cases (between 32 and 88 years) with no known neurological, psychiatric or neuropathological disorders were used in this study (Mirra et al. 1991). Post-mortem intervals ranged between 2 and 6 h. Tissue blocks were obtained from human frontal cortex (Broadmann’s area 9). The cerebral cortex was separated by dissection in white and grey matter. Three types of samples were obtained from each case, one was frozen at −80°C for RNA or protein extraction, and others were fixed in paraformaldehyde in 0.1 phosphate buffer (pH 7.4) for neuropathological diagnosis and immuno- or in situ hybrido-histochemical studies.
The present study was conducted according to the Declaration of Helsinki and has been approved by the local ethics committee.
Western blot analysis
Samples of grey and white matter from BA9 and samples from human breast cyst fluid (as a positive apo D control) were electrophoresed on a 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis in a Bio-Rad Miniprotean III electrophoresis unit. Subsequently, proteins were electrotransferred to a nitrocellulose membrane (Enhanced chemiluminescence (ECL), Hybond GE healthcare) at 50 V for 60 min. For avoiding non-specific reaction, membranes were blocked with Tris-NaCl-Tween 20 (TNT) supplemented with 1% bovine serum albumin (BSA) for 2 h at room temperature and then incubated with primary antibody against human apo D [1:10,000 dilution; antibody was a gift from Dr. Carlos López Otín, Departamento de Bioquímica y Biología Molecular, Universidad de Oviedo (see Díez-Itza et al. 1994; López-Boado et al. 1994; Navarro et al. 1998, 2003, 2008; Ordóñez et al. 2006)] overnight at 4°C. After three washes of 10 min each in TNT, membranes were incubated for 1 h at room temperature with anti-rabbit IgG horseradish peroxidase (HRP) secondary antibody (Chemicon, 1:20,000 dilution). Membranes were washed again as we described above, and proteins were detected by chemiluminescence using ECL reagent (GE healthcare) according to the manufacturer’s instructions on a Kodak X-Omat film.
Protein slot-blotting
Apo D levels were quantified using a slot-blot technique. Samples from human breast cyst fluid were also included as a positive control of apo D and ExtrAvidin-Alkaline Phosphatase (Sigma, E26366) as an internal control. Briefly, 2, 1, 0.5, 0.25, 0.12 and 0.06 μg of total protein were slot-blotted under vacuum in a Bio-Dot (Bio-Rad) apparatus. Nitrocellulose membranes were then immunostained for apo D protein according to the following protocol: non-specific binding was blocked by incubation with 1% BSA. Then, they were incubated for 1 h at room temperature with the antibody against human apo D (1:10,000 dilution). After three washes of 15 min each in phosphate-buffered saline (PBS), membranes were incubated for 30 min at room temperature with biotinylated monoclonal anti-rabbit IgG (Sigma, B-5283) diluted 1:10,000, washed again as before and then incubated with ExtrAvidin-Alkaline Phosphatase (Sigma, E26366) diluted 1:10,000. After three washes of 15 min each in PBS, enzyme activity was shown by incubation with Sigma Fast 5-bromo-4-chloro-3-indolyl phosphate (BCIP)/nitro blue tetrazolium chloride (NBT; Sigma, B5655) solution (2 h at room temperature).
mRNA slot-blotting
Total RNA were extracted from dissected brain regions by homogenised guanidium thiocyanate-phenol-chloroform extraction method (Chomczynski and Sacchi 1987) and treated with RNase-free DNase I (GE Healthcare) in order to remove genomic DNA. ExtrAvidin-Alkaline Phosphatase was also included as an internal control. Briefly, 2, 1, 0.5, 0.25, 0.12 and 0.06 μg of total RNA were denatured in 150 μl of 20 × SSC for 5 min at 70°C and slot-blotted under vacuum in a Bio-Dot (Bio-Rad) apparatus. Nylon membranes (Hybond-N + GE Healthcare) were then prehybridized at 56°C for 2 h in Rapid-hyb buffer (GE Healthcare). Hybridization in the same buffer was then performed overnight using 5 μl of digoxigenin-labelled RNA probe for human apo D (obtained from Dr. Carlos López-Otín, Dpto. Bioquímica y Biología Molecular, Universidad de Oviedo; see Del Valle et al. 2003; Díez-Itza et al. 1994; López-Boado et al. 1994). After several washes in TNT (3 × 15 min), hybridised probe was detected with an AP-coupled anti-DIG antibody (Roche 1093274) diluted 1:10,000 for 30 min at room temperature. For signal amplification, the membranes were rinsed with TNT (3 × 10 min) and treated with biotinylated universal antibody (BA-1300, Vector Laboratories, Burlingame, CA, USA) diluted 1:10,000 and ExtrAvidin-Alkaline Phosphatase (Sigma, E26366) diluted 1:10,000, both for 30 min at room temperature. Enzyme activity was shown by incubation with Sigma Fast BCIP/NBT (Sigma, B5655) solution (2 h at room temperature).
The blots of both protein and mRNA of apo D were dried, and bands were quantified as a relative optical densities (arbitrary units) using a digital scanner (Nikon AX-110, Nikon) and NIH Image 1.57 free software (Scion Corp).
Hybridohistochemistry and immunohistochemistry
After fixation, blocks were washed in distilled water, dehydrated, cleared in butyl acetate and embedded in paraffin. Sections were cut at 10 μm in thickness and mounted on “SuperFrost Plus” (Mentzel-Glasse) slides, dried at 36°C for 24 h, deparaffined in xylene and rehydrated by successive alcohols for 2 min each. Endogenous peroxidase was blocked by subsequent incubation in 3% hydrogen peroxide solution.
ISH was performed on sections dried at room temperature for 5 min, digested with 2 μg/ml of proteinase K in 0.001 M PBS at 37°C for 5 min, and rinsed in 0.001 M PBS. Twenty microlitre of ISH solution (Sigma Hybridization Solution, H-7782) were applied on each section for 10 min. Twenty microlitres of digoxigenin-labelled RNA probe for human apo D were applied on each of the sections that subsequently were incubated in a moist chamber for 16 h at 55°C. The hybridised probe was visualised by two methods of detection. (1) The slides were rinsed in PBS, and the hybridised probe was detected with an alkaline phosphatase-coupled anti-DIG antibody (Roche 1093274) diluted 1:1,000 for 60 min at room temperature. Then, slides were rinsed with PBS and incubated in Sigma Fast BCIP/NBT (Sigma B5655) solution (14 h at room temperature). (2) The slides were rinsed in PBS, and the hybridised probe was detected with an HRP-coupled anti-DIG antibody (Roche 1207733) diluted 1:500 for 60 min at room temperature. For the signal amplification, the sections were rinsed with PBS and treated with “Vectastain Universal Quick kit” (PK-8800, Vector Laboratories). Peroxidase activity was shown by incubation with sigma Fast DAB (Sigma D4168) at room temperature for 30 min.
For immunohistochemistry, sections were treated with triton X (0.1%, 5 min), washed in distilled water, treated with H2O2 (3%, 5 min), washed in distilled water and treated with PBS. Non-specific binding was blocked by incubation with BSA (30 min). Incubation with a specific antibody against human apo D (1:2,000 dilution) was carried out overnight at 4°C. The immunoreactivity was detected using the ExtrAvidin-biotin-peroxidase staining kit (Sigma Extra-3), and peroxidase activity was shown as it was described above for hybridohistochemistry.
Finally, the sections visualised with DAB were counterstained with a modification of formaldehyde-thionin method (Tolivia et al. 1994), dehydrated, cleared in eucalyptol and mounted with Eukitt. The sections revealed with NBT were mounted in aqueous mounting medium. The usual specificity control tests for both hybridohistochemistry and immunohistochemistry were carried out.
Statistical analyses
The data were subjected to a statistic study using Statistical Package for the Social Sciences 15.0 for Windows. The test of Kolmogorov–Smirnov with the correction of Lilliefors was used to evaluate the fit of the data to a normal distribution. In view of no homogeneity of variance, we performed the Mann–Whitney test to compare the variables (apo D protein and apo D mRNA) between white and grey matter. P values <0.05 were considered as statistically significant. Finally, a correlation analysis and a lineal regression were done to detect the association between age and protein and mRNA apo D levels, respectively.
Results
Western blot for apo D
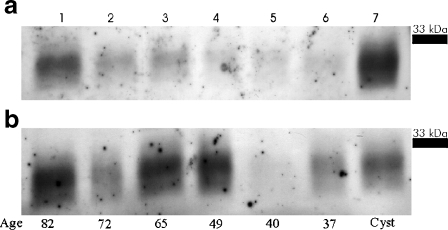
In the present work, we have measured apo D gene expression and its cellular and tissue localization in human frontal cerebral cortex during adult lifespan and ageing, in white and grey matter samples. As it is shown in Fig. 1, western blot technique clearly demonstrates a band between 29 and 31 kDa, corresponding to apo D, in all cerebral extracts analysed. Our results (Fig. 1a, b) show that apo D is expressed in all samples, white and grey matter, young and elders.
Fig. 1.
Western blot analysis in grey (a) and white (b) matter of apo D in cerebral cortex from six subjects of different age (82–37 years old). Line 7 was charged with breast cyst fluid
In the grey matter, a clear tendency to an apo D increment related with age can be observed (Fig. 1a). In spite of this, bias is also present in the white matter samples; it shows more oscillations than in grey matter (Fig. 1b). When samples of the same subject are compared, the white matter shows the higher amount of apo D (Fig. 1a, b).
Apo D protein levels
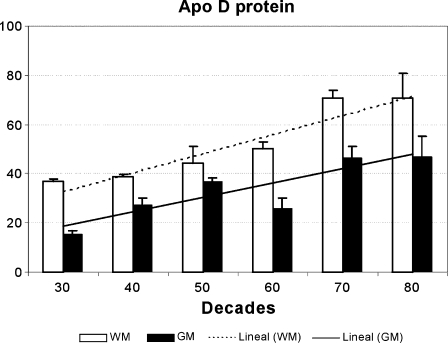
The levels of expression of apo D were measured in all frontal cortex samples to study direct effects of age. Slot-blot technique showed similar results to that observed in the western blot (Fig. 2). The analysis of the densitometric values of the slots showed that apo D protein levels are increased with a gradual pattern during ageing in both grey and white matter. However, apo D is expressed in grey and white matter in different manner. The highest levels of protein were always seen in white matter samples (Fig. 3), just as we had appreciated in western and slot-blot images. In addition, grey matter presented more individual oscillations than white matter (Fig. 3). A positive correlation was found in both white and grey matter between apo D level and age (r = 0.860, p < 0.001 and r = 0.606, p < 0.01, respectively). In all decades, a statistically significant difference in apo D levels between white and grey matter is observed, being the protein levels in the white matter higher in all the cases. Notwithstanding, the differences are less pronounced in the middle age decades.
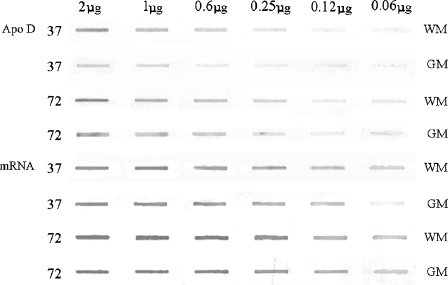
Fig. 2.
Slot-blot analysis of apo D protein and for its mRNA from two subjects (32 and 72 years old). Samples from white and grey matter are included, and the differences during ageing and between nervous regions can be clearly observed
Fig. 3.
Levels of apo D, in white (WM) and grey matter (GM), in samples from 26 subjects between 32 and 88 years old. The histogram shows the densitometry analysis of slot blots. Data presented as means of relative optical densities (ROD) with standard error of the mean shown by vertical bars. The lineal correlation with age is also shown (r = 0.860, p < 0.001 for the white matter and r = 0.606, p < 0.01 for the grey matter)
Tissue distribution of apo D protein
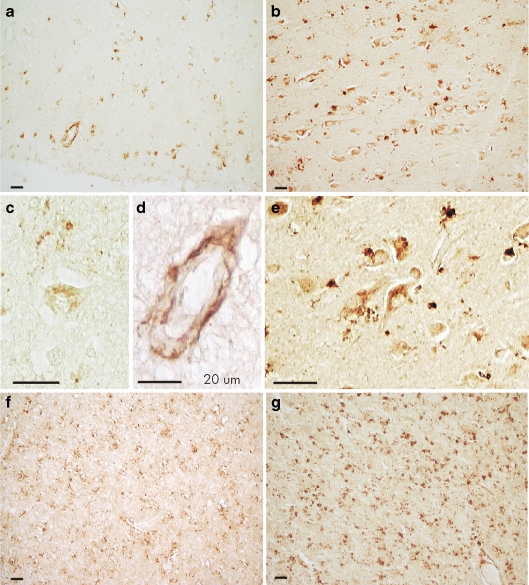
The protein slot-blot results showed that apo D is present in white and grey matter in human frontal cortex in all ages under study, so we decided to examine the distribution and cellular localization of this protein using immunohistochemistry on representative sections of the same brains. In general, a clear tendency to the increment of apo D positive staining with age is observed (Fig. 4). In young subjects, apo D immunostaining in grey matter is scarce and mainly located in glial and meningeal cells as well as in some perivascular cells (Fig. 4a), whereas in white matter, it is higher, and oligodendrocytes are the main labelled cells (Fig. 4f). During ageing, the number of immunoreactive cells and the intensity of extra-cellular signal are increased in the white matter (Fig. 4g). Immunoreactivity for apo D in the grey matter is found in a small number of astrocytes and oligodendrocytes down to the middle age (Fig. 4a). However, in elders, an increase in the number of oligodendrocytes and astrocytes labelled for apo D can be clearly observed, and the intensity of signal is higher than in the young ones (Fig. 4b).
Fig. 4.
Immunocytochemistry for apo D on grey (a–e) and white matter (f, g). a Cerebral cortex of a young subject; some glial cells and one vessel are marked. b Cerebral cortex of an old subject; a great number of neurons and glial cells are positives for apo D. c One neuron in the grey matter of a young subject shows a few grains of apo D. d Vessel in the cerebral cortex (a) showing positive signal in perivascular cells but not in endothelial cells. e Positive neurons in cerebral cortex of an old subject; some glial cells can be also observed. f White matter from a young subject; a general positive signal for apo D can be observed. g White matter from an old subject; an intense signal for apo D principally located in the oligodendrocytes is showed
There are no apo D reactive neurons in the youngest samples, but in some individual cases, a weak signal for apo D can be detected (Fig. 4c); with ageing, some neurons, principally pyramidal cells, appear labelled; and over the sixth decade, a great number of neuronal cells are immunopositives for apo D (Fig. 4b, e). Positive neurons are not usually linked to any ageing or pathological-related features and always show microscopical characteristics of a normal neuron (vesicular nucleus, large nucleolus and the presence of granular or striated Nissl substance in the cytoplasm; Fig. 4c, e). Apo D immunoreactivity was also found in blood vessels, mainly in pial and subpial layer (Fig. 4a) and occasionally in cortical and sub-cortical layer. Number and signal intensity of pial and subpial blood vessels are increased during ageing, while cortical and sub-cortical vessels are not. Apo D signal was uniform around small blood vessels and was discontinue in big vessels. It could be observed inside perivascular cells (perycites and fibroblasts) but is undetectable in endothelial cells (Fig. 4d).
mRNA slot-blot
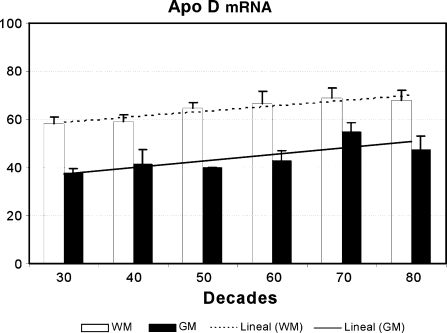
Gene expression of apo D in the frontal cortex in all subjects was measured using slot-blot technique (Fig. 2). When the densitometric values of the slots were analysed, our results showed a gradual and significant increase of apo D mRNA during ageing in both grey and white matter but in different fashion. The highest levels of mRNA were always seen in white matter samples as we had also observed for the protein (Fig. 5). In addition, white matter levels of mRNA presented less individual oscillations than grey matter ones. Data demonstrated that the increase of apo D mRNA with age is statistically significant in grey (r = 0.555, p < 0.05) as well as in white matter (r = 0.518, p < 0.01). Comparative studies of expression for apo D showed statistical differences between grey and white matter in all decades, being levels always higher in white matter.
Fig. 5.
Levels of apo D mRNA, in white (WM) and grey matter (GM), in samples from 26 subjects between 32 and 88 years old. The histogram shows the densitometry analysis of slot blots. Data presented as means of relative optical densities with standard error of the mean shown by vertical bars. The lineal correlation with age is also shown (r = 0.555, p < 0.05 for the grey matter and r = 0.518, p < 0.01 for the white matter)
Tissue distribution of the apo D mRNA
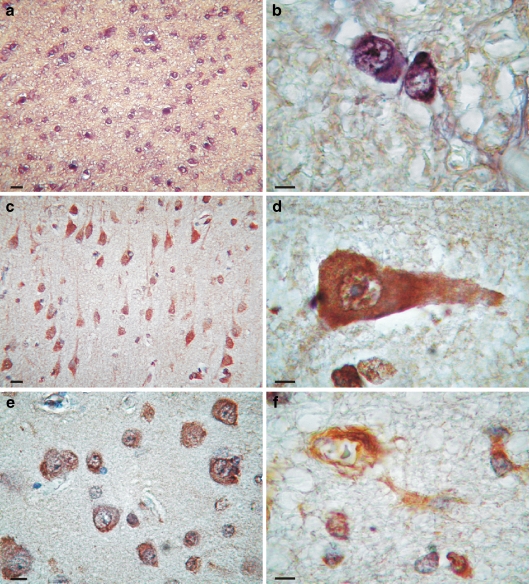
In situ non-radioactive hybridization was subsequently performed to determine localization of apo D mRNA in human frontal cortex. In younger subjects, the signal in this cortical region is restricted to the white matter, while grey matter presents faint signal. In the white matter, the number of labelling glial cells is increased with age, showing a stronger signal in elders (Fig. 6a). These cells are mainly oligodendrocytes, recognised for their morphological characteristics (Fig. 6b). In the grey matter, we also observed an increase of labelled cells with ageing. These cells were identified as neurons and astrocytes (Fig. 6c–e), but expression signal was also detected in microglia and pericytes (Fig. 6f). Neurons showing mRNA for apo D are principally pyramidal cells (Fig. 6c, d), but granular neurons could be also detected (Fig. 6e). Positive neurons always show microscopical characteristics of a normal neuron (vesicular nucleus and large nucleolus; Fig. 6d, e). The deposits of chromogen (Fig. 6d) mask the presence of granular or striated Nissl substance in the cytoplasm. The numbers of hybridised neurons increased with ageing as we also have seen with immunohistochemistry.
Fig. 6.
Hybridocytochemistry for apo D mRNA on white (a, b) and grey matter (c–f). a White matter from an old subject; an intense expression of apo D can be observed. b White matter from an old subject; two oligodendrocytes expressing apo D are showed. c Cerebral cortex of an old subject; a great number of pyramidal neurons expressing apo D can be observed. d Pyramidal neuron expressing apo D in which the normal morphology of nucleus and nucleoli can be observed. e Granular neurons in cerebral cortex of an old subject expressing apo D. f Pericyte and astrocyte cells expressing apo D in an old subject
Protein and mRNA relation
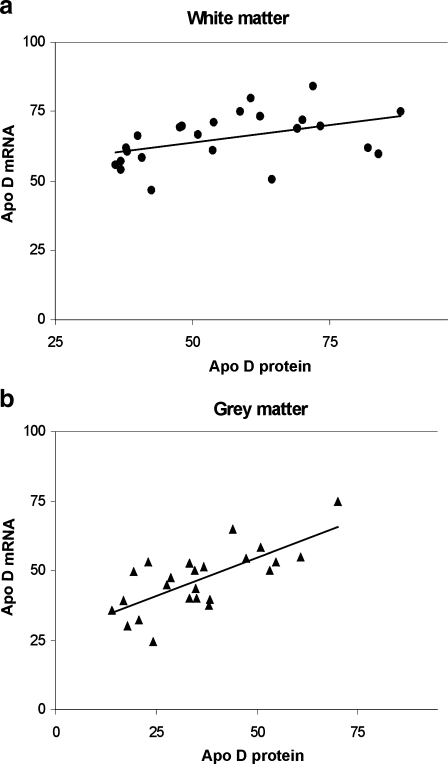
To complete the study, we achieved a statistical analysis to determine the correlation between mRNA and protein levels in white and grey matter (Fig. 7a, b). The result of protein closely corresponds with mRNA immunoblots in white (r = 0.454, p = 0.05) and grey matter (r = 0.522, p = 0.01).
Fig. 7.
Correlation between mRNA and apo D protein levels during ageing in white (a; r = 0.454, p = 0.05) and grey matter (b; r = 0.522, p = 0.01)
Discussion
Several studies relate increments in the expression of apo D in brain regions affected by neurological diseases. This relation has led to the hypothesis that apo D could be a marker for brain neuropathology. Increases in apo D expression selectively within CNS-injured regions suggest a focal compensatory response of apo D (Franz et al. 1999; Terrisse et al. 1999; Thomas et al. 2001). However, few data have been reported about the amounts of apo D in brain of normal subjects. The use of human tissue as study material has very important associated difficulties derived from each particular case (e.g., cause of death, life styles and differences of medication); however, it is required to obtain direct information about how apo D behaves during lifetime. The value of our work lies in to take samples in multiple time points during human development and normal ageing and to perform quantitative and qualitative analyses of apo D protein and mRNA in all of them. Tissue blocks were divided in grey and white matter that allowed us to obtain separate results of both structures.
Protein measurement by immunoblotting showed that in spite of quantitative differences being found between levels of apo D in the white and grey matter in the frontal cortex, both increase with age. Immunohistochemical analyses showed the expected cellular localization in oligodendrocytes, astrocytes and neurons, and an increment in the number of positive cells with ageing. According to Kalman et al. (2000), these results suggest that apo D protein is involved in normal ageing process. Senile plaques and vascular amyloid were also stained although neurofibrillar tangles were not. Apo D immunoreactivity was found mainly in the non-congophilic parts of vessels and plaques as was previously published (Navarro et al. 2003). The presence of apo D in some pathological markers of AD could be an indirect consequence of the ageing process more than a contribution of apo D to the pathophysiology of the disease.
Studies carried out by Northern blot and isotopic ISH in pre-frontal cortex of elders with and without AD showed apo D labelling for all cases, and regions under study always linked to white matter and its glial cells (Belloir et al. 2001; Provost et al. 1991). Moreover, increments of mRNA occur only in those subjects with the pathology (Belloir et al. 2001). Our study shows an increase of apo D mRNA during ageing; this increment is bigger in grey matter where we have seen the percentage of apo D-labelled astrocytes and neurons grow. The anisotopic signal found in neurons show, for the first time, that apo D could be expressed and synthesised by those nervous cells. That expression was supposed, but not clearly confirmed, by other authors (Smith et al. 1990; Belloir et al. 2001) in monkey and humans using isotopic hybridization methods. Our results suggest that neurons of some encephalic regions not only can obtain apo D from the surrounding glia but also express this protein as response to changes in the cellular homeostasis during ageing. However, some neuronal nuclei or individual neurons cannot express apo D, as was previously published by our group (Navarro et al. 2008; Ordóñez et al. 2006), and these nervous cells show a great vulnerability to the stressors during ageing and under pathologic situations.
In our study, western and slot blots showed that the amount of apo D protein and mRNA measured was always higher in the white that in the grey matter. In addition, an increment in the amount of protein and mRNA is observed during lifespan. Using microarray technology, Loerch et al. (2008) have shown a strong up-regulation of the apo D gene in ageing, not only in humans but also in rhesus monkey and mouse, being the most phylogenetically conserved in the three species among the so-called “ageing genes”. The presence and expression of apo D in oligodendrocytes and the increased signal observed in this cellular type in the elderly subjects suggest a fundamental role for these cells in the normal ageing process.
In conclusion, the present work describes a progressive increment in both apo D protein and mRNA levels in frontal cortex (Broadmann’s area 9) of normal subjects during the normal ageing process. Increments of apo D and/or its mRNA have been reported in the CSF, hippocampus, frontal and temporal cortex and entorhinal cortex of AD subjects, whereas in other areas, as parietal cortex and cerebellum, quantitative differences were not observed (Belloir et al. 2001; Kalman et al. 2000; Navarro et al. 1998; Thomas et al. 2003). Thus, some brain regions habitually present apo D whereas others do not. The region-specific distribution for apo D expression was introduced by previous studies of our group (Navarro et al. 1998) and later reported by Thomas et al. (2001, 2003). These authors found that the increase of apo D level was associated in some brain regions with the pathophysiology of AD, schizophrenia and bipolar disorder. However, these data are controversial because no statistical differences were found by immunoblotting when comparing AD with age-matched control subjects (Kalman et al. 2000). Recent studies show that those differences in expression of apo D exist among neuronal nuclei located in the same region (Ordóñez et al. 2006) or among neurons of the same area (Navarro et al. 2008). Based in these observations, to keep an apo D profile of brain regions is important to interpret experimental and pathological data.
A great number of studies show that the normal ageing, as well as some neurodegenerative disorders, are related with the oxidative metabolism of different molecules. The new molecules formed can damage, directly or indirectly, the cellular homeostasis and as a consequence, the cell dies. In the last years, it has been proved that apo D is a protein directly related with the oxidative stress. Studies achieved in Drosophila show that its absence reduces lifespan and stress resistance (Sánchez et al. 2006) and, on the contrary, the over-expression of apo D extends lifespan and increases stress resistance (Muffat et al. 2008). These results are in concordance with other studies that show that its expression or over-expression has a neuroprotective effect and protect nervous cells from some damages (Navarro et al. 2008; Ordóñez et al. 2006; Sánchez et al. 2006; Walker et al. 2006). These authors say that apo D could play an important role in the reduction of products derivates of the lipid peroxidation. The over-expression of apo D with ageing, in the cerebral cortex, can be included in a global mechanism of cellular protection used by the nervous cells to prevent the deleterious effects of lipid peroxidation caused by ageing.
Acknowledgments
This work was supported by MEC and FEDER (SAF2007-64076/) grant. CO is a pre-doctoral fellow from “Gobierno del Principado de Asturias”, Spain. EM is a pre-doctoral fellow from “Ministerio de Educación y Ciencia”, Spain.
Conflict of interest All authors of the present work disclose that there are no actual and potential conflicts of interest.
Footnotes
The study achieved in the present work has been approved by the Ethical Committee of Clinical Investigation of Asturias (Spain).
References
- Belloir B, Kovari E, Surini-Demiri M, Savioz A. Altered apolipoprotein D expression in the brain of patients with Alzheimer’s disease. J Neurosci Res. 2001;64:61–69. doi: 10.1002/jnr.1054. [DOI] [PubMed] [Google Scholar]
- Boyles JK, Notterpek LM, Anderson LJ. Accumulation of apolipoproteins in the regenerating and remyelinating mammalian peripheral nerve. Identification of apolipoprotein D, apolipoprotein A-IV, apolipoprotein E and apolipoprotein A-I. J Biol Chem. 1990;265:17805–17815. [PubMed] [Google Scholar]
- Boyles JK, Notterpek LM, Wardell MR, Rall SC., Jr Identification, characterization, and tissue distribution of apolipoprotein D in the rat. J Lipid Res. 1990;31:2243–2256. [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1016/0003-2697(87)90021-2. [DOI] [PubMed] [Google Scholar]
- Dandoy-Dron F, Guillo F, Benboudjema L, Deslys JP, Lasmézas C, Dormont D, Tovey MG, Dron M. Gene expression in scrapie. Cloning of a new scrapie-responsive gene and the identification of increased levels of seven other mRNA transcripts. J Biol Chem. 1998;273:7691–7697. doi: 10.1074/jbc.273.13.7691. [DOI] [PubMed] [Google Scholar]
- Signore A, Sanctis V, Mauro E, Negri R, Perrone-Capano C, Paggi P. Gene expression pathways induced by axotomy and decentralization of rat superior cervical ganglion neurons. Eur J NeuroSci. 2006;23:65–74. doi: 10.1111/j.1460-9568.2005.04520.x. [DOI] [PubMed] [Google Scholar]
- Valle E, Navarro A, Astudillo A, Tolivia J. Apolipoprotein D expression in human brain astrocytes. J Histochem Cytochem. 2003;51:1285–1290. doi: 10.1177/002215540305101005. [DOI] [PubMed] [Google Scholar]
- Desai PP, Ikonomovic MD, Abrahamson EE, Hamilton RL, Isanski BA, Hope CE, Klunk WE, DeKosky ST, Kamboh MI. Apolipoprotein D is a component of compact but not diffuse amyloid-beta plaques in Alzheimer’s disease temporal cortex. Neurobiol Dis. 2005;20:574–582. doi: 10.1016/j.nbd.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Díez-Itza I, Vizoso F, Merino AM, Sánchez LM, Tolivia J, Fernández J, Ruibal A, López-Otín C. Expression and prognostic significance of apolipoprotein D in breast cancer. Am J Pathol. 1994;144:310–320. [PMC free article] [PubMed] [Google Scholar]
- Do Carmo S, Séguin D, Milne R, Rassart E. Modulation of apolipoprotein D and apolipoprotein E mRNA expression by growth arrest and identification of key elements in the promoter. J Biol Chem. 2002;277:5514–5523. doi: 10.1074/jbc.M105057200. [DOI] [PubMed] [Google Scholar]
- Drayna D, Fielding C, McLean J, Baer B, Castro G, Chen E, Comstock L, Henzel W, Kohr W, Rhee L, Wion K, Lawn K. Cloning and expression of human apolipoprotein D cDNA. J Biol Chem. 1986;261:16535–16539. [PubMed] [Google Scholar]
- Drayna DT, McLean JW, Wion KL, Trent JM, Drabkin HA, Lawn RM. Human apolipoprotein D gene: gene sequence, chromosome localization, and homology to the alpha 2micro-globulin superfamily. DNA. 1987;6:199–204. doi: 10.1089/dna.1987.6.199. [DOI] [PubMed] [Google Scholar]
- Flower DR. The lipocalin protein family: structure and function. Biochem J. 1996;318:1–14. doi: 10.1042/bj3180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz G, Reindl M, Patel SC, Beer I, Unterrichter I, Berger T, Schmutzhard E, Poewe W, Kampfl A. Increased expression of apolipoprotein D following experimental traumatic brain injury. J Neurochem. 1999;73:1615–1625. doi: 10.1046/j.1471-4159.1999.0731615.x. [DOI] [PubMed] [Google Scholar]
- Ganfornina MD, Do Carmo S, Lora JM, Torres-Schumann S, Vogel M, Allhorn M, González C, Bastiani MJ, Rassart E, Sanchez D. Apolipoprotein D is involved in the mechanisms regulating protection from oxidative stress. Aging Cell. 2008;7:506–515. doi: 10.1111/j.1474-9726.2008.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling W, Zucker SD. Role of apolipoprotein D in the transport of bilirubin in plasma. Am J Physiol Gastrointest Liver Physiol. 2000;279:G356–G365. doi: 10.1152/ajpgi.2000.279.2.G356. [DOI] [PubMed] [Google Scholar]
- Kalman J, McConathy W, Araoz C, Kasa P, Lacko AG. Apolipoprotein D in the aging brain and in Alzheimer’s dementia. Neurol Res. 2000;22:330–336. doi: 10.1080/01616412.2000.11740678. [DOI] [PubMed] [Google Scholar]
- Kang MK, Kameta A, Shin KH, Baluda MA, Kim HR, Park NH. Senescence-associated genes in normal human oral keratinocytes. Exp Cell Res. 2003;287:272–281. doi: 10.1016/S0014-4827(03)00061-2. [DOI] [PubMed] [Google Scholar]
- Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat Genet. 2000;25:294–297. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- Loerch PM, Lu T, Dakin KA, Vann JM, Isaacs A, Geula C, Wang J, Pan Y, Gabuzda DH, Li C, Prolla TA, Yankner BA. Evolution of the aging brain transcriptome and synaptic regulation. PLoS ONE. 2008;3:e3329. doi: 10.1371/journal.pone.0003329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Boado YS, Tolivia J, López-Otín C. Apolipoprotein gene induction by retinoic acid is concomitant with grow arrest and cell differentiation in human breast cancer cells. J Biol Chem. 1994;269:26871–26878. [PubMed] [Google Scholar]
- McConathy WJ, Alaupovic P. Isolation and partial characterization of apolipoprotein D: a new protein moiety o f the human plasma lipoprotein system. FEBS Lett. 1973;37:178–182. doi: 10.1016/0014-5793(73)80453-3. [DOI] [PubMed] [Google Scholar]
- McConathy WJ, Alaupovic P. Studies on isolation and partial characterization of apolipoprotein D and lipoprotein D of human plasma. Biochemistry. 1976;15:515–520. doi: 10.1021/bi00648a010. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Montpied P, Bock F, Lerner-Natoli M, Bockaert J, Rondouin G. Hippocampal alterations of apolipoprotein E and D mRNA levels in vivo and in vitro following kainate excitotoxicity. Epilepsy Res. 1999;35:135–146. doi: 10.1016/S0920-1211(99)00003-0. [DOI] [PubMed] [Google Scholar]
- Morais-Cabral JH, Atkins GL, Sánchez LM, Lopez-Boado YS, Lopez-Otin C, Sawyer L. Arachidonic acid binds to apolipoprotein D: implications for the protein’s function. FEBS Lett. 1995;366:53–56. doi: 10.1016/0014-5793(95)00484-Q. [DOI] [PubMed] [Google Scholar]
- Muffat J, Walker DW, Benzer S. Human ApoD, an apolipoprotein up-regulated in neurodegenerative diseases, extends lifespan and increases stress resistance in Drosophila. Proc Natl Acad Sci USA. 2008;105:7088–7093. doi: 10.1073/pnas.0800896105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro A, Tolivia J, Astudillo A, Valle E. Pattern of apolipoprotein D immunoreactivity in human brain. Neurosci Lett. 1998;254:17–20. doi: 10.1016/S0304-3940(98)00639-9. [DOI] [PubMed] [Google Scholar]
- Navarro A, Astudillo A, Valle E, González del Rey C, Tolivia J. Immunohistochemical presence of Apolipoprotein D in senile plaques. J Histotechnol. 2001;24:45–48. [Google Scholar]
- Navarro A, Valle E, Astudillo A, González del Rey C, Tolivia J. Immunohistochemical study of distribution of apolipoproteins E and D in human cerebral beta amyloid deposits. Exp Neurol. 2003;184:697–704. doi: 10.1016/S0014-4886(03)00315-7. [DOI] [PubMed] [Google Scholar]
- Navarro A, Ordóñez C, Martínez E, Pérez C, Astudillo A, Tolivia J. Apolipoprotein D expression absence in degenerating neurons of human central nervous system. Histol Histopathol. 2008;23:995–1001. doi: 10.14670/HH-23.995. [DOI] [PubMed] [Google Scholar]
- Ong WY, He Y, Suresh S, Patel SC. Differential expression of apolipoprotein D and apolipoprotein E in the kainic acid-lesioned rat hippocampus. Neurosci. 1997;79:359–367. doi: 10.1016/S0306-4522(96)00608-2. [DOI] [PubMed] [Google Scholar]
- Ordóñez C, Navarro A, Pérez C, Astudillo A, Martínez E, Tolivia J. Apolipoprotein D expression in substantia nigra of Parkinson disease. Histol Histopathol. 2006;21:361–366. doi: 10.14670/HH-21.361. [DOI] [PubMed] [Google Scholar]
- Patel SC, Asotra K, Patel YC, McConathy WJ, Patel RC, Suresh S. Astrocytes synthesize and secrete the lipophilic ligand carrier apolipoprotein D. NeuroReport. 1995;6:653–657. doi: 10.1097/00001756-199503000-00017. [DOI] [PubMed] [Google Scholar]
- Pearlman WH, Gueriguian JL, Sawyer ME. A specific progesterone-binding component of human breast cyst fluid. J Biol Chem. 1973;248:5736–5741. [PubMed] [Google Scholar]
- Provost PR, Villeneuve L, Weech PK, Milne RW, Marcel YL, Rassart E. Localization of the major sites of rabbit apolipoprotein D gene transcription by in situ hybridization. J Lipid Res. 1991;32:1959–1970. [PubMed] [Google Scholar]
- Rassart E, Berdirian A, Do Carmo S, Guinard O, Sirois S, Terrisse L, Milne R. Apolipoprotein D (review) Biochim Biophys Acta. 2000;1482:185–198. doi: 10.1016/s0167-4838(00)00162-x. [DOI] [PubMed] [Google Scholar]
- Rickhag M, Deierborg T, Patel S, Ruscher K, Wieloch T. Apolipoprotein D is elevated in oligodendrocytes in the peri-infarct region after experimental stroke: influence of enriched environment. J Cereb Blood Flow Metab. 2008;28:551–562. doi: 10.1038/sj.jcbfm.9600552. [DOI] [PubMed] [Google Scholar]
- Sánchez D, Ganfornina MD, Martinez S. Expression pattern of the lipocalin apolipoprotein D during mouse embryogenesis. Mech Dev. 2002;110:225–229. doi: 10.1016/S0925-4773(01)00578-0. [DOI] [PubMed] [Google Scholar]
- Sánchez D, López-Arias B, Torroja L, Canal I, Wang X, Bastiani MJ, Ganfornina MD. Loss of glial lazarillo, a homolog of apolipoprotein D, reduces lifespan and stress resistance in Drosophila. Curr Biol. 2006;16:680–686. doi: 10.1016/j.cub.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Seguin D, Desforges M, Rassart E. Molecular characterization and differential mRNA tissue distribution of mouse apolipoprotein D. Mol Brain Res. 1995;30:242–250. doi: 10.1016/0169-328X(95)00008-G. [DOI] [PubMed] [Google Scholar]
- Smith KM, Lawn RM, Wilcox JN. Cellular localization of apolipoprotein D and lecithin: cholesterol acyltransferase mRNA in rhesus monkey tissues by in situ hybridization. J Lipid Res. 1990;31:995–1004. [PubMed] [Google Scholar]
- Spreyer P, Schaal H, Kuhn G, Rothe T, Unterbeck A, Olek K, Muller HW. Regeneration-associated high level expression of apolipoprotein D mRNA in endoneurial fibroblasts of peripheral nerve. EMBO J. 1990;9:2479–2484. doi: 10.1002/j.1460-2075.1990.tb07426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyrer E, Kostner GM. Activation of lecithin-cholesterol acyltitransferase by apolipoprotein D: comparison of proteoliposomes containing apolipoprotein D, A-I or C-I. Biochim Biophys Acta. 1988;958:484–491. doi: 10.1016/0005-2760(88)90235-4. [DOI] [PubMed] [Google Scholar]
- Terrisse L, Poirier J, Bertrand P, Merched A, Siest G, Milne RW, Rassart E. Increased levels of apolipoprotein D in cerebrospinal fluid and hippocampus of Alzheimer’s patients. J Neurochem. 1998;71:1643–1650. doi: 10.1046/j.1471-4159.1998.71041643.x. [DOI] [PubMed] [Google Scholar]
- Terrisse L, Seguin D, Bertrand P, Poirier J, Milne RW, Rassart E. Modulation of apolipoprotein D and apolipoprotein E mRNA expression in rat hippocampus after enthorinal cortex lesion. Mol Brain Res. 1999;70:26–35. doi: 10.1016/S0169-328X(99)00123-0. [DOI] [PubMed] [Google Scholar]
- Thomas EA, Dean B, Pavey G, Sutcliffe JG. Increased CNS levels of apolipoprotein D in schizophrenic and bipolar subjects: implications for the pathophysiology of psychiatric disorders. Proc Natl Acad Sci USA. 2001;98:4066–4071. doi: 10.1073/pnas.071056198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas EA, Laws SM, Sutcliffe JG, Harper C, Dean B, McClean C, Masters C, Lautenschlager N, Gandy SE, Martins RN. Apolipoprotein D levels are elevated in prefrontal cortex of subjects with Alzheimer’s disease: no relation to apolipoprotein E expression or genotype. Biol Psychiatry. 2003;54:136–141. doi: 10.1016/S0006-3223(02)01976-5. [DOI] [PubMed] [Google Scholar]
- Tolivia J, Navarro A, Tolivia D. Differential staining of nerve cells and fibres for sections of paraffin-embedded material in mammalian central nervous system. Histochemistry. 1994;102:101–104. doi: 10.1007/BF00269013. [DOI] [PubMed] [Google Scholar]
- Trieu VN, Uckun FM. Apolipoprotein E and apolipoprotein D expression in a murine model of singlet oxygen-induced cerebral stroke. Biochem Biophys Res Commun. 2000;268:835–841. doi: 10.1006/bbrc.2000.2205. [DOI] [PubMed] [Google Scholar]
- Walker DW, Muffat J, Rundel C, Benzer S. Overexpression of a Drosophila homolog of apolipoprotein D leads to increased stress resistance and extended lifespan. Curr Biol. 2006;16:674–679. doi: 10.1016/j.cub.2006.01.057. [DOI] [PubMed] [Google Scholar]
- Zeng C, Spielman AI, Vowels BR, Leyden JJ, Biemann K, Preti G. A human axillary odorant is carried by apolipoprotein D. Proc Natl Acad Sci USA. 1996;93:6626–6630. doi: 10.1073/pnas.93.13.6626. [DOI] [PMC free article] [PubMed] [Google Scholar]