Abstract
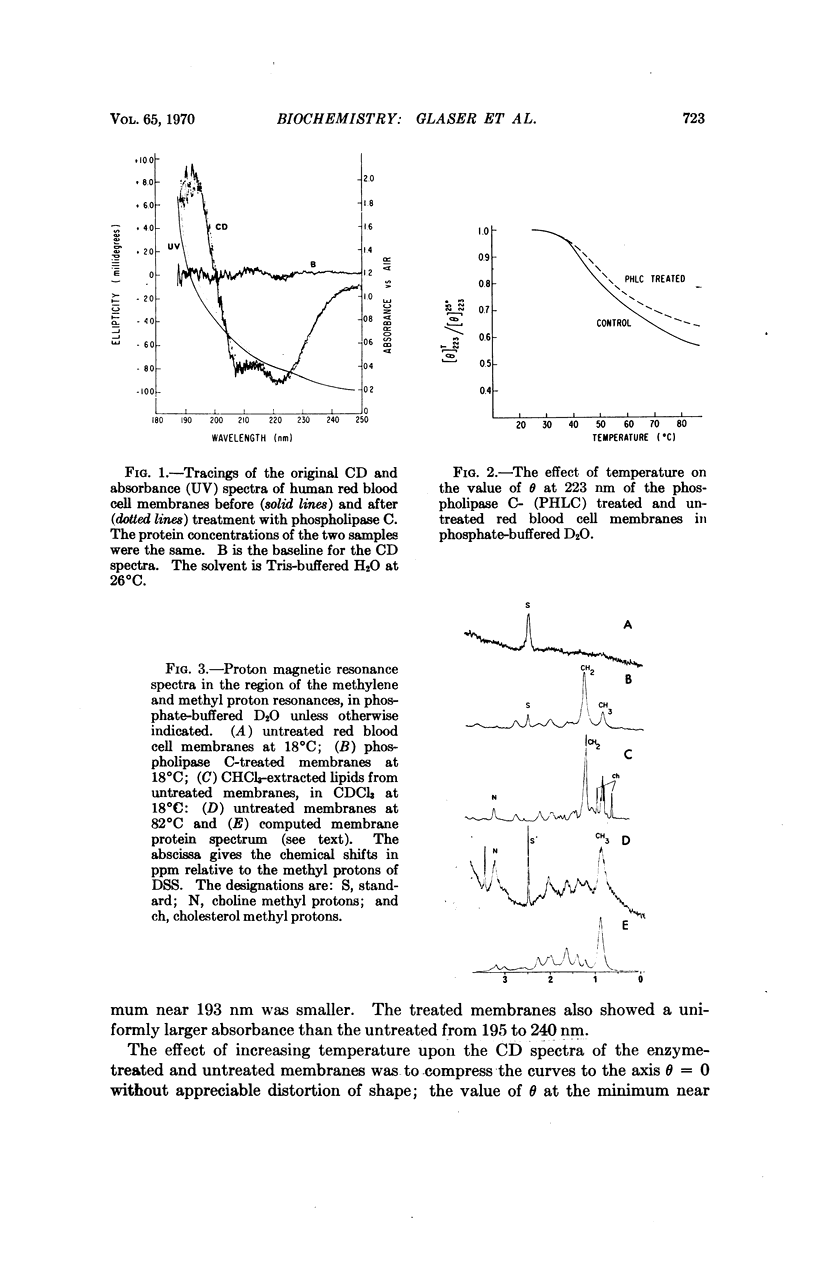
The effects of temperature and of the action of a purified phospholipase C enzyme preparation on human red blood cell membranes has been investigated by chemical analyses, circular dichroism, and proton magnetic resonance measurements. The results indicate that a substantial fraction of the phospholipids and the proteins of the membranes can change structure independently of one another, suggesting a mosaic pattern for the organization of the lipids and proteins in membranes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABEL L. L., LEVY B. B., BRODIE B. B., KENDALL F. E. A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J Biol Chem. 1952 Mar;195(1):357–366. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Chapman D., Kamat V. B., de Gier J., Penkett S. A. Nuclear magnetic resonance studies of erythrocyte membranes. J Mol Biol. 1968 Jan 14;31(1):101–114. doi: 10.1016/0022-2836(68)90058-2. [DOI] [PubMed] [Google Scholar]
- Chapman D., Leslie R. B., Hirz R., Scanu A. M. 220 MHz nuclear magnetic resonance spectra of high density serum lipoproteins. Nature. 1969 Jan 18;221(5177):260–261. doi: 10.1038/221260a0. [DOI] [PubMed] [Google Scholar]
- Chapman D., Penkett S. A. Nuclear magnetic resonance spectroscopic studies of the interaction of phospholipids with cholesterol. Nature. 1966 Sep 17;211(5055):1304–1305. doi: 10.1038/2111304a0. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Finean J. B., Martonosi A. The action of phospholipase C on muscle microsomes: a correlation of electron microscope and biochemical data. Biochim Biophys Acta. 1965 Jun 1;98(3):547–553. doi: 10.1016/0005-2760(65)90151-7. [DOI] [PubMed] [Google Scholar]
- Gordon A. S., Wallach D. F., Straus J. H. The optical activity of plasma membranes and its modification by lysolecithin, phospholipase A and phospholipase C. Biochim Biophys Acta. 1969;183(3):405–416. doi: 10.1016/0005-2736(69)90155-2. [DOI] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Motion of steroid spin labels in membranes. Proc Natl Acad Sci U S A. 1969 May;63(1):16–22. doi: 10.1073/pnas.63.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat V. B., Chapman D. Nuclear resonance studies of erythrocyte ghosts at 220 Mcycles-sec. Biochim Biophys Acta. 1968 Nov 5;163(3):411–414. doi: 10.1016/0005-2736(68)90126-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lenard J., Singer S. J. Protein conformation in cell membrane preparations as studied by optical rotatory dispersion and circular dichroism. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1828–1835. doi: 10.1073/pnas.56.6.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenard J., Singer S. J. Structure of membranes: reaction of red blood cell membranes with phospholipase C. Science. 1968 Feb 16;159(3816):738–739. doi: 10.1126/science.159.3816.738-a. [DOI] [PubMed] [Google Scholar]
- Macfarlane M. G. The biochemistry of bacterial toxins: 2. The enzymic specificity of Clostridium welchii lecithinase. Biochem J. 1948;42(4):587–590. [PMC free article] [PubMed] [Google Scholar]
- McDonald C. C., Phillips W. D. Proton magnetic resonance spectra of proteins in random-coil configurations. J Am Chem Soc. 1969 Mar 12;91(6):1513–1521. doi: 10.1021/ja01034a039. [DOI] [PubMed] [Google Scholar]
- Ottolenghi A. C. Phospholipase C from Bacillus cereus, a zinc-requiring metalloenzyme. Biochim Biophys Acta. 1965 Dec 2;106(3):510–518. doi: 10.1016/0005-2760(65)90067-6. [DOI] [PubMed] [Google Scholar]
- RICHARDSON S. H., HULTIN H. O., GREEN D. E. STRUCTURAL PROTEINS OF MEMBRANE SYSTEMS. Proc Natl Acad Sci U S A. 1963 Nov;50:821–827. doi: 10.1073/pnas.50.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. A., Guidotti G. The protein of human erythrocyte membranes. I. Preparation, solubilization, and partial characterization. J Biol Chem. 1968 Apr 25;243(8):1985–1992. [PubMed] [Google Scholar]
- SNYDER F., STEPHENS N. A simplified spectrophotometric determination of ester groups in lipids. Biochim Biophys Acta. 1959 Jul;34:244–245. doi: 10.1016/0006-3002(59)90255-0. [DOI] [PubMed] [Google Scholar]
- Steim J. M., Tourtellotte M. E., Reinert J. C., McElhaney R. N., Rader R. L. Calorimetric evidence for the liquid-crystalline state of lipids in a biomembrane. Proc Natl Acad Sci U S A. 1969 May;63(1):104–109. doi: 10.1073/pnas.63.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W., Ji T. H. Distortions in circular dichroism patterns of particulate (or membranous) systems. Arch Biochem Biophys. 1968 Dec;128(3):802–807. doi: 10.1016/0003-9861(68)90088-x. [DOI] [PubMed] [Google Scholar]
- Wallach D. F., Zahler P. H. Protein conformations in cellular membranes. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1552–1559. doi: 10.1073/pnas.56.5.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zull J. E., Greanoff S., Adam H. K. Interaction of egg lecithin with cholesterol in the solid state. Biochemistry. 1968 Dec;7(12):4172–4176. doi: 10.1021/bi00852a005. [DOI] [PubMed] [Google Scholar]




