Abstract
Tumid lupus erythematosus is a rare variant of chronic cutaneous lupus erythematosus that is characterized clinically by smooth, nonscarring, pink to violaceous papules or plaques without evidence of surface change. Histopathologic features include superficial and deep lymphocytic infiltration in a perivascular and periadnexal distribution, with dermal interstitial mucin deposition and focal or absent dermoepidermal junction involvement. These clinical and histopathologic features can be challenging to differentiate from other cutaneous diseases. This is particularly true because patients with tumid lupus erythematosus usually do not have other manifestations of systemic lupus erythematosus or cutaneous lupus erythematosus. We present two cases of tumid lupus erythematosus, one associated with concomitant systemic lupus erythematosus and the other occurring concurrently with discoid lupus erythematosus. Furthermore, we demonstrate the rare occurrence of a patient with tumid LE occurring below the waist at a photoprotected site.
Keywords: tumid lupus erythematosus, systemic lupus erythematosus, discoid lupus erythematosus, chronic cutaneous lupus erythematosus
Introduction
Lupus erythematosus (LE) is a multifaceted disease with a wide spectrum of manifestations, ranging from purely skin lesions in some cases of cutaneous lupus erythematosus (CLE) to the most severe form with multiple organ involvement in systemic lupus erythematosus (SLE). Cutaneous lupus erythematosus encompasses a number of distinct clinical presentations that can be divided into acute, subacute, and chronic cutaneous LE. Some of the common types of CLE are categorized under chronic cutaneous lupus erythematosus, including localized and generalized discoid lupus erythematosus (DLE), lupus panniculitis, and hypertrophic LE. Tumid lupus erythematosus, the subject of this case report, is an unusual form of chronic CLE that can have histologic features that are challenging to differentiate from other cutaneous diseases such as pseudolymphoma, polymorphous light eruption, reticular erythematous mucinosis, erythema migrans, and lymphocytic infiltrate of Jessner and Kanof. It is uncommon for patients with tumid LE to have other manifestations of CLE or SLE. We present two cases of tumid LE, one associated with concomitant SLE and the other occurring concurrently with DLE. Furthermore, we demonstrate the rare occurrence of a patient with tumid LE occurring below the waist at a photoprotected site. The clinical, histopathologic, and laboratory findings of these two cases are discussed in this article.
Case Reports
CASE 1
A 31-year-old Caucasian female presented with a new, slightly pruritic rash on her upper thighs bilaterally (Figure 1). Her medical history was significant for systemic lupus erythematosus (SLE), diagnosed when she presented with glomerulonephritis. The complications associated with the patient’s SLE included the presence of antiphospholipid antibody syndrome, as manifested by multiple pregnancy losses, deep vein thrombosis (DVT), a transient ischemic attack (TIA) and a seizure disorder. Additionally, she had lupus nephritis requiring cyclophosphamide, arthritis, and a history of some redness on her central face. The patient did not have a history of any chronic cutaneous lupus erythematosus lesions, aside from the new rash on her thighs.
Figure 1.
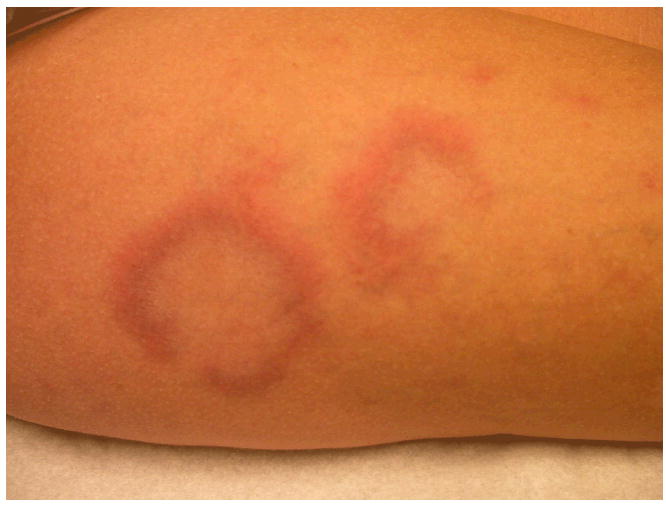
Annular dermal plaques on thigh
Physical exam revealed pink to purple annular dermal plaques on her thighs bilaterally. The lesions showed no evidence of scale. Her labs included an antinuclear antibody (ANA) titer of >1:2560 (normal <1:40) with a speckled pattern, anti-Ro/SS-A autoantibodies 186 (normal ≤ 100), anti-La/SS-B autoantibodies 586 (normal ≤ 100), anti-Smith antibodies 379 (normal < 100), anti-RNP antibodies 176 (normal < 100), anticardiolipin IgM autoantibodies 20 MPL U/mL (normal < 10) and IgG autoantibodies >100 GPL U/mL (normal < 10), C3 55 (normal 70-176), C4 <10 (normal 14-45), and CH50 24 (normal 26-58). A four-mm punch biopsy of one of the dermal plaques, including the underlying adipose tissue, illustrated superficial and deep periadnexal and perivascular lymphocytic infiltrates, no changes at the dermal-epidermal junction, with increased dermal mucin, favoring tumid lupus erythematosus (Figures 2 and 3). There was no evidence of panniculitis.
Figure 2.
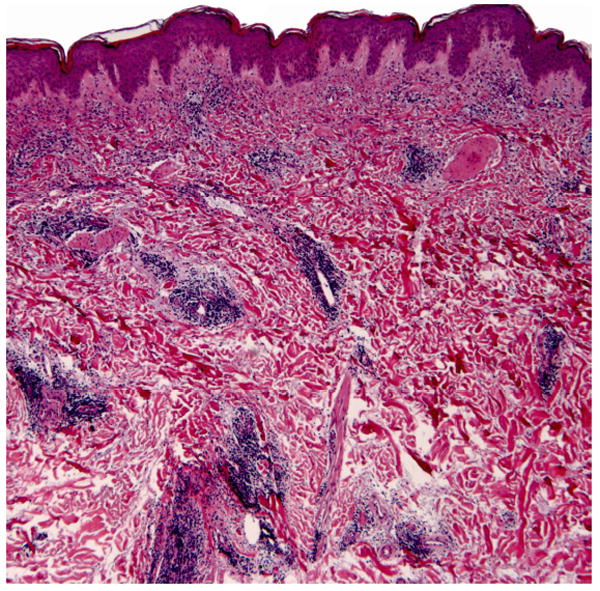
Superficial and deep dermal perivascular and periadnexal lymphocytic infiltrate with increased interstitial dermal mucin
Figure 3.
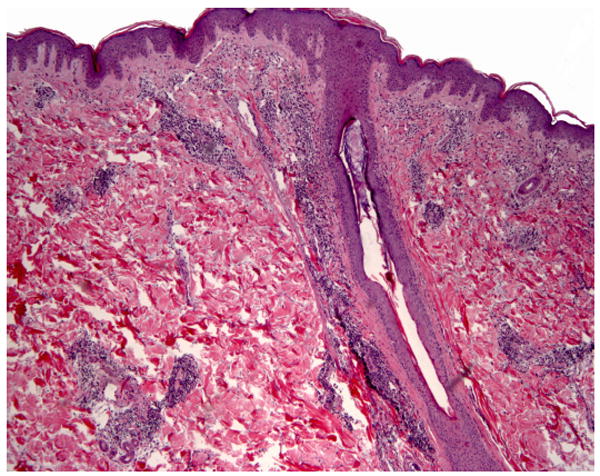
Perivascular and perifollicular dermal lymphocytic infiltrate with no significant epidermal involvement
Given that the patient was already on plaquenil at the time of presentation, we added clobetasol 0.05% cream to treat the focal areas of tumid LE. The skin lesions cleared gradually with the topical regimen and the patient has not had a recurrence while remaining on plaquenil.
CASE 2
A 34-year-old Caucasian female presented with “bumps” on her chest (Figure 4), which worsened with sun exposure. Her medical history was significant for discoid lupus erythematosus (DLE) confirmed by biopsy in February 2005, with evidence of follicular scarring around her mouth. Although she had no recent alopecia or active DLE in the scalp, she did present with prominent hyperpigmentation on her right scalp. She also had a history of arthralgias, myalgias, xerophthalmia, and xerostomia.
Figure 4.
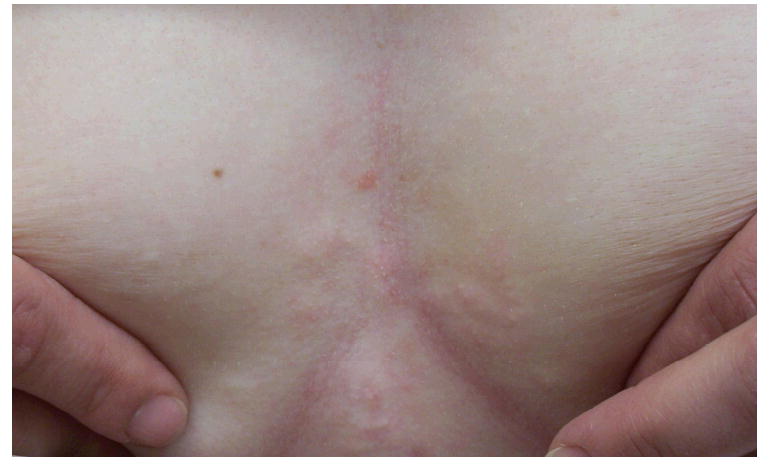
Discrete erythematous papules on the central chest.
Physical exam revealed numerous pink to flesh colored firm papules scattered on her anterior chest. Her labs consisted of an antinuclear antibody titer of 1:40 (normal <1:40) with a speckled pattern, anti-Ro/SS-A autoantibodies 145 (normal ≤ 100) and normal anti-La/SS-B autoantibodies. A four-mm punch biopsy of a chest papule demonstrated superficial and mid-dermal perivascular and perifollicular lymphocytic infiltrates with an associated increase in dermal mucinous ground substance (Figures 5 and 6). No interface dermatitis was appreciated and the findings were consistent with tumid lupus erythematosus.
Figure 5.
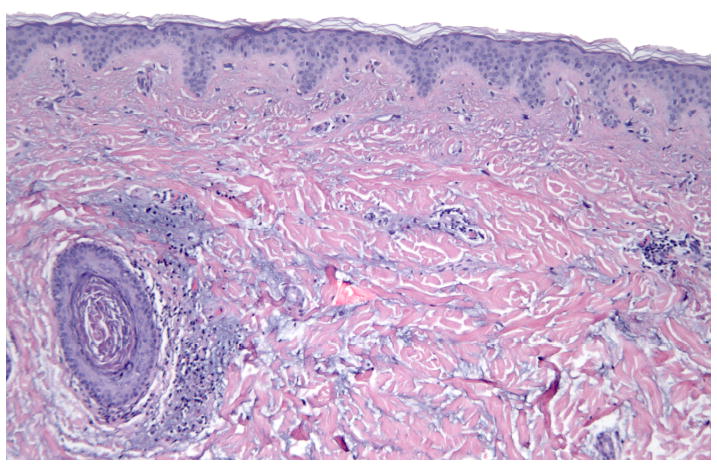
Perivascular and perifollicular dermal lymphocytic infiltrate with increased interstitial dermal mucin
Figure 6.
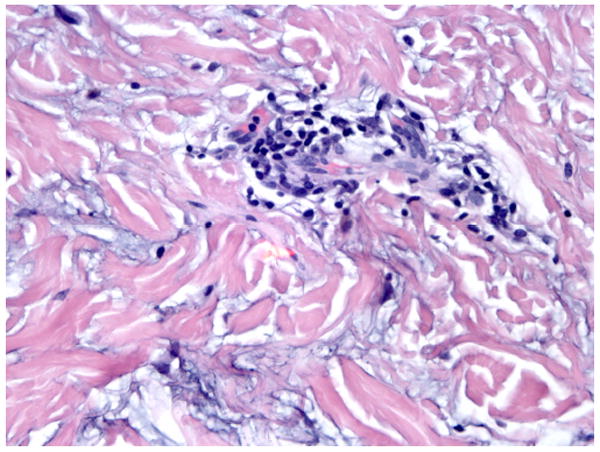
Perivascular lymphocytic infiltrate with increased interstitial dermal mucin
At the time of presentation, the patient was on a regimen of hydroxychloroquine 200 mg daily for treatment of her DLE lesions. The dose was increased to 300 mg daily and, at 4 months follow-up, the patient’s appearance was improved.
Comment
Tumid lupus erythematosus was first reported by Erich Hoffman in 1909 at a meeting of the Berlin Dermatological Society. The first citation was in 1930 and the entity has been documented infrequently ever since (1). Tumid LE is characterized by smooth, erythematous to violaceous papules, plaques, or nodules (2) with no surface changes, such as follicular plugging, atrophy, or scale. Lesions are easily induced with provocative phototesting and characteristically appear on sun-exposed areas of the face, upper back, V area of the neck, trunk, and upper extremities, with little documented evidence of lower extremity involvement (2-5). Similar to other forms of cutaneous lupus erythematosus, the lesions of tumid LE characteristically appear in a symmetrical distribution, although Pacheco, et al described one case of unilateral tumid LE following the lines of Blaschko (6). The lesions normally clear without scarring. The spontaneous clearing of lesions inspired Gourgerot to describe this phenomenon in tumid LE as “the eclipse” (1,5).
Histopathologic features of tumid LE include moderate to dense superficial and deep lymphocytic inflammation in a perivascular and periadnexal distribution, with interstitial mucin deposition between collagen bundles (2,3,7). Involvement of the dermoepidermal junction is focal or absent (3). Tumid LE can be difficult to distinguish from other dermal lymphocytic infiltrates on histopathologic criteria alone. Conditions to consider in the differential diagnosis include pseudolymphoma, polymorphous light eruption (PMLE), reticular erythematous mucinosis (REM), erythema migrans, and lymphocytic infiltrate of Jessner and Kanof. Of these, REM most closely resembles tumid LE despite having more mucin and less inflammation. Pseudolymphoma often shows germinal center formation and PMLE has marked papillary dermal edema, while erythema migrans is characterized by a tightly cuffed lymphoplasmacytic infiltrate with minimal interstitial involvement (8). Immunohistochemical studies have demonstrated a predominance of CD-3+ and CD-4+ lymphocytes in tumid LE lesions, with a low percentage of CD-8+ lymphocytes (3). The ratio of CD4 to CD8 cells was found to be approximately 3:1, differentiating tumid LE from other cutaneous diseases, such as pseudolymphoma which shows variable numbers of B and T cells in the inflammatory infiltrate and polymorphous light eruption which demonstrates the opposite CD marker ratio with CD8+ lymphocytes predominating over CD4+ cells (3,9,10,11).
Historically, the serologic findings in tumid LE are unremarkable. Dekle, et al. retrospectively studied four cases of documented tumid LE, reporting ANA titers as weakly positive in two patients and negative in the remaining two. Anti-Ro/SS-A and anti-La/SS-B measurements were negative in all cases, apart from one anti-La/SS-B documented as “equivocal” (5). Kuhn, et al. measured ANA titers greater than 1:160 in 10% of patients, anti-Ro/SS-A and anti-La/SS-B antibodies in 5%, and anti-double-stranded DNA (dsDNA) antibodies in 3% (2). Our patient with concomitant SLE had a positive ANA titer of 1:2560 along with low complement levels and positive anti-Ro/SS-A, anti-La/SS-B, anti- RNP, anti-Smith, and anticardiolipin antibody titers. Our second patient with coexistent DLE had an ANA titer of 1:40 with positive anti-Ro/SS-A and normal anti-La/SS-B antibodies. The diversity of these measurements elucidates the range of potential serologic findings in patients presenting with tumid LE alone or in combination with other forms of LE.
The precise classification of tumid LE remains a topic of debate throughout the dermatology literature. In recent years, few studies have attempted to characterize the specific clinical and histopathologic features of tumid LE in order to define comprehensive criteria to distinguish it from other conditions and emphasize its value as a unique entity within the cutaneous LE spectrum. While most published reports suggest that tumid LE is a form of chronic cutaneous LE, several propose that due to the lack of serology, epidermal change, and interface dermatitis on histopathologic evaluation, it may belong grouped with other lupus non-specific skin lesions.
The literature contains conflicting reports regarding the possibility of tumid LE occurring simultaneously with SLE or other forms of cutaneous LE (2,12). Although the association with systemic disease is low, there have been a number of documented reports of tumid LE coexisting with either DLE or SLE (1,3,4,5). We describe two patients who were diagnosed as having tumid LE and concomitant SLE or DLE. Additionally, we demonstrate the rare occurrence of a patient with tumid LE occurring below the waist. Our patient, who had an earlier diagnosis of SLE with significant organ involvement, subsequently developed tumid LE. She presented with annular dermal plaques on her thighs bilaterally. Although rare, this patient demonstrates the potential for tumid LE to occur in photoprotected sites like the upper thigh.
The two cases presented reveal the definite association of tumid LE with other subtypes of LE, including SLE and DLE. The presence of this rare variant of cutaneous lupus erythematosus amid the more common forms of SLE and DLE needs to be considered when managing patients with various forms of LE. This awareness should enhance early diagnosis and emphasize that tumid LE can occur together with other forms of chronic cutaneous LE, as well as SLE.
Acknowledgments
Funding Source: None
Footnotes
Conflicts of Interest: None
References
- 1.Gougerot H, Burnier R. Lupus erythemateux “tumidus.”. Bull Soc Fr Dermatol Syph. 1930;37:1291–1292. [Google Scholar]
- 2.Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus Erythematosus Tumidus: A Neglected Subset of Cutaneous Lupus Erythematosus: Report of 40 cases. Archives of Dermatology. 2000;136:1033–1041. doi: 10.1001/archderm.136.8.1033. [DOI] [PubMed] [Google Scholar]
- 3.Alexiades-Armenakas MR, Baldassano M, Bince B, et al. Tumid Lupus Erythematosus: Criteria for Classification With Immunohistochemical Analysis. Arthritis and Rheumatism. 2003;49:494–500. doi: 10.1002/art.11206. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz H, Sánchez J. Tumid Lupus Erythematosus. The American Journal of Dermatopathology. 1999;21:356–360. doi: 10.1097/00000372-199908000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Dekle CL, Mannes KD, Davis LS, et al. Lupus Tumidus. J Am Acad Dermatol. 1999;41:250–253. doi: 10.1016/s0190-9622(99)70056-3. [DOI] [PubMed] [Google Scholar]
- 6.Pacheco T, Spates S, Lee L. Unilateral tumid lupus erythematosus. Lupus. 2002;11:388–391. doi: 10.1191/0961203302lu208cr. [DOI] [PubMed] [Google Scholar]
- 7.Kuhn A, Sonntag M, Ruzicka T, et al. Histopathologic findings in lupus erythematosus Tumidus: Review of 80 patients. J Am Acad Dermatol. 2003;48:901–908. doi: 10.1067/mjd.2003.435. [DOI] [PubMed] [Google Scholar]
- 8.Elder DE, Elenitsas R, Johnson B, et al. Atlas and Synopsis of Lever’s Histopathology of the Skin. Philadelphia, PA: Lippincott Williams and Wilkins; 2007. [Google Scholar]
- 9.Ackerman BA, Chongchitnint N, Sanchez J, et al. Histologic diagnosis of inflammatory skin diseases: an algorithmic method based on pattern analysis. 2. Baltimore: Williams and Wilkins; 1997. [Google Scholar]
- 10.Rijlaarsdam JU, Nieboer C, De Vries E, et al. Characterization of the dermal infiltrates in Jessner’s lymphocytic infiltrate of the skin, polymorphous light eruption and cutaneous lupus erythematosus: differential diagnostic and pathogenic aspects. J Cutan Pathol. 1990;17:2–8. doi: 10.1111/j.1600-0560.1990.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 11.Alexiades-Armenakas M. Tumid lupus erythematosus. Dermatology Online Journal. 2:7–14. [PubMed] [Google Scholar]
- 12.Pramatarov KD. Chronic Cutaneous Lupus Erythematosus – Clinical Spectrum. Clinics in Dermatology. 2004;22:113–120. doi: 10.1016/j.clindermatol.2003.12.016. [DOI] [PubMed] [Google Scholar]


