Abstract
Background
The epidemiology of heart failure (HF) in young adults is poorly understood.
Methods
The Coronary Artery Risk Development in Young Adults (CARDIA) study evaluated 5115 black and white men and women, age 18–30 at baseline, for the development of heart disease over 20 years. We examined predictors of hospitalization or death from HF using Cox models.
Results
In 20 years, 27 participants developed HF (mean age, 39±6). All but one were black, with a cumulative incidence before age 50 in black women of 1.1% (0.6–1.7%), black men=0.9% (0.5–1.4%), white women=0.08% (0.0–0.5%), white men=0.0% (0.0–0.4%; p=0.001 for blacks vs. whites). In blacks, independent predictors at age 18–30 of HF occurring 15 years later included higher diastolic blood pressure (HR per 10.0 mmHg=2.1, 1.4–3.1), higher body mass index (HR per 5.7 kg/m2=1.4, 1.0–1.9), lower HDL cholesterol (HR per 13.3 mg/dL=0.6, 0.4–1.0), and kidney disease (HR 20, 4.5–87). Three quarters of those who subsequently developed HF had hypertension by age 40; one quarter had depressed systolic function on study echocardiogram at age 23–35. Systolic function was associated with subsequent HF 10 years later (abnormal: HR=37, 6.9–200; borderline: HR=3.5, 1.2–10). Myocardial infarction, drug use, or alcohol use were not associated with HF risk.
Conclusions
Incident HF before age 50 is substantially more common among blacks than whites and is not rare. Hypertension, obesity, kidney disease, and systolic dysfunction before age 35 are antecedents of HF 1–2 decades later and may be important for screening and treatment aimed at HF prevention.
Introduction
Heart failure (HF) is a major public health problem in the US, causing substantial morbidity and mortality in the later decades of life. The risk of HF rises sharply with age, with rates doubling every 10 years among older adults.1, 2 Less is known about the incidence of HF before age 50; one estimate suggests the five-year risk of HF among white 40 year-olds is only 0.1–0.2%.3
Blacks have a higher prevalence of HF than others in the US, and present at younger ages with HF symptoms.4, 5 The reason for the greater propensity for HF among blacks is not fully understood; higher burden of risk factors such as hypertension, genetic predisposition to cardiomyopathy, and exposures to toxins including drugs and alcohol have all been postulated to play a role.4–6 No study has prospectively determined HF incidence in a young adult population that includes blacks or explored the mechanisms for HF development in this age group.
In this report we describe HF incidence and its antecedents in the Coronary Artery Risk Development in Young Adults (CARDIA) study. CARDIA is a well-characterized cohort of black and white men and women, aged 18–30 at the time of enrollment and followed for twenty years, with periodic risk factor assessments including echocardiography, and adjudicated cardiovascular outcomes.
Methods
The CARDIA Study
CARDIA is a multicenter study designed to investigate the development of coronary disease in young adults. CARDIA began in 1985-6 with 5115 black and white men and women age 18–30 recruited from Birmingham, AL, Chicago, IL, Minneapolis, MN, and Oakland, CA. The cohort is balanced by race (52% black), sex (55% female), and education (40% with ≤12 years of education).7 Baseline and additional measurements were repeated at Years 2, 5, 7, 10, 15, and 20. CARDIA has achieved a high retention rate with 87.5% of the original cohort completing the Year 20 annual telephone interview for outcome ascertainment and 72% completing the Year 20 in-person examination.
Incident heart failure
Participants were asked about overnight hospitalizations during their scheduled study exams and yearly telephone interviews, and records were requested for suspected cardiovascular events. Deaths were reported to the field centers every 6 months and records requested with consent from next-of-kin. Two members of the Endpoints Committee reviewed each record to determine the primary cause; disagreements were resolved by consensus. Hospitalization for HF was a pre-specified CARDIA endpoint and required both 1) final diagnosis of HF by a physician, and 2) medical treatment for HF during hospitalization (use of a diuretic, and either digitalis or an afterload reducer, such as nitroglycerin, hydralyzine, ACE inhibitor or angiotensin receptor blocker). HF was not a pre-specified category for primary cause of death; we defined a HF-associated death as one in which the adjudicated cause was cardiovascular and an ICD-9 code for HF (428xx) or cardiomyopathy (425xx) was noted as a contributory cause. We also reviewed endpoint records for co-morbid conditions at the time of HF presentation; these data were not used in the analyses (Tables 1–3) but are described in Table 4.
Table 1.
Characteristics of CARDIA participants at baseline, by subsequent heart failure status
|
White participants1 |
Black participants |
|||||
|---|---|---|---|---|---|---|
| No heart failure N=2477 | No heart failure N=2611 | Heart failure N=26 | p-value2 | p-value3 | ||
| DEMOGRAPHIC characteristics | ||||||
| Age (years) | 25 ± 3 | 24 ± 4 | 26 ± 3 | 0.12 | 0.06 | |
| Sex (% male) | 47% | 44% | 38% | 0.56 | 0.69 | |
| Education- | Less than high school | 6% | 13% | 23% | 0.03 | 0.31 |
| High school graduate | 21% | 38% | 38.5% | |||
| More than high school | 73% | 49% | 38.5% | |||
| CLINICAL characteristics4 | ||||||
| Diastolic blood pressure (mmHg) | 68.4 ± 9.2 | 68.7 ± 9.6 | 78.7 ± 11.5 | <0.001 | <0.001 | |
| Systolic blood pressure (mmHg) | 109.3 ± 109 | 111.4 ± 10.9 | 120.6 ± 10.8 | <0.001 | <0.001 | |
| Clinical hypertension | 2% | 3% | 19% | <0.001 | <0.001 | |
| Body mass index (kg/m2) | 23.6 ± 4.0 | 25.2 ± 5.6 | 32.0 ± 9.7 | <0.001 | 0.001 | |
| Obesity | 6% | 16% | 34% | 0.002 | 0.03 | |
| Diabetes | 1% | 2% | 12% | 0.008 | 0.01 | |
| HDL cholesterol (mg/dL) | 51.8 ± 13.0 | 54.6 ± 13.3 | 45.6 ± 11.2 | 0.004 | <0.001 | |
| LDL cholesterol (mg/dL) | 108.5 ± 30.0 | 109.5 ± 32.2 | 113.5 ± 46.7 | 0.62 | 0.65 | |
| Chronic kidney disease | 6% | 1% | 8% | 0.19 | 0.01 | |
| Family history coronary disease | 14% | 10% | 15% | 0.54 | 0.31 | |
| Current tobacco use | 27% | 34% | 38% | 0.40 | 0.67 | |
| Excessive alcohol use | 16% | 9% | 8% | 0.76 | 1.0 | |
| Illicit drug use | 52% | 29% | 35% | 0.69 | 0.52 | |
| Prior pregnancy5 | 40% | 63% | 69% | 0.21 | 0.80 | |
| ECHOCARDIOGRAPHIC characteristics6 | ||||||
| Ejection fraction | 0.63 ± 0.06 | 0.63 ± 0.15 | 0.54 ± 0.09 | 0.02 | 0.07 | |
| Systolic dysfunction7 | Borderline | 9% | 10% | 19% | <0.001 | <0.001 |
| Abnormal | 0.2% | 0.3% | 8% | |||
| Left ventricular mass index (gm/m2.7) | 33.5 ± 8.5 | 36.5 ± 9.6 | 47.4 ± 19.6 | <0.001 | <0.001 | |
| % with left ventricular hypertrophy | 4% | 7% | 26% | 0.003 | 0.01 | |
The one white female with heart failure was excluded from this analysis
p value for Fisher exact test of proportions (for categorical variables) or Students t-test of means (for continuous variables), comparing blacks with heart failure to the entire cohort without heart failure.
p value for Fisher exact test of proportions (for categorical variables) or Students t-test of means (for continuous variables), comparing blacks with heart failure to blacks without heart failure.
All measured at baseline, with the exception of illicit drug use, which was measured at Year 2
Proportions and comparisons are restricted to women.
From study echocardiogram at Year 5 among 4230 participants
Borderline” defined by ejection fraction 40–60% or qualitative assessment as borderline systolic function; “abnormal” defined by ejection fraction <40% or qualitative assessment of abnormal systolic function.
Table 3.
Echocardiographic measures at year 5 (age 23–35) associated with subsequent heart failure among blacks
| Bivariate model |
Multivariate model (adjusted for the other echocardiographic measure)1 |
Multivariate model (additionally adjusted for clinical measures)2 |
||||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-value | Hazard Ratio (95% CI) | p-value | Hazard Ratio (95% CI) | p-value | |
| Systolic function | ||||||
| Abnormal (EF <40%) | 34 (7.6–154) | <0.001 | 27 (5.7–128) | <0.001 | 37 (6.9–200) | <0.001 |
| Borderline (EF 40–60%) | 3.1 (1.1–9.0) | 0.04 | 3.0 (1.1–8.5) | 0.04 | 3.5 (1.2–10) | 0.02 |
| Left ventricular hypertrophy | 6.0 (2.2–17) | <0.001 | 5.0 (1.7–15) | 0.003 | 1.9 (0.4–7.4) | 0.33 |
Models for systolic function adjusted for left ventricular hypertrophy; models for left ventricular hypertrophy adjusted for systolic function
All models adjusted diastolic blood pressure, body mass index, HDL cholest.
Table 4.
Characteristics of incident heart failure events from medical and death records1
| Type of Event | Age | EF2 | Co-morbid conditions at presentation2 | |
|---|---|---|---|---|
| 1 | Death | 21–25 | Autopsy – DCM, LVH (630 gm), no CHD | |
| 2 | Death | 21–25 | Autopsy – DCM, LVH (530 gm), no CHD | |
| 3 | Death | 36–40 | ESRD | |
| 4 | Death | 36–40 | Autopsy – mild CHD, DCM, LVH (430 gm) | |
| 5 | Hosp./death | 31–35 | 16% | HTN, CKD, alcohol, Autopsy – DCM, mod CHD, LVH (825 gm) |
| 6 | Hospitalization | 31–35 | 15% | HTN, anemia, family hx |
| 7 | Hospitalization | 36–40 | 15% | HTN, CKD, DM |
| 8 | Hospitalization | 41–45 | 15% | HTN |
| 9 | Hospitalization | 31–35 | 24% | CKD, OSA, cath – no CHD |
| 10 | Hospitalization | 36–40 | 25% | Atrial fibrillation |
| 11 | Hospitalization | 41–45 | 25% | DM, cath – no CHD |
| 12 | Hospitalization | 46–50 | 25% | HTN, ESRD, DM, cath – mild CHD |
| 13 | Hospitalization | 41–45 | 30% | HTN, CKD, A. Fib, alcohol |
| 14 | Hospitalization | 46–50 | 30% | HTN, OSA, anemia |
| 15 | Hospitalization | 31–35 | 30% | Postpartum, Family hx |
| 16 | Hospitalization | 46–50 | 39% | HTN, CKD, alcoholism |
| 17 | Hospitalization | 41–45 | 40% | HTN |
| 18 | Hospitalization | 46–50 | 40% | HTN, family hx, new DM, cath- no CHD |
| 19 | Hospitalization | 41–45 | 48% | HTN, A. fib |
| 20 | Hospitalization | 36–40 | >50% | HTN |
| 21 | Hospitalization | 41–45 | >50% | HTN, CKD, cocaine |
| 22 | Hospitalization | 41–45 | >50% | Echo – MS, cath – no CHD |
| 23 | Hospitalization | 46–50 | >50% | HTN, DM |
| 24 | Hospitalization | 36–40 | NR | HTN, ESRD, DM |
| 25 | Hospitalization | 36–40 | NR | ESRD, sickle cell disease |
| 26 | Hospitalization | 41–45 | NR | HTN, peripartum |
| 27 | Hospitalization | 41–45 | NR | HTN, OSA |
To protect the anonymity of participants, age is presented in 5-year ranges and race and sex have been omitted.
Codes: EF = ejection fraction; HTN = hypertension; CKD= chronic kidney disease; A. fib = atrial fibrillation; OSA = obstructive sleep apnea; DM = diabetes; DCM = dilated cardiomyopathy; LVH = left ventricular hypertrophy; ESRD = end stage renal disease; CHD = coronary heart disease; Hx = history; NR = not recorded
Clinical antecedents measured at each exam
Race was determined by participant self-report. We used the average of the second and third of three blood pressure measurements (taken at 1-minute intervals after sitting quietly), and defined hypertension as systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure (DBP) ≥90 mmHg or the use of anti-hypertensive medications. Weight in kilograms was measured in light clothing using a standard balance beam scale and indexed to height in meters2. Diabetes was defined by fasting blood sugar ≥126 mg/dL or the use of diabetes medications. Total cholesterol and HDL cholesterol were measured and LDL cholesterol calculated using the Friedewald equation.8 Creatinine was measured at Year 0, 10, 15, and 20; glomerular filtration rate (GFR) was estimated using the Modification of Diet in Renal Disease equation and chronic kidney disease (CKD) defined as GFR<60 ml/min. Education, family history of premature coronary disease, tobacco, alcohol, and illicit drug use were all determined by self-report at each exam. Consumption of more than 14 drinks per week in men or more than seven in women was considered excessive alcohol use; illicit drug use was defined as any lifetime use of cocaine, amphetamines, or heroin.
Echocardiographic antecedents
During the Year 5 exam, CARDIA participants underwent 2-dimensional, guided M-mode echocardiography and Doppler study of transmitral flow velocities performed on an Acuson cardiac ultrasound machine.9 All studies were recorded and read at a reading center at the University of California, Irvine. Systolic function was assessed as a continuous measure of ejection fraction (EF, in %) in 1893 participants, and as a qualitative EF rating in the remainder; we categorized systolic function as “abnormal” (EF<40% or qualitative rating abnormal), “borderline” (EF 40–60% or qualitative rating borderline) or “normal.” Left ventricular mass in grams was derived from the formula of Devereux,10 indexed to height in meters2.7 and expressed as left ventricular mass index (gm/m2.7).11 Left ventricular hypertrophy (LVH) was defined as left ventricular mass index ≥51 gm/m2.7, a cutpoint that has been previously validated for both blacks and whites.12 Of the 4351 participants available for this exam, 121 were missing variables for either EF or LVH, leaving 4230 available for this analysis.
Analysis
We compared baseline risk factors among participants who did and did not go on to develop HF using t-, chi-square, and Fisher’s exact tests as appropriate. We used Cox proportional hazards models to analyze associations between candidate risk factors and HF among blacks in unadjusted analyses. We developed two multivariate models, the first using baseline predictors only, and the second using time-varying covariates, with predictor values updated at the time of each study visit. Because of the small number of outcomes, we chose predictors for the adjusted analyses using forward selection, retaining potential risk factors if they remained associated with HF at p<0.05. Finally, we explored the association between systolic dysfunction and LVH on Year 5 echocardiogram and risk of subsequent HF in bivariate and multivariate analyses.
Results
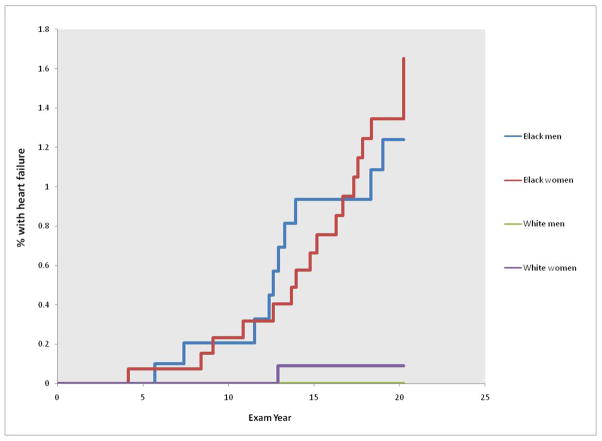
In 20 years of follow-up of 5115 participants, incident HF was more common than MI (observed in 16 participants). Twenty seven men and women developed HF, and with the exception of one white women, all participants with HF were black (Figure 1; p=0.001). HF was observed in black women (cumulative incidence 1.1%, 0.6–1.7%) and black men (0.9%, 0.5–1.4%), with a mean age at onset of 39±6 years. The cumulative incidence of HF among white women was 0.08% (0.0–0.5%) and among white men was 0% (0.0–0.4%). HF was fatal in three black men (4.5% of all deaths among black men), and two black women (7.7% of all deaths among black women).
Figure 1. Incident heart failure in the black and white men and women in CARDIA.
Kaplan-Meier curves for incident heart failure over 20 years of follow-up in CARDIA. P-value for comparison of blacks and whites = 0.001.
At baseline, when participants were age 18–30, blacks who subsequently developed HF differed from black and white participants who did not (Table 1). Compared to all those who did not develop HF, blacks with subsequent HF had higher SBP and DBP and were more likely to have clinical hypertension. They were also more likely to be obese, diabetic, or have CKD. Alcohol and drug use did not differ significantly between blacks with HF and participants who did not develop HF. Blacks who subsequently developed HF were more likely to have borderline or abnormal systolic function or LVH.
Because HF occurred almost exclusively in blacks, we restricted the Cox regression analyses to blacks. In bivariate models of baseline predictors (Table 2), higher blood pressure, higher body mass index (BMI), lower educational attainment, lower HDL cholesterol, CKD, and diabetes were all associated with subsequent HF. In multivariate models, DBP, BMI, HDL cholesterol, and CKD were each independently associated with HF risk. Each standard deviation increase in DBP (10.0 mmHg) among blacks age 18–30 doubled the hazard of future HF occurring on average 15 years later.
Table 2.
Baseline antecedents (at age 18–30 years) of subsequent heart failure among 2637 black CARDIA participants
| Cardiovascular risk factor at baseline | Bivariate models |
Multivariate model1 |
|||
|---|---|---|---|---|---|
| Hazard Ratio | p-value | Hazard Ratio | p-value | ||
| Age (years) (per SD, 3.8 years) | 1.5 (1.0–2.3) | 0.05 | |||
| Sex (% male) | 0.9 (0.4–2.0) | 0.81 | |||
| Education | Less than high school | 2.6 (1.0–7.2) | 0.06 | ||
| High school graduate | 1.4 (0.6–3.4) | 0.42 | |||
| More than high school | --- | --- | |||
| Diastolic blood pressure (mmHg)2 (per SD, 10.0 mmHg) | 2.5 (1.8–3.5) | < 0.001 | 2.1 (1.4–3.1) | <0.001 | |
| Systolic blood pressure (mmHg)2 (per SD, 10.9 mmHg) | 1.9 (1.4–2.6) | < 0.001 | |||
| Body mass index (kg/m2) (per SD, 5.7 kg/m2) | 2.0 (1.6–2.5) | < 0.001 | 1.4 (1.0–1.9) | 0.02 | |
| Diabetes | 6.7(2.0–22) | 0.002 | |||
| HDL cholesterol (mg/dL) (per SD, 13.3 mg/dL) | 0.4 (0.2–0.7) | < 0.001 | 0.6 (0.4–1.0) | 0.05 | |
| LDL cholesterol (mg/dL) (per SD, 32.4 mg/dL) | 1.1(0.7–1.6) | 0.70 | |||
| Chronic kidney disease | 13.8 (3.2–59) | < 0.001 | 19.8 (4.5–87) | < 0.001 | |
| Family history premature coronary disease | 1.6 (0.5–4.6) | 0.40 | |||
| Current tobacco use | 1.4 (0.6–3.1) | 0.41 | |||
| Excessive alcohol use | 0.9 (0.2–3.8) | 0.88 | |||
| Prior pregnancy (among women) | 1.3 (0.4–3.7) | 0.63 | |||
Variables selected by forward selection and retained if associated with heart failure at p<0.05
Systolic blood pressure and diastolic blood pressure are collinear. Each is significant in the multivariate models without the other present. When both are presented for forward selection (as they are in the multivariable models above) diastolic blood pressure is retained in the final model. Replacing diastolic blood pressure with systolic blood pressure would leave the hazard ratios for the other covariates unchanged; the adjusted HR for systolic blood pressure per 10.9 mmHg = 1.5, 95% CI 1.1–2.1, p=0.01
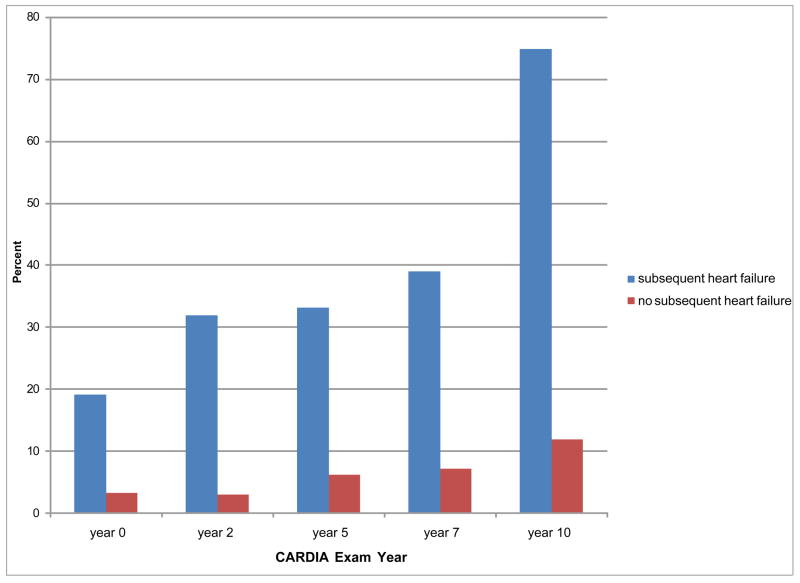
During the first 10 years of CARDIA, clinical hypertension was more common among black participants who subsequently developed HF than blacks who did not (Figure 2). By Year 10 when participants were age 28–40, 75% of those who subsequently developed HF had clinical hypertension (versus 12% of those who did not develop HF; p<0.001). Most participants with hypertension had untreated or had poorly controlled blood pressure. At baseline, 75% (66/88) of all black participants with hypertension were untreated and another 9% (8/88) were poorly controlled on medications; all black participants with hypertension who subsequently developed HF had untreated or poorly controlled hypertension. By year 10, 57% (137/239) of black participants with hypertension were not on medications and 19% (45/239) were poorly controlled; among those who subsequently developed HF, 87% had untreated or poorly controlled hypertension.
Figure 2. Proportion of blacks with clinical hypertension over the first 10 years of CARDIA, by subsequent heart failure status.
By Exam Year 10, 75% of blacks who subsequently developed heart failure had clinical hypertension, compared with 12% of blacks who did not develop heart failure (p<0.001).
To examine antecedents more proximal to the onset of HF, we used data from each exam and time-varying covariates. Over the entire 20 years, higher DBP (HR per 10.0 mmHg=1.8, 1.5–2.2, p<0.001), higher SBP (HR per 10.9 mmHg=1.7, 1.4–2.0, p<0.001), higher BMI (HR per 5.7 kg/m2=1.7, 1.3–2.1, p<0.001), diabetes (HR=5.5, 2.4–13, p<0.001), and CKD (HR=7.7, 1.8–33, p=0.006) were all associated with HF in bivariate models. Neither alcohol (HR=1.0, 0.4–2.5, p=0.97) nor drug use (HR=1.1, 0.5–2.4, p=0.82) was associated with HF. In multivariate models, SBP (HR per 10.9 mmHg=1.7, 1.4–2.0, p<0.001) and diabetes (HR=4.9, 2.1–12, p<0.001) each remained independently associated with HF, as was DBP in models substituting DBP for SBP (HR per 10 mmHg=1.8, 1.5–2.2, p<0.001).
Systolic dysfunction and LVH on the study echocardiogram at Year 5 were each independently associated with HF on average 10 years later (Table 3). After additional adjustment for clinical variables, systolic dysfunction remained strongly associated with subsequent HF, while the magnitude of the LVH association with subsequent HF was markedly diminished and not statistically significant.
Clinical records revealed co-morbid conditions present at the time of HF presentation (Table 4) that were consistent with study measurements of clinical antecedents (Tables 1–3). Hypertension (17/22), CKD (9/22) and diabetes (5/22) were commonly noted in the records of HF hospitalizations among blacks. Coronary disease was uncommon; none of the HF records noted concurrent or prior MI, and most of the cardiac catheterizations and autopsy records (8/9) noted mild or absent coronary disease. Although excessive alcohol was noted in three of 10 HF cases in men and cocaine use in one of the 10, none of the 16 HF records in black women commented on these factors. When systolic function was available in the record, this was most often recorded as EF<50% (14/17) or there was evidence of dilated cardiomyopathy on autopsy (3/3).
Discussion
In this large cohort of black and white young adults, incident HF was substantially more common among blacks and was not rare. One in 100 black men and black women in CARDIA developed HF before the age of 50, twenty times the incidence in whites. HF in blacks occurred at an average age of 39, and was predicted by the presence of hypertension, obesity, CKD and depressed systolic function 10–15 years earlier, findings that have important implications for efforts aimed at preventing and treating this important disease in this high risk population.
Prior work on HF epidemiology has focused on older adults.2, 3, 13 HF incidence up to 2 times higher in blacks compared with whites have recently been described;14–16 these populations are largely elderly, with a mean age at the start of observation ranging from 55–74 years. The one large cohort to include younger adults and to describe the low rates of incident HF before age 50 is predominantly white;1, 3 the low rate of HF that we observe in white CARDIA participants is consistent with the estimates from this cohort. National data have been used to compare HF prevalence across various demographic groups over the entire age spectrum; 2, 4 prevalence may understate the burden of disease given the high fatality rate associated with HF (5 of the 26 cases of HF in CARDIA resulted in death). Our work is consistent with this prior literature and extends it by making the important and novel observation of substantial rates of incident HF among young black men and women early in adulthood.
We have identified potentially modifiable antecedents of HF in blacks present more than a decade before the onset of clinical HF. Each 10 mmHg increase in DBP among blacks in their 20’s doubles the likelihood of developing HF in their forties, and three quarters of those who develop HF have prior clinical hypertension. Obesity also contributes to HF risk, possibly directly17 or through the associated rise in blood pressure and development of Type 2 diabetes. This latter possibility is consistent with our observation that increased BMI is an early independent risk factor for HF, with diabetes confounding or (more likely) mediating this association when the entire 20 years of follow-up is considered. Low HDL cholesterol in young adulthood may play a role in cardiac remodeling particularly in hypertensive disease.18, 19 CKD is a strong predictor of HF, and black men who are disproportionately affected by declining kidney function in young adulthood.20 Our finding that clinical factors increase HF risk even many years later is consistent with observations in older cohorts21–24 and suggests that these factors may be targets for HF prevention in young adults.
We find that ten years prior to the clinical manifestation, blacks who develop HF were more likely to have systolic dysfunction and LVH. These structural and functional cardiac changes may be the consequence of underlying clinical factors such as hypertension and obesity and may mediate the association between these factors and HF.25, 26 Interestingly, systolic dysfunction remains a strong risk factor for HF independent of the other clinical risk factors including blood pressure, raising with the possibility of other causal pathways contributing to the development of systolic dysfunction. Several studies have identified polymorphisms that appear to be linked to HF and systolic dysfunction in blacks, and some have found the risk associated with these polymorphisms to be greatest among blacks with hypertension.4, 5, 27, 28 Most of these studies are limited by the small numbers of black participants and lack of longitudinal data to determine how clinical and genetic factors may interact in the development of this disease, which is an important area for further study.
Our results have implications for the identification of high risk individuals and prevention of this disease. Current guidelines recommend initiating treatment for asymptomatic systolic dysfunction with ACE inhibitors and beta blockers before the onset of HF symptoms.4, 29–31 Although screening for systolic dysfunction in the general population has several limitations,32 screening high risk groups (such as those with hypertension) to target therapies may be an important tool for HF prevention.33, 34 Young adults have not been included in clinical trials of preventive therapies or screening strategies and the benefits and harms of these approaches in a young at-risk population are not known. However, the high rate of borderline or abnormal systolic function we observed in both races (13% among blacks and 9% among whites), and its strong association with subsequent clinical HF before age 50 among blacks, underscores the importance of this area of investigation.
Our study also highlights the potential for preventing HF by modifying important risk factors when present in young adulthood. Recent national data suggest that young adults with hypertension are far less likely than their middle-aged counterparts to be aware of this diagnosis or to be on treatment.35, 36 The reasons for low hypertension treatment rates in young adulthood may include barriers in access to medical care.37, 38 Some have suggested that blood pressure control may be more difficult to achieve among black patients, although a recent consensus statement on treating hypertension in blacks found that failure of health professionals to initiate therapy early in accordance with guidelines was the major obstacle to effective blood pressure control.39, 40 When treated according to these guidelines, blacks and whites appear to achieve similar control rates.41
Physicians may be reluctant to treat younger patients because of the perceived large number needed to treat to prevent cardiovascular outcomes that are still rare and often far in the future. In 20 years of CARDIA follow-up, the cumulative incidence of HF among blacks with hypertension at baseline (when their average age was 24) was 5.6%, compared with 0.8% among those without hypertension at baseline. Most of these hypertensive individuals were untreated. Blood pressure treatment trials in older populations that have included blacks have demonstrated considerable reductions in the risk of HF with blood pressure treatment, particularly with diuretic therapy.42 Thus, while treatment of hypertension and reversal of HF risk have not been studied in this age group, our data suggest that the number of young black hypertensive patients one would need to treat to prevent one case of HF before age 50 could be as low as 21.
The small number of outcomes in our study limits the precision of our descriptive observations, as well as our ability to explore a broader range of clinical antecedents and mediators. The single HF outcome in whites prevents us from assessing whether racial differences in HF risk factors account for the differences in HF incidence between blacks and whites that we observe. Because we define incident HF by hospitalization or death with this diagnosis, HF identified in the outpatient setting is missed in these analyses. The retention rate for outcome ascertainment is 87.5% by Year 20, which is a limitation; black men are the demographic group most likely to be lost-to-follow-up leaving open the possibility that we have underestimated the HF incidence particularly in this group.
Despite these limitations, the clear strength of this study is the large, well-characterized cohort of black and white young adults with rich longitudinal clinical and echocardiographic data and adjudicated HF outcomes. HF occurs at a disproportionately high rate among young and middle-aged blacks compared with whites, and is not rare. Elevated blood pressure, obesity, CKD and systolic dysfunction early in adulthood are important antecedents that could become targets for screening and interventions aimed at HF prevention. Studies are needed to examine the benefits and harms of these early approaches to preventing this serious disease in black young adults.
Acknowledgments
CARDIA is supported (or partially supported) by these contracts: University of Alabama at Birmingham, Coordinating Center, N01-HC-95095; University of Alabama at Birmingham, Field Center, N01-HC-48047; University of Minnesota, Field Center and Diet Reading Center (Year 20 Exam), N01-HC-48048; Northwestern University, Field Center, N01-HC-48049; Kaiser Foundation Research Institute, N01-HC-48050; University of California, Irvine, Echocardiography Reading Center (Year 5 & 10), N01-HC-45134; Harbor-UCLA Research Education Institute, Computed Tomography Reading Center (Year 15 Exam) N01-HC-05187; Wake Forest University (Year 20 Exam), N01-HC-45205; New England Medical Center (Year 20 Exam), N01-HC-45204 from the National Heart, Lung and Blood Institute.
K. Bibbins-Domingo has been supported by grants from the Robert Wood Johnson Amos Faculty Development Program, a diversity supplement to the CARDIA contract to the University of Alabama Coordinating Center (N01-HC-95095), R01 grants from the NIDDK (1R01DK078124) and the NHLBI (R01 HL081257), and the UCSF Hellman Family Faculty Award.
We gratefully acknowledge the administrative assistance of Tekeshe Mekonnen, MS in the submission of this manuscript.
References
- 1.Kannel WB. Incidence and epidemiology of heart failure. Heart Fail Rev. 2000;5:167–73. doi: 10.1023/A:1009884820941. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Furie K, et al. Heart Disease and Stroke Statistics--2008 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–72. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 4.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 5.Yancy CW. Heart failure in African Americans. Am J Cardiol. 2005;96:3i–12i. doi: 10.1016/j.amjcard.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 6.Schocken DD, Benjamin EJ, Fonarow GC, et al. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117:2544–65. doi: 10.1161/CIRCULATIONAHA.107.188965. [DOI] [PubMed] [Google Scholar]
- 7.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–16. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 8.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 9.Gardin JM, Wagenknecht LE, Anton-Culver H, et al. Relationship of cardiovascular risk factors to echocardiographic left ventricular mass in healthy young black and white adult men and women. The CARDIA study. Coronary Artery Risk Development in Young Adults. Circulation. 1995;92:380–7. doi: 10.1161/01.cir.92.3.380. [DOI] [PubMed] [Google Scholar]
- 10.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 11.de Simone G, Daniels SR, Devereux RB, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–60. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- 12.Nunez E, Arnett DK, Benjamin EJ, et al. Optimal threshold value for left ventricular hypertrophy in blacks: the Atherosclerosis Risk in Communities study. Hypertension. 2005;45:58–63. doi: 10.1161/01.HYP.0000149951.70491.4c. [DOI] [PubMed] [Google Scholar]
- 13.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–50. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 14.Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the Incidence of Congestive Heart Failure by Ethnicity: The Multi-Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168:2138–45. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart Failure Incidence and Survival (from the Atherosclerosis Risk in Communities Study) The American Journal of Cardiology. 2008;101:1016–22. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 16.Kalogeropoulos A, Georgiopoulou V, Kritchevsky SB, et al. Epidemiology of Incident Heart Failure in a Contemporary Elderly Population: The Health, Aging, and Body Composition StudyThe Health, Aging, and Body Composition Study. Archives of Internal Medicine. 2008 doi: 10.1001/archinternmed.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 18.Horio T, Miyazato J, Kamide K, Takiushi S, Kawano Y. Influence of low-high density lipoprotein cholesterol on left ventricular hypertrophy and diastolic function in essential hypertension. Am J Hypertens. 2003;16:938–44. doi: 10.1016/s0895-7061(03)01015-x. [DOI] [PubMed] [Google Scholar]
- 19.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22:6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 20.Stehman-Breen CO, Gillen D, Steffes M, et al. Racial differences in early-onset renal disease among young adults: the coronary artery risk development in young adults (CARDIA) study. J Am Soc Nephrol. 2003;14:2352–7. doi: 10.1097/01.asn.0000083392.11042.14. [DOI] [PubMed] [Google Scholar]
- 21.Bibbins-Domingo K, Chertow GM, Fried LF, et al. Renal function and heart failure risk in older black and white individuals: the Health, Aging, and Body Composition Study. Arch Intern Med. 2006;166:1396–402. doi: 10.1001/archinte.166.13.1396. [DOI] [PubMed] [Google Scholar]
- 22.Haider AW, Larson MG, Franklin SS, Levy D. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2003;138:10–6. doi: 10.7326/0003-4819-138-1-200301070-00006. [DOI] [PubMed] [Google Scholar]
- 23.Lee DS, Massaro JM, Wang TJ, et al. Antecedent blood pressure, body mass index, and the risk of incident heart failure in later life. Hypertension. 2007;50:869–76. doi: 10.1161/HYPERTENSIONAHA.107.095380. [DOI] [PubMed] [Google Scholar]
- 24.Sarnak MJ, Katz R, Stehman-Breen CO, et al. Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med. 2005;142:497–505. doi: 10.7326/0003-4819-142-7-200504050-00008. [DOI] [PubMed] [Google Scholar]
- 25.Lauer MS, Anderson KM, Kannel WB, Levy D. The impact of obesity on left ventricular mass and geometry. The Framingham Heart Study. JAMA. 1991;266:231–6. [PubMed] [Google Scholar]
- 26.Lauer MS, Anderson KM, Levy D. Influence of contemporary versus 30-year blood pressure levels on left ventricular mass and geometry: the Framingham Heart Study. J Am Coll Cardiol. 1991;18:1287–94. doi: 10.1016/0735-1097(91)90549-o. [DOI] [PubMed] [Google Scholar]
- 27.Bleumink GS, Knetsch AM, Sturkenboom MC, et al. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J. 2004;25:1614–9. doi: 10.1016/j.ehj.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 28.Konstam MA. Comment--Val-HeFT and angiotensin-receptor blockers in perspective: A tale of the blind man and the elephant. J Card Fail. 2002;8:56–8. doi: 10.1054/jcaf.2002.32950. [DOI] [PubMed] [Google Scholar]
- 29.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–18. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 30.Konstam MA, Kronenberg MW, Rousseau MF, et al. Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dilatation in patients with asymptomatic systolic dysfunction. SOLVD (Studies of Left Ventricular Dysfunction) Investigators. Circulation. 1993;88:2277–83. doi: 10.1161/01.cir.88.5.2277. [DOI] [PubMed] [Google Scholar]
- 31.Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327:669–77. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 32.Wang TJ, Levy D, Benjamin EJ, Vasan RS. The epidemiology of “asymptomatic” left ventricular systolic dysfunction: implications for screening. Ann Intern Med. 2003;138:907–16. doi: 10.7326/0003-4819-138-11-200306030-00012. [DOI] [PubMed] [Google Scholar]
- 33.Struthers AD, Morris AD. Screening for and treating left-ventricular abnormalities in diabetes mellitus: a new way of reducing cardiac deaths. Lancet. 2002;359:1430–2. doi: 10.1016/S0140-6736(02)08358-7. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg LR, Jessup M. Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction. Circulation. 2006;113:2851–60. doi: 10.1161/CIRCULATIONAHA.105.600437. [DOI] [PubMed] [Google Scholar]
- 35.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 36.Gu Q, Paulose-Ram R, Dillon C, Burt V. Antihypertensive medication use among US adults with hypertension. Circulation. 2006;113:213–21. doi: 10.1161/CIRCULATIONAHA.105.542290. [DOI] [PubMed] [Google Scholar]
- 37.Ostchega Y, Hughes JP, Wright JD, McDowell MA, Louis T. Are demographic characteristics, health care access and utilization, and comorbid conditions associated with hypertension among US adults? Am J Hypertens. 2008;21:159–65. doi: 10.1038/ajh.2007.32. [DOI] [PubMed] [Google Scholar]
- 38.Victor RG, Leonard D, Hess P, et al. Factors associated with hypertension awareness, treatment, and control in Dallas County, Texas. Arch Intern Med. 2008;168:1285–93. doi: 10.1001/archinte.168.12.1285. [DOI] [PubMed] [Google Scholar]
- 39.Bakris GL, Weir MR, Shanifar S, et al. Effects of blood pressure level on progression of diabetic nephropathy: results from the RENAAL study. Arch Intern Med. 2003;163:1555–65. doi: 10.1001/archinte.163.13.1555. [DOI] [PubMed] [Google Scholar]
- 40.Douglas JG, Bakris GL, Epstein M, et al. Management of high blood pressure in African Americans: consensus statement of the Hypertension in African Americans Working Group of the International Society on Hypertension in Blacks. Arch Intern Med. 2003;163:525–41. doi: 10.1001/archinte.163.5.525. [DOI] [PubMed] [Google Scholar]
- 41.Racial/ethnic disparities in prevalence, treatment, and control of hypertension--United States, 1999–2002. MMWR Morb Mortal Wkly Rep. 2005;54:7–9. [PubMed] [Google Scholar]
- 42.Wright JT, Jr, Dunn JK, Cutler JA, et al. Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA. 2005;293:1595–608. doi: 10.1001/jama.293.13.1595. [DOI] [PubMed] [Google Scholar]