Abstract
Lipotoxicity, which is triggered when cells are exposed to elevated levels of free fatty acids, involves cell dysfunction and apoptosis and is emerging as an underlying factor contributing to various pathological conditions including disorders of the central nervous system and diabetes. We have shown that palmitic acid (PA)-induced lipotoxicity (PA-LTx) in nerve growth factor-differentiated PC12 (NGFDPC12) cells is linked to an augmented state of cellular oxidative stress (ASCOS) and apoptosis, and that these events are inhibited by docosahexanoic acid (DHA). The mechanisms of PA-LTx in nerve cells are not well understood, but our previous findings indicate that it involves ROS generation, mitochondrial membrane permeabilization (MMP), and caspase activation. The present study used nerve growth factor differentiated PC12 cells (NGFDPC12 cells) and found that lysosomal membrane permeabilization (LMP) is an early event during PA-induced lipotoxicity that precedes MMP and apoptosis. Cathepsin L, but not cathepsin B, is an important contributor in this process since its pharmacological inhibition significantly attenuated LMP, MMP, and apoptosis. In addition, co-treatment of NGFDPC12 cells undergoing lipotoxicity with DHA significantly reduced LMP, suggesting that DHA acts by antagonizing upstream signals leading to lysosomal dysfunction. These results suggest that LMP is a key early mediator of lipotoxicity, and underscore the value of interventions targeting upstream signals leading to LMP for the treatment of pathological conditions associated with lipotoxicity.
Keywords: apoptosis, cathepsin L, docosahexanoic acid, lipotoxicity, lysosomal dysfunction
1. Introduction
Lipotoxicity is caused when non-adipose cells are exposed to chronic elevation of free fatty acids, and is believed to play an important role in the development of diabetes, cardiomyopathy, and nonalcoholic fatty liver disease (Gomez-Lechon et al., 2007; Saunders et al., 2008; Unger, 2008). Of potential significance is that lipotoxicity exacerbates cellular damage associated with traumatic injuries in the nervous system (CNS) and neurological disorders (Adibhatla and Hatcher, 2008). Further, acute brain injury caused by stroke or trauma generates high levels of free fatty acid as a consequence of the degradation of membrane phospholipids by activated intracellular phospholipases, which can result in cellular lipid overload (Farooqui and Horrocks, 1998). This pathological accumulation of FFA may be sustained from days to weeks, and can pose a serious threat to normal cellular homeostasis (Cooper, 1985; Farooqui and Horrocks, 2006). In addition to stimulated phospholipid hydrolysis, abnormally high intracellular lipid concentration could also result from dysregulation of FFA/lipid metabolism due to an unbalance between uptake and utilization of FFA as in the case of a high fat diet or other metabolic conditions. The lipotoxicity hypothesis suggests that this high fat diet may be responsible for the development of type 2 diabetes (Unger and Orci, 2001). We have previously shown that saturated fatty acid overload triggers a series of events that leads to apoptosis in NGFDPC12 and primary cortical cells (Ulloth et al., 2003; Almaguel et al., 2009). The mechanisms by which FFA induce apoptosis are not fully identified yet and are expected to be cell type-dependent due to intrinsic differences in lipid metabolism. However, FFA overloading has been associated with reactive oxygen species (ROS) generation (Listenberger et al., 2001), de novo ceramide synthesis (Shimabukuro et al., 1998), nitric oxide production (Kumar and Das, 1993), and mitochondrial dysfunction (Maestre et al., 2003).
During the past decade, the mitochondria has been established as the central hub of cellular life and death decisions (Kroemer et al., 2007). Two main pathways of caspase-dependent apoptotic cell death have been characterized, the extrinsic and intrinsic pathways (Logue and Martin, 2008), and mitochondria plays a critical role in orchestrating both pathways. The intrinsic pathway is initiated as a result of various stress signals, such as ROS, UV radiation, hypoxia, endoplasmic reticulum stress, serum starvation, and cytotoxic drugs. Key events in this pathway are mitochondrial membrane permeabilization (MMP), followed by release of cytochrome C (cyt-C) and other pro-apoptotic effectors, and subsequent activation of initiator caspase-9 and effector caspases-3, and -7 (Kroemer et al., 2007; Logue and Martin, 2008). The extrinsic pathway is initiated by extracellular signals through the interaction of death receptors with ligands such as Fas, TNF, and TRAIL, leading to activation of initiation caspases-8, and -10, and effector caspases-3, -6, and -7 (Logue and Martin, 2008). Crosstalk between both pathways is mediated by caspase-8-induced cleavage of Bid into tBid, which provokes the release of cytochrome c from the mitochondria by stimulating the oligomerization of Bak and/or Bax to form channels in the mitochondrial outer membrane, leading to MMP and apoptosis (Logue and Martin, 2008).
More recently, the lysosomes have emerged as a second hub for orchestrating cellular life and death decisions. Induction of lysosomal membrane permeabilization (LMP) by agents such as ROS, sphingosine, and FFA is associated with both caspase-dependent and independent cell death, and involves the release of cathepsins B, D, and L, which retain their activity at neutral pH in the cytosol (Boya et al., 2003; Kirkegaard and Jaattela, 2009). These proteases contribute to cell death by activating effectors such as mitochondria-associated proteins, caspases, apoptosis-inducing factor (AIF), or by directly cleaving nuclear and cytoplasmic factors (Boya et al., 2003; Kirkegaard and Jaattela, 2009). Cathepsins have been implicated in CNS apoptosis following ischemia or during neurodegenerative processes. For instance, cathepsin B released from compromised lysosomes into the cytoplasm was crucial for the post-ischemic neuronal death in vivo (Seyfried et al., 1997: Yamashima et al., 1998), and in vitro studies suggested that this process was dependent on NMDA-mediated calcium influx and ROS production (Windelborn and Lipton, 2008). Cathepsin L was also identified as an important mediator of the ß-amyloid protein-induced apoptosis in cultured cortical neurons (Boland and Campbell, 2004). Of particular interest is that lysosomal destablization was evident in FFA-induced hepatic apoptosis (Feldstein et al., 2004; Wu et al., 2008).
We have reported previously that exposure of nerve growth factor-differentiated PC12 (NGFDPC12) cells to palmitic acid (PA)/BSA (2:1 ratio) triggers apoptotic cell death via both intrinsic and extrinsic pathways (Almaguel et al., 2009; Ulloth et al., 2003). PA-induced lipotoxicity correlates with early ROS generation concomitant with upregulation of Fas receptor, Fas ligand and BNIP3 mRNAs, followed by MMP, and activation of caspases-3 and -8, ultimately leading to cleavage of intracellular substrates such as lamin B and PARP (Almaguel et al., 2009; Ulloth et al., 2003). As part of an ongoing investigation of the specific mechanisms by which FFA induce caspase independent neuronal cell death, we provide evidence in this study for the involvement of the lysosomal apoptotic pathway in PA-induced lipotoxicity. Further, we also show that docosahexaenoic acid (DHA) rescues NGFDPC12 cells from PA-induced lipotoxicity by decreasing LMP.
2. Results
2.1. PA induces lysosomal and mitochondrial membrane permeabilization in NGFDPC12 cells
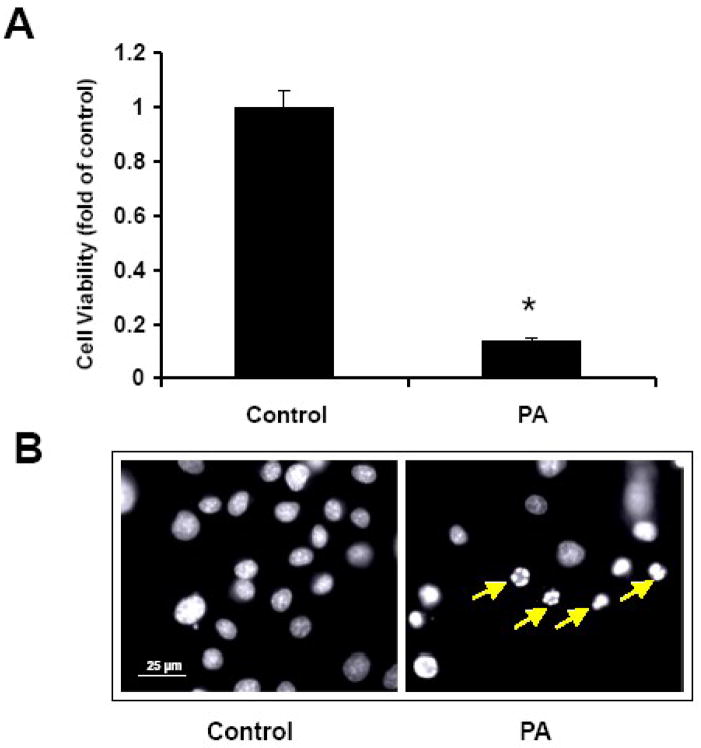
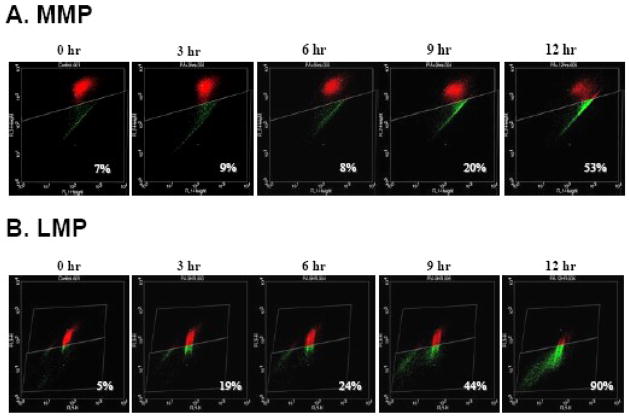
Treatment of NGFDPC12 cells with PA/BSA at 2:1 ratio for 24 h resulted in 80% loss of cell viability (Fig. 1A). The nuclear morphology of PA-treated cells was characteristic of apoptotic cell death (Fig. 1B). We investigated how early PA triggers MMP, using the JC-1 flow cytometry assay. Fig. 2A shows that non-treated control NGFDPC12 cells displayed only a 7% decrease in FL2 red fluorescence (R2) compared to 9%, 8%, 20% and 53% decreases in cells undergoing PA-induced lipotoxicity for 3, 6, 9 and 12 h, respectively. These results suggest that PA induced MMP approximately 9 h after exposure. We also determined the PA-mediated LMP in the same time-dependent manner, using the acridine orange (AO) flow cytometry assay. Fig. 2B illustrates that non-treated control NGFDPC12 cells displayed only a 5% decrease in FL3 red fluorescence (R2). Surprisingly, a dramatic decrease (19%) was observed as early as 3 h after PA exposure. Decreases in FL3 red fluorescence (R2) of 24%, 44% and 90% were observed in cells undergoing PA-induced lipotoxicity for 6, 9 and 12 h, respectively. Therefore, PA induced both MMP and LMP in NGFDPC12 cells, with LMP preceding MMP.
Figure 1. PA induces lipotoxicity in NGFDPC12 cells.
Cells were treated with PA: BSA, 2:1 molar ratio (PA), or BSA alone (Control) for 24 hr. A: Cell viability was determined by WST-1 assay (see Materials and Methods). The data represent mean ± SEM of 3 independent experiments. Significance (*) was determined at p<0.05 compared to control group. B: Nuclear morphology was analyzed with Hoechst staining and examined under fluorescent microscopy. Arrows indicate representative nuclei showing chromatin condensation and fragmentation.
Figure 2. PA induces mitochondrial and lysosomal membrane permeabilization in NGFDPC12 cells.
Cells were treated with PA/BSA (2:1) for 0, 3, 6, 9 and 12 hr. A: Mitochondrial membrane permeabilization (MMP) was determined by flow cytometry using JC-1. Cells with MMP showing reduced FL2 reading are presented in green, whereas cells with intact mitochondria are shown in red. The percents of cells with MMP (green) were calculated. B: Lysosomal membrane permeabilization (LMP) was analyzed with flow cytometry using acridine orange (AO). Cells with LMP showing reduced FL2 reading are presented in green, whereas cells with intact lysosomes are shown in red. The percents of cells with LMP (green) were calculated. Representative flow cytometric plots of three independent experiments are shown.
2.2. Inhibition of Cathepsin L reverses PA-induced loss of viability and LMP in NGFDPC12 cells
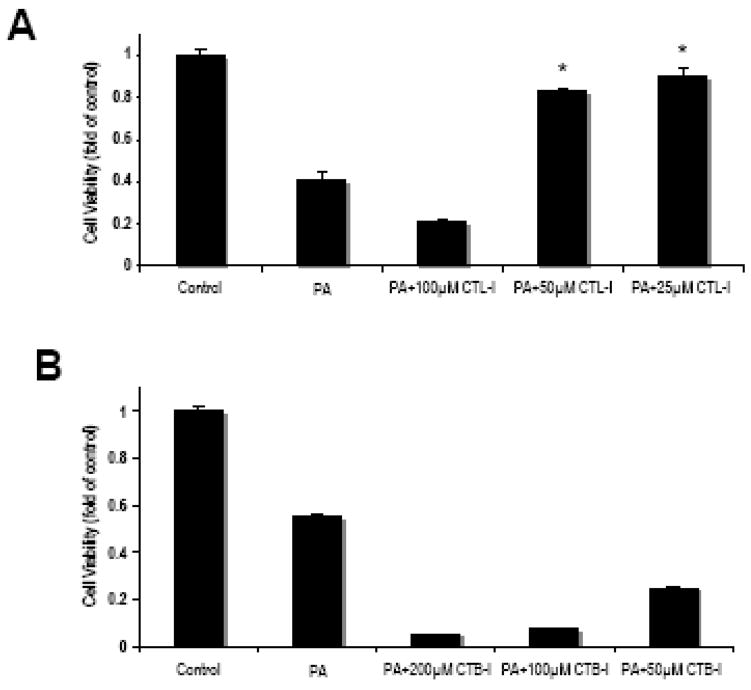
To further characterize the lysosomal dysfunction in PA-induced lipotoxicity, we used specific inhibitors for cathepsin L (CTL-I) and cathepsin B (CTB-I). As shown in Fig. 3A, inhibition of cathepsin L with 25 and 50 μM of CTL-I significantly attenuated PA-induced lipotoxicity. However, at higher concentrations (100 μM) CTL-I appeared to exacerbate PA-induced cell death, most likely due to its toxicity. On the other hand, CTB-I, used at concentrations ranging from 50 to 200 μM not only failed to protect against PA-induced NGFDPC12 cell death but appeared to exacerbate cell death, even through such toxicity was not observed in other experimental systems (Li et al., 2008; Newman et al., 2009). These results implicated cathepsin L, but not cathepsin B, in PA-induced lipotoxicity in NGFDPC12 cells. Cathepsin D inhibitor (pepstatin A) was also examined in similar experiments and did not show significant protective effect (data not shown) suggesting that cathepsin D may not be involved in PA-mediated lipotoxicity in NGFDPC12 cells under the conditions described in this study.
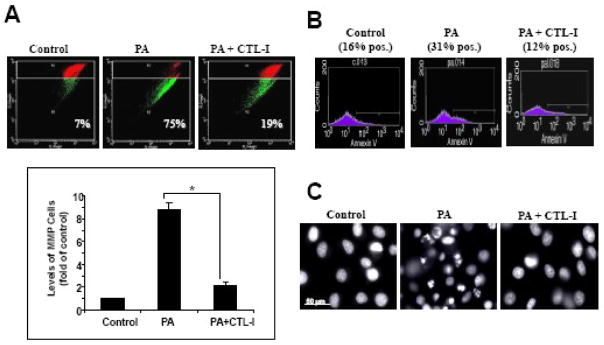
Figure 3. Pharmacological inhibition of cathepsin L attenuates PA-induced cell death in NGFDPC12 cells.
A: Cells were treated with PA/BSA (2:1) or BSA alone (Control) for 24 hr in the presence or absence of cathepsin L inhibitor at 25, 50 or 100 μM. Cell viability was determined by WST-1 assay. B: NGFDPC12 cells were treated with PA/BSA (2:1) or BSA alone (CTL) for 24 hr in the presence or absence of cathepsin B inhibitor at 50, 100 or 200 μM. Cell viability was determined by WST-1 assay. The data represent mean ± SEM of 3 independent experiments. Significance (*) was determined at p<0.05 when compared to PA group.
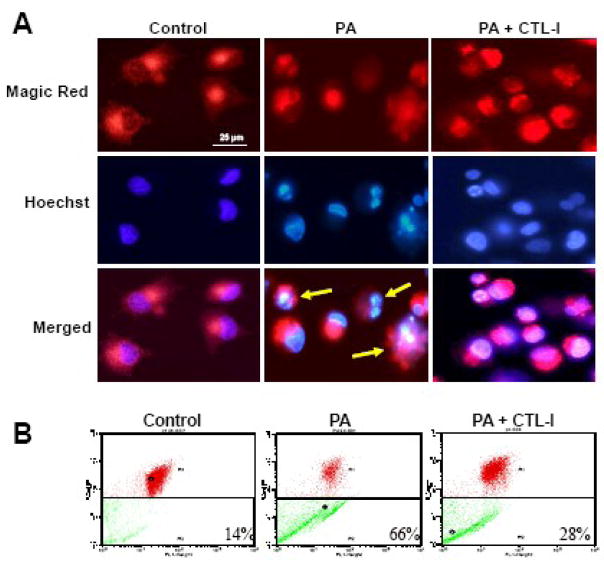
To further confirm a role for cathepsin L in PA-induced lipotoxicity, the activity of this protease in NGFDPC12 cells was analyzed using the Magic Red fluorogenic substrate. As expected, control untreated cells displayed punctuate fluorescence confined to the cytoplasm, typical of intact lysosomes (Fig. 4A). After exposure to PA, fluorescence was diffused within the dead cells (Fig. 4A, merged panel with yellow arrows), indicating leakage of cathepsin L into the cytosolic compartment. Consistent with the results presented in Fig. 3A, CTL-I (25 μM) prevented PA-induced LMP. The protective effect of CTL-I was also examined by AO flow cytometric assay. As shown in Fig. 4B, non-treated control NGFDPC12 cells displayed only a 14% decrease in FL3 red fluorescence compared to a 66% decrease in PA-treated cells and 28% in cells treated with PA in the presence of CTL-I (25 μM).
Figure 4.
Inhibition of cathepsin L activity attenuates lysosomal membrane permeabilization during PA-induced lipotoxicity in NGFDPC12 cells. Cells were treated with BSA alone (Control), PA/BSA (2:1) or PA/BSA with 25μM cathepsin L inhibitor (PA+CTL-I) for 24 hr. A: Cathepsin L activity in cells was examined with Magic Red and detected by fluorescent microscopy. Cells were counterstained with Hoechst 33342. Merged images are also shown. B: LMP was determined by flow cytometry using acridine orange. The percents of cells with LMP were calculated. Representative flow cytometric plots of three independent experiments are shown.
2.3. Inhibition of Cathepsin L reverses PA-induced MMP and apoptosis in NGFDPC12 cells
The results presented above indicated that LMP precedes MMP in PA-induced lipotoxicity, and implicated cathepsin L in this cell death process. To further examine the role of cathepsin L in PA-induced lipotoxicity, we examined if inhibition of this protease with CTL-I could reverse MMP in PA-treated NGFDPC12 cells. Fig. 5A shows that PA elicited a nine-fold increase of MMP and that CTL-I (25 μM) significantly reduced this effect. In addition, PA-induced apoptosis in NGFDPC12 cells was abolished by CTL-I (25 μM), as determined by the reduction in Annexin V binding to externalized phosphatidylserine (Fig. 5B), and apoptotic nuclear morphology (Fig. 5C) in cells treated with PA in the presence of CTL-I. Taken together, the above results indicated that LMP is an early event in PA-induced lipotoxicity of NGFDPC12 cells that is followed by the release of active cathepsin L, which in turn acts as an upstream mediator of the apoptotic process.
Figure 5. Cathepsin L inhibitor abolished PA-induced mitochondrial membrane permeabilization and apoptosis.
NGFDPC12 cells were treated with BSA alone (Control), PA/BSA (2:1) or PA/BSA with 25μM cathepsin L inhibitor (PA+CTL-I) for 24 hr. A: MMP was determined by flow cytometry using JC-1. The top panel shows flow cytometric plots and the bottom panel shows the quantitative analysis of percent cells with MMP under different treatments. The data represent mean ± SEM of 3 independent experiments. Significance (*) was determined at p<0.05 when compared to PA group. B: Apoptosis was examined by flow cytometry using Annexin V assay, and the percents of Annexin V-positive cells were determined. C: Nuclear morphology after each treatment was visualized with Hoechst 33342 staining. Representative flow cytometric plots and fluorescent micrographs are shown.
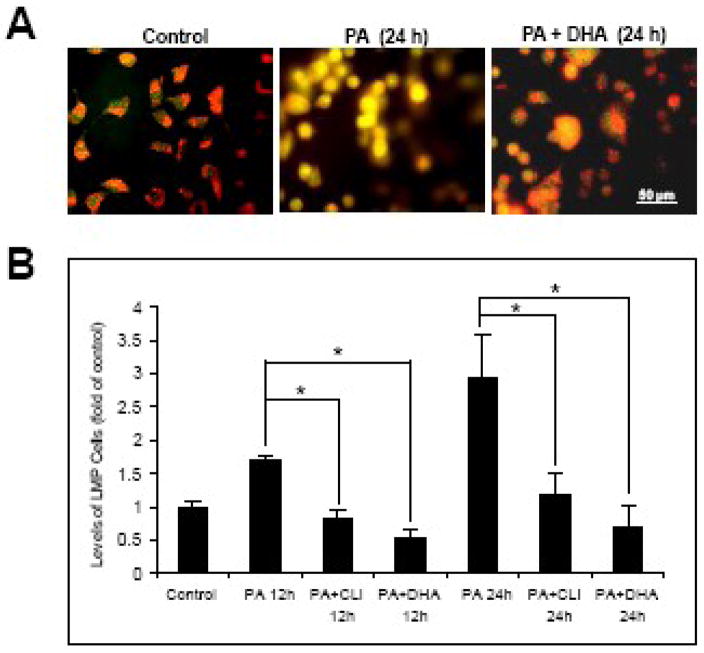
2.4. DHA protects NGFDPC12 cells from PA-induced lipotoxicity by attenuating LMP
In a previous report we showed that DHA protects NGFDPC12 cells from PA-induced mitochondrial disruption and apoptosis (Almaguel et al., 2009). We sought to determine the effect of DHA on PA-induced LMP in NGFDPC12 cells. As shown in Fig. 6A, control, untreated NGFDPC12 cells displayed AO staining associated with intact lysosomes, i.e. red fluorescence corresponding to lysosomes and green fluorescence in other cellular compartments. However, cells exposed to PA for 24 h underwent LMP, as indicated by the observed strong yellow fluorescence. Co-incubation of cells with DHA and PA prevented lipotoxicity and LMP, as evidenced by the red and green fluorescence (Fig. 6A). Using AO flow cytometric assay, we performed a quantitative analysis of lysosomal membrane integrity after treating NGFDPC12 cells with PA for 12 and 24 h, in the absence and presence of DHA. PA induced 1.7 and 3 fold increases of LMP at 12 and 24 h respectively (Fig. 6B). DHA, as well as CTL-I, were able to reduce the LMP to levels comparable to those seen in control cells (Fig. 6B).
Figure 6. DHA protects NGFDPC12 cells against PA-induced lysosomal membrane permeabilization.
A: NGFDPC12 cells were treated with BSA alone (Control), PA/BSA (2:1) or PA/BSA+DHA for 24 hr. LMP was determined by acridine orange staining followed by fluorescent microscopy. Representative fluorescent micrographs are shown. B: NGFDPC12 cells were treated with BSA alone (Control), PA/BSA alone, PA/BSA+DHA or PA/BSA with 25μM cathepsin L inhibitor (PA+CLI) for 12 or 24 hr. LMP was analyzed by flow cytometry using Acridine Orange. The percents of cells with LMP were calculated and values for control cells were normalized to one. The data represent mean ± SEM of 3 independent experiments. Significance (*) was determined at p<0.05.
3. Discussion
In previous studies we have demonstrated that PA-LTx in NGFDPC12 cells occurs via both caspase-dependent and independent cell death pathways and that DHA can be protective (Ulloth et al., 2003; Almaguel et al., 2009). The present study shows that: (1) lysosomal and mitochondrial dysfunction are prominent events in lipotoxicity induced by PA/BSA at 2:1 ratio, and LMP occurred prior to MMP; (2) cathepsin L seems to mediate the PA-LTx apoptosis since cathepsin L inhibitor prevented downstream apoptotic events such as MMP, phosphatidylserine externalization, and nuclear condensation and fragmentation; and (3) treatment with DHA stabilizes lysosomal membranes and protects NGFDPC12 cells from PA-LTx. These observations suggest the existence of a lysosomal-mitochondrial crosstalk associated with PA-LTx cell death.
Pathology associated with traumatic brain injury (TBI) is primarily caused by transient global ischemia and subsequent secondary damage occurring hours to days after the primary injury (Conti et al., 1998; Cooper, 1985). This second phase of TBI shows prominent release of excitatory amino acids, increased intracellular Ca2+ levels, phospholipid degradation, local FFA accumulation, lactic acid accumulation, free radical formation, and lipid peroxidation (Newcomb et al., 1999) and current research is focused in evaluating the respective contribution to tissue damage and recovery. For instance, there is strong correlation between the magnitude of FFAs accumulation and the severity of tissue injury (Bazan et al., 1971; Dhillon et al., 1999; Zhang and Sun, 1995), which suggest that this increase can be a reliable marker of secondary brain injury. FFA accumulation is most likely by the action of phospholipase-A2 (PLA-2), which is activated by increased intracellular Ca2+, and phospholipase C (PLC), which is activated by excitatory amino acids (Farooqui and Horrocks, 1998).
Exposing NGFPC12 cells to high levels of saturated FFA (i.e., PA and stearic acid) is more damaging than unsaturated FFA (i.e., arachidonic acid and oleic acid) (Ulloth et al., 2003), a phenomenon that has been also observed in other cell types, and differential reincorporation of FFA into membrane phospholipids has been proposed to play an important role (Rabin et al., 1998; Tone et al., 1987; El-Assaad et al., 2003; Hardy et al., 2003; Li et al., 2008). Further, unesterified PA, as a precursor of de novo ceramide synthesis, may act at least partially via ceramide-dependent or -independent pathways (Listenberger et al., 2003; Shimabukuro et al., 1998).
In agreement with the LMC observed during apoptosis reported by others (Li et al., 2008; Zhao et al., 2003), the present study shows that LMP is an upstream early event in PA-LTx and cell death in NGFDPC12 cells. The release of cathepsin L from disrupted lysosomes appeared to be required for subsequent MMP, since inhibition of cathepsin L, but not cathepsin B, prevented subsequent MMP and apoptosis. This LMC during PA-LTx is likely to be mediated by cleavage of Bid, which is a known substrate of cathepsins B, D, and L in different cell models (Blomgran et al., 2007; Cirman et al., 2004). Interestingly, anti-apoptotic mitochondrial proteins such as Bcl-2, Bcl-xL and Mcl-1 are degraded by lysosomal cathepsins, which, combined with cathepsin-mediated Bid activation, can trigger MMP and apoptosis (Droga-Mazovec et al., 2008). Cathepsins D and L have also been shown to directly activate caspase-8 in some cell models (Baumgartner et al., 2007; Conus et al., 2008), which may result in Bid cleavage. Our current and previous results indicate that PA-LTx in NGFDPC12 cells is cathepsin L-dependent and involves the activation of caspase-8 (Ulloth et al., 2003). Therefore, we hypothesize that early lysosomal disruption during PA-LTx leads to cathepsin L leakage, which in turn may directly cleave Bid to activate the intrinsic apoptotic pathway. Alternatively, cathepsin L may activate caspase-8, which in turns cleaves and activates Bid.
PA-LTx in NGFDPC12 cell death involves a caspase-independent pathway, as evidenced by the observation that inhibition of caspase activity with the pan-caspase inhibitor z-VAD did not prevent cell death (Ulloth et al., 2003). Interestingly, LMP and cathepsins release have been shown to mediate both caspase-dependent and -independent cell death (Chen et al., 2005; Feldstein et al., 2006; Zhao et al., 2003). Also significant is that cathepsins can mediate the activation of PLA2, which may trigger damage to mitochondrial and lysosomal membranes (Zhao et al., 2001). Furthermore, overactive PLA2 can induce FFA release and accumulation, thus contributing to lipotoxicity (Farooqui and Horrocks, 2006).
Our previous studies indicated an increase of ROS, Fas-R, Fas-L, and Bnip3 during the early stages of PA-induced lipotoxicity in NGFDPC12 cells (Almaguel et al., 2009; Ulloth et al., 2003). In addition to exhibiting classic nuclear apoptotic structural features, PA-LTx also induced cleavage of PARP into the 85 kD apoptotic signature cleavage fragment generated by caspase-3, and not the 50 kD fragment associated with necrotic cell death (Ulloth et al., 2003). These observations suggest that LMP can be induced through different pathways. For instance, ROS-induced LMP can lead to cell damage and apoptosis (Dare et al., 2001; Ostenfeld et al., 2005; Yeung et al., 2006) and ROS-dependent LMP and cathepsin release causes ischemic damage in the rat hippocampal slice after oxygen-glucose deprivation (Windelborn and Lipton, 2008). LMP was also observed in Jurkat T-cells shortly after treatment with H2O2, and an intralysosomal iron-catalyzed oxidative reaction was presumed to be responsible for the LMP since an iron chelator specifically directed into lysosomes was able to reduce lysosomal leakage (Brunk and Svensson 1999; Antunes et al., 2001). Cathepsins leaked from partially ruptured lysosomes cause downstream MMP and stimulate more ROS generation, which in turn triggers further lysosomal damage (Ghavami et al., 2008; Li et al., 2008). Lysosomal destabilization was also observed in apoptosis induced by the death receptor pathway (Brunk and Svensson, 1999; Nagaraj et al., 2007; Werneburg et al., 2004). In a hepatocyte model, activation of TNF receptors led to activation of caspase 8 and release of cathepsin B, which in turn induced cytochrome C release from mitochondria (Guicciardi et al., 2000). Additional and even stronger activation of caspase 8 occured after induction of MMP, generating a feedback loop causing further LMP and exacerbation of apoptosis (Baumgartner et al., 2007; Guicciardi et al., 2000). Bax, a pro-apoptotic member of the Bcl-2 family, is well recognized for its pore formation activity, which is responsible for MMP (Qian et al., 2008). PA was shown to activate and translocate Bax to lysosomes, leading to LMP and subsequent cell death (Feldstein et al., 2006). Since this activation/translocation of Bax is independent of cathepsin B, Bax could be responsible for initial events leading to LMP.
DHA, the major n-3 polyunsaturated fatty acid in the brain, is critical for normal nervous system development and exerts neuroprotective effect in various neuronal injury models (Kaur et al., 2008). DHA protects NGFDPC12 cells from PA-induced lipotoxicity (Almaguel et al., 2009) but the mechanism remains to be elucidated. The present study shows that co-treatment of cells with DHA and PA led to a marked decrease in LMP. As a highly unsaturated fatty acid, the neuroprotective effect of DHA may be attributed to its free radical scavenging capacity (Bas et al., 2007; Shimazawa et al., 2009). Interestingly, our previous data did not show signficant attenuation of ROS by DHA (Almaguel et al., 2009). This is an area of interest to the field and our data is consistent with findings reporting that treatment of human fibroblasts with DHA increased ROS while simultaneously inducing a strong antioxidant response as evident by the upregulation of the activities of antioxidant enzymes gamma-glutamyl-cysteinyl ligase and glutathione reductase (Arab et al., 2006). Alternativelly, DHA can exert protective actions through DHA-derived metabolites such as Neuroprotectin D1 has been shown to protect human retinal pigment epithelial cells from oxidative stress-induced cell death (Mukherjee et al., 2004). Although our studies did not examine Neuroprotectin D1 effects, we do not rule out a potential neuroprotective role during PA-induced lipotoxiciy. Additional ways by which DHA could lead to neuroprotection is by stabilizing lysosomal membranes and altering their fluidity following incorporation into the cell membrane (Hulbert and Else, 1999 ; Valentine and Valentine, 2004), or by preventing PA-induced lipotoxicity by channeling excess of PA into triglyceride pools and away from pathways leading to apoptosis (Listenberger et al., 2003).
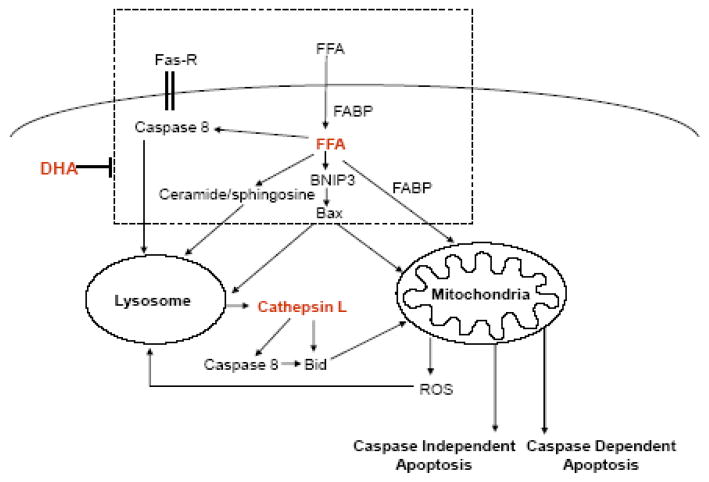
In conclusion, our data suggest that LMP is an early event during PA-induced lipotoxicity in NGFDPC12 cells that is associated with cathepsin L activation and that precedes MMP and apoptosis (Fig. 7). The reduction of LMP by DHA suggests that DHA acts by blocking upstream signals leading to lysosomal dysfunction. Studies are underway to identify these signals. These results underscore the value of interventions targeting upstream signals that lead to LMP for the treatment of conditions associated with lipotoxicity.
Figure 7. Model depicting the potential lysosomal-mitochondrial crosstalk during saturated free fatty acid-induced apoptosis.
FFAs are transported by albumin in the plasma and by fatty acid binding proteins intracellularly. FFA overload leads to: 1) increase of Fas receptor (FAS-R) and activation of caspase 8; 2) enhanced formation of ceramide/sphingosine; and 3) up-regulation of BNIP3 and Bax expression. All these upstream events can contribute to an early lysosomal membrane permeabilization (LMP) and subsequent release of lysosomal enzyme cathepsin L. Released cathepsin L can cleave/activate pro-apoptotic Bid directly or via caspase 8. Translocation of pro-apoptotic proteins to the mitochondrial outer membrane induces mitochondrial membrane permeabilization (MMP), which results in caspase-dependent and independent apoptotic cell death. Reactive oxygen species (ROS) generated by mitochondria can promote LMP and enhance apoptosis. DHA, an n-3 polyunsaturated fatty acid, inhibits FFA-induced apoptosis by blocking upstream signaling cascade and prevents LMP.
4. Experimental Procedures
4.1. Cell culture and treatment with fatty acids
Undifferentiated PC12 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% horse serum, 5% fetal bovine serum (FBS), 2 mM L-glutamine, 100 units/mL penicillin and 100 μg/mL streptomycin (Mediatech, Herndon, VA), at 37°C with 95% air/5% CO2. Differentiation of PC12 cells was achieved by exposure to 50 ng/mL of 2.5S (grade II) nerve growth factor (NGF, Alomone Laboratories, Jerusalem, Israel) for 7–14 days in DMEM supplemented with 1% FBS, penicillin/streptomycin and L-glutamine (low serum medium). Culture medium was replaced every 2–3 days. NGFDPC12 cells were re-plated at a density of 12,000 cells/cm2 24–36 hrs before exposure to FFA. NGFDPC12 cells were treated with levels of PA comparable to those found during ischemic injury (PA:BSA, 2:1 molar ratio) as described previously (Almaguel et al., 2009). PA and DHA (Sigma, St. Louis, MI) were first dissolved/diluted in 100% ethanol and further diluted to 300 μM in warm low serum medium with 150 μM fatty acid-free BSA (EMD Biosciences, La Jolla, CA). The final concentration of ethanol was 0.1%. Prior to treatment, the PA- or (PA+DHA)- containing medium was supplemented with NGF and cathepsin B inhibitor (CA-074Me) or cathepsin L inhibitor I (EMD Biosciences, San Diego, CA) and sterilized using a 0.22 μM filter.
4.2. Determination of cell viability
NGFDPC12 cells were harvested and re-plated in 96-well tissue culture plates. The columns for the standard curve were plated at 500, 1000, 2000, 4000 and 8000 cells per well, and columns for experimental treatments were plated at 4000 cells per well. Cells were grown for 24 h before treatment. At the end of treatments, culture medium was removed from the wells followed by addition to each well of 100 μL of phenol red free-media containing 10 μL of WST-1 (Roche Applied Science, Indianapolis, IN). Optical density at 450 nm was determined after 3 h.
4.3. Analysis of nuclear morphology
Nuclear morphology was assessed using the Hoechst 33342 fluorescent dye (Molecular Probes, Eugene, OR). Hoechst was added to the culture medium (2.5 μg/mL) and incubated in the dark for 10 min at 37°C. Stained cell nuclei visualized under fluorescent microscopy (Olympus BX50) using an UMPlanPI 60X/0.90W water immersion objective (Olympus, 400× magnification). Images were acquired using a digital Spot camera system (Diagnostic Instruments, Sterling Heights, MI). Apoptotic cells were identified by the presence of highly condensed or fragmented nuclei.
4.4. Assessment of apoptosis by flow cytometric analysis of Annexin V binding
NGFDPC12 cells were collected and resuspended in 40 μL of Annexin V binding buffer containing 2 μL of Annexin V-FITC suspension (BD Biosciences Pharmingen, San Diego, CA). After incubation for 15 min in the dark at room temperature, 160 μL of Annexin V binding buffer were added, followed by 5 min incubation. Before flow cytometry analysis another 200 μL of Annexin V binding buffer were added. Annexin V fluorescence was detected in the FL-1 channel using a Becton-Dikinson FACSCalibur® flow cytometer (Becton-Dickinson, San Francisco, CA). A total of 100,000 events were measured per sample.
4.5. Analysis of mitochondrial membrane permeabilization (MMP)
Disruption of MMP was assessed using the lipophilic cationic probe 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylben-zimidazol-carbocyanine iodide (JC-1 MitoScreen kit, BD Biosciences). Briefly, unfixed cells were washed and resuspended in PBS supplemented with 10 μg/mL of JC-1. Cells were then incubated for 15 min at room temperature in the dark, washed, and resuspended in PBS for immediate FACSCalibur® flow cytometry analysis. The percentage of cells with disrupted mitochondrial membrane potential was calculated using the CellQuest software.
4.6. Analysis of lysosomal membrane permeabilization (LMP)
The Acridine Orange (AO, Sigma Aldrich, St. Louis, MO) method was used to analyze LMP as described previously (Pacheco et al., 2005). Due to proton trapping, this vital dye accumulates mainly in the acidic vacuolar apparatus, preferentially in lysosomes. When excited by blue light, AO emits red/orange fluorescence at high concentrations (lysosomes) and green fluorescence at low concentrations (nucleus and cytoplasm). Cells with intact lysosomes appear red and green while cells with compromised lysosomes show a yellow color. Briefly, NGFDPC12 cells growing in 6-well culture plates were exposed to AO (5 μg/mL) and counterstained with Hoescht 33342 (1 μg/mL) for 20 minutes at 37°C. Cells were then examined under an Olympus BX50 epifluorescence microscope using a water immersion objective. Images were acquired using a digital Spot camera system.
LMP was also measured by flow cytometry. Briefly, NGFDPC12 cells were stained with AO (5 μg/mL) in DMEM medium for 15 min at 37 C. Cells were then washed, resuspended in PBS and the green (FL1) and red (FL3) fluorescence of 10,000 cells was recorded on a logarithmic scale using a BectonDickinson FACScan instrument (no band-pass filters) while excited at 488 nm (argon laser). Using this technique, early alterations of lysosomal stability were assayed, as evident by decrease in FL3 red fluorescence.
4.7. Detection of cathepsin L activity
Cathepsin L activity was detected using the fluorogenic substrate-based assay Magic Red MR-(FR)2 (Immunochemistry Technologies, Bloomington, MN). Briefly, cultured NGFDPC12 cells were exposed for 30 min to the cathepsin L substrate MR-(FR)2, rinsed twice with medium, and directly examined under the fluorescence microscope. Cells were counterstained with Hoechst 33342 for nuclear visualization.
4.9. Statistical analysis
All the experiments were repeated independently at least three times. Values represent means ± SE. Statistical comparisons were made using one-way ANOVA. Significance was accepted at p values < 0.05.
Acknowledgments
This work has been funded in part by awards and NIH 5P20MD001632, and 5R25GM060507.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adibhatla RM, Hatcher JF. Altered lipid metabolism in brain injury and disorders. Subcell Biochem. 2008;49:241–68. doi: 10.1007/978-1-4020-8831-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almaguel FG, Liu JW, Pacheco FJ, Casiano CA, De Leon M. Activation and reversal of lipotoxicity in PC12 and rat cortical cells following exposure to palmitic acid. J Neurosci Res. 2009;87:1207–18. doi: 10.1002/jnr.21918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes F, Cadenas E, Brunk UT. Apoptosis induced by exposure to a low steady-state concentration of H2O2 is a consequence of lysosomal rupture. Biochem J. 2001;356:549–55. doi: 10.1042/0264-6021:3560549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arab K, Rossary A, Flourié F, Tourneur Y, Steghens JP. Docosahexaenoic acid enhances the antioxidant response of human fibroblasts by upregulating gamma-glutamyl-cysteinyl ligase and glutathione reductase. Br J Nutr. 2006;95:18–26. doi: 10.1079/bjn20051626. [DOI] [PubMed] [Google Scholar]
- Bas O, Songur A, Sahin O, Mollaoglu H, Ozen OA, Yaman M, Eser O, Fidan H, Yagmurca M. The protective effect of fish n-3 fatty acids on cerebral ischemia in rat hippocampus. Neurochem Int. 2007;50:548–54. doi: 10.1016/j.neuint.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Baumgartner HK, Gerasimenko JV, Thorne C, Ashurst LH, Barrow SL, Chvanov MA, Gillies S, Criddle DN, Tepikin AV, Petersen OH, Sutton R, Watson AJ, Gerasimenko OV. Caspase-8-mediated apoptosis induced by oxidative stress is independent of the intrinsic pathway and dependent on cathepsins. Am J Physiol Gastrointest Liver Physiol. 2007;293:G296–307. doi: 10.1152/ajpgi.00103.2007. [DOI] [PubMed] [Google Scholar]
- Bazan NG, Jr, de Bazan HE, Kennedy WG, Joel CD. Regional distribution and rate of production of free fatty acids in rat brain. J Neurochem. 1971;18:1387–93. doi: 10.1111/j.1471-4159.1971.tb00003.x. [DOI] [PubMed] [Google Scholar]
- Blomgran R, Zheng L, Stendahl O. Cathepsin-cleaved Bid promotes apoptosis in human neutrophils via oxidative stress-induced lysosomal membrane permeabilization. J Leukoc Biol. 2007;81:1213–23. doi: 10.1189/jlb.0506359. [DOI] [PubMed] [Google Scholar]
- Boland B, Campbell V. Abeta-mediated activation of the apoptotic cascade in cultured cortical neurones: a role for cathepsin-L. Neurobiol Aging. 2004;25:83–91. doi: 10.1016/s0197-4580(03)00034-4. [DOI] [PubMed] [Google Scholar]
- Boya P, Andreau K, Poncet D, Zamzami N, Perfettini JL, Metivier D, Ojcius DM, Jaattela M, Kroemer G. Lysosomal membrane permeabilization induces cell death in a mitochondrion-dependent fashion. J Exp Med. 2003;197:1323–34. doi: 10.1084/jem.20021952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk UT, Svensson I. Oxidative stress, growth factor starvation and Fas activation may all cause apoptosis through lysosomal leak. Redox Rep. 1999;4:3–11. doi: 10.1179/135100099101534675. [DOI] [PubMed] [Google Scholar]
- Chen W, Li N, Chen T, Han Y, Li C, Wang Y, He W, Zhang L, Wan T, Cao X. The lysosome-associated apoptosis-inducing protein containing the pleckstrin homology (PH) and FYVE domains (LAPF), representative of a novel family of PH and FYVE domain-containing proteins, induces caspase-independent apoptosis via the lysosomal-mitochondrial pathway. J Biol Chem. 2005;280:40985–95. doi: 10.1074/jbc.M502190200. [DOI] [PubMed] [Google Scholar]
- Cirman T, Oresic K, Mazovec GD, Turk V, Reed JC, Myers RM, Salvesen GS, Turk B. Selective disruption of lysosomes in HeLa cells triggers apoptosis mediated by cleavage of Bid by multiple papain-like lysosomal cathepsins. J Biol Chem. 2004;279:3578–87. doi: 10.1074/jbc.M308347200. [DOI] [PubMed] [Google Scholar]
- Conti AC, Raghupathi R, Trojanowski JQ, McIntosh TK. Experimental brain injury induces regionally distinct apoptosis during the acute and delayed post-traumatic period. J Neurosci. 1998;18:5663–72. doi: 10.1523/JNEUROSCI.18-15-05663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conus S, Perozzo R, Reinheckel T, Peters C, Scapozza L, Yousefi S, Simon HU. Caspase-8 is activated by cathepsin D initiating neutrophil apoptosis during the resolution of inflammation. J Exp Med. 2008;205:685–98. doi: 10.1084/jem.20072152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper PR. Delayed brain injury: secondary insults. In: Becker DP, Povlishock JT, editors. Central Nervous System Trauma Status Report, National Institute of Neurological and Communicative Disorders and Stroke, National Institute of Health. Bethesda, MD: 1985. pp. 217–228. [Google Scholar]
- Dare E, Li W, Zhivotovsky B, Yuan X, Ceccatelli S. Methylmercury and H(2)O(2) provoke lysosomal damage in human astrocytoma D384 cells followed by apoptosis. Free Radic Biol Med. 2001;30:1347–56. doi: 10.1016/s0891-5849(01)00526-3. [DOI] [PubMed] [Google Scholar]
- Dhillon HS, Carman HM, Zhang D, Scheff SW, Prasad MR. Severity of experimental brain injury on lactate and free fatty acid accumulation and Evans blue extravasation in the rat cortex and hippocampus. J Neurotrauma. 1999;16:455–69. doi: 10.1089/neu.1999.16.455. [DOI] [PubMed] [Google Scholar]
- Droga-Mazovec G, Bojic L, Petelin A, Ivanova S, Romih R, Repnik U, Salvesen GS, Stoka V, Turk V, Turk B. Cysteine cathepsins trigger caspase-dependent cell death through cleavage of bid and antiapoptotic Bcl-2 homologues. J Biol Chem. 2008;283:19140–50. doi: 10.1074/jbc.M802513200. [DOI] [PubMed] [Google Scholar]
- El-Assaad W, Buteau J, Peyot ML, Nolan C, Roduit R, Hardy S, Joly E, Dbaibo G, Rosenberg L, Prentki M. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology. 2003;144:4154–63. doi: 10.1210/en.2003-0410. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Horrocks LA. Lipid peroxides in the free radical pathophysiology of brain diseases. Cell Mol Neurobiol. 1998;18:599–608. doi: 10.1023/A:1020625717298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqui AA, Horrocks LA. Phospholipase A2-generated lipid mediators in the brain: the good, the bad, and the ugly. Neuroscientist. 2006;12:245–60. doi: 10.1177/1073858405285923. [DOI] [PubMed] [Google Scholar]
- Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, Burgart LJ, Gores GJ. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–94. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- Feldstein AE, Werneburg NW, Li Z, Bronk SF, Gores GJ. Bax inhibition protects against free fatty acid-induced lysosomal permeabilization. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1339–46. doi: 10.1152/ajpgi.00509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghavami S, Asoodeh A, Klonisch T, Halayko AJ, Kadkhoda K, Kroczak TJ, Gibson SB, Booy EP, Naderi-Manesh H, Los M. Brevinin-2R(1) semi-selectively kills cancer cells by a distinct mechanism, which involves the lysosomal-mitochondrial death pathway. J Cell Mol Med. 2008;12:1005–22. doi: 10.1111/j.1582-4934.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lechon MJ, Donato MT, Martinez-Romero A, Jimenez N, Castell JV, O’Connor JE. A human hepatocellular in vitro model to investigate steatosis. Chem Biol Interact. 2007;165:106–16. doi: 10.1016/j.cbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C, Kaufmann SH, Gores GJ. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest. 2000;106:1127–37. doi: 10.1172/JCI9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S, El-Assaad W, Przybytkowski E, Joly E, Prentki M, Langelier Y. Saturated fatty acid-induced apoptosis in MDA-MB-231 breast cancer cells. A role for cardiolipin. J Biol Chem. 2003;278:31861–70. doi: 10.1074/jbc.M300190200. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Else PL. Membranes as possible pacemakers of metabolism. J Theor Biol. 1999;199:257–74. doi: 10.1006/jtbi.1999.0955. [DOI] [PubMed] [Google Scholar]
- Kaur P, Heggland I, Aschner M, Syversen T. Docosahexaenoic acid may act as a neuroprotector for methylmercury-induced neurotoxicity in primary neural cell cultures. Neurotoxicology. 2008;29:978–87. doi: 10.1016/j.neuro.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Kirkegaard T, Jaattela M. Lysosomal involvement in cell death and cancer. Biochim Biophys Acta. 2009;1793:746–54. doi: 10.1016/j.bbamcr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- Kumar KV, Das UN. Are free radicals involved in the pathobiology of human essential hypertension? Free Radic Res Commun. 1993;19:59–66. doi: 10.3109/10715769309056499. [DOI] [PubMed] [Google Scholar]
- Li Z, Berk M, McIntyre TM, Gores GJ, Feldstein AE. The lysosomal-mitochondrial axis in free fatty acid-induced hepatic lipotoxicity. Hepatology. 2008;47:1495–503. doi: 10.1002/hep.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listenberger LL, Ory DS, Schaffer JE. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J Biol Chem. 2001;276:14890–5. doi: 10.1074/jbc.M010286200. [DOI] [PubMed] [Google Scholar]
- Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100:3077–82. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue SE, Martin SJ. Caspase activation cascades in apoptosis. Biochem Soc Trans. 2008;36:1–9. doi: 10.1042/BST0360001. [DOI] [PubMed] [Google Scholar]
- Maestre I, Jordan J, Calvo S, Reig JA, Cena V, Soria B, Prentki M, Roche E. Mitochondrial dysfunction is involved in apoptosis induced by serum withdrawal and fatty acids in the beta-cell line INS-1. Endocrinology. 2003;144:335–45. doi: 10.1210/en.2001-211282. [DOI] [PubMed] [Google Scholar]
- Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A. 2004;101:8491–6. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj NS, Vigneswaran N, Zacharias W. Hypoxia inhibits TRAIL-induced tumor cell apoptosis: involvement of lysosomal cathepsins. Apoptosis. 2007;12:125–39. doi: 10.1007/s10495-006-0490-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb JK, Zhao X, Pike BR, Hayes RL. Temporal profile of apoptotic-like changes in neurons and astrocytes following controlled cortical impact injury in the rat. Exp Neurol. 1999;158:76–88. doi: 10.1006/exnr.1999.7071. [DOI] [PubMed] [Google Scholar]
- Newman ZL, Leppla SH, Moayeri M. CA-074Me protection against anthrax lethal toxin. Infect Immun. 2009;77:4327–36. doi: 10.1128/IAI.00730-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostenfeld MS, Fehrenbacher N, Hoyer-Hansen M, Thomsen C, Farkas T, Jaattela M. Effective tumor cell death by sigma-2 receptor ligand siramesine involves lysosomal leakage and oxidative stress. Cancer Res. 2005;65:8975–83. doi: 10.1158/0008-5472.CAN-05-0269. [DOI] [PubMed] [Google Scholar]
- Pacheco FJ, Servin J, Dang D, Kim J, Molinaro C, Daniels T, Brown-Bryan TA, Imoto-Egami M, Casiano CA. Involvement of lysosomal cathepsins in the cleavage of DNA topoisomerase I during necrotic cell death. Arthritis Rheum. 2005;52:2133–45. doi: 10.1002/art.21147. [DOI] [PubMed] [Google Scholar]
- Qian S, Wang W, Yang L, Huang HW. Structure of transmembrane pore induced by Bax-derived peptide: evidence for lipidic pores. Proc Natl Acad Sci U S A. 2008;105:17379–83. doi: 10.1073/pnas.0807764105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin O, Chang MC, Grange E, Bell J, Rapoport SI, Deutsch J, Purdon AD. Selective acceleration of arachidonic acid reincorporation into brain membrane phospholipid following transient ischemia in awake gerbil. J Neurochem. 1998;70:325–34. doi: 10.1046/j.1471-4159.1998.70010325.x. [DOI] [PubMed] [Google Scholar]
- Saunders J, Mathewkutty S, Drazner MH, McGuire DK. Cardiomyopathy in type 2 diabetes: update on pathophysiological mechanisms. Herz. 2008;33:184–90. doi: 10.1007/s00059-008-3115-3. [DOI] [PubMed] [Google Scholar]
- Seyfried D, Han Y, Zheng Z, Day N, Moin K, Rempel S, Sloane B, Chopp M. Cathepsin B and middle cerebral artery occlusion in the rat. J Neurosurg. 1997;87:716–23. doi: 10.3171/jns.1997.87.5.0716. [DOI] [PubMed] [Google Scholar]
- Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci U S A. 1998;95:2498–502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazawa M, Nakajima Y, Mashima Y, Hara H. Docosahexaenoic acid (DHA) has neuroprotective effects against oxidative stress in retinal ganglion cells. Brain Res. 2009;1251:269–75. doi: 10.1016/j.brainres.2008.11.031. [DOI] [PubMed] [Google Scholar]
- Tone O, Miller JC, Bell JM, Rapoport SI. Regional cerebral palmitate incorporation following transient bilateral carotid occlusion in awake gerbils. Stroke. 1987;18:1120–7. doi: 10.1161/01.str.18.6.1120. [DOI] [PubMed] [Google Scholar]
- Ulloth JE, Casiano CA, De Leon M. Palmitic and stearic fatty acids induce caspase-dependent and -independent cell death in nerve growth factor differentiated PC12 cells. J Neurochem. 2003;84:655–68. doi: 10.1046/j.1471-4159.2003.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH, Orci L. Diseases of liporegulation: new perspective on obesity and related disorders. FASEB J. 2001;15:312–21. doi: 10.1096/fj.00-0590. [DOI] [PubMed] [Google Scholar]
- Unger RH. Reinventing type 2 diabetes: pathogenesis, treatment, and prevention. JAMA. 2008;299:1185–7. doi: 10.1001/jama.299.10.1185. [DOI] [PubMed] [Google Scholar]
- Valentine RC, Valentine DL. Omega-3 fatty acids in cellular membranes: a unified concept. Prog Lipid Res. 2004;43:383–402. doi: 10.1016/j.plipres.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Werneburg N, Guicciardi ME, Yin XM, Gores GJ. TNF-alpha-mediated lysosomal permeabilization is FAN and caspase 8/Bid dependent. Am J Physiol Gastrointest Liver Physiol. 2004;287:G436–43. doi: 10.1152/ajpgi.00019.2004. [DOI] [PubMed] [Google Scholar]
- Windelborn JA, Lipton P. Lysosomal release of cathepsins causes ischemic damage in the rat hippocampal slice and depends on NMDA-mediated calcium influx, arachidonic acid metabolism, and free radical production. J Neurochem. 2008;106:56–69. doi: 10.1111/j.1471-4159.2008.05349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zhang L, Gurley E, Studer E, Shang J, Wang T, Wang C, Yan M, Jiang Z, Hylemon PB, Sanyal AJ, Pandak WM, Jr, Zhou H. Prevention of free fatty acid-induced hepatic lipotoxicity by 18beta-glycyrrhetinic acid through lysosomal and mitochondrial pathways. Hepatology. 2008;47:1905–15. doi: 10.1002/hep.22239. [DOI] [PubMed] [Google Scholar]
- Yamashima T, Kohda Y, Tsuchiya K, Ueno T, Yamashita J, Yoshioka T, Kominami E. Inhibition of ischaemic hippocampal neuronal death in primates with cathepsin B inhibitor CA-074: a novel strategy for neuroprotection based on ‘calpain-cathepsin hypothesis’. Eur J Neurosci. 1998;10:1723–33. doi: 10.1046/j.1460-9568.1998.00184.x. [DOI] [PubMed] [Google Scholar]
- Yeung BH, Huang DC, Sinicrope FA. PS-341 (bortezomib) induces lysosomal cathepsin B release and a caspase-2-dependent mitochondrial permeabilization and apoptosis in human pancreatic cancer cells. J Biol Chem. 2006;281:11923–32. doi: 10.1074/jbc.M508533200. [DOI] [PubMed] [Google Scholar]
- Zhang JP, Sun GY. Free fatty acids, neutral glycerides, and phosphoglycerides in transient focal cerebral ischemia. J Neurochem. 1995;64:1688–95. doi: 10.1046/j.1471-4159.1995.64041688.x. [DOI] [PubMed] [Google Scholar]
- Zhao M, Brunk UT, Eaton JW. Delayed oxidant-induced cell death involves activation of phospholipase A2. FEBS Lett. 2001;509:399–404. doi: 10.1016/s0014-5793(01)03184-2. [DOI] [PubMed] [Google Scholar]
- Zhao M, Antunes F, Eaton JW, Brunk UT. Lysosomal enzymes promote mitochondrial oxidant production, cytochrome c release and apoptosis. Eur J Biochem. 2003;270:3778–86. doi: 10.1046/j.1432-1033.2003.03765.x. [DOI] [PubMed] [Google Scholar]