Abstract
AIM
The optimal dosage of proton pump inhibitor in bleeding peptic ulcers remains controversial.
The aim was to compare the clinical effectiveness of two doses of infusional pantoprazole in peptic ulcer bleeding.
METHODS
Peptic ulcer patients (n= 120) with bleeding stigmata were enrolled after successful endoscopic therapy. After an initial bolus injection of 80 mg pantoprazole, patients were randomized to receive continuously infused pantoprazole at either 192 mg day−1 or 40 mg every 6 h (i.e. 160 mg day−1) for 3 days. Clinical outcomes between the two groups within 14 days were compared, with 14-day recurrent bleeding regarded as the primary end-point.
RESULTS
Both groups (n= 60 each) were well matched in demographic and clinical factors upon entry. Bleeding totally recurred in 11 (9.2%) patients, with six (10%) in the 192 mg day−1 group and five (8.3%) in the 160 mg day−1 group (relative risk of bleeding recurrence between two treatments 1.2; 95% CI 0.39, 3.72). All secondary outcomes between the two groups were similar, including the amount of blood transfusion (mean 1179 ml vs. 1203 ml, P > 0.1), hospital stay (mean 9.5 days vs. 9.9 days, P > 0.1), need for surgery (n= 1 vs. n= 0, P > 0.1), and mortality (n= 1 vs. n= 0, P > 0.1).
CONCLUSIONS
Following endoscopic haemostasis, infusional pantoprazole at either 192 mg day−1 or 40 mg every 6 h appear similar.
Keywords: pantoprazole, peptic ulcer bleeding, proton pump inhibitor, recurrent bleeding
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
An adjunct to endoscopy, proton pump inhibitor (PPI) is effective pharmacotherapy in high-risk patients with peptic ulcer bleeding (PUB).
An intravenous 80-mg bolus and 192 mg day−1 successive infusion for 3 days is the currently recommended dosing modality in administering PPI.
WHAT THIS STUDY ADDS
Clinical outcomes are not different in PUB patients receiving infusional pantoprazole at either 192 mg or 160 mg day−1 for 3 days.
The effective dosage of PPI may not be as high as currently recommended.
In view of cost-effectiveness, a lower dosage (160 mg day−1) of infusional PPI may be adopted in the management of PUB.
Introduction
Peptic ulcer bleeding (PUB) remains a serious medical problem worldwide, with significant morbidity and mortality [1, 2]. Endoscopic therapy reduces recurrent bleeding, surgery and mortality in PUB patients, and has been recommended as the standard haemostatic modality [3, 4]. Following endoscopic therapy, proton pump inhibitor (PPI) can reduce re-bleeding and surgery [5, 6].
The therapeutic efficacy of PPI is related to its potent inhibition of gastric acid [7], because acid and acid-dependent protease activity impair blood clotting [8, 9]. Although randomized trials have shown evidence of the clinical effectiveness of high-dose continuous infusional PPI over placebo or histamine type 2 receptor antagonist [7, 10–14], the optimal dosage of PPI remains controversial [5, 15–20]. Current practice guidelines recommend an 80-mg bolus, followed by continuous infusion of 8 mg h−1 for 3 days (192 mg day−1) as the standard regimen. However, the lowest effective dose is unclear [21, 22]. Because of the costly management of PUB [23], it is clinically relevant to investigate whether a lower dose of PPI may be as effective. Our previous randomized trials have already confirmed continuous infusion of omeprazole 40 mg every 6 h (160 mg day−1) as an effective dosing method [7, 24].
Hence, this study aimed to determine if a lower PPI dose was as effective as the current standard by directly comparing two dosing modalities (192 mg day−1vs. 160 mg day−1) of pantoprazole as adjunct to endoscopic therapy in PUB.
Methods
Setting and patients
This open-label, randomized, controlled trial was conducted in a regional teaching hospital (Lotung Poh-Ai Hospital) in north-eastern Taiwan. The hospital's Institutional Review Board approved the study protocol (ClinicalTrial.gov, NCT00731601). Patients who presented with haematemesis, melena, haematochezia, unexplained anaemia or other symptoms suggestive of upper gastrointestinal (UGI) bleeding were screened for eligibility. Patients were enrolled if they were aged >18 years, underwent UGI endoscopy within 24 h of presentation, and had peptic ulcers with high-risk stigmata confirmed by endoscopy. Endoscopic stigmata included active spurting (Forrest IA), oozing (Forrest IB), nonbleeding visible vessel (NBVV, Forrest IIA), or adherent blood clot (Forrest IIB). Patients were included after successful endoscopic haemostasis and after providing written informed consent.
Patients were excluded if they were pregnant, did not obtain initial haemostasis with endoscopic therapy, did not give written informed consent, had bleeding tendencies (i.e. platelet count <50 × 109 l−1, serum prothrombin <30% of normal, or using anticoagulants), used PPI within 7 days, had severe renal insufficiency (uraemia with or without dialysis), or bled from gastric cancers.
Endoscopic procedures
All endoscopic procedures were carried out by experienced endoscopists using standard video-endoscopes (GIF-XQ240 or GIF-XQ260; Olympus, Tokyo, Japan). Bleeding stigmata of peptic ulcers was evaluated by applying the Forrest classification [25]. An NBVV (Forrest IIA) was defined as a discrete protuberance at the ulcer base, and with an adherent clot (Forrest IIB) resistant to forceful washing or suction. Endoscopic therapy with multi-polar electro-coagulation (Force™ 2-2PCH; Valley Laboratory, Elgin, IL, USA) was done and the probe acted as a tamponade on the bleeders, which were electro-coagulated 10 s per pulse for three to four pulses. On the other hand, endoscopic injection of diluted epinephrine (1:10 000) was optional and left at the discretion of the treating endoscopists.
The bleeder was observed for 3 min and successful haemostasis was required for entry to the study. Infection by Helicobacter pylori was examined by rapid urease test during endoscopy (CLO test; Kimberly-Clark, Fullerton, CA, USA). Urease-positive patients received a 1-week course of standard triple therapy (pantoprazole 40 mg twice daily, clarithromycin 500 mg twice daily, and amoxicillin 1000 mg twice daily) after discharge.
Randomization process and intervention
The study patients were randomly divided with 1:1 proportions into two groups, using sealed envelopes containing a therapeutic option derived from a computer-generated random table. The envelope was not opened until treatment was assigned to a patient. After an initial intravenous (i.v.) bolus of 80 mg pantoprazole, study participants received continuously infused pantoprazole at either 192 mg day−1 (192 mg day−1 or Group 192) or 40 mg every 6 h (160 mg day−1 or Group 160), both for 3 days. After completing the i.v. therapy, both groups received oral pantoprazole of 40 mg once daily for 2 months.
Follow-up monitoring and outcome measurements
After enrolment, the patients' vital signs were checked every hour for the first 12 h, every 2 h for the second 12 h, every 4 h for the next 24 h, and then four times daily. Haemoglobin and haematocrit levels were checked at least once daily and blood transfusion was given if the haemoglobin became <90 g l−1 or if the patient's vital signs deteriorated. Shock was defined as systolic blood pressure <100 mmHg or pulse rate >100 beats min−1 accompanied by cold sweating, pallor, or oliguria.
Re-bleeding was suspected when there were unstable vital signs, continuous tarry or bloody stools, drop of haemoglobin level >20 g l−1, or little increase (<10 g l−1) of haemoglobin level despite two or more units of blood transfusion. Emergency endoscopy was immediately performed in such cases. Re-bleeding was confirmed if active bleeding, fresh blood or blood clots in the stomach were found. All patients with re-bleeding were treated with electro-coagulation unless they refused.
The primary end-point was recurrent bleeding within 14 days, while secondary end-points were hospital stay, volume of blood transfusion, need for surgery, and all-cause mortality at day 14.
Statistical analysis
Sample size estimation was based on detecting a 20% difference in favour of the 192 mg day−1 pantoprazole dosing with a type I error of 0.05 and type II error of 0.2. At least 59 patients were required for each group.
Unpaired Student's t-test was used to compare continuous variables between the two groups, and the χ2 test, with or without Yates' correction, was used for the categorical variables. Fisher's exact test was used if the expected value was <10. All statistical tests were two-tailed with a P-value < 0.05 considered as significant.
Results
Between May 2008 and March 2009, 1258 patients who presented at the emergency department with symptoms suggestive of gastrointestinal bleeding were screened (Figure 1). Among the 132 peptic ulcer patients with bleeding stigmata documented by emergency endoscopy, 12 were excluded for lack of written consent (n= 2), bleeding tendency (n= 2), poor cooperation (n= 2), gastric malignancy (n= 2), failure to achieve haemostasis (n= 1), and prior use of PPI (n= 3). The two groups were well matched in terms of baseline demographic and clinical factors (Table 1).
Figure 1.
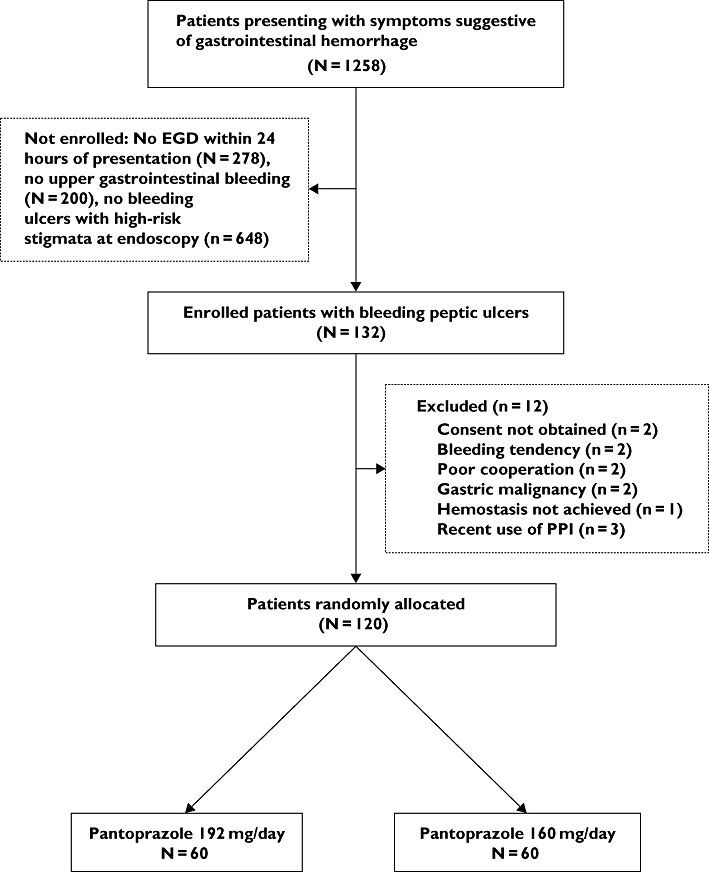
Flowchart of this study
Table 1.
Demographic and clinical variables of the study patients upon entry
| Pantoprazole 192 mg day−1 | Pantoprazole 160 mg day−1 | |
|---|---|---|
| (n= 60) | (n= 60) | |
| Age, mean (years) | 65.5 (20.4–82.7) | 64.5 (20.2–80.7) |
| Male gender (%) | 40 (66.7%) | 42 (70%) |
| Location of ulcer (%) | ||
| Stomach | 43 (71.7%) | 38 (63.3%) |
| Duodenum | 17 (28.3%) | 22 (36.7%) |
| Endoscopic findings (%) | ||
| Spurting | 5 (8.3%) | 7 (11.7%) |
| Oozing | 22 (36.7%) | 18 (30%) |
| NBVV | 25 (41.7%) | 27 (45%) |
| Clot | 8 (13.3%) | 8 (13.3%) |
| Gastric contents (%) | ||
| Blood | 20 (33.3%) | 22 (36.7%) |
| Coffee grounds | 25 (41.7%) | 24 (40%) |
| Clear | 15 (25%) | 14 (23.3%) |
| Shock (%) | 12 (20%) | 10 (16.7%) |
| Ulcer size (cm) | 1.05 (0.4–2.0) | 1.10 (0.5–2.1) |
| Helicoibacter pylori infection (%) | 38 (63.3%) | 40 (66.7%) |
| Rockall score | 5.05 (3.8–7.0) | 4.98 (3.5–6.9) |
Numerical variables expressed as mean with 95% confidence interval of distribution. There was no statistically significant difference between the two groups. NBVV, nonbleeding visible vessel.
Clinical outcomes were summarized in Table 2. The two treatment groups were not different in either primary or secondary outcomes (Table 2). Overall recurrent bleeding occurred in 11 study participants (9.2%) within 14 days, with six (10%) from Group 192 and five (8.3%) from Group 160 (relative risk of bleeding recurrence between two treatments 1.2; 95% CI 0.39, 3.72). Ten of the 11 re-bleeding patients were successfully managed by rescue endoscopic electro-coagulation. Unfortunately, massive and uncontrolled bleeding recurred in one patient in Group 192 and mortality ensued despite emergency surgery. There was neither surgical nor fatal outcome in Group 160 (P > 0.1 for both comparisons).
Table 2.
Clinical outcomes of patients according to PPI regimen
| Pantoprazole 192 mg day−1 | Pantoprazole 160 mg day−1 | |
|---|---|---|
| (n= 60) | (n= 60) | |
| Recurrent bleeding (%) | 6 (10%) | 5 (8.3%) |
| Hospital stay (days) | 9.5 (8.4–10.6) | 9.9 (8.3–10.7) |
| Volume of blood transfusion after therapy (ml) | 1179 (487–1995) | 1203 (492–2009) |
| Surgery (%) | 1 (1.7%) | 0 |
| Death (%) | 1 (1.7%) | 0 |
Numerical variables expressed as mean with 95% confidence interval of distribution. There was no statistically significant difference between the two groups.
The mean volume of blood transfusion was 1179 ml [95% confidence interval (CI) 487, 1995] in Group 192 and 1203 ml (492, 2009) in Group 160 (P > 0.1). On average, patients in Group 192 and Group 160 stayed in the hospital for 9.5 days (95% CI 8.4, 10.6) and 9.9 days (95% CI 8.3, 10.7), respectively (P > 0.1).
Discussion
This randomized, controlled trial has demonstrated the comparable clinical effectiveness of two doses of infusional pantoprazole in patients with high-risk bleeding peptic ulcers. By head-to-head comparison, the re-bleeding rate, mortality rate, need for surgery, blood transfusion and length of hospital stay were similar whether pantoprazole was continuously infused at 192 mg day−1 or at 40 mg every 6 h. These findings add to the growing body of evidence suggesting that effective dosages of PPI may not necessarily be as high as currently recommended [21, 22]. This is of important clinical relevance in view of cost-effectiveness in managing such a common but serious disease as PUB.
After Lau and colleagues reported their landmark trial in 2000 [10], their infusional method of an initial 80-mg bolus injection and successive infusion of 8 mg h−1 for 72 h has become the recommended dosing modality in administering PPI to PUB patients after successful endoscopic haemostasis [21, 22, 26]. However, convincing evidence supporting the superiority of 8 mg h−1 over other infusional methods with lower dosages has not been available to date. Simon-Rudlere et al.[18] retrospectively compared high-dose omeprazole (80-mg bolus followed by 8 mg h−1) with low-dose omeprazole (40 mg once daily) and reported that high-dose PPI reduced recurrent bleeding, surgery and bleeding-related mortality. However, because their comparison was derived from historical cohorts (the low-dose receivers were patients managed between 1997 and 2001 and the high-dose receivers those managed between 2001 and 2004), probable bias and confounding factors could not be overlooked.
In contrast, several comparative trials directly examining the clinical effectiveness of different doses of PPI have revealed that higher and lower doses of PPI were equally effective in PUB patients [15–17, 20], consistent with our findings. Moreover, pooled analysis of randomized trails comparing PPI with placebo or H2-receptor antagonist has shown that the therapeutic benefit of PPI was independent of dosage [5, 19].
It may not be necessary to keep high serum concentrations of PPI to achieve therapeutic efficacy because it exerts its potent suppression of acid secretion by forming irreversible disulphide bonding with a cysteine residue of the proton pump. Therefore, the inhibitory effect of PPI on gastric acid secretion can be restored only on turnover of the pumps [27]. In a randomized controlled trial comparing high-dose (80 mg bolus followed by 8 mg h−1) and low-dose regimens of pantoprazole (80 mg bolus followed by 40 mg every 12 h) [17], Hung et al. demonstrated that both clinical outcomes and pH control were not different between the two groups. Interestingly, the low-dose group achieved an intragastric pH value > 4 in 76.8% of the study period (85.9% in the 8 mg h−1 group, P= 0.12), and >6 in 49% of the duration (59% in the 8 mg h−1 group, P= 0.18).
Furthermore, it remains undetermined to what extent and for what duration the inhibition of acid secretion is sufficient to facilitate haemostasis after optimal endoscopic therapy. A remarkably lower dose of PPI and thus significantly poorer inhibition of acid secretion (still pH > 4 at most durations) may still be regarded as clinically sufficient [16, 28]. Whilst the lowest effective dose of PPI remains to be determined, this study and our previous trials [7, 24] suggest the dosing method of an 80-mg bolus followed by 40 mg every 6 h can be recommended in Asian PUB patients with high-risk stigmata. Nonetheless, caution should be exercised in extrapolating this recommendation in specific patient populations, such as those with comorbidities [29]. Although it is reasonable that a larger dose and stronger gastric acid suppression will be beneficial in patients with higher risks, more research is warranted to provide direct evidence. Future studies with adequate statistical power and standardized optimal endoscopic therapy are needed to determine the settings (presumably the most at-risk patients) in which high-dose infusional PPI is preferred.
The route of administration is another unsettled issue regarding PPI dosing in PUB. Randomized controlled trials have demonstrated that oral PPI is more effective than placebo in reducing recurrent bleeding, blood transfusion and need for surgery in bleeding peptic ulcer patients [30–32]. Indirect comparison by meta-analysis studies have found no evidence suggesting oral administration to be inferior to the i.v. route [5, 19]. Pharmacological trials directly comparing oral and i.v. lansoprazole revealed equivalent efficacy of the two routes in controlling intragastric pH [33, 34]. Furthermore, our open-label randomized trial demonstrated that oral rabeprazole (20 mg twice daily) was as effective as infusional omeprazole (40 mg every 12 h) in the management of PUB [35]. Considering cost-effectiveness and convenience, more studies are expected to resolve the controversy surrounding the optimal route of PPI.
The strengths of this study were clear inclusion criteria to enrol only those at high risk of recurrent bleeding, and universally applicable outcome measurements to observe clinical end-points. Moreover, the endoscopic therapy adopted in this study was thermo-coagulation with or without epinephrine injection, which was recognized as the optimal method in performing endoscopic haemostasis [36, 37]. Indeed, the present study addressed several shortcomings of previous research, such as inclusion of peptic ulcers with low risk of re-bleeding (Forrest IIc ulcers) [16], nonstandardized endoscopic therapy that allowed epinephrine injection alone [15–17, 20], scheduled second-look endoscopy that was not universally applied [15], and enrolment confined to severely comorbid patients with noticeably high re-bleeding rates (overall, 34.41%) [20].
Nonetheless, this study has several limitations. First, intragastric pH value was not monitored, so that it could not be concluded that both regimens resulted in similar control of acid suppression. However, there was no need to reaffirm the antisecretory efficacy of infusional PPI 40 mg every 6 h, which had already been demonstrated in our previous study [7]. Furthermore, whether or not these two regimens differed in inhibiting gastric acid would not change the conclusion because it was clinical effectiveness that was being investigated. Second, since genetic polymorphism of cytochrome P450 2C19, which determined pharmacokinetics and serum level of pantoprazole, was different between Asian and Western populations, this study did not address the issue of pharmacogenetics [38, 39]. Moreover, it has been observed that the therapeutic effectiveness of PPI appeared more pronounced in Asian PUB patients than in their Western counterparts [40]. Therefore, whether our results are applicable to Whites remains unknown and requires prospective validation in future research. Finally, this was an open-label trial without specific blinding methods for either patients or treating physicians. Consequently, there might be concerns about bias resulting from knowledge of treatment allocation. However, we believe this limitation would not change our results, because the predefined primary as well as secondary outcomes were measured and evaluated on the basis of objective definition, leaving little room for subjective judgment.
In conclusion, this comparative trial has revealed that continuously infused pantoprazole 40 mg every 6 h following an initial 80-mg bolus is similar to the current standard modality in patients with high-risk PUB. Clinical outcomes, including recurrent bleeding, blood transfusion, length of hospitalization, surgery and mortality are all similar. Our findings add to existing evidence that questions the current recommended dosage and have important clinical implication in terms of cost-effectiveness.
Competing interests
Y-C.H. reports receiving a subsidy from AstraZeneca for attending Digestive Disease Week 2009. No other potential conflict of interest was reported.
This study was supported by the Tomorrow Medical Foundation Grant no. 98-4. The authors express their gratitude to Miss Betty Tzu-en Lin, Miss Tze Yu Tung and Mr Alex Jen-hao Lin for their assistance.
REFERENCES
- 1.Lim CH, Vani D, Shah SG, Everett SM, Rembacken BJ. The outcome of suspected upper gastrointestinal bleeding with 24-hour access to upper gastrointestinal endoscopy: a prospective cohort study. Endoscopy. 2006;38:581–5. doi: 10.1055/s-2006-925313. [DOI] [PubMed] [Google Scholar]
- 2.van Leerdam ME, Vreeburg EM, Rauws EA, Geraedts AA, Tijssen JG, Reitsma JB, Tytgat GN. Acute upper GI bleeding: did anything change? Time trend analysis of incidence and outcome of acute upper GI bleeding between 1993/1994 and 2000. Am J Gastroenterol. 2003;98:1494–9. doi: 10.1111/j.1572-0241.2003.07517.x. [DOI] [PubMed] [Google Scholar]
- 3.Cook DJ, Guyatt GH, Salena BJ, Laine LA. Endoscopic therapy for acute non-variceal upper gastro-intestinal hemorrhage: a meta-analysis. Gastroenterology. 1992;102:139–48. doi: 10.1016/0016-5085(92)91793-4. [DOI] [PubMed] [Google Scholar]
- 4.Adler DG, Leighton JA, Davila RE, Hirota WK, Jacobson BC, Qureshi WA, Rajan E, Zuckerman MJ, Fanelli RD, Hambrick RD, Baron T, Faigel DO. ASGE guideline: the role of endoscopy in acute non-variceal upper-GI hemorrhage. Gastrointest Endosc. 2004;60:497–504. doi: 10.1016/s0016-5107(04)01568-8. [DOI] [PubMed] [Google Scholar]
- 5.Leontiadis GI, Sharma VK, Howden CW. Proton pump inhibitor treatment for acute peptic ulcer bleeding. Cochrane Database Syst Rev. 2006;(1) doi: 10.1002/14651858.CD002094.pub3. CD002094. [DOI] [PubMed] [Google Scholar]
- 6.Leontiadis GI, Sharma VK, Howden CW. Proton pump inhibitor therapy for peptic ulcer bleeding: Cochrane collaboration meta-analysis of randomized controlled trials. Mayo Clin Proc. 2007;82:286–96. doi: 10.4065/82.3.286. [DOI] [PubMed] [Google Scholar]
- 7.Lin HJ, Lo WC, Lee FY, Perng CL, Tseng GY. A prospective randomized comparative trial showing that omeprazole prevents rebleeding in patients with bleeding peptic ulcer after successful endoscopic therapy. Arch Intern Med. 1998;158:54–8. doi: 10.1001/archinte.158.1.54. [DOI] [PubMed] [Google Scholar]
- 8.Green FW, Jr, Kaplan MM, Curtis LE, Levine PH. Effect of acid and pepsin on blood coagulation and platelet aggregation. A possible contributor prolonged gastro-duodenal mucosal hemorrhage. Gastroenterology. 1978;74:38–43. [PubMed] [Google Scholar]
- 9.Patchett SE, Enright H, Afdhal N, O'Connell W, O'Donoghue DP. Clot lysis by gastric juice: an in vitro study. Gut. 1989;30:1704–7. doi: 10.1136/gut.30.12.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau JY, Sung JJ, Lee KK, Yung MY, Wong SK, Wu JC, Chan FK, Ng EK, You JH, Lee CW, Chan AC, Chung SC. Effect of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. N Engl J Med. 2000;343:310–6. doi: 10.1056/NEJM200008033430501. [DOI] [PubMed] [Google Scholar]
- 11.Hasselgren G, Lind T, Lundell L, Aadland E, Efskind P, Falk A, Hyltander A, Soderlund C, Eriksson S, Fernstrom P. Continuous intravenous infusion of omeprazole in elderly patients with peptic ulcer bleeding. Results of a placebo-controlled multi-center study. Scand J Gastroenterol. 1997;32:328–33. doi: 10.3109/00365529709007680. [DOI] [PubMed] [Google Scholar]
- 12.Schaffalitzky de Muckadell OB, Havelund T, Harling H, Boesby S, Snel P, Vreeburg EM, Eriksson S, Fernstrom P, Hasselgren G. Effect of omeprazole on the outcome of endoscopically treated bleeding peptic ulcers. Randomized double-blind placebo-controlled multi-centre study. Scand J Gastroenterol. 1997;32:320–7. doi: 10.3109/00365529709007679. [DOI] [PubMed] [Google Scholar]
- 13.Sung JJ, Barkun A, Kuipers EJ, Mossner J, Jensen DM, Stuart R, Lau JY, Ahlbom H, Kilhamn J, Lind T. Intravenous esomeprazole for prevention of recurrent peptic ulcer bleeding: a randomized trial. Ann Intern Med. 2009;150:455–64. doi: 10.7326/0003-4819-150-7-200904070-00105. [DOI] [PubMed] [Google Scholar]
- 14.Zargar SA, Javid G, Khan BA, Yattoo GN, Shah AH, Gulzar GM, Sodhi JS, Mujeeb SA, Khan MA, Shah NA, Shafi HM. Pantoprazole infusion as adjuvant therapy to endoscopic treatment in patients with peptic ulcer bleeding: prospective randomized controlled trial. J Gastroenterol Hepatol. 2006;21:716–21. doi: 10.1111/j.1440-1746.2006.04292.x. [DOI] [PubMed] [Google Scholar]
- 15.Andriulli A, Loperfido S, Focareta R, Leo P, Fornari F, Garripoli A, Tonti P, Peyre S, Spadaccini A, Marmo R, Merla A, Caroli A, Forte GB, Belmonte A, Aragona G, Imperiali G, Forte F, Monica F, Caruso N, Perri F. High- versus low-dose proton pump inhibitors after endoscopic hemostasis in patients with peptic ulcer bleeding: a multicentre, randomized study. Am J Gastroenterol. 2008;103:3011–8. doi: 10.1111/j.1572-0241.2008.02149.x. [DOI] [PubMed] [Google Scholar]
- 16.Udd M, Miettinen P, Palmu A, Heikkinen M, Janatuinen E, Pasanen P, Tarvainen R, Kairaluoma MV, Lohman M, Mustonen H, Julkunen R. Regular-dose versus high-dose omeprazole in peptic ulcer bleeding: a prospective randomized double-blind study. Scand J Gastroenterol. 2001;36:1332–8. doi: 10.1080/003655201317097218. [DOI] [PubMed] [Google Scholar]
- 17.Hung WK, Li VK, Chung CK, Ying MW, Loo CK, Liu CK, Lam BY, Chan MC. Randomized trial comparing pantoprazole infusion, bolus and no treatment on gastric pH and recurrent bleeding in peptic ulcers. ANZ J Surg. 2007;77:677–81. doi: 10.1111/j.1445-2197.2007.04185.x. [DOI] [PubMed] [Google Scholar]
- 18.Simon-Rudler M, Massard J, Bernard-Chabert B, Di Martino V, Ratziu V, Poynard T, Thabut D. Continuous infusion of high-dose omeprazole is more effective than standard-dose omeprazole in patients with high-risk peptic ulcer bleeding: a retrospective study. Aliment Pharmacol Ther. 2007;25:949–54. doi: 10.1111/j.1365-2036.2007.03286.x. [DOI] [PubMed] [Google Scholar]
- 19.Andriulli A, Annese V, Caruso N, Pilotto A, Accadia L, Niro AG, Quitadamo M, Merla A, Fiorella S, Leandro G. Proton-pump inhibitors and outcome of endoscopic hemostasis in bleeding peptic ulcers: a series of meta-analyses. Am J Gastroenterol. 2005;100:207–19. doi: 10.1111/j.1572-0241.2005.40636.x. [DOI] [PubMed] [Google Scholar]
- 20.Cheng HC, Kao AW, Chuang CH, Sheu BS. The efficacy of high- and low-dose intravenous omeprazole in preventing rebleeding for patients with bleeding peptic ulcers and comorbid illnesses. Dig Dis Sci. 2005;50:1194–201. doi: 10.1007/s10620-005-2759-6. [DOI] [PubMed] [Google Scholar]
- 21.Barkun A, Bardou M, Marshall JK. Consensus recommendations for managing patients with non-variceal upper gastro-intestinal bleeding. Ann Intern Med. 2003;139:843–57. doi: 10.7326/0003-4819-139-10-200311180-00012. [DOI] [PubMed] [Google Scholar]
- 22.Non-variceal upper gastro-intestinal hemorrhage: guidelines. Gut. 2002;51(Suppl. 4):iv1–6. doi: 10.1136/gut.51.suppl_4.iv1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiegel BM, Ofman JJ, Woods K, Vakil NB. Minimizing recurrent peptic ulcer hemorrhage after endoscopic hemostasis: the cost-effectiveness of competing strategies. Am J Gastroenterol. 2003;98:86–97. doi: 10.1111/j.1572-0241.2003.07163.x. [DOI] [PubMed] [Google Scholar]
- 24.Lin HJ, Lo WC, Cheng YC, Perng CL. Role of intravenous omeprazole in patients with high-risk peptic ulcer bleeding after successful endoscopic epinephrine injection: a prospective randomized comparative trial. Am J Gastroenterol. 2006;101:500–5. doi: 10.1111/j.1572-0241.2006.00399.x. [DOI] [PubMed] [Google Scholar]
- 25.Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastro-intestinal bleeding. Lancet. 1974;2:394–7. doi: 10.1016/s0140-6736(74)91770-x. [DOI] [PubMed] [Google Scholar]
- 26.Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med. 2008;359:928–37. doi: 10.1056/NEJMra0706113. [DOI] [PubMed] [Google Scholar]
- 27.Sachs G, Shin JM, Howden CW. Review article: the clinical pharmacology of proton pump inhibitors. Aliment Pharmacol Ther. 2006;23(Suppl. 2):2–8. doi: 10.1111/j.1365-2036.2006.02943.x. [DOI] [PubMed] [Google Scholar]
- 28.Udd M, Toyry J, Miettinen P, Vanninen E, Mustonen H, Julkunen R. The effect of regular and high doses of omeprazole on the intra-gastric acidity in patients with bleeding peptic ulcer treated endoscopically: a clinical trial with continuous intra-gastric pH monitoring. Eur J Gastroenterol Hepatol. 2005;17:1351–6. doi: 10.1097/00042737-200512000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Cheng HC, Chang WL, Yeh YC, Chen WY, Tsai YC, Sheu BS. Seven-day intravenous low-dose omeprazole infusion reduces peptic ulcer rebleeding for patients with comorbidities. Gastrointest Endosc. 2009;70:433–9. doi: 10.1016/j.gie.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 30.Khuroo MS, Yattoo GN, Javid G, Khan BA, Shah AA, Gulzar GM, Sodi JS. A comparison of omeprazole and placebo for bleeding peptic ulcer. N Engl J Med. 1997;336:1054–8. doi: 10.1056/NEJM199704103361503. [DOI] [PubMed] [Google Scholar]
- 31.Javid G, Masoodi I, Zargar SA, Khan BA, Yatoo GN, Shah AH, Gulzar GM, Sodhi JS. Omeprazole as adjuvant therapy to endoscopic combination injection sclerotherapy for treating bleeding peptic ulcer. Am J Med. 2001;111:280–4. doi: 10.1016/s0002-9343(01)00812-9. [DOI] [PubMed] [Google Scholar]
- 32.Kaviani MJ, Hashemi MR, Kazemifar AR, Roozitalab S, Mostaghni AA, Merat S, Alizadeh-Naini M, Yarmohammadi H. Effect of oral omeprazole in reducing re-bleeding in bleeding peptic ulcers: a prospective, double-blind, randomized, clinical trial. Aliment Pharmacol Ther. 2003;17:211–6. doi: 10.1046/j.1365-2036.2003.01416.x. [DOI] [PubMed] [Google Scholar]
- 33.Metz DC, Amer F, Hunt B, Vakily M, Kukulka MJ, Samra N. Lansoprazole regimens that sustain intragastric pH >6.0: an evaluation of intermittent oral and continuous intravenous infusion dosages. Aliment Pharmacol Ther. 2006;23:985–95. doi: 10.1111/j.1365-2036.2006.02850.x. [DOI] [PubMed] [Google Scholar]
- 34.Laine L, Shah A, Bemanian S. Intragastric. pH with oral vs. intravenous bolus plus infusion proton-pump inhibitor therapy in patients with bleeding ulcers. Gastroenterology. 2008;134:1836–41. doi: 10.1053/j.gastro.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Tsai JJ, Hsu YC, Perng CL, Lin HJ. Oral or intravenous proton pump inhibitor in patients with peptic ulcer bleeding after successful endoscopic epinephrine injection. Br J Clin Pharmacol. 2009;67:326–32. doi: 10.1111/j.1365-2125.2008.03359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sung JJ, Tsoi KK, Lai LH, Wu JC, Lau JY. Endoscopic clipping vs. injection and thermo-coagulation in the treatment of non-variceal upper gastro-intestinal bleeding: a meta-analysis. Gut. 2007;56:1364–73. doi: 10.1136/gut.2007.123976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barkun AN, Martel M, Toubouti Y, Rahme E, Bardou M. Endoscopic hemostasis in peptic ulcer bleeding for patients with high-risk lesions: a series of meta-analyses. Gastrointest Endosc. 2009;69:786–99. doi: 10.1016/j.gie.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 38.Lam SK, Hasan M, Sircus W, Wong J, Ong GB, Prescott RJ. Comparison of maximal acid output and gastrin response to meals in Chinese and Scottish normal and duodenal ulcer subjects. Gut. 1980;21:324–8. doi: 10.1136/gut.21.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caraco Y, Lagerstrom PO, Wood AJ. Ethnic and genetic determinants of omeprazole disposition and effect. Clin Pharmacol Ther. 1996;60:157–67. doi: 10.1016/S0009-9236(96)90131-9. [DOI] [PubMed] [Google Scholar]
- 40.Leontiadis GI, Sharma VK, Howden CW. Systematic review and meta-analysis: enhanced efficacy of proton-pump inhibitor therapy for peptic ulcer bleeding in Asia – a post hoc analysis from the Cochrane Collaboration. Aliment Pharmacol Ther. 2005;21:1055–61. doi: 10.1111/j.1365-2036.2005.02441.x. [DOI] [PubMed] [Google Scholar]


