Abstract
AIMS
To evaluate putative associations between drugs and dilated cardiomyopathy.
METHODS
We used the case/noncase method in the French PharmacoVigilance Database (FPVD). Cases were all the observations with dilated cardiomyopathy registered into the FPVD between 1 January 1990 and 30 June 2007. Noncases were all other reports other than those studied. Anthracyclines were used as positive controls. Data were expressed as reporting odds ratio (ROR) with their 95% confidence intervals.
RESULTS
Out of the 258 729 adverse drug reaction (ADR) reports recorded in the FPVD between 1 January 1990 and 30 June 2007, 47 (22 men, mean age 49 years) were defined as dilated cardiomyopathy. In these 47 patients, 67 drugs were ‘suspect’. A significant ROR was found with cytotoxic (epirubicin, mitoxantrone, cyclophosphamide, gemcitabine, fluorouracil) and antiretroviral (lamividune, zidovudine, abacavir) but also with isotretinoin, prednisone, appetite suppressant (clobenzorex) and psychotropic [antipsychotic (clozapine, olanzapine), lithium, antidepressant (clomipramine, amitriptyline, fluvoxamine)] drugs.
CONCLUSIONS
The present study describes an association between some drugs and reports of dilated cardiomyopathies. This relationship involves not only some already suspected drugs (anthracyclines, antiretrovirals), but also other drugs (antipsychotics, lithium, antidepressants, retinoids) less known to induce such an ADR. Despite the mandatory limits of this kind of study (underreporting, confounding factors …), these data represent a pharmacovigilance signal and could contribute to establish further prospective studies in order to confirm such signals.
Keywords: adverse drug reactions, anthracyclines, dilated cardiomyopathy, pharmacoepidemiology, pharmacovigilance
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Dilated cardiomyopathy is a frequent disease, responsible for 40–50% of cases of heart failure.
Several aetiologies have been reported: idiopathic, familial/genetic, viral and/or immune but also toxic agents (alcohol, cobalt, carbon monoxide, lead, cocaine, mercury or drugs).
Among drugs, anthracyclines are well known to induce such an adverse drug reaction.
WHAT THIS STUDY ADDS
This study describes an association with already suspected drugs (anthracyclines, antiretrovirals).
It also found a signal with other drugs (antipsychotics, lithium, antidepressants, retinoids).
This pharmacovigilance signal should be confirmed by further prospective studies.
Introduction
Dilated cardiomyopathy is defined by the presence of left ventricular systolic dysfunction in the absence of abnormal loading conditions (arterial hypertension, valve disease) or coronary artery disease sufficient to cause global systolic impairment [1]. It is a frequent disease, known to be responsible for 40–50% of cases of heart failure. Right ventricular dilation and dysfunction may be present but are not necessary for the diagnosis. Dilated cardiomyopathy is characterized by ventricular remodelling producing chamber dilation, with normal or decreased wall thickness and diminution in systolic function [2]. Congestive heart failure may or may not supervene. Impairment of systolic function can involve the left, right or both ventricles with an ejection fraction defined as <40% [3]. Presentation with disturbances of ventricular or atrial rhythm is common and death could occur at any stage. Its prevalence in the general population remains unknown, but at least 25% of patients in Western populations have evidence for familial disease with predominantly autosomal dominant inheritance [1]. Several aetiologies have been reported for dilated cardiomyopathies: idiopathic cardiomyopathy is a primary, global, myocardial disorder of unknown cause. Dilated cardiomyopathy could also be familial/genetic, viral and/or immune and related to some toxic agents [4, 5]. Besides alcohol, cobalt, carbon monoxide, lead, cocaine and mercury, exogenous agents potentially inducing dilated cardiomyopathy could be drugs: the main pharmacological class known to induce such a serious adverse drug reaction (ADR) is anthracyclines [5, 6]. Some cases reports or publications have also discussed the involvement of other drugs [5]. However, data remain conflicting.
Thus, the aim of the present study was to evaluate associations between drugs and reports of dilated cardiomyopathy, using the case/noncase method in the French PharmacoVigilance Database (FPVD).
Methods
Case/noncase method
The case/noncase approach measures disproportionality of combination between a drug and a particular ADR in a PharmacoVigilance Database [7–11]. Cases are reports corresponding to the ADR and noncases all other reports. The method allows comparison of drug exposure among cases and noncases and calculation of an ADR reporting odds ratio (ROR) with its 95% confidence interval (CI).
Source: the French PharmacoVigilance Database
The reporting of ADRs has been compulsory in France since 1984. According to the law, physicians must report ‘serious’ or ‘unexpected’ ADRs to their regional pharmacovigilance centre (31 in France). All suspected ADRs are registered in the FPVD [12]. For each report, information about patient (age, gender, medical history) and drug exposure (suspected and other associated nonsuspected drugs) are recorded in the FPVD. Causality assessment (imputation) is performed according to the French method used by all the Regional Centres of PharmacoVigilance [12]. A detailed summary of clinical description is added at the end of each pharmacovigilance case report. ADRs are coded according to ADR terminology of the World Health Organization (WHO-ART) [13]. ‘Serious’ ADRs were defined as reports leading to death, hospitalization (or prolongation of hospitalization), persistent or significant disability or incapacity, or being life threatening [14]. We investigated ADRs recorded in the FPVD between 1 January 1990 and 30 June 2007.
Selection of ‘cases’ and ‘noncases’
As a first step, we identified all cases recorded during this period and including a code related to a cardiomyopathy (WHO-ART code 0425.001). All these reports were carefully read. Second, we included in the study only reports in accordance with the diagnosis of dilated cardiomyopathy according to the WHO/International Society and Federation Cardiology task force on the definition and classification of cardiomyopathies [4]. Analysis of each report was carefully performed by a specialist in clinical cardiology (G.M.). Only drugs for which two or more reports of dilated cardiomyopathy have been registered in the FPVD were investigated in the case/noncase analysis. When the reporting form was not sufficiently informative or when the characteristics of ADRs could not be completely defined, the report was not included in the analysis.
‘Noncases’ were defined as all other reports (i.e. all other reports of ADRs, not defined as dilated cardiomyopathy) registered between 1 January 1990 and 30 June 2007 in the FPVD. We included all reports (coded according to the International Classification of Diseases 9th edn) of whatever age or gender.
Drug exposition (coded according to Anatomical Therapeutic Chemical classification) was defined by the presence in the report of the drug of interest, whatever the level of causality assessment. In our analysis, we did not include drug doses, since it is not exhaustively recorded in reports.
Statistical analysis
Characteristics of patients, number and type of drugs were compared between reports defined as dilated cardiomyopathy (named ‘cases’) and all other reports in the database (named ‘noncases’). We calculated an ADR ROR in order to compare the risk of exposure to the different classes of drugs in ‘cases’ and ‘noncases’. RORs were given with their 95% CI as crude RORs [15].
Results
Characteristics of cases
Of the 258 729 reports recorded in the FPVD between 1 January 1990 and 30 June 2007, 47 were finally validated as dilated cardiomyopathy (Figure 1). Among them, 22 (46.8%) were observed in men. Mean age was 49.1 years (median 49.0 years).
Figure 1.
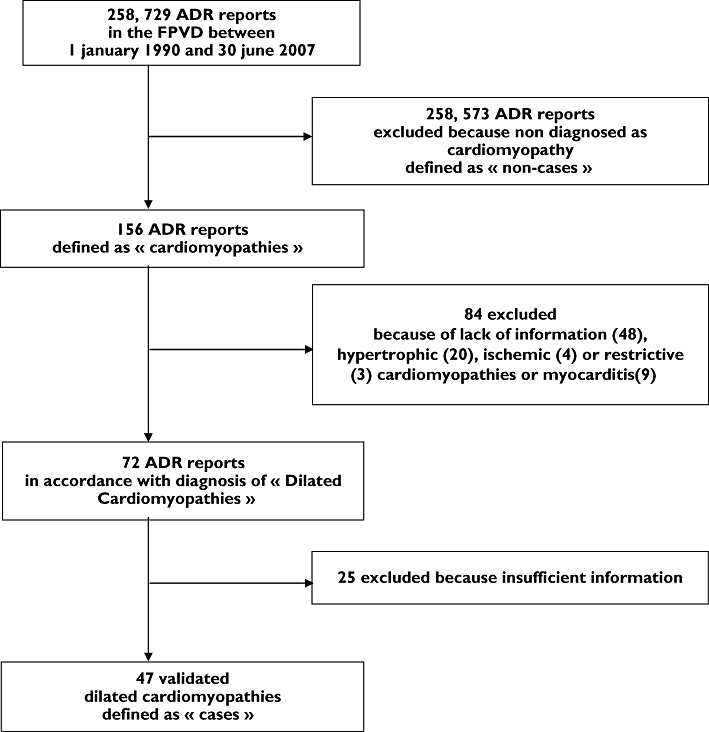
Flow chart of the study. FPVD, French PharmacoVigilance Database; ADR, adverse drug reaction
Figure 2 shows that the maximal number of cases (n= 14) occurred between 40 and 49 years. No case was reported after 79 years.
Figure 2.
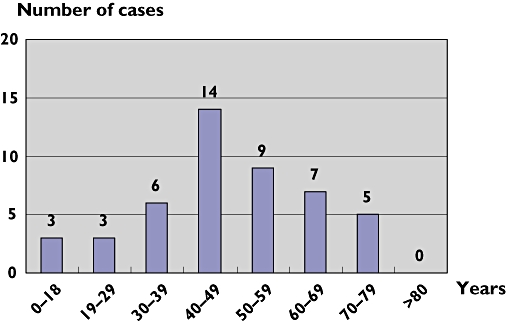
Repartition of the 47 cases of dilated cardiomyopathies according to age
Among the 47 case reports, 67 drugs were ‘suspect’. In 36 observations, only one drug was defined as ‘suspect’. In five cases, two different drugs were involved: epirubicin + fluorouracil (twofold), fluvoxamine + lithium (onefold), fluvoxamine + olanzapine (onefold) and zidovudine + lamivudine (onefold). Five other case reports involved three suspected drugs: zidovudine + abacavir (fourfold) and zidovudine + lamivudine + didanosine (onefold), whereas one notification involved four suspected drugs: didanosine + lamivudine + lopinavir + ritonavir.
Case/noncase comparison
The case/noncase comparison included the 47 patients (‘cases’) and the 258 729 reports (‘noncases’), i.e. the 84 + 25 patients excluded for lack of information on other causes of cardiomyopathies were not included in the study. In these 47 patients, 67 drugs were defined as ‘suspect’ (i.e. possibly associated with the occurrence of ADR whatever the level of causality assessment). Table 1 indicates the values of ROR between occurrence of dilated cardiomyopathy and exposure to drugs in the FPVD.
Table 1.
Risk of exposure to drugs in cases (n= 47 with 67 suspect drugs) and noncases (n= 258 664) and occurrence of dilated cardiomyopathy in the French PharmacoVigilance Database (FPVD)
| Pharmacological classes | Drugs | Number of dilated cardiomyopathies in the FPVD | Number of ADRs in the FPVD | ROR (95%CI) |
|---|---|---|---|---|
| Appetite suppressants | Clobenzorex | 2 | 67 | 122.4 (29.3, 510,4)* |
| Antineoplastics | ||||
| Anthracyclines and derivatives | Epirubicin | 4 | 256 | 65.1 (23.5, 180.2)* |
| Alkylating | Mitoxantrone | 2 | 209 | 38.4 (9.3, 157.9)* |
| Antimetabolites | Cyclophosphamide | 7 | 1126 | 26.8 (12.2, 58.8)* |
| Gemcitabine | 2 | 565 | 14.1 (3.4, 57.7)* | |
| Fluorouracil | 4 | 1494 | 10.9 (3.9, 30.1)* | |
| Antipsychotics | Clozapine | 4 | 866 | 18.9 (6.9, 52.2)* |
| Olanzapine | 3 | 821 | 14.7 (4.6, 47.1)* | |
| Mood stabilizers | Lithium carbonate | 3 | 745 | 16.2 (5.1, 51.9)* |
| Antidepressants | ||||
| Imipraminic | Clomipramine | 4 | 1418 | 11.5 (4.2, 31.7)* |
| Amitriptyline | 3 | 1129 | 10.7 (3.3, 34.1)* | |
| Serotoninergic | Fluvoxamine | 2 | 820 | 9.7 (2.3, 39.6)* |
| Retinoids | Isotretinoin | 3 | 873 | 13.8 (4.3, 44.3)* |
| Glucocorticoids | Prednisone | 3 | 1849 | 6.5 (2.0, 20.8)* |
| Antiretrovirals | ||||
| Nucleoside and nucleotide | Lamivudine | 7 | 4436 | 6.7 (3.0, 14.6)* |
| reverse transcriptase inhibitors | Zidovudine | 6 | 4344 | 5.7 (2.4, 13.3)* |
| Abacavir | 4 | 1919 | 8.5 (3.0, 23.4)* | |
| Didanosine | 2 | 2113 | 3.7 (0.9, 15.2) |
P < 0.01, ROR, reporting odds ratio; 95% CI, 95% confidence interval. Only drugs for which two or more reports of dilated cardiomyopathy have been registered in the FPVD were included in the study.
Significant RORs were found for appetite suppressant (clobenzorex), cytotoxic (epirubicin, mitoxantrone, cyclophosphamide, gencitabine, fluorouracil), antipsychotic (clozapine, olanzapine), lithium, antidepressant (clomipramine, amitriptyline, fluvoxamine), isotretinoin, prednisone and antiretroviral (lamivudine, zidovudine, abacavir) drugs. In contrast, no significant association was found for didanosine.
Discussion
In order to better define involvement of drugs in dilated cardiomyopathy (a ‘rare’ ADR), the present study investigated putative associations between reports of dilated cardiomyopathy and exposure to drugs. We worked in the FPVD, a large PharmacoVigilance database (>350 000 case reports from 1985) allowing such investigations. Cases were carefully selected by a specialized clinical cardiologist in order to restrict our analysis to true validated dilated cardiomyopathies. Positive associations were found with some expected drugs (anorexic, antineoplasic and antiretroviral drugs) but also with other unexpected drugs used in psychiatric, dermatological or endocrinological diseases.
Our study suffers from some compulsory methodological drawbacks. The first problem is underreporting, a well-known phenomenon in pharmacovigilance surveys. Estimation could be biased according to several factors, one of the most important being the characteristic of ‘seriousness’ of the ADR [16]. In fact, it is known that a ‘serious’ and/or ‘unexpected’ ADR occurring with a new drug is more likely to be reported than another kind of ADR. Moreover, ascribing a cardiac event (like dilated cardiomyopathy) to a drug is not easy. However, it is important to underline that the work was performed in a context of signal detection: thus, we chose to be sensible rather than unable to identify a true association. In fact, the values of the RORs should be considered only as a signal and not as a true risk value.
The choice of the control group is another important limitation of case/noncase methodology. Results should be inferred only to patients who have suffered from an ADR. In fact, one of the limitations of the present study is that we did not account for some important factors such as smoking, alcohol, cardiac or familial antecedents (they were not always as fully informed in cases as well in noncases) … Alcohol is known to be an important factor in the pathophysiology of dilated cardiomyopathies [5]. Finally, the present study should be considered as exploratory. However, despite its mandatory limits, use of case/noncase methodology is now recognized as very useful in order to generate signals in PharmacoVigilance and drug safety research [17].
Taking into account these inherent limits, our study suggests that some cytotoxic and antiretroviral drugs, but also psychotropic, dermatological and endocrine drugs, could be associated with dilated cardiomyopathy. These findings may have three explanations: the underlying disease for which these drugs are used could be risk factor for dilated cardiomyopathy; the drug could be an innocent bystander; or the relation could be causal. In fact, with the notable exceptions of human immunodeficiency virus (HIV) infection (which could induce, at least partly, such a cardiac disease) and glucocorticoids, the two first explanations are unlikely due to the strong association found with most of the drugs. Moreover, careful revision of cases allows us to exclude other co-prescribed drugs in the pathophysiology of these dilated cardiomyopathies.
It is interesting to discuss the results from a pharmacodynamic point of view. First, our study confirms the risk of dilated cardiomyopathy with anthracyclines. This pharmacological class was selected as positive control in this study, as methodologically required [7–12]. This kind of cardiac ADR could occur in patients treated with anthracyclines but previously free of cardiac disorders [18–21]. In our study, association was found to be significant with epirubicin and mitoxantrone. However, no case was reported to the FPVD with other anthracycline antibiotics (such as daunorubicin or doxorubicin) known to induce such a serious ADR [22]. The reason remains unclear: underreporting, less marked use in France …
Other antineoplasic agents were found to be associated with dilated cardiomyopathy in our study: an alkylating agent (cyclophosphamide) and two antimetabolites (gemcitabine and fluorouracil). Dilated cardiomyopathies were not previously described with cyclophosphamide, a nitrogen mustard. In contrast, myocarditis induced by this alkylating agent is well documented and unknown myocarditis could lead to dilated cardiomyopathies. Incidence of myocarditis (after high doses of cyclophosphamide) has been estimated to 7–25% in adults and 5% in children [21]. In some cases, it could be related to total administered dose. The onset of myocarditis is acute and no late toxicity (>3 weeks) was reported. The precise mechanism is unknown and histopathological examination shows increased heart weight, thickening of the left ventricular wall and haemorrhagic myocardial necrosis [21]. In our study, no case was found with ifosfamide.
Among antimetabolites, an association between dilated cardiomyopathies and two pyrimidine analogues (fluorouracil and gemcitabine) was found in the present work. Fluorouracil-related cardiac events are complex and range from chest pain to massive myocardial infarction leading to cardiogenic shock and death. The topic is much discussed in the literature [20, 21]. Our work indicates that some cardiological complications of fluorouracil use could be dilated cardiomyopathy and also allows adding gemcitabine to the list of drugs potentially inducing this ADR.
It should be underlined that, in the present study, no case involved herceptin (a recombinant humanized anti-HER2 antibody), used in the treatment of breast cancer and known for its cardiac toxicity [20]. In fact, we found one cardiac case report inside the FPVD: however, the clinical description was not in accordance with the diagnosis of dilated cardiomyopathy and the observation was not included in the present study.
We should stress that the above-discussed antineoplasic drugs are frequently part of combination treatment regimens: thus, other associated antineoplasic drugs could also, at least in part, be responsible for the ADR (although they were not registered as ‘suspect’ in the case reports). However, in our study, ciclosporin was alone in two cases out of the seven and the two cases with gemcitabine only involved this drug.
One of the interesting results of our study was an association between some classes of psychotropic drugs (antipsychotics, lithium and antidepressants) and dilated cardiomyopathy. Among antipsychotics, a significant association was found with clozapine and olanzapine. In fact, myocarditis associated with clozapine has been known for several years. A recent paper reviewed 116 case reports submitted to the Australian Adverse Drug Reactions Unit between 1993 and 2003: calculated incidence was between 0.7 and 1.2% of treated patients. Patients were relatively young (mean age 30 years). More than 10% of the patients died [22]. Our results extend the conclusion of this large paper, indicating that clozapine could also induce dilated cardiomyopathy. The exact mechanism remains unknown, but this kind of ADR could be a class effect since it was also found with olanzapine, another antipsychotic drug structurally related to clozapine.
Among antidepressants, a significant association was found with both imipraminic (clomipramine, amitriptyline) and serotoninergic (fluvoxamine) antidepressants. In fact, some dilated cardiomyopathies with heart failure have already been reported with imipraminic antidepressants [20, 23–26], especially with high dosages [18]. Usually, symptoms disappeared after drug withdrawal. One publication described a positive rechallenge [26]. Pharmacological data suggest that the myocardial effects of antidepressants could be mediated by several mechanisms: atropinic and quinidine-like effects, interference with reuptake of adrenergic amines, alteration of membrane permeability or direct myocardial depression [20]. The observation of a significant association with fluvoxamine is more surprising, since this serotoninergic antidepressant is supposed to exert less direct effects on myocardial function than imipraminic compounds [27]. Thus, the results with antidepressants could represent a pharmacovigilance signal, which should be confirmed by other further studies.
Another finding involves corticosteroids (and particularly prednisone). These drugs could exacerbate heart failure: this effect can be explained by their lateral mineralocorticoid properties, particularly after long-term use [18]. However, we cannot assume, from the results of the present study, that the described relationship with prednisone was causal: as discussed above, it could only be a indication of the underlying disease (infectious diseases in two cases and immunological disorder in one case) justifying prednisone prescription. In fact, the value of prednisone ROR was lower than that found with other drugs.
Antiretroviral agents are listed as one of the classical aetiologies of dilated cardiomyopathies [5]. In fact, this ADR has been reported with nucleoside and nucleotide reverse transcriptase inhibitors, including mainly zidovudine [27–30]. One case of dilated cardiomyopathy was previously published in a woman treated with zidovudine, lamivudine and nelfinavir: the dilated cardiomyopathy resolved after discontinuation of the three drugs and cardiac function remained normal with lamivudine, nelfinavir and abacavir, suggesting an important role for zidovudine [28]. Our study found such an association with zidovudine but also with lamivudine and abacavir (but not with didanosine). Dilated cardiomyopathy induced by antiretroviral agents is usually reversible, either spontaneously or with treatment. The mechanism of the dilated cardiomyopathy related to nucleoside and nucleotide reverse transcriptase inhibitors could involve drug-induced mitochondrial toxicity [30, 31]. However, one should stress that HIV infection alone is also able to induce such dilated cardiomyopathies [5]. Thus, the association found with antiretroviral drugs could be multifactorial, involving both the underlying disease and drugs.
One of the interests of this kind of research is to find new associations between occurrence of dilated cardiomyopathy and exposure to drugs. For example, we found only one case of dilated cardiomyopathy in the literature with clobenzorex [32] (an appetite-suppressant drug related to amphetamine drugs and known to induce primary pulmonary hypertension [33] but also cardiac valvulopathies [34]). The mechanism of this ADR remains unknown, although the description of such dilated cardiomyopathies with methamphetamine [35] could suggest involvement of catecholamine release and direct cardiac toxicity of catecholamine release.
Similarly, we were also unable to find, in literature, any report with isotretinoin (13- cis-retinoid acid), a vitamin A derivative which belongs to first-generation retinoids. Isotretinoin cardiac ADRs remain poorly described, even if one case of cardiomyopathy was previously found in a multicentre Phase II trial in association with interleukin (IL)-2 and IL-α in metastatic renal cell carcinoma [36]. Recently, two cases of isolated myocarditis were reported with tretinoin (all-trans-retinoid acid), another first-generation retinoid [37]. The proposed mechanism was suggested to be leucocyte infiltration and cytokine release causing endothelial damage and extravasations of acute promyelocytic leukaemia [37].
The present case/noncase study has also described an association with lithium. The effects of lithium on skeletal muscle are represented mainly by varying degrees of weakness and tremor. Acute or subacute painful proximal myopathy causing myalgia, cramps, myokymia or weakness has also been described. As far as we know, few case reports of cardiomyopathy associated with lithium have been published in the literature [38]. However, using the Bayesian confidence propagation network in the WHO database, Coulter et al.[39] found a significant association between lithium exposure and cardiomyopathy (but not myocarditis). The nature of the relationship (causal or incidental) remains a matter of discussion [38, 39].
In conclusion, the present study has described an association between some drugs and the reporting of dilated cardiomyopathies. This relationship involves, not only some already suspected drugs (anthracyclines, antiretrovirals), but also other drugs (antipsychotics, lithium, antidepressants, retinoids) less known to induce such an ADR. Despite the mandatory limits of this kind of study (underreporting, confounding factors …), these data represent a pharmacovigilance signal and could contribute to establishing further prospective studies in order to confirm such signals. From a practical point of view, the present study indicates that a systematic anamnesis about drugs should be involved in each aetiological research of dilated cardiomyopathy. Finally, these data also underline the interest of data mining in order to detect new signals of rare ADRs in a pharmacovigilance database.
Competing interests
None to declare.
The study was performed as a medical thesis by G.M.
REFERENCES
- 1.Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kühl U, Maisch B, McKenna WJ, Monserrat L, Pankuweit S, Rapezzi C, Seferovic P, Tavazzi L, Keren A. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on myocardial and pericardial diseases. Eur Heart J. 2008;29:270–6. doi: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 2.Rodkey SM, Ratliff NB, Youg JB. Cardiomyopathy and myocardial failure. In: Topol EJ, editor. Comprehensive Cardiovascular Medicine. Philadelphia, PA: Lippincott-Raven Publishers; 1998. pp. 2589–620. [Google Scholar]
- 3.Feild BJ, Baxley WA, Russel RO, Jr, Hood WP, Jr, Holt JH, Dowling JT, Rackley CE. Left ventricular function and hypertrophy in cardiomyopathy with depressed ejection fraction. Circulation. 1973;47:1022–31. doi: 10.1161/01.cir.47.5.1022. [DOI] [PubMed] [Google Scholar]
- 4.Oakley CM. Report of the WHO/ISFC task force on the definition and classification of cardiomyopathies. Br Heart J. 1980;44:672–3. doi: 10.1136/hrt.44.6.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dec GW, Fuster V. Idiopathic dilated cardiomyopathy. N Engl J Med. 1994;331:1564–75. doi: 10.1056/NEJM199412083312307. [DOI] [PubMed] [Google Scholar]
- 6.Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf. 2000;22:263–302. doi: 10.2165/00002018-200022040-00002. [DOI] [PubMed] [Google Scholar]
- 7.Egberts AC, Meyboon RH, De Koning FH, Bakker A, Leufkens HG. Non puerperal lactation associated with antidepressant drug use. Br J Clin Pharmacol. 1997;44:277–81. doi: 10.1046/j.1365-2125.1997.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore N, Kreft-Jais C, Haramburu F, Noblet C, Andrejak M, Ollagnier M, Begaud B. Reports of hypoglycaemia associated with the use of ACE inhibitors and other drugs: a case/non-case study in the French pharmacovigilance system database. Br J Clin Pharmacol. 1998;44:513–8. doi: 10.1046/j.1365-2125.1997.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egberts AC, Meyboom RH, van Puijenbroek EP. Use of measures of disproportionality in pharmacovigilance: three Dutch examples. Drug Saf. 2002;25:453–8. doi: 10.2165/00002018-200225060-00010. [DOI] [PubMed] [Google Scholar]
- 10.Stricker BH, Tijssen JG. Serum sickness-like reactions to cefaclor. J Clin Epidemiol. 1992;45:1177–84. doi: 10.1016/0895-4356(92)90158-j. [DOI] [PubMed] [Google Scholar]
- 11.Lugardon S, Lapeyre-Mestre M, Montastruc JL. The French Network of PharmacoVigilance Centres. Upper gastrointestinal adverse drug reactions and cyclooxygenase- 2 inhibitors (celecoxib and rofecoxib): a case/non-case study from the French Pharmacovigilance Database. Eur J Clin Pharmacol. 2004;60:673–7. doi: 10.1007/s00228-004-0813-5. [DOI] [PubMed] [Google Scholar]
- 12.Moore N, Noblet C, Kreft-Jais C, Lagier G, Ollagnier M, Imbs JL. French PharmacoVigilance Database system: examples of utilization. Therapie. 1995;50:557–62. [PubMed] [Google Scholar]
- 13.WHO. International Monitoring of Adverse Reactions to Drugs: Adverse Reaction Terminology. Uppsala: WHO Collaborating Centre for International Drug Monitoring; 1992. [Google Scholar]
- 14.Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis and management. Lancet. 2000;356:1255–9. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 15.Ewans SJW. Statistics: analysis and presentation of safety data. In: Talbot J, Waller P, editors. Stephens' Detection of New Adverse Drug Reactions. 5th. Chichester: Wiley; 2004. pp. 301–28. [Google Scholar]
- 16.Van der Heijden PG, Van Puijenbroek EP, Van Buuren S, Van der Hofstede JX. On the assessment of adverse drug reactions from spontaneous reporting systems: the influence of underreporting on odds ratios. Stat Med. 2002;21:2027–44. doi: 10.1002/sim.1157. [DOI] [PubMed] [Google Scholar]
- 17.Wilson AM, Thabane L, Holbrook A. Application of data mining data in pharmacovigilance. Br J Clin Pharmacol. 2004;57:127–34. doi: 10.1046/j.1365-2125.2003.01968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy CA, Dargie HJ. Drug-induced cardiovascular disorders. Drug Saf. 2007;30:783–804. doi: 10.2165/00002018-200730090-00005. [DOI] [PubMed] [Google Scholar]
- 19.Late cardiotoxicity of anthracyclines. Prescrire Int. 1999;8:145–6. No authors listed. [PubMed] [Google Scholar]
- 20.Feenstra J, Grobbee DF, Remme WJ, Stricker BHCh. Drug-induced heart failure. J Am Coll Cardiol. 1999;33:1152–62. doi: 10.1016/s0735-1097(99)00006-6. [DOI] [PubMed] [Google Scholar]
- 21.Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents. Drug Saf. 2000;22:263–302. doi: 10.2165/00002018-200022040-00002. [DOI] [PubMed] [Google Scholar]
- 22.Maas SJ, Hill R, Krum H, Liew D, Tonkin A, Demos L, Stephan K, McNeil J. Clozapine-associated myocarditis: a review of 116 cases of suspected myocarditis associated with the use of clozapine in Australia during 1993–2003. Drug Saf. 2007;30:47–57. doi: 10.2165/00002018-200730010-00005. [DOI] [PubMed] [Google Scholar]
- 23.Howland JS, Poe TE, Keith JF., Jr Cardiomyopathy associated with tricyclic antidepressants. South Med J. 1983;76:1455–6. doi: 10.1097/00007611-198311000-00039. [DOI] [PubMed] [Google Scholar]
- 24.Dallack GW, Roose SP, Glassman AH. Tricyclics and heart failure. Am J Psychiatry. 1991;148:1601. doi: 10.1176/ajp.148.11.1601a. [DOI] [PubMed] [Google Scholar]
- 25.Marti V, Ballester M, Obrador D, Udina C, Moya C, Pons-llado G. Reversal of dilated cardiomyopathy after chronic antidepressant drug withdrawal. Int J Cardiol. 1995;48:192–4. doi: 10.1016/0167-5273(94)02231-7. [DOI] [PubMed] [Google Scholar]
- 26.Briec F, Delaire C, Bouhour JB, Trochu JN. Recurrence of dilated cardiomyopathy after reintroduction of a tricyclic antidepressant. Arch Mal Coeur Vaiss. 2006;99:933–5. [PubMed] [Google Scholar]
- 27.Roos JC. Cardiac effects of antidepressant drugs. A comparison of the tricyclic antidepressants and fluvoxamine. Br J Clin Pharmacol. 1983;15(Suppl 3):439S–445S. doi: 10.1111/j.1365-2125.1983.tb02135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monsuez JJ, Gallet B, Escaut L, Pulik M, Charniot JC, Merad M, Slama M, Weber S, Vittecoq D. Cardiac side effects of anti-HIV agents. Arch Mal Coeur Vaiss. 2000;93:835–40. [PubMed] [Google Scholar]
- 29.Frerichs FC, Dingemans KP, Brinkman K. Cardiomyopathy with mitochondrial damage associated with nucleoside reverse-transcriptase inhibitors. N Engl J Med. 2002;347:1895–6. doi: 10.1056/NEJM200212053472320. [DOI] [PubMed] [Google Scholar]
- 30.Tanuma J, Ishizaki A, Gatanaga H, Kikuchi Y, Kimura S, Hiroe M, Oka S. Dilated cardiomyopathy in adult human immunodeficiency virus type 1-positive patient treated with a zidovudine-containing antiretroviral regimen. Clin Infect Dis. 2003;37:109–11. doi: 10.1086/377609. [DOI] [PubMed] [Google Scholar]
- 31.Miro O, Lopez S, Cardellach F, Casademont J. Mitochondrial studies in HAART-related lipodystrophy: from experimental hypothesis to clinical findings. Antivir Ther. 2005;10(Suppl 2):M73–81. [PubMed] [Google Scholar]
- 32.Cornaert P, Camblin J, Graux P, Anaye B, Dutoit A, Croccel L. Congestive cardiomyopathy in addition to clobenzorex, an anorexigenic drug. Arch Mal Coeur Vaiss. 1986;79:515–8. [PubMed] [Google Scholar]
- 33.Abenhaim L, Moride Y, Brenot F, Rich S, Benichou J, Kurz X, Higenbottam T, Oakley C, Wouters E, Aubier M, Simonneau G, Begaud B. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. International Primary Pulmonary Hypertension Study Group. N Engl J Med. 1996;335:609–16. doi: 10.1056/NEJM199608293350901. [DOI] [PubMed] [Google Scholar]
- 34.Weissman NJ. Appetite suppressants and valvular heart disease: a systematic review. BMC Clin Pharmacol. 2002;2:6. doi: 10.1186/1472-6904-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobs LJ. Reversible dilated cardiomyopathy induced by methamphetamine. Clin Cardiol. 1989;12:725–7. doi: 10.1002/clc.4960121211. [DOI] [PubMed] [Google Scholar]
- 36.Stadler WM, Kuzel T, Dumas M, Vogelzang NJ. Multicenter phase II trial of interleukin-2, interferon-alpha, and 13-cis-retinoic acid in patients with metastatic renal cell carcinoma. J Clin Oncol. 1998;16:1820–5. doi: 10.1200/JCO.1998.16.5.1820. [DOI] [PubMed] [Google Scholar]
- 37.Klein SK, Biemond BJ, Van Oers MHJ. Two cases of isolated symptomatic myocarditis induced by all-trans retinoic acid (ATRA) Ann Hematol. 2007;86:917–8. doi: 10.1007/s00277-007-0333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aichhorn W, Huber R, Stuppaech C, Whitworth AB. Cardiomyopathy after long-term treatment with lithium: more than a coincidence? J Psychopharmacol. 2006;20:589–91. doi: 10.1177/0269881106059696. [DOI] [PubMed] [Google Scholar]
- 39.Coulter DM, Bate A, Meyboom RHB, Lindquist M, Edwards IR. Antipsychotic drugs and heart muscle disorder in international pharmacovigilance: data mining study. BMJ. 2001;322:1207–9. doi: 10.1136/bmj.322.7296.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]


