Abstract
Diabetic pregnancies are characterized by chronic metabolic insults, including iron deficiency, that place the developing brain at risk and for memory impairment later in life. A behavioral recall paradigm coupled with electrophysiological measures was used to assess the longevity of these effects in 40 3½-year-old children. When memory demands were high, recall was significantly impaired in the at-risk group and correlated with perinatal measures of iron. Electrophysiological results suggested both encoding and retrieval processes were compromised. These findings support the hypothesis that prenatal iron deficiency leads to alterations in neural development that have a lasting impact on memory ability.
Outcome studies of infants of diabetic mothers (IDMs) demonstrate short- and long-term neurobehavioral morbidities that include altered auditory recognition memory processing at birth (Siddappa et al., 2004), reduced visual, cross-modal, and imitative memory performance in the first postnatal year (DeBoer, Wewerka, Bauer, Georgieff, & Nelson, 2005; Nelson et al., 2000; Nelson, Wewerka, Borscheid, deRegnier, & Georgieff, 2003), poorer performance on tests of general development in infancy and toddlerhood (deRegnier, Nelson, Thomas, Wewerka, & Georgieff, 2000; Rizzo, Metzger, Burns, & K, 1991), and inferior school performance in elementary school (Rizzo, Metzger, Dooley, & Cho, 1997). While diminished general development and school performance could represent any number of neuropathologies, both behavioral (DeBoer et al., 2005) and electrophysiological studies demonstrate abnormalities in memory processing and strongly suggest alterations in hippocampal development and function (deRegnier et al., 2000; Nelson et al., 2000; Nelson et al., 2003; Siddappa et al., 2004; see Nelson, 2007 for review), a hypothesis that is consistent with research from animal models (Rao et al., 1999).
Pregnancies complicated by diabetes mellitus are characterized by chronic fetal hypoxia, fetal hyper- and hypoglycemia, and fetal iron deficiency (Nold & Georgieff, 2004). Previous research has described the cascading effects of these metabolic abnormalities; specifically, how altered glucose handling leads to fetal iron deficiency through chronic hypoxia (Georgieff et al., 1990), with the quality of maternal glycemic control inversely proportional to the amount of risk experienced by the fetus. These abnormalities result in a fetal environment that places the growing brain at risk for perturbations in development (de Ungria et al., 2000; Jorgenson, Sun, O’Connor, & Georgieff, 2005; Jorgenson, Wobken, & Georgieff, 2003; Petry et al., 1992; Raman, Hamilton, Gewirtz, & Rao, 2008; Raman et al., 2005; Rao et al., 1999; Rao, Tkac, Townsend, Gruetter, & Georgieff, 2003; Schmidt, Waldow, Grove, Salinas, & Georgieff, 2007; Siddappa et al., 2004).
One region that has been shown to be particularly vulnerable to these specific metabolic abnormalities is the hippocampus. First, this structure has a prolonged developmental trajectory with significant changes occurring late gestation and early neonatal life in both humans and rodents. Second, its high metabolic demands place it at risk when gestational and neonatal conditions compromise the delivery of vital substrates such as glucose, oxygen, and iron. Studies using animal models have demonstrated that deficiencies of these substrates cause metabolic (de Ungria et al., 2000; Raman et al., 2005; Rao et al., 2003), structural (Jorgenson et al., 2003; Raman et al., 2008), and electrophysiological (Jorgenson et al., 2005; Yamada et al., 2004) changes in the CA1 region, an area that is crucial to recognition memory processing. Moreover, research using behavioral methods has supported these findings by reporting impairments in both short- and long-term hippocampally dependent memory abilities following these early life substrate deficiencies (Carlson et al., in press; McEchron, Cheng, Liu, Connor, & Gilmartin, 2005; Raman et al., 2008; Schmidt et al, 2007).
Glucose tolerance, hypoxia, and iron deficiency are inextricably linked in IDMs (Georgieff et al., 1990); thus there is no way to isolate the effects of each risk factor in a human model. However, because animal studies utilize a rodent model of iron deficiency anemia (not a diabetic model) these studies have been able to isolate the effects of each factor independent of the others. Results from this line of research directly implicate iron as an important predictor of outcome. The considerable effects of fetal and neonatal iron deficiency in mediating the short- (while iron deficient) and long- (following iron repletion) term hippocampal-specific abnormalities in the rodent have been delineated from the genome (Carlson, Stead, Neal, Petryk, & Georgieff, 2007) through electrophysiology (Jorgenson et al., 2005) to behavior (McEchron et al., 2005; Schmidt et al., 2007). Consistent with these findings, a previous study in human infants has also described an association between extent of perinatal iron deficiency and memory performance at 12 months of age (DeBoer, et al., 2005).
Taken together, these results suggest that prenatal iron deficiency can lead to alterations in hippocampal development and result in memory impairments later in life. The present research sought to extend these previous findings by investigating, in humans, 1) whether memory impairments caused by prenatal iron deficiency persist into early childhood, 2) if so, under what conditions are they detectable, and 3) what cognitive mechanisms (e.g., encoding or retrieval) underlie these impairments. Given the known metabolic cascade that accompanies the diabetic pregnancy (Georgieff et al., 1990), IDMs represent a model population in which to examine these questions.
Memory outcomes were examined in IDMs between 3 and 4 years of age using a modified and age-appropriate elicited/delayed imitation paradigm. This unique paradigm utilizes both behavioral and electrophysiological measures (Carver, Bauer, & Nelson, 2000) to examine functions that are dependent on the hippocampus (Adlam, Vargha-Khadem, Mishkin, & de Haan, 2005; McDonough, Mandler, McKee, & Squire, 1995) long after any prenatal metabolic deficiencies have resolved (Georgieff, Wewerka, Nelson, & deRegnier, 2002). The dependent measures consisted of elicited imitation (i.e., immediate recall, which served as a measure of encoding) as well as delayed imitation (i.e., one-week delayed recall, which served as a measure of memory) in combination with event-related potential (ERP) indices of recognition memory. Task difficulty was manipulated by varying the amount of internal structure within the to-be-remembered material. This manipulation allowed for the examination of outcomes as a function of the demands placed on memory processes and underlying neural circuitry.
Based on findings from previous research, we hypothesized that memory would be impaired in the IDM group relative to age-matched controls, that this impairment would be observed in both the behavioral and electrophysiological measures, and would be significantly related to the extent of iron deficiency experienced during the prenatal period. Exploratory aspects of this investigation included assessment of whether memory outcomes were modulated by the extent of demands placed on the circuitry, and examination of sources of variation within electrophysiological measures representing the underlying time course and neural activation patterns associated with recognition.
Method
Participants
Participants’ mothers were recruited before giving birth for participation in an ongoing longitudinal research project examining cognitive development (see Nelson, 2007 for a recent summary of previous reports on the sample). The subjects composing both groups were recruited from the same hospitals and matched for age and gender. An initial report from this cohort included data from 60 infants at 6 months of age: 26 IDMs and 34 Controls (Nelson et al., 2000). In accordance with the ethical principles expressed in the Declaration of Helsinki, informed consent was obtained from the guardians of the child participants, and the University’s Institutional Review Board approved all procedures prior to the start of the investigation.
Forty 3- to 4-year-old children (mean age 3 years, 6 months) participated in this follow-up investigation. Twenty children (10 male, 10 female) were born to mothers diagnosed with diabetes mellitus and 20 children (8 male, 12 female) were born to mothers who did not have any form of diabetes during pregnancy (see Riggins, Miller, Bauer, Georgieff, & Nelson, 2009). Of the 20 mothers diagnosed with diabetes, 75% (15) were diagnosed with gestational diabetes, 15% (3) with Type 1 diabetes, and 10% (2) with Type 2 diabetes. Regardless of the type of diabetes present, the pathophysiology driven by maternal hyperglycemia occurs in all three conditions (see Nold & Georgieff, 2004 for a useful diagram). Parents were asked to provide race and ethnicity information of the children in the current sample. Thirty-four children (85%) were of Caucasian, non-Hispanic or Latino decent, 3 children (7.5%) were of Caucasian, Hispanic or Latino decent, 2 children (5%) were of African American decent, and 1 child (2.5%) was of Asian decent. All participants met the following medical criteria: lack of intrauterine growth restriction, no significant maternal hypertension, no chromosomal syndromes/non-chromosomal congenital anomalad sequences or congenital infectious agents (TORCH: Toxoplasmosis, Other Agents, Rubella, Cytomegalovirus, Herpes Simplex), normal labor and delivery, no significant heart rate decelerations, 5 minute Apgar scores greater than 6, and an uncomplicated neonatal course including no mechanical ventilation and no indication of acute perinatal or neonatal insult (such as asphyxia, sepsis, seizures, meningitis, or intracranial hemorrhage). In order to compare the groups on perinatal complications, an obstetrics complication scale was used (Prechtl, 1967). Consistent with previous reports (deRegnier et al., 2000), points given for diabetes or insulin use were removed prior to group comparison. There were no differences between groups in perinatal complications unrelated to diabetes. In addition, there was no difference in maternal age at time of birth between IDM (mean = 32.15 years, SD = 7.07) and control (mean = 31.74, SD = 3.89) groups.
At time of delivery, infants in both groups were assessed for signs of perinatal iron deficiency by means of cord serum ferritin concentrations (Siddappa, Rao, Long, Widness, & Georgieff, 2007) and exposure to hypoxia and hyperinsulinemia by means of neonatal macrosomia (Akin et al., 2002; Morris, Grandis, & Litton, 1985). Ferritin values less than 76μg/L were considered deficient in fetal iron stores. This value has both biologic and physiologic relevance as it represents the cut-off for the bottom quartile of cord blood ferritin levels and has been used in previous reports demonstrating group differences (DeBoer, et al., 2005; Tamura et al., 2002b). For example, Tamura and colleagues demonstrated that cord blood serum ferritin concentrations in the lowest quartile were a risk factor for poorer school-age performance (Tamura et al., 2002b). Size for dates (i.e., birthweight z-scores) greater than 2 standard deviations (SDs) above the population mean suggests the presence of chronic fetal hypoxia and hyperinsulinemia and was used as an index of diabetic risk (Nold & Georgieff, 2004).
Comparisons between the groups indicated that more children in the IDM group (9 out of 20, or 45%) had newborn ferritin concentrations below 76μg/L, compared to the control group (2 out of 16, or 12.5%), χ2 (1, N = 36) = 4.43, p <.05; and more children in the IDM group (10 out of 20, or 50%) had birthweight z-scores greater than 2 standard deviations above the mean, compared to the control group (1 out of 20 or 5%), χ2 (1, N = 40) = 10.16, p =.001. There were no differences between the groups in gestational age or 5 minute Apgar scores. Finally, the Wechsler Preschool and Primary Scale of Intelligence-Revised (WPPSI-R) was administered at a separate session when children were 4 years of age. Three children in the control group did not provide data for this measure due to missed appointments. Consistent with previous reports (Tamura et al., 2002a), the IDM group’s performance (Full Scale IQ: mean = 100, SD = 13) was below that of controls (Full Scale IQ: mean = 112, SD = 13); however, no child’s score was below the average range. See Table 1 for a summary of sample characteristics.
Table 1.
Summary of group characteristics
| Characteristic | Control n=20 | IDM n=20 | Assessment of Group Differences |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Note: df corrected for violation of equality of variance assumption | |
| Age at test (months) | 42.65 (5.78) | 42.10 (5.75) | ns |
| Gestational age (weeks) | 38.75 (2.05) | 38.05 (2.04) | ns |
| Apgar scores (5 minute) | 8 (1) | 8 (1) | ns |
| Birthweight (g) | 3528.60 (56.99) | 3720.15 (807.89) | ns |
| Birthweight z-scores | 0.63 (.88) | 1.54 (.97) | t(26.02) = 1.86, p = .07 |
| Birthweight z-scores > 2 | 1/20 or 5% | 10/20 or 50% | χ2 (1, N = 40) = 10.16, p =.001 |
| Ferritin from cord serum at birth (μg/L) | 134.81 (56.99) | 97.00 (75.10) | t(33.93) = 1.72, p =.095 |
| Ferritin < 76 μg/L | 2/16 or 12.5% | 9/20 or 45% | χ2 (1, N = 36) = 4.43, p <.05 |
| Full-Scale IQ WPPSI-R | 112 (13) | 100 (13) | t(33.68) = 2.87, p <.01 |
| Verbal IQ | 108 (13) | 98 (10) | t(34.42) = 2.84, p <.01 |
| Perceptual IQ | 112 (11) | 102 (16) | t(28.63) = 2.30, p <.05 |
WPPSI-R = Wechsler Preschool and Primary Intelligence Scale-Revised
ns = not significant
Although prenatal iron stores are well below normal in IDMs, previous studies have shown that these deficiencies resolve shortly after birth (Georgieff et al., 2002). Moreover, this postnatal recovery is spontaneous as no supplemental iron is necessary. Although iron status was not collected at the time of the present study, 9 of the 20 IDM children in the present report provided follow-up assessments of iron status between 6 and 12 months of age as part of a previous investigation (Beard, de Regnier, Shaw, Rao, & Georgieff, 2007; Georgieff et al., 2002). All measured ferritin concentrations between 6 and 12 months of age were within the normal range i.e., >10 μg/L (actual range: 21–54μg/L; Georgieff et al., 2002). Thus, no evidence of postnatal iron deficiency was indicated, even in infants born with low iron stores. This characterization is important as it points to pre- or perinatal iron deficiency as the source of any group differences. Previous investigations of the long-term effects of postnatal dietary iron deficiency have implicated alterations in monamine metabolism and myelination (Lozoff & Georgieff, 2006) as well as alterations in affect and motivation, reduced speed of processing, and abnormal frontal lobe function related to striatal-frontal circuitry (Algarin, Peirano, Garrido, Pizarro, & Lozoff, 2003; Lozoff et al., 2007; Lozoff, Jimenez, & Smith, 2006).
There were no age differences between the groups at the time of assessment. Out of the 40 children who participated in the present investigation, analyses of behavioral memory performance included data from 37 children (17 IDM, 20 controls); analyses of electrophysiological recordings included data from 33 children (13 IDM, 20 controls). Behavioral data were excluded due to recording equipment failure (n = 2), or incomplete data due to refusal to complete a portion of the paradigm (n = 1). Electrophysiological data were excluded due to refusal to wear the cap (n = 2), excessive movement artifact resulting in an insufficient number of trials (n = 3), or refusal to complete ERP paradigm (n = 2). In order to ensure that the results from the electrophysiological data revealing group differences were not attributable to differences in sample size, data were reanalyzed with 13 controls. Results regarding group differences from these analyses with equal sample sizes did not differ; for the sake of brevity only the former are reported. All analyses were conducted with SPSS, version 14.0 (SPSS Inc., Chicago, IL). Repeated measures analyses employed listwise deletion procedures; therefore, sample sizes vary slightly between tasks and degrees of freedom were adjusted accordingly. Greenhouse-Geisser corrections were used to correct for violations in the assumption of sphericity.
Materials
Event sequences
Each child was shown three different nine-item event sequences. Event sequences contained novel objects that were used to produce nine different individual actions related to a common theme (e.g., going camping). Within each sequence, memory for both individual items (e.g., baiting a hook or catching a fish or setting up the tent) and the order of the items (e.g., baiting the hook before catching the fish and then setting up the tent) was assessed. To induce variability in the difficulty of ordered recall, the internal organization of the event sequences was manipulated by altering the number of enabling connections between items (Bauer, Hertsgaard, Dropik, & Daly, 1998). Enabling relations exist when it is necessary to complete the individual items in the correct temporal order to achieve the desired end state (for example, baiting a hook before catching a fish). However, each individual item can be completed independent of the others (i.e., one could attempt to catch the fish without bait, but would not be successful). Event sequences with more enabling relations have greater internal organization, which leads to a more organized representation (Bauer, 1992). Sequences with more enabling relations are easier to recall because each item in the event serves as a reminder of subsequent items. The differential effects of the reminders are thought to be associated with the strength of the organization of the event representation. This argument implies that if an event representation is sufficiently well organized to support reliable ordered recall, then there should exist the potential for recollection to facilitate memory for the event (see Bauer et al., 1998 for elaboration).
Because there were nine items in the event sequences, all sequences contained eight possible relations between items. In our manipulation, event sequences that had “high” internal organization contained six enabling relations, sequences with “medium” internal organization contained three enabling relations, and sequences with “low” internal organization contained zero enabling relations (i.e., all relations between items were arbitrary). Three event sequences (one of each organization level) were randomly selected from an existing pool of 15 sequences for each participant.
ERP stimuli
For ERP testing, stimuli consisted of digital photographs of a woman’s hand completing each individual item from the three event sequences observed in behavioral testing and three novel event sequences matched for the number of enabling relations. Pictures of the entire group of props for each sequence were also presented, resulting in 10 unique stimuli per event. Pictures of the old and new sequences were randomly presented blocked by sequence type. The order of the blocks was counterbalanced between participants and each picture was presented twice for a total of 120 trials over the entire session.
Procedure
Data were collected in two separate sessions spaced approximately one week apart. All sessions were conducted by the same experimenter and in the same location. The first session consisted of orientation to the task, baseline assessment of children’s spontaneous behaviors with the event sequences, modeling of the sequences, and measurement of immediate recall. Orientation was used to ensure understanding of the task. Baseline measures were used as a control for problem-solving abilities and/or fortuitous production of the items. Modeling of the event sequences was done twice with verbal narration by the experienced experimenter. Immediate recall of the individual items and temporal order was used as an index of encoding.
After the one-week delay (M = 7 days, SD = 1 day, range 4–10 days), children returned to the laboratory and both electrophysiological and behavioral measures of memory were obtained. There was no significant difference between length of delay for the IDM and control groups. During the electrophysiological assessment, children were seated in a dimly lit room and passively viewed pictures of three familiar and three novel sequences while event-related potentials (ERPs) were recorded using ERPw version 3.2 (Pearson Technical Software, Minneapolis, MN) from 32 sites on the scalp using Electro-Caps (Electro-Cap International, Inc.). A Neurodata acquisition system (Model 12C-32-235) and Grass amplifier (Model 12A5) were used to record and filter EEG and electrooculogram (EOG) signals. In accordance with previous studies (for review see DeBoer, Scott, & Nelson, 2005), EEG amplifier gain was set to 20,000 and EOG gain was set to 5,000. Lower and upper cutoff frequencies were set to 0.1 and 30 Hz respectively, and a 60Hz notch filter was in place. EEG was sampled at 100 Hz, referenced to Cz, and rereferenced offline to mathematically linked mastoids. EOG activity was also recorded from a transverse position above and below the eye to allow for detection and deletion of blink artifacts. Impedances at all leads were kept below 10 k. The duration of each stimulus was 1000ms with a pretrial baseline of 100 and a variable intertrial interval. Immediately following the ERP session, children behaviorally recalled the event sequences. The fixed order of presentation of the tasks was necessary because (a) the constraints of ERP methodology are such that behavioral recall could not be done simultaneously, (b) behavioral testing could have influenced the ERP response, whereas (c) prior research has demonstrated no effect of ERP exposure on subsequent recall performance (Carver & Bauer, 1999; Carver et al., 2000).
Data Reduction and Analysis
Behavioral sessions were videotaped and scored off-line by experienced observers masked in regard to group status and unaware of the hypotheses of the study. Production of both individual items (maximum = 9 per sequence) and pairs of items in the correct temporal order (maximum = 8 per sequence) were assessed for each sequence at baseline, immediate, and one-week delayed recall. Only the first production of each individual item was recorded to reduce the possibility of successful reproduction of order information due to trial and error (Bauer, Wenner, Dropik, & Wewerka, 2000). Twenty-five percent of the tapes were re-coded by a second experienced observer to ensure reliability. Inter-rater reliability for behavioral coding was 93.2%. Coding disagreements were resolved by discussion.
Electrophysiological data were evaluated automatically using ERPw version 3.2 (Pearson Technical Software, Minneapolis, MN). Artifacts due to eye movements or blinks were corrected using a computerized algorithm (Gratton, Coles, & Donchin, 1983). Following this procedure, data were excluded if the EEG signal exceeded +/−150 μV in any 100 ms window. Individual averages were created for each condition (familiar, novel) and sequence type (six, three, or zero enabling relations) with the constraint that an equal number of trials were included for each of the six conditions (M = 16 trials, SD = 4 per condition). Based on previous reports, two components of the ERP response were identified for analysis (de Haan, 2007; DeBoer et al., 2005). First was the well-defined, negative amplitude, middle latency component (occurring 400–600 ms after stimulus onset). This component has been related to attentional processes (Courchesne, Granz, & Norcia, 1981; Nelson & Collins, 1991) and modulated by memory (Bauer et al., 2006; Bauer, Wiebe, Carver, Waters, & Nelson, 2003; Carver et al., 2000; de Haan & Nelson, 1997). A recent study using source analysis in infants (Reynolds & Richards, 2005) indicated that this component may be generated by areas within the prefrontal and frontal pole regions (see de Haan, 2007 for discussion). In the present investigation, both the maximum negative amplitude and latency to peak amplitude were calculated and used as the dependent measures. The second ERP component occurred later in the waveform (approximately 900 ms after stimulus onset), was positive in amplitude, relatively more distributed over a 500–600ms window, and has been referred to as positive slow wave activity or PSW (Nelson, 1994). PSW is typically thought to be invoked by stimuli that have been seen previously and have been partially encoded. In a recent study with 3- and 4-year-old children, PSW was associated with recollective processes that are hippocampally mediated (Riggins et al., 2009). Consistent with this suggestion, results from source analysis have linked PSW activity to right temporal regions (Reynolds & Richards, 2005). Area under the curve was calculated and used as the dependent measure for this component of interest. To examine laterality differences in both components, midline and lateral leads were analyzed separately.
Results
Behavioral Analyses
Memory for individual items
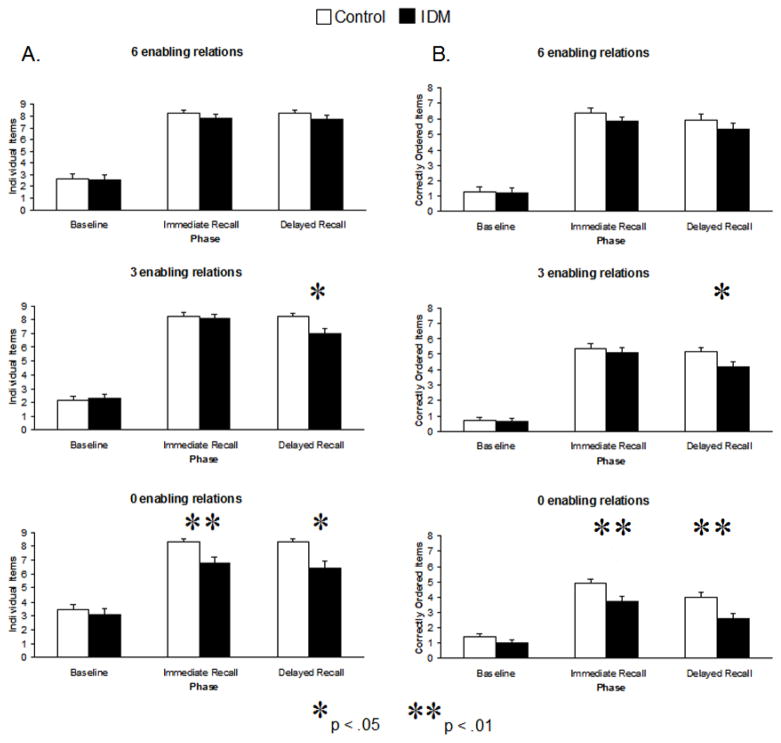
Mean numbers of individual items produced by each group are displayed in Figure 1A. Performance was examined using a 3 Phase (baseline, immediate recall, delayed recall) × 3 Sequence Type (6 enabling relations, 3 enabling relations, 0 enabling relations) repeated measures ANOVA with group (IDM, control) as a between subjects factor. This analysis revealed significant main effects of phase, F(2, 70) = 502.72, p < .001, ηp2 = .94 and group, F(1, 35) = 8.65, p < .01, ηp2 = .20, which were qualified by significant interactions between phase and sequence type, F(4, 140) = 5.38, p < .01, ηp2 = .13, phase and group, F(2, 70) = 4.67, p < .05, ηp2 = .12, and sequence type and group F(2, 70) = 4.22, p < .05, ηp2 = .11. Descriptions of the main effects and follow-up tests used to examine significant interactions are provided below.
Figure 1.
Mean number of (A) individual items and (B) correctly ordered items (+1 SEM) produced at all three phases for both controls (open bars) and IDMs (colored bars) for sequences with six, three, and zero enabling relations. * p<.05
All children produced more actions at both immediate and delayed recall compared to baseline. Follow-up analyses indicated that although performance during baseline was low for all sequence types (M = 2 individual items out of 9 possible), there was a greater production of individual items for the sequences with zero enabling relations compared to the sequences with three enabling relations across groups, F(2, 70) = 4.49, p < .05, ηp2 = .11. Thus, baseline measures were entered as a covariate in all follow-up tests examining immediate and delayed recall of individual items. These analyses revealed that immediate recall varied as a function of group, F(1, 32) = 5.25, p < .05, ηp2 = .14, and sequence type, F(2, 64) = 3.98, p = .05, ηp2 = .09. Although both groups learned the sequences with six and three enabling relations with equal proficiency, immediately after modeling IDMs produced fewer individual items on the sequences with zero enabling relations compared to the control group, F(1, 34) = 9.19, p < .01, ηp2 = .21, suggesting a deficit in encoding processes. Delayed recall also varied as a function of group, F(1, 32) = 12.59, p = .001, ηp2 = .28 and sequence type, F(2, 64) = 3.84, p < .05, ηp2 = .11. Follow-up analyses indicated that recall of items for sequences with three and zero enabling relations was lower in IDMs compared to controls, F (1, 34) = 5.48, 14.64 respectively, ps < .05, ηp2 = .14 and .30. The group difference for recall of items for sequences with zero enabling relations remained even when controlling for differences in both baseline and immediate recall performance, F (1, 33) = 5.70, p <.05, ηp2 = .15.
Together, these results suggest a deficit in IDM’s memory performance for event sequences with both three and zero enabling relations. For sequences with three enabling relations, IDMs and controls displayed similar levels of recall immediately after modeling, indicative of similar levels of encoding. However, when required to recall the sequences after a one-week delay, IDMs produced fewer individual items than controls, a pattern indicative of forgetting. For sequences with zero enabling relations, IDMs learned fewer items than controls, suggesting diminished encoding. IDMs also recalled fewer individual items than controls after the one-week delay, a result that remained significant even after controlling for differences in encoding (i.e., immediate recall). Finally, for sequences with six enabling relations, there were no differences between controls and IDMs memory performance.
Memory for items in the correct temporal order
Mean number of correctly ordered items are displayed in Figure 1B. Performance was examined using a 3 Phase (baseline, immediate recall, delayed recall) × 3 Sequence Type (6 enabling relations, 3 enabling relations, 0 enabling relations) repeated measures ANOVA with group (IDM, control) as a between subjects factor. This analysis revealed significant main effects of phase, F(2, 70) = 319.61, p < .001, ηp2 = .90, sequence type, F(2, 70) = 30.21, p < .001, ηp2 = .46, and group, F(1, 35) = 10.38, p < .05, ηp2 = .23, which was qualified by significant interactions between phase and sequence type, F(4, 140) = 12.18, p < .001, ηp2 = .26. Descriptions of the main effects and follow-up tests used to examine significant interactions are provided below.
All children learned the order of the sequences within the lab setting, and remembered the order across the delay. As predicted, ordered recall was highest for sequences with six enabling relations, followed by sequences with three and lastly zero enabling relations. In addition, ordered recall in the IDM group was lower than the control group. This group difference was observed at both immediate and delayed recall assessments, F(1, 35) = 5.34, p < .05, ηp2 = .13 and F(1, 35) = 7.51, p = .01, ηp2 = .17 respectively, but not at baseline. At the immediate recall assessment, IDMs produced fewer correctly ordered items than controls on sequences with zero enabling relations, F(1, 35) = 7.48, p = .01, η2 = .18. At the delayed recall assessment, IDMs produced fewer correctly ordered items than controls on both sequences with three and zero enabling relations, F(1, 35) = 4.44, p < .05, η2 = .11 and F(1, 35) = 8.66, p = .01, η2 = .19.
Finally, to examine whether differences in ordered recall were solely attributed to differences in production of individual items, analyses were repeated with memory for individual items as a covariate. In the context of this analysis, there were no differences in production of correctly ordered items as a function of sequence type or group. Thus, it is not possible in the present report to separate the unique effects of differences in memory for individual items and differences in memory for temporal order.
Associations between Behavioral Recall and Perinatal Measures
We then examined whether any of the perinatal characteristics were predictive of performance on the behavioral measures of memory. A series of correlational analyses were conducted between newborn indices of risk and recall performance across both groups. Ferritin levels from cord serum at birth were correlated with performance on the imitation task. Across all sequence types, ferritin was related to both immediate and one-week delayed recall of individual items (immediate: r(33) = .51, p < .01, delayed: r(34) = .40, p < .01), and immediate ordered recall, r(33) = .52, p < .01. These positive correlations suggest that, regardless of group status, lower ferritin levels from cord serum at birth are associated with worse memory performance at 3 1/2 years of age. Birthweight z-scores were not related to performance on the task.
ERP Analyses
Midline leads - Fz, Cz, Pz
Three dependent variables: peak amplitude of the middle latency component, latency to the peak amplitude of the middle latency component, and area under the curve for the slow wave activity were analyzed for each sequence type at the midline leads using a 2 condition (novel, familiar) × 2 hemisphere (left, right) × 3 lead (Fz, Cz, Pz) repeated measures ANOVA with group (IDM, control) as a between subjects factor. Results from analyses conducted with average amplitude of the middle latency component were similar to results with peak amplitude; due to space considerations, only the latter are reported. On the basis of results from the elicited/delayed imitation analyses, ERP data from each sequence type were analyzed separately; results are provided. Results that were similar across all three sequence types are presented first, followed, in order, by specific results for sequences with six, three, and zero enabling relations.
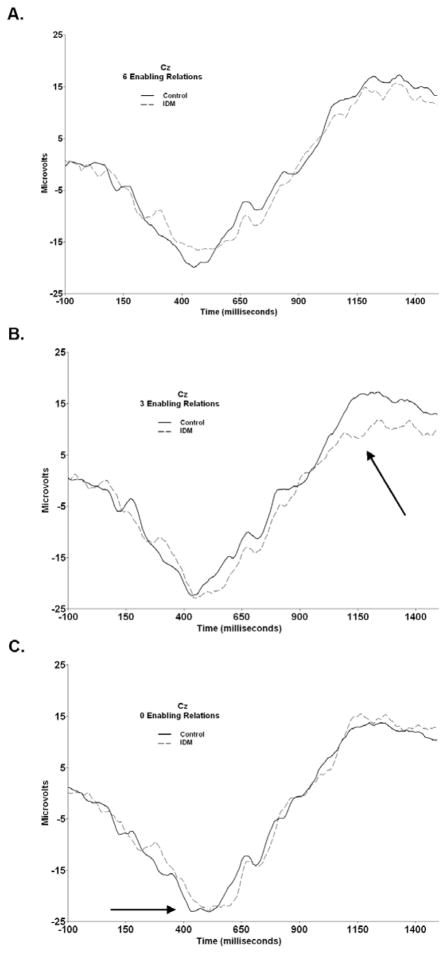
Figure 2 illustrates average ERPs for both groups for each sequence type. Consistent with previous reports, ERP responses were greatest over frontal/central midline leads, indicating a fronto-central distribution of the middle-latency component for all sequence types: six enabling relations, F(2, 62) = 23.30, p < .001, ηp2 = .43, three enabling relations, F(2, 62) = 22.34, p < .001, ηp2 = .42, and zero enabling relations: F(2, 62) = 28.23, p < .001, ηp2 = .48. Analyses of sequences with six enabling relations revealed no significant effects as a function of group. Sequences with three enabling relations revealed group differences in positive slow wave activity, F(1, 31) = 4.52, p < .05, ηp2 = .13. Across the three midline leads, controls (M = 6762.55, SD = 995.90) evidenced greater positive slow wave activity than IDMs (M = 3707.51, SD = 1235.27). Sequences with zero enabling relations showed group differences in latency to peak of the middle latency component, F(1, 31) = 4.94, p < .05, ηp2 = .14. Latency to peak was shorter in the control group (M = 475.25ms, SD = 55.48ms) than in the IDM group (M = 502.67, SD = 60.38).
Figure 2.
ERP waveforms recorded from the vertex for both control (solid) and IDM (dashed) groups as a function of sequence type (as determined by internal organization). Arrows indicate significant group difference.
Lateral leads
Based on the distribution of the components, parallel analyses using a 2 condition (novel, familiar) × 2 hemisphere (left, right) repeated measures ANOVA with group (IDM, control) as a between subjects factor were computed for two groupings of fronto-central lateral leads (Left fronto-central: F7, F3, Fc5, Fc1, C3, Right fronto-central: F8, F4, Fc6, Fc2, C4). The same dependent measures were used (peak amplitude of the middle latency component, latency to the peak amplitude of the middle latency component, and area under the curve for the slow wave activity) and each sequence type was analyzed separately. Results that were similar across all three sequence types are presented first, followed, in order, by specific results for sequences with six, three, and zero enabling relations.
For all three sequence types, peak amplitude of the middle latency component was greater over the right compared to the left hemisphere indicating a right hemisphere bias for the middle latency component (six enabling relations: right M = −29.51μV, SD = 9.88μV, left M = −24.17μV, SD = 8.97μV, F(1,31) = 28.45, p < .001, ηp2 = .48; three enabling relations right M = −28.26μV, SD = 8.39μV, left M = −21.81μV, SD = 10.19μV, F(1,31) = 32.56, p < .001, ηp2 = .51; zero enabling relations right M = −26.30μV, SD = 10.94μV, left M = −22.15μV, SD = 10.02μV), F(1,31) = 14.28, p = .001, ηp2 = .32.
There were no significant effects of group or condition for event sequences with six enabling relations. For sequences with three enabling relations, there was a trend toward a group by condition interaction for positive slow wave activity, F(1, 31) = 3.82, p = .06,, ηp2 = .11. In the right hemisphere, positive slow wave activity was greater for familiar (M = 6937.89 μV/ms, SD = 5622.83) compared to novel sequences (M = 4739.34 μV/ms, SD = 6859.29). However, in the left hemisphere, positive slow wave activity was similar for familiar (M = 6593.34 μV/ms, SD = 4728.21) and novel sequences (M = 6178.79 μV/ms, SD = 6125.91). Although this finding does not meet conventional levels of statistical significance, it is included in this report as it is consistent with 1) previous reports (see Riggins et al., 2009), and 2) is similar to the group difference reported below for sequences with zero enabling relations.
For sequences with zero enabling relations, positive slow wave activity varied as a function of condition and hemisphere F(1, 31) = 4.32, p = .05, ηp2 = .12. Similar to the trend reported for sequences with three enabling relations, in the right hemisphere, positive slow wave activity was greater for familiar (M = 6796.50 μV/ms, SD = 6572.86) compared to novel sequences (M = 5488.29μV/ms, SD = 5446.38). However, in the left hemisphere, positive slow wave activity was similar for familiar (M = 5878.46μV/ms, SD = 5455.69) and novel sequences (M = 6072.53 μV/ms, SD = 4498.52). ERP responses to sequences with zero enabling relations also revealed a main effect of condition for peak amplitude of the middle latency component; across the lateral leads, peak amplitude to novel stimuli (M = −26.16μV, SD = 9.99μV) was greater than peak amplitude to familiar stimuli (M = −22.28μV, SD = 10.96μV), F(1, 31) = 5.02, p < .05, ηp2 = .14.
Correlations between Behavioral Recall and ERPs
In order to determine how differences in behavioral recall of the event sequences related to processing at the neural level, a series of correlational analyses were conducted. Statistical tests were constrained by previous findings (Bauer et al., 2006; Bauer et al., 2003; Riggins et al., 2009) and only included behavioral measures that showed differences between the two groups in memory performance and ERP measures at the vertex (where activity was maximal). Immediate recall of individual items (i.e., encoding) predicted latency to peak of the middle latency component to novel stimuli, r (31) = −.35, p = .05. PSW to novel stimuli was related to behavioral recall of individual items after the one-week delay, r (32) = .37, p < .05. Thus, longer latency to peak of the middle latency component to novel stimuli was associated with reduced levels of encoding of the individual items at Session 1; decreased PSW activity (to novel stimuli) was related to reductions in processing of previously encoded material, which may have contributed to lower levels of behavioral recall at Session 2. These results are similar to previous reports (Bauer et al., 2006; Bauer et al., 2003; Riggins et al., 2009) and correspond with group difference findings from the ERP analyses. Specifically, IDMs evidenced longer latencies and less PSW activity in comparison with controls, which suggests impairments in both encoding and subsequent recollective processes.
Discussion
Results from the present investigation extend previous research in several ways. First, we report long-term impairments in recall memory in IDMs during early childhood, years after initial risk factors have resolved. Previous investigations used global behavioral outcome measures to assess development of IDMs long-term (Rizzo, Metzger, Burns, & K, 1991; Rizzo, Metzger, Dooley, & Cho, 1997). In order to further explicate the neurocognitive aspects underlying these findings, behavioral and electrophysiological assays were used to assess the functioning of circuitry that has been shown to be altered at the genomic, biochemical, structural, and electrophysiological levels in rodent models of gestational iron deficiency (Carlson et al., 2007; de Ungria et al., 2000; Jorgenson et al., 2005; Jorgenson et al., 2003; Rao et al., 2003). Critically, the observed deficits in our study were related to the extent of iron deficiency experienced prenatally (as indexed by serum ferritin concentrations in cord blood at birth). Although a similar association has been reported in human infants at 12 months of age, the current finding is unique in that it demonstrates the persisting effects of neonatal iron status on hippocampally-based memory circuits and behavior into childhood. This finding highlights the importance of the prenatal environment for subsequent memory performance and provides empirical support for the connection between iron deficiency during the prenatal period, the development of memory circuitry, and behavioral recall performance well downstream from the proposed insult in humans.
Second, based on findings from the combined use of behavioral and electrophysiological measures, we can also begin to elaborate on the nature of these deficits both in terms of extent and underlying mechanisms. Consistent with animal models, the observed deficits are not absolute (Schmidt et al., 2007), but rather result in slight dysfunctions in performance that can be overcome through enabling the learning process (Fuglestad, Rao, & Georgieff, 2008). When cognitive demands were greatest (i.e., zero enabling relations between items), impairments occurred at the initial stage of memory processing, during encoding, which was reflected by both behavioral (i.e., immediate imitation) and electrophysiological (i.e., latency to peak of the middle-latency component) measures. In contrast, when cognitive demands were moderate (three enabling relations between items), IDMs were able to successfully encode items in the event sequences; however, they were not able to recall these items after the one-week delay. This difference was also apparent in the ERP response in the form of decreased positive slow wave activity, which is thought to reflect hippocampally-mediated recollective processes (Riggins et al., 2009; Reynolds & Richards, 2005). This finding is also consistent with animal models of gestational iron deficiency that indicate the largest metabolic defects occur in the hippocampus and the prefrontal cortex, areas that are critical for recollection (de Ungria et al., 2000). Finally, when memory demands were low (six enabling relations between items), there were no observable differences in performance of IDMs and controls, at either behavioral or electrophysiological levels, indicating that IDMs clearly could perform the task when external support for the to-be-remembered items was high. Thus, the dysfunction appears to be modulated by the nature and extent of the demands placed on the circuitry (cf. de Haan, Mishkin, Baldeweg, & Vargha-Khadem, 2006).
The extent of IDMs’ reduced capacity is likely a function of the timing and degree of the initial insult, the swiftness of resolution as it relates to sensitive periods of development, and the subsequent remaining plasticity of the system (Fuglestad et al., 2008; Kretchmer, Beard, & Carlson, 1996). The hippocampus, which is central to recognition and recall memory function, develops rapidly during late fetal life, is highly dependent on adequate iron status for its development, and is vulnerable to the metabolic insults extant in diabetic pregnancies: iron deficiency, chronic hypoxia and hypoglycemia. These alterations in the prenatal metabolic environment result directly from the lack of maternal glycemic control (Georgieff, 2006; Nold & Georgieff, 2004). Therefore, one implication from these findings is that prevention is essential. If diabetes and maternal glucose levels are well controlled, we do not predict that the cascade of events described will occur and risk will be minimal. However, when prevention is not possible, findings in other cognitive domains suggest that early intervention is likely the best course of action (Shonkoff & Phillips, 2000). Early intervention is predicated on early identification, and our work collectively demonstrates that we can identify very early in life individuals who might be at risk for memory impairments using proxy measures of the metabolic prenatal environment, such as neonatal or cord blood ferritin, to estimate the extent of iron deficiency and the likelihood of a long-term impact on neurobehavioral development.
The subsequent step would be to develop targeted interventions to be administered early in development. Regions of the hippocampus continue to be remolded throughout the life span, largely through experience-dependent synaptic plasticity (see Markham & Greenough, 2004 and Nelson, 2000 for elaboration). Our results suggest that strategies that enable cognitive performance may assist high-risk children later in development. Specifically, when the internal structure of the event sequences was high, memory demands were low and IDM performance was comparable to that of the control group. This finding is similar to a recent report examining effects of iron deficiency in a rodent model (Carlson et al., in press). In this investigation, iron-deficient mice were able to solve the Morris water maze when specific enabling supports were in place (e.g., using a non-moving platform in the target quadrant, or using a larger platform; Carlson et al., in press). Thus, learning and recall in IDMs can, on some level, be supported externally and brought to levels similar to that of controls, which is a potentially important strategy for teachers and parents of such children. These supports may include providing additional time to encode and retrieve information or informational support platforms to enhance specific learning. Factors that have been found to facilitate recall in typically developing infants and young children include repeated experience with events prior to imposition of a delay (Bauer, Wiebe, Waters, & Bankston, 2001) and the use of verbal and nonverbal reminders at the time of recall (Bauer et al., 2000). These findings may be incorporated into the development of specific interventions in collaboration with pediatric neuropsychologist and educators. However, at present, no such interventions exist.
Beyond the clinical implications, these findings contribute to our understanding of the development of the neural bases of memory. The concurrent use of behavioral and electrophysiological methods allowed us to elucidate more specific circuit-function relationships underlying these memory impairments (Bauer et al., 2006; Bauer et al., 2003; Carver et al., 2000; Nelson, 1994), and suggest that early hippocampal damage results in both encoding and retrieval deficits. This conclusion remains tentative, however, as the true extent of damage to the hippocampus is unknown and likely varies within the IDM group. In addition, although the sample is relatively well-characterized, the somewhat small sample size precluded multivariate analysis of effects of all possible outcome variables and interactions with environmental factors (e.g., socioeconomic status). Future investigations should attempt to address these questions. We strongly recommend the use of ERP measures in future investigations as they appear sensitive to differences in underlying neural circuitry and can be used to further probe differences in cognitive processing. Moreover, these methods can be used in the first days of life when behavioral assessments of cognition are limited (deRegnier et al., 2000; Nelson, 2007; Nelson et al., 2000).
Given the known pathophysiology associated with the diabetic pregnancy, our findings support the hypothesis that iron deficiency during the prenatal period leads to alterations in neural development and has a lasting negative impact on memory ability. These impairments vary as a function of the extent of iron deficiency experienced during the prenatal period. Follow-up of this group is currently underway to confirm the presence, degree, and selectivity of hippocampal damage using magnetic resonance imaging techniques (Nelson, 2007). We will continue to assess whether the observed impairments reported here fade with further development of the hippocampus and its associated memory system, or if other protective developmental factors will be revealed as we continue to track this sample over the next few years.
Acknowledgments
The authors thank Jennifer Haight, Lindsay Lewis, and the members of the Cognition in Transition Laboratory for their assistance in coding the elicited imitation data, the members of the Center for Neurobehavioral Development for their assistance with data collection, Sandi Wewerka for assistance with study design, and the children and families who participated in the study. This research was conducted at the Center for Neurobehavioral Development at the University of Minnesota and was supported by grants from NIH to Charles A. Nelson (NS34458) and Michael K. Georgieff (HD29421), a grant from the NICHD to Patricia J. Bauer (HD28425), and a grant from the NIH National Center for Research Resources (RR00400).
Footnotes
Portions of these data were presented at the meeting of the Society for Research Child Development in Boston, MA, March 2007 and in Riggins et al., 2009.
References
- Adlam AL, Vargha-Khadem F, Mishkin M, de Haan M. Deferred imitation of action sequences in developmental amnesia. Journal of Cognitive Neuroscience. 2005;17:240–248. doi: 10.1162/0898929053124901. [DOI] [PubMed] [Google Scholar]
- Akin M, Ceran O, Atay E, Atay Z, Akin F, Akturk Z. Postpartum maternal levels of hemoglobin A1c and cord C-peptide in macrosomic infants of non-diabetic mothers. Journal of Maternal-Fetal and Neonatal Medicine. 2002;12:274–276. doi: 10.1080/jmf.12.4.274.276. [DOI] [PubMed] [Google Scholar]
- Algarin C, Peirano P, Garrido M, Pizarro F, Lozoff B. Iron deficiency anemia in infancy: Long-lasting effects on auditory and visual system functioning. Pediatric Research. 2003;53(2):217–223. doi: 10.1203/01.PDR.0000047657.23156.55. [DOI] [PubMed] [Google Scholar]
- Bauer PJ. Holding it all together: How enabling relations facilitate young children’s event recall. Cognitive Development. 1992;7:1–28. [Google Scholar]
- Bauer PJ, Hertsgaard LA, Dropik P, Daly BP. When even arbitrary order becomes important: Developments in reliable temporal sequencing of arbitrarily ordered events. Memory. 1998;6:165–198. doi: 10.1080/741942074. [DOI] [PubMed] [Google Scholar]
- Bauer PJ, Wiebe SA, Carver LJ, Lukowski AF, Haight JC, Waters JM, et al. Electrophysiological indices of encoding and behavioral indices of recall: Examining relations and developmental change late in the first year of life. Developmental Neuropsychology. 2006;29:293–320. doi: 10.1207/s15326942dn2902_2. [DOI] [PubMed] [Google Scholar]
- Bauer PJ, Wiebe SA, Carver LJ, Waters JM, Nelson CA. Developments in long-term explicit memory late in the first year of life: Behavioral and electrophysiological indices. Psychological Science. 2003;14:629–635. doi: 10.1046/j.0956-7976.2003.psci_1476.x. [DOI] [PubMed] [Google Scholar]
- Bauer PJ, Wiebe SA, Waters JM, Bankston SK. Reexposure breeds recall: Effects of experience on 9-month-olds’ ordered recall. Journal of Experimental Child Psychology. 2001;80(2):174–200. doi: 10.1006/jecp.2000.2628. [DOI] [PubMed] [Google Scholar]
- Beard JL, de Regnier RA, Shaw M, Rao R, Georgieff MK. Diagnosis of iron deficiency in infants. Lab Medicine. 2007;38:103–108. [Google Scholar]
- Carlson ES, Stead JD, Neal CR, Petryk A, Georgieff MK. Perinatal iron deficiency results in altered developmental expression of genes mediating energy metabolism and neuronal morphogenesis in hippocampus. Hippocampus. 2007;17(8):679–691. doi: 10.1002/hipo.20307. [DOI] [PubMed] [Google Scholar]
- Carlson ES, Tkac I, Magid R, ‘Connor MB, Nadrews NC, Schallert T, et al. Iron is essential for neuron development and memory function in mouse hippocampus. Journal of Nutrition. doi: 10.3945/jn.108.096354. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver LJ, Bauer PJ. When the event is more than the sum of its parts: Nine-month-olds’ long-term ordered recall. Memory. 1999;7:147–174. doi: 10.1080/741944070. [DOI] [PubMed] [Google Scholar]
- Carver LJ, Bauer PJ, Nelson CA. Associations between infant brain activity and recall memory. Developmental Science. 2000;3:234–246. [Google Scholar]
- Courchesne E, Granz L, Norcia AM. Event-related brain potentials to human faces in infants. Child Development. 1981;52:804–811. [PubMed] [Google Scholar]
- de Haan M. Visual attention and recognition memory in infancy. In: de Haan M, editor. Infant EEG and Event-Related Potentials. East Sussex: Psychology Press; 2007. pp. 101–144. [Google Scholar]
- de Haan M, Mishkin M, Baldeweg T, Vargha-Khadem F. Human memory development and its dysfunction after early hippocampal injury. Trends in Neurosciences. 2006;29(7):374–381. doi: 10.1016/j.tins.2006.05.008. [DOI] [PubMed] [Google Scholar]
- de Haan M, Nelson CA. Recognition of the mother’s face by 6-month-old infants: A neurobehavioral study. Child Development. 1997;68:187–210. [PubMed] [Google Scholar]
- de Ungria M, Rao R, Wobken JD, Luciana M, Nelson CA, Georgieff MK. Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selective regions of neonatal rat brain. Pediatric Research. 2000;48:169–176. doi: 10.1203/00006450-200008000-00009. [DOI] [PubMed] [Google Scholar]
- DeBoer T, Miller NC, Bauer PJ, Georgieff MK, Nelson CA. Electrophysiological indices of memory for temporal order in early childhood: Implications for the development of recollection. Developmental Science. doi: 10.1111/j.1467-7687.2008.00757.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBoer T, Scott LS, Nelson CA. Event-related potentials in developmental populations. In: Handy T, editor. Methodological Handbook for Research Using Event-related Potentials. Cambridge, MA: The MIT Press; 2005. pp. 263–297. [Google Scholar]
- DeBoer T, Wewerka S, Bauer PJ, Georgieff MK, Nelson CA. Neurobehavioral sequelae of infants of diabetic mothers: Deficits in explicit memory at 1 year of age. Developmental Medicine and Child Neurology. 2005;47:525–531. doi: 10.1017/s0012162205001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deRegnier RA, Nelson CA, Thomas K, Wewerka S, Georgieff MK. Neurophysiologic evaluation of auditory recognition memory in healthy newborn infants and infants of diabetic mothers. Journal of Pediatrics. 2000;137:777–784. doi: 10.1067/mpd.2000.109149. [DOI] [PubMed] [Google Scholar]
- Fuglestad AJ, Rao R, Georgieff MK. The role of nutrition in cognitive development. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. 2. Cambridge, MA: MIT Press; 2008. [Google Scholar]
- Georgieff MK. The effect of maternal diabetes during pregnancy on the neurodevelopment of offspring. Minnesota Medical Association, Minnesota Medicine. 2006;89 [PubMed] [Google Scholar]
- Georgieff MK, Landon MB, Mills MM, Hedlund BE, Faassen AE, Schmidt RL, et al. Abnormal iron distribution in infants of diabetic mothers: Spectrum and maternal antecedents. Journal of Pediatrics. 1990;117:455–461. doi: 10.1016/s0022-3476(05)81097-2. [DOI] [PubMed] [Google Scholar]
- Georgieff MK, Wewerka SW, Nelson CA, deRegnier RA. Follow-up iron status of infants with low iron stores at birth. Journal of Pediatrics. 2002;141:405–409. doi: 10.1067/mpd.2002.127090. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. A new method of off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Jorgenson LA, Sun M, O’Connor M, Georgieff MK. Fetal iron deficiency disrupts the maturation of synaptic function and efficacy in area CA1 of the developing rat hippocampus. Hippocampus. 2005;15(18):1094–1102. doi: 10.1002/hipo.20128. [DOI] [PubMed] [Google Scholar]
- Jorgenson LA, Wobken JD, Georgieff MK. Perinatal iron deficiency alters apical dendritic growth in hippocampal CA-1 pyramidal neurons. Developmental Neuroscience. 2003;25:412–420. doi: 10.1159/000075667. [DOI] [PubMed] [Google Scholar]
- Kretchmer N, Beard JL, Carlson S. The role of nutrition in the development of normal cognition. American Journal of Clinical Nutrition. 1996;63(6):997S–1001S. doi: 10.1093/ajcn/63.6.997. [DOI] [PubMed] [Google Scholar]
- Lozoff B, Corapci F, Burden MJ, Kaciroti N, Angulo-Barroso R, Sazawal S, et al. Preschool-aged children with iron deficiency anemia show altered affect and behavior. Journal of Nutrition. 2007;137:683–689. doi: 10.1093/jn/137.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B, Georgieff MK. Iron deficiency and brain development. Seminars in Pediatric Neurology. 2006;13:158–165. doi: 10.1016/j.spen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Lozoff B, Jimenez E, Smith JB. Double burden of iron deficiency in infancy and low socioeconomic status: A longitudidnal analysis of cognitive test scores to age 19 years. Archives of Pediatrics & Adolescent Medicine. 2006;160(11):1108–1113. doi: 10.1001/archpedi.160.11.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Greenough WT. Experience-driven brain plasticity: Beyond the synapse. Neuron Glia Biology. 2004;1(4):351–363. doi: 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough L, Mandler JM, McKee RD, Squire LR. The deferred imitation task as a nonverbal measure of declarative memory. Proceedings of the National Academy of Sciences. 1995;92:7580–7584. doi: 10.1073/pnas.92.16.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEchron MD, Cheng AY, Liu H, Connor JR, Gilmartin MR. Perinatal nutritional iron deficiency permanently impairs hippocampus-dependent trace fear conditioning in rats. Nutrition Neuroscience. 2005;8(3):195–206. doi: 10.1080/10284150500162952. [DOI] [PubMed] [Google Scholar]
- Morris MA, Grandis AS, Litton JC. Glycosylated hemoglobin concentration in early gestation associated with neonatal outcome. American Journal of Obstetrics & Gynecology. 1985;153:651–654. doi: 10.1016/s0002-9378(85)80253-2. [DOI] [PubMed] [Google Scholar]
- Nelson CA. Neural correlates of recognition memory in the first postnatal year of life. In: Dawson G, Fischer K, editors. Human Behavior and the Developing Brain. New York: Guilford Press; 1994. pp. 269–313. [Google Scholar]
- Nelson CA. Neural plasticity and human development: the role of early experience in sculpting memory systems. Developmental Science. 2000;3(2):115–136. [Google Scholar]
- Nelson CA. A developmental cognitive neuroscience approach to the study of atypical development: A model system involving infants of diabetic mothers. In: Dawson G, Fischer K, Coch D, editors. Human behavior and the developing brain. 2. New York: Guilford Press; 2007. [Google Scholar]
- Nelson CA, Collins PF. Event-related potential and looking time analysis of infants’ responses to familiar and novel events: Implications for visual recognition memory. Developmental Psychology. 1991;27:50–58. [Google Scholar]
- Nelson CA, Wewerka S, Thomas KM, Tribby-Walbridge S, deRegnier RA, Georgieff MK. Neurocognitive sequelae of Infants of Diabetic Mothers. Behavioral Neuroscience. 2000;114:950–956. [PubMed] [Google Scholar]
- Nelson CA, Wewerka SS, Borscheid AJ, deRegnier RA, Georgieff MK. Electrophysiologic evidence of impaired cross-modal recognition memory in 8-month-old infants of diabetic mothers. Pediatrics. 2003;142(5):575–582. doi: 10.1067/mpd.2003.210. [DOI] [PubMed] [Google Scholar]
- Nold JL, Georgieff MK. Infants of diabetic mothers. Pediatric Clinics of North America. 2004;51:619–637. doi: 10.1016/j.pcl.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Petry CD, Eaton MA, Wobken JD, Mills MM, Johnson DE, Georgieff MK. Iron deficiency of liver, heart, and brain in newborn infants of diabetic mothers. Journal of Pediatrics. 1992;112:109–114. doi: 10.1016/s0022-3476(05)82554-5. [DOI] [PubMed] [Google Scholar]
- Prechtl HF. Neurologic sequelae of prenatal and perinatal complications. British Medical Journal. 1967;4:763–767. doi: 10.1136/bmj.4.5582.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman L, Hamilton K, Gewirtz J, Rao R. Effects of chronic hypoxia in developing rats on dendritic morphology of the CA1 subarea of the hippocampus and on fear potentiated startle. Brain Research. 2008;1190C:167–174. doi: 10.1016/j.brainres.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman L, Tkac I, Ennis K, Georgieff MK, Gruetter R, Rao R. In vivo effect of chronic hypoxia on the neurochemical profile of the developing rat hippocampus. Developmental Brain Research. 2005;156:202–209. doi: 10.1016/j.devbrainres.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Rao R, de Ungria M, Sullivan D, Wu P, Wobken JD, Nelson CA, et al. Perinatal iron deficiency increases the vulnerability of rat hippocampus to hypoxic ischemic insult. Journal of Nutrition. 1999;129:199–206. doi: 10.1093/jn/129.1.199. [DOI] [PubMed] [Google Scholar]
- Rao R, Tkac I, Townsend EL, Gruetter R, Georgieff MK. Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. Journal of Nutrition. 2003;133:3215–3221. doi: 10.1093/jn/133.10.3215. [DOI] [PubMed] [Google Scholar]
- Reynolds GD, Richards JE. Familiarization, attention, and recognition memory in infancy: An event-related potential and coritcal source localization study. Developmental Psychology. 2005;41(4):598–615. doi: 10.1037/0012-1649.41.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T, Miller NC, Bauer PJ, Georgieff MK, Nelson CA. Electrophysiological indices of memory for temporal order in early childhood: Implications for the development of recollection. Developmental Science. 2009;12(2):209–219. doi: 10.1111/j.1467-7687.2008.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo TA, Metzger BE, Burns WJ, KB Correlations between antepartum maternal metabolism and intelligence in offspring. New England Journal of Medicine. 1991;325:911–916. doi: 10.1056/NEJM199109263251303. [DOI] [PubMed] [Google Scholar]
- Rizzo TA, Metzger BE, Dooley SL, Cho NH. Early malnutrition and child neurobehavioral development: Insights from the study of children of diabetic mothers. Child Development. 1997;68:26–38. [PubMed] [Google Scholar]
- Schmidt AT, Waldow KJ, Grove WM, Salinas JA, Georgieff MK. Dissociating the long-term effects of fetal/neonatal iron deficiency on three types of learning in the rat. Behavioral Neuroscience. 2007;121(3):475–482. doi: 10.1037/0735-7044.121.3.475. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Phillips DA. From neurons to neighborhoods: The science of early childhood development. Washington, D.C: National Academic Press; 2000. [PubMed] [Google Scholar]
- Siddappa AM, Georgieff MK, Wewerka S, Worwa C, Nelson CA, deRegnier RA. Iron deficiency alters auditory recognition memory in newborn infants of diabetic mothers. Pediatric Research. 2004;55(6):1034–1041. doi: 10.1203/01.pdr.0000127021.38207.62. [DOI] [PubMed] [Google Scholar]
- Siddappa AM, Rao R, Long JD, Widness JA, Georgieff MK. The assessment of newborn iron stores at birth: A review of the literature and standards for ferritin concentrations. Neonatology. 2007;92:73–82. doi: 10.1159/000100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T, Goldenberg R, Hou J, Johnston K, Cliver S, Ramey S, et al. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. Journal of Pediatrics. 2002a;140(2):165–170. doi: 10.1067/mpd.2002.120688. [DOI] [PubMed] [Google Scholar]
- Tamura T, Goldenberg R, Hou J, Johnston K, Cliver S, Ramey S, et al. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. Journal of Pediatrics. 2002b;140(2):165–170. doi: 10.1067/mpd.2002.120688. [DOI] [PubMed] [Google Scholar]
- Yamada KA, Rensing N, Izumi Y, De Erausquin GA, Gazit V, Dorsey DA, et al. Repetitive hypoglycemia in young rats impairs hippocampal long-term potentiation. Pediatric Research. 2004;55:372–379. doi: 10.1203/01.PDR.0000110523.07240.C1. [DOI] [PubMed] [Google Scholar]