Abstract
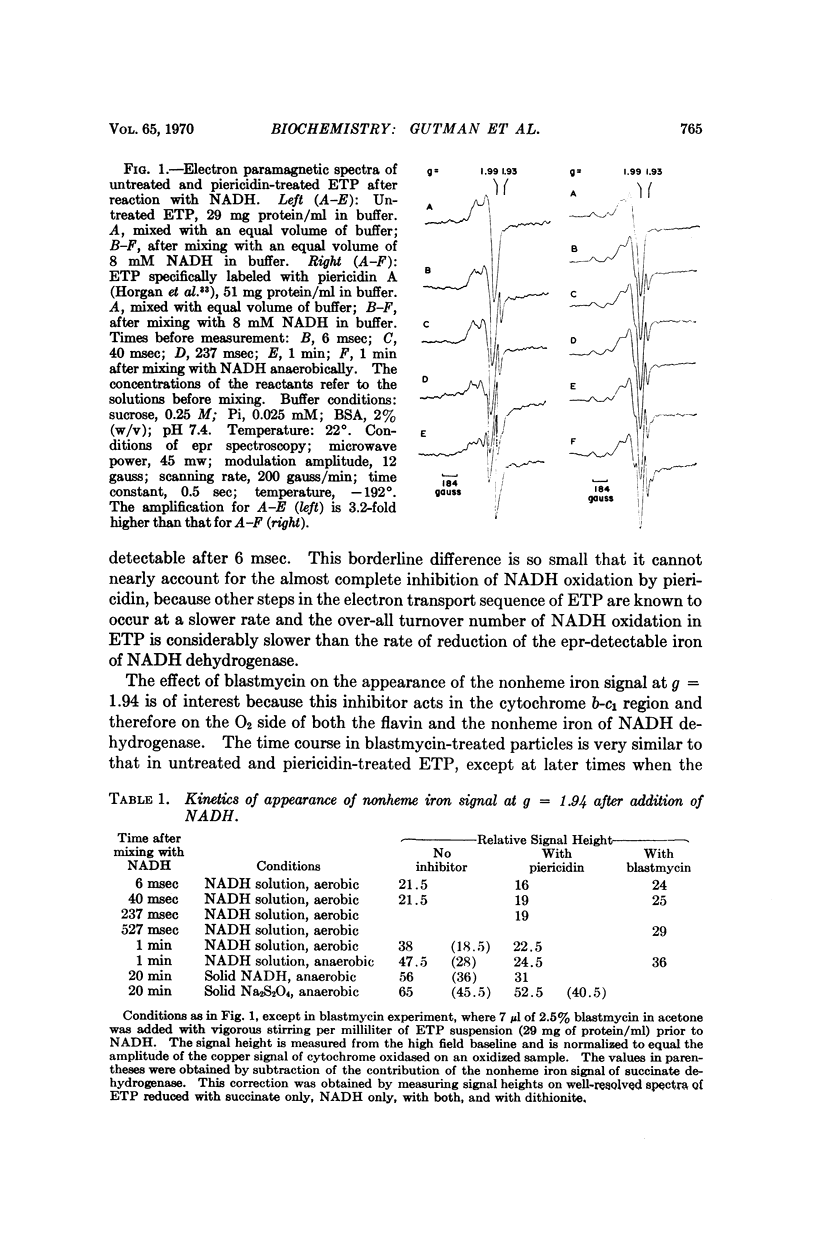
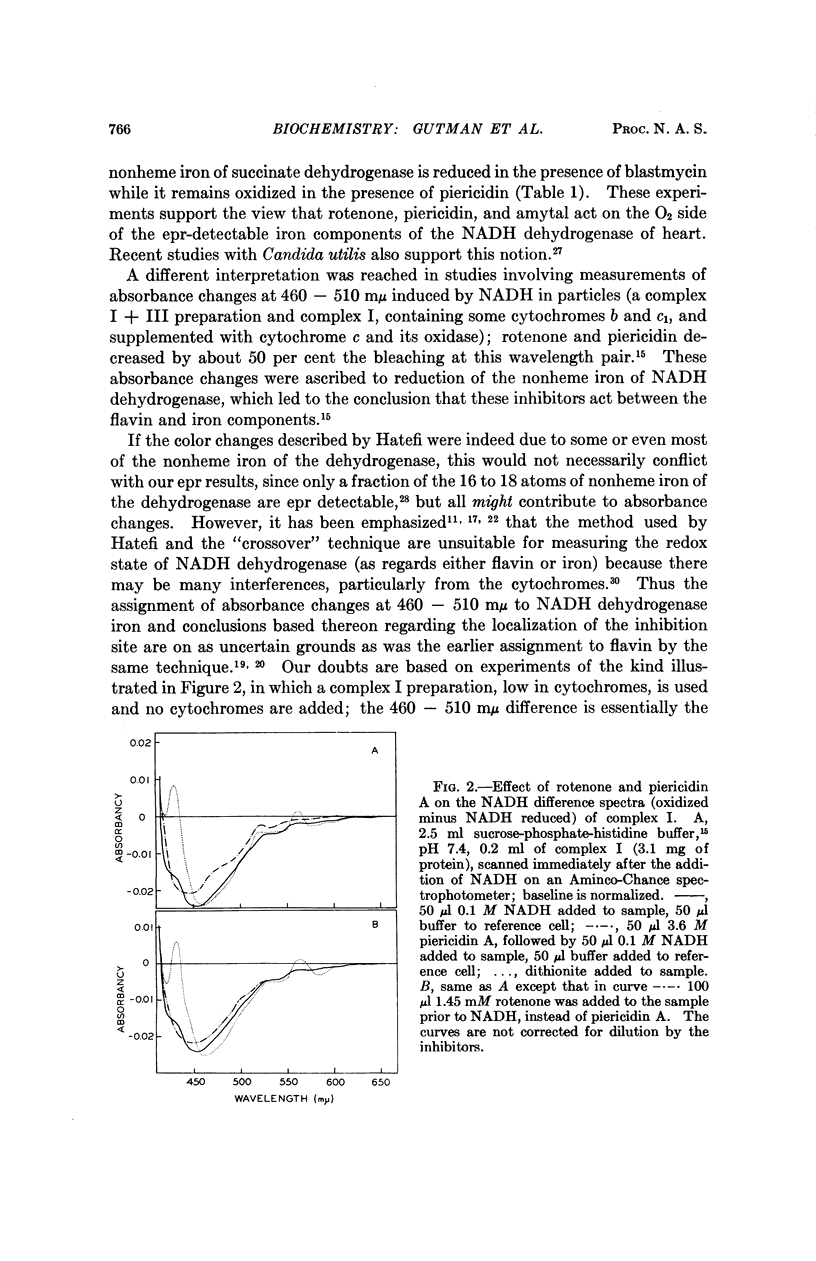
The locus of inhibition of nicotinamide adenine dinucleotide, reduced form (NADH) oxidation in mitochondria by rotenone, piercidin A, and barbiturates is considered in the light of available information. Most lines of evidence indicate that the point of inhibition is on the O2 side of NADH dehydrogenase. Kinetic experiments on the substrate-induced appearance of the electron paramagnetic resonance signal at g = 1.94 in membrane preparations (ETP) reveal that these inhibitors do not interfere with the reduction of the electron paramagnetic resonance detectable iron by NADH. Our spectrophotometric studies on complex I give no evidence for absorbance differences between untreated and rotenone or piericidin inhibited preparations, which can be attributed to nonheme iron. Whatever changes were observed appear to be due to cytochromes. These experiments, therefore, do not support the idea that in inhibited preparations electron transport is interrupted between the flavin and nonheme iron components of NADH dehydrogenase.
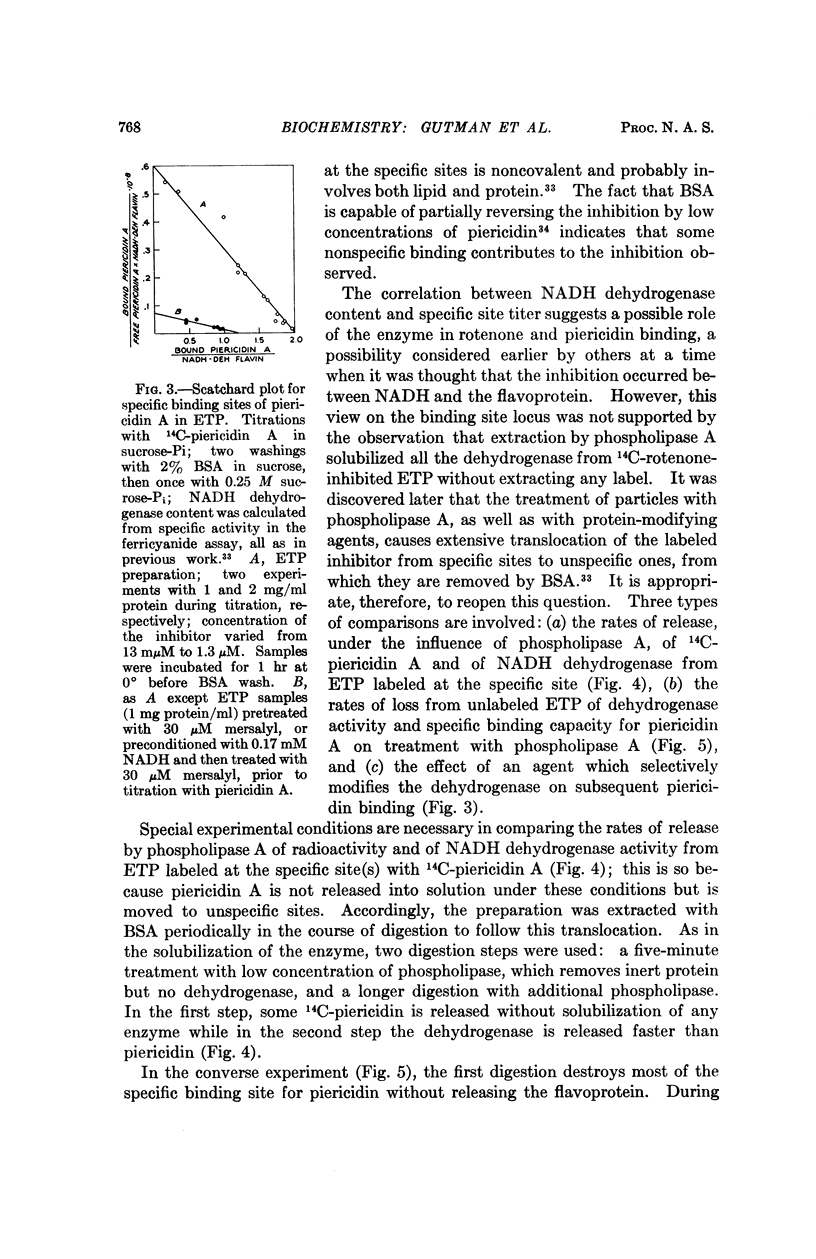
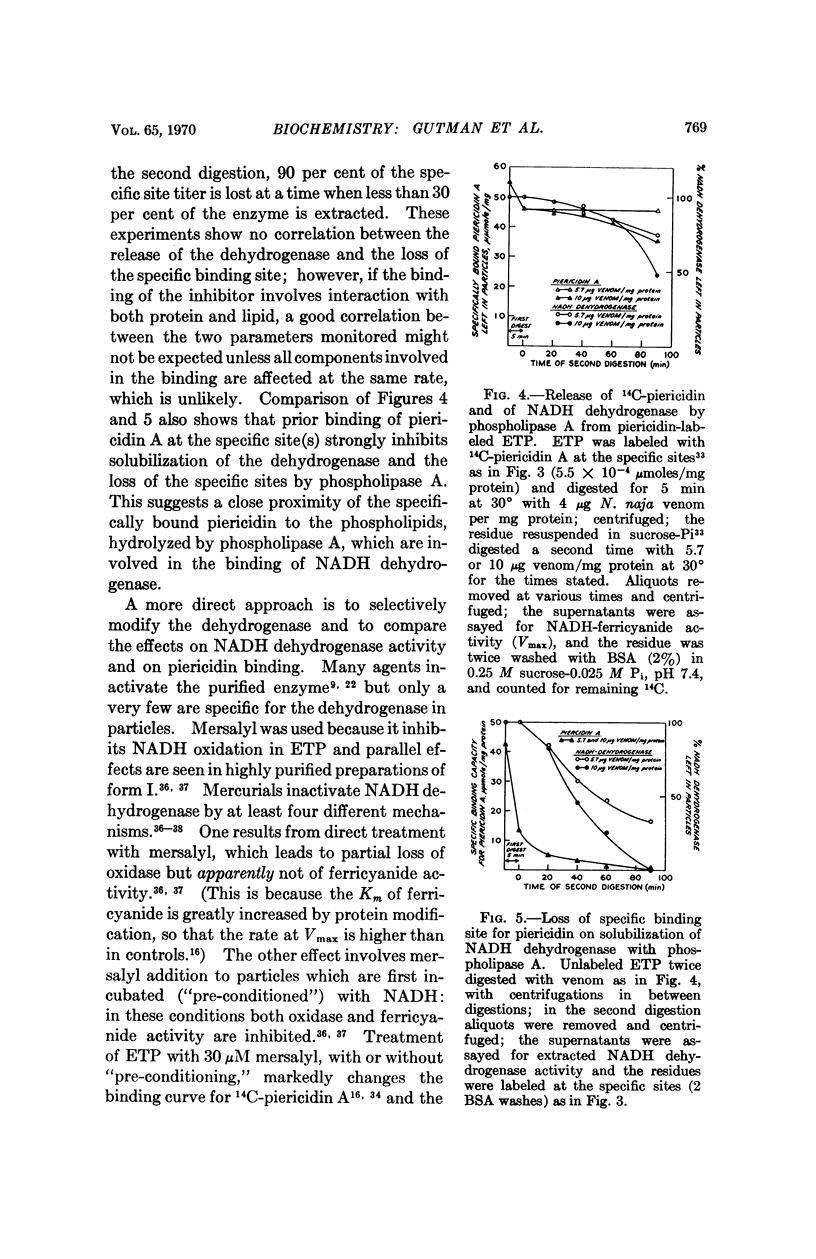
The specific binding of rotenone and piericidin seems to involve both lipid and protein. The possibility that NADH dehydrogenase participates in the binding is suggested by the apparent stoichiometric relation between specific binding site titer and NADH dehydrogenase content and the profound effect of mersalyl inhibition of the enzyme on piericidin binding capacity.
ETP, electron transport particle.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEINERT H., GRIFFITHS D. E., WHARTON D. C., SANDS R. H. Properties of the copper associated with cytochrome oxidase as studied by paramagnetic resonance spectroscopy. J Biol Chem. 1962 Jul;237:2337–2346. [PubMed] [Google Scholar]
- Bois R., Estabrook R. W. Nonheme iron protein as a possible site of rotenone inhibition of mitochondrial NADH dehydrogenase. Arch Biochem Biophys. 1969 Jan;129(1):362–369. doi: 10.1016/0003-9861(69)90187-8. [DOI] [PubMed] [Google Scholar]
- CREMONA T., KEARNEY E. B., VILLAVICENCIO M., SINGER T. P. STUDIES ON THE RESPIRATORY CHAIN-LINKED DPNH DEHYDROGENASE. V. TRANSFORMATION OF DPNH DEHYDROGENASE TO DPNH-CYTOCHROME REDUCTASE AND DIAPHORASE UNDER THE INFLUENCE OF HEAT, PROTEOLYTIC ENZYMES, AND UREA. Biochem Z. 1963;338:407–442. [PubMed] [Google Scholar]
- Cremona T., Kearney E. B. Studies on the respiratory chain-linked reduced nicotinamide adenine dinucleotide dehydrogenase. X. Reactions with sulfhydryl reagents. J Biol Chem. 1965 Sep;240(9):3645–3652. [PubMed] [Google Scholar]
- ERNSTER L., LEE C. P. BIOLOGICAL OXIDOREDUCTIONS. Annu Rev Biochem. 1964;33:729–790. doi: 10.1146/annurev.bi.33.070164.003501. [DOI] [PubMed] [Google Scholar]
- HATEFI Y., HAAVIK A. G., GRIFFITHS D. E. Studies on the electron transfer system. XL. Preparation and properties of mitochondrial DPNH-coenzyme Q reductase. J Biol Chem. 1962 May;237:1676–1680. [PubMed] [Google Scholar]
- Hatefi Y. Flavoproteins of the electron transport system and the site of action of amytal, rotenone, and piericidin A. Proc Natl Acad Sci U S A. 1968 Jun;60(2):733–740. doi: 10.1073/pnas.60.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatefi Y., Stempel K. E. Resolution of complex I (DPNH-coenzyme Q reductase) of the mitochondrial electron transfer system. Biochem Biophys Res Commun. 1967 Feb 8;26(3):301–308. doi: 10.1016/0006-291x(67)90122-2. [DOI] [PubMed] [Google Scholar]
- Horgan D. J., Ohno H., Singer T. P. Studies on the respiratory chain-linked reduced nicotinamide adenine dinucleotide dehydrogenase. XV. Interactions of piericidin with the mitochondrial respiratory chain. J Biol Chem. 1968 Nov 25;243(22):5967–5976. [PubMed] [Google Scholar]
- Horgan D. J., Singer T. P., Casida J. E. Studies on the respiratory chain-linked reduced nicotinamide adenine dinucleotide dehydrogenase. 13. Binding sites of rotenone, piericidin A, and amytal in the respiratory chain. J Biol Chem. 1968 Feb 25;243(4):834–843. [PubMed] [Google Scholar]
- KING T. E., HOWARD R. L. The preparation and some properties of a reduced diphosphopyridine nucleotide dehydrogenase from the snake venom digest of a heartmuscle preparation. J Biol Chem. 1962 May;237:1686–1698. [PubMed] [Google Scholar]
- Light P. A., Ragan C. I., Clegg R. A., Garland P. B. Iron-limited growth of torulopsis utilis, and the reversible loss of mitochondrial energy conservation at site 1 and of sensitivity to rotenone and piericidin A. FEBS Lett. 1968 Jul;1(1):4–8. doi: 10.1016/0014-5793(68)80004-3. [DOI] [PubMed] [Google Scholar]
- MACHINIST J. M., SINGER T. P. REACTIONS OF COENZYME Q IN THE DPNH DEHYDROGENASE SEGMENT OF THE RESPIRATORY CHAIN. Proc Natl Acad Sci U S A. 1965 Feb;53:467–474. doi: 10.1073/pnas.53.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKLER B. Studies of DPNH oxidase: properties of a soluble DPNH dehydrogenase. Biochim Biophys Acta. 1961 Jun 10;50:141–146. doi: 10.1016/0006-3002(61)91070-8. [DOI] [PubMed] [Google Scholar]
- MAHLER H. R., SARKAR N. K., VERNON L. P. Studies on diphosphopyridine nucleotide-cytochrome c reductase. II. Purification and properties. J Biol Chem. 1952 Dec;199(2):585–597. [PubMed] [Google Scholar]
- Mersmann H., Luthy J., Singer T. P. On the diversity of -SH groups in DPNH dehydrogenase and their tentative localization. Biochem Biophys Res Commun. 1966 Oct 5;25(1):43–48. doi: 10.1016/0006-291x(66)90637-1. [DOI] [PubMed] [Google Scholar]
- Nicholls P., Malviya A. N. Inhibition of nonphosphorylating electron transfer by zinc. The problem of delineating interaction sites. Biochemistry. 1968 Jan;7(1):305–310. doi: 10.1021/bi00841a038. [DOI] [PubMed] [Google Scholar]
- OBERG K. E. The site of the action of rotenone in the respiratory chain. Exp Cell Res. 1961 Jun;24:163–164. doi: 10.1016/0014-4827(61)90263-4. [DOI] [PubMed] [Google Scholar]
- Ohnishi T., Schleyer H., Chance B. Studies on non-heme iron proteins and the piericidin A binding site of submitochondrial particles from Candida utilis cells grown in media of varying iron concentrations. Biochem Biophys Res Commun. 1969 Aug 7;36(3):487–493. doi: 10.1016/0006-291x(69)90591-9. [DOI] [PubMed] [Google Scholar]
- Palmer G., Horgan D. J., Tisdale H., Singer T. P., Beinert H. Studies on the respiratory chain-linked reduced nicotinamide adenine dinucleotide dehydrogenase. XIV. Location of the sites of inhibition of rotenone, barbiturates, and piericidin by means of electron paramagnetic resonance spectroscopy. J Biol Chem. 1968 Feb 25;243(4):844–847. [PubMed] [Google Scholar]
- Pharo R. L., Sordahl L. A., Edelhoch H., Sanadi D. R. Studies on dihydronicotinamide adenine dinucleotide ubiquinone reductase. II. Purication and properties. Arch Biochem Biophys. 1968 May;125(2):416–428. doi: 10.1016/0003-9861(68)90598-5. [DOI] [PubMed] [Google Scholar]
- Pharo R. L., Sordahl L. A., Vyas S. R., Sanadi D. R. Studies on dihydronicotinamide adenine dinucleotide ubiquinone reductase. I. Assay of ubiquinone reductase activity in submitochondrial particles and extracts. J Biol Chem. 1966 Oct 25;241(20):4771–4780. [PubMed] [Google Scholar]
- RINGLER R. L., MINAKAMI S., SINGER T. P. Studies on the respiratory chain-linked reduced nicotinamide adenine dinucleotide dehydrogenase. II. Isolation and molecular properties of the enzyme from beef heart. J Biol Chem. 1963 Feb;238:801–810. [PubMed] [Google Scholar]
- ROSSI C., CREMONA T., MACHINIST J. M., SINGER T. P. STUDIES ON THE RESPIRATORY CHAIN-LINKED REDUCED NICOTINAMIDE ADENINE DINUCLEOTIDE DEHYDROGENASE.8. INACTIVATION, FRAGMENTATION, AND PROTECTION BY SUBSTRATES. J Biol Chem. 1965 Jun;240:2634–2643. [PubMed] [Google Scholar]
- Salach J., Singer T. P., Bader P. Studies on the respiratory chain-linked reduced nicotinamide adenine dinucleotide dehydrogenase. XI. Transformation of the dehydrogenase to reduced nicotinamide adenine dinucleotide-coenzyme Q reductase. J Biol Chem. 1967 Oct 25;242(20):4555–4562. [PubMed] [Google Scholar]



