Abstract
Background
At present, there is no safe and effective hemoglobin-based oxygen carrier (HBOC) to substitute for red blood cell transfusion. It is uncertain whether a deficiency of endothelial nitric oxide bioavailability (endothelial dysfunction) prevents or augments the HBOC-induced vasoconstriction.
Methods
Hemodynamic effects of infusion of PolyHeme (1.08 g hemoglobin/kg, Northfield Laboratories, Evanston, IL) or murine tetrameric hemoglobin (0.48 g hemoglobin/kg) were determined in awake healthy lambs, awake mice and anesthetized mice. In vitro, a cumulative dose-tension response was obtained by sequential addition of PolyHeme or tetrameric hemoglobin to phenylephrine-precontracted murine aortic rings.
Results
Infusion of PolyHeme did not cause systemic hypertension in awake lambs, but produced acute systemic and pulmonary vasoconstriction. Infusion of PolyHeme did not cause systemic hypertension in healthy wild-type mice, but induced severe systemic vasoconstriction in mice with endothelial dysfunction (either db/db mice or high-fat fed wild-type mice for 4–6 weeks). The db/db mice were more sensitive to systemic vasoconstriction than wild-type mice after the infusion of either tetrameric hemoglobin or PolyHeme. Murine aortic ring studies confirmed that db/db mice have an impaired response to an endothelial-dependent vasodilator and an enhanced vasoconstrictor response to a HBOC.
Conclusions
Reduction of low molecular weight hemoglobin concentrations to less than 1% is insufficient to abrogate the vasoconstrictor effects of HBOC infusion in healthy awake sheep or in mice with reduced vascular nitric oxide levels associated with endothelial dysfunction. These findings suggest that testing HBOCs in animals with endothelial dysfunction can provide a more sensitive indication of their potential vasoconstrictor effects.
Introduction
The mortality for patients who hemorrhage without receiving a red blood cell transfusion is high.1 There is a critical unmet medical need for an alternative to red blood cell transfusion, when red blood cells are not available. At present, after decades of laboratory and clinical research, there is no safe and effective hemoglobin-based oxygen carrier (HBOC) to substitute for red blood cell transfusion in the treatment of hemorrhagic shock. Ideally, a HBOC would provide vital tissues and organs with oxygen transport after major hemorrhage in the field, and before typed and cross-matched blood is available for transfusion. But clinical application of HBOCs has been stymied by the noxious side effects of nitric oxide scavenging.2 A recent meta-analysis reviewed sixteen trials of 5 HBOC products (1,927 subjects who received HBOCs) and demonstrated that those patients receiving a HBOC had a statistically increased risk of death and myocardial infarction (MI).3
Understanding the mechanisms responsible for HBOC-induced vasoconstriction and learning how to prevent it are crucial to developing safe and effective HBOC-based therapies. Scavenging of endothelium-derived nitric oxide by cell-free hemoglobin appears to be responsible for HBOC-induced vasoconstriction,4,5 since mice that are congenitally-deficient in nitric oxide synthase 3 do not undergo vasoconstriction when challenged with either a HBOC or tetrameric hemoglobin.6 However, it is unknown whether an acquired reduction of nitric oxide synthase 3 activity, such as that seen in individuals with endothelial dysfunction associated with diabetes mellitus or atherosclerosis, reduces or increases the vasoconstrictor response to HBOCs.
A variety of strategies have been developed to minimize the scavenging of nitric oxide by HBOCs. In one such strategy, hemoglobin is extensively crosslinked, and the fraction of low molecular weight hemoglobin is markedly reduced. We previously reported that administration of a cross-linked bovine hemoglobin containing 3% low molecular weight hemoglobin, HBOC-201 (Biopure Corporation, Cambridge, MA), induced systemic hypertension in both awake mice and sheep.6
In the current study, we tested the hypothesis that administration of a HBOC containing less than 1% low molecular weight hemoglobin would not cause hypertension. Unfortunately, cross-linked bovine hemoglobin containing less than 1% low molecular weight hemoglobin was not available for our studies. Instead, we examined the systemic and pulmonary vascular effects of a cross-linked human hemoglobin containing less than 1% low molecular weight hemoglobin, PolyHeme (Northfield Laboratories, Evanston, IL). We report that intravenous administration of PolyHeme did not increase systemic blood pressure in healthy awake mice or sheep. However, PolyHeme administration caused pulmonary hypertension and reduced cardiac output in awake sheep. Moreover, PolyHeme induced systemic hypertension in mice with endothelial dysfunction due to either diabetes mellitus (db/db mice) or in wild-type (WT) mice fed a high-fat diet. This hypertension and vasoconstriction was prevented by pretreatment with inhaled nitric oxide.
Materials and Methods
Animal preparation
Mice
This study was approved by the Subcommittee on Research Animal Care of Massachusetts General Hospital, Boston, MA. We studied 8- to 10-week-old male C57BL/6 WT mice (total of 68) and B6.Cg-m+/+Leprdb/J (C57BL6/J background) db/db mice (total of 74). Additional WT mice (total of 11) were fed a high-fat diet (60 kcal% fat, Research Diets, Inc., New Brunswick, NJ) for 4–6 weeks. All mice were obtained from Jackson Laboratory (Bar Harbor, ME).
Lambs
Fifteen Suffolk lambs weighing 23.9±2.9 kg (mean±SD) were anesthetized. A tracheostomy was performed, and monitoring catheters were placed as previously described.7 After emergence from general anesthesia, all lambs were allowed to recover for at least 2 h in a large-animal mobile restraint unit (Lomir, Malone, NY) before starting the awake study while spontaneously breathing via the tracheostomy in the cage. Mean arterial pressure, mean pulmonary arterial pressure (PAP), and central venous pressure were continuously monitored. Heart rate and cardiac output were measured every 15–30 min.
Preparation of murine tetrameric hemoglobin solution and PolyHeme
Murine tetrameric hemoglobin solution (4 g/dl, methemoglobin ≤ 2%) was prepared as previously described.6 PolyHeme (9–10 g/dl, methemoglobin ≤ 8%, containing <1% tetrameric hemoglobin) is a preparation of glutaraldehyde-polymerized pyridoxylated human hemoglobin and was obtained from Northfield Laboratories (Evanston, IL).
Hemodynamic effects of PolyHeme infusion in awake lambs
Three groups of lambs were studied. One group (n=7) received an iv infusion of utologous whole blood warmed to 37°C (1.44 g hemoglobin/kg over 20 min) while spontaneously breathing via a tracheostomy at inspired oxygen fraction of (FiO2) 0.30. Autologous blood was donated and stored in heparin anticoagulant 2 days before the infusion experiment as previously described.7 A second group (n=4) received an infusion of PolyHeme (1.08 g hemoglobin/kg over 20 min) while breathing at FiO2=0.30. A third group (n=4) breathed 80 ppm nitric oxide for 1 h at FiO2=0.30, followed by continuously breathing at a lower level of nitric oxide (5 ppm) during and after an infusion of PolyHeme (1.08 g hemoglobin/kg over 20 min) for 2 h. At 2 h, nitric oxide inhalation was acutely discontinued, and pulmonary and systemic hemodynamics were continuously monitored while lambs breathed at FiO2=0.30.
Hemodynamic effects of PolyHeme infusion in awake mice
Systolic blood pressure (SBP) was measured with a non-invasive blood pressure system (XBP 1000, Kent Scientific, Torrington, CT). The mask delivery system for nitric oxide (80 ppm) was previously described.6
Four groups of WT mice were studied. Each mouse was given a 16% of blood volume (approximately 0.3 ml in a 25 g mouse) infusion (a “topload”) via a tail vein. A control group of WT mice received an iv infusion of murine whole blood (1.44 g/kg). A second group received an infusion of PolyHeme (1.08 g/kg). A third group of WT mice was fed a high-fat diet (containing 60 kcal% fat) for 4–6 weeks and then received an infusion of PolyHeme (1.08 g/kg). The fourth group of WT mice received various concentrations of murine tetrameric hemoglobin (0.048, 0.12, 0.24, and 0.48 g/kg in the same total volume) at one dose iv per mouse.
Four groups of db/db mice were studied. The first received an iv infusion of murine whole blood and served as a control group. A second group received an infusion of PolyHeme (1.08 g/kg). The third was pretreated with inhaled nitric oxide at 80 ppm (FiO2=0.21) for 1 h followed by discontinuation of nitric oxide breathing and infusion of PolyHeme (1.08 g/kg). A fourth group received various concentrations of iv murine tetrameric hemoglobin (0.048, 0.12, 0.24, and 0.48 g/kg in the same total volume) at one dose per mouse.
Invasive hemodynamic measurements in anesthetized mice
Mice were anesthetized with an intraperitoneal injection of ketamine (120 mg/kg) and fentanyl (225 μg/kg) as previously described.6 Briefly, a thoracotomy was performed and a Millar pressure-volume catheter (size 1F, model PVR-1030, Millar Instruments Inc., Houston, TX) was inserted via the apex into the left ventricle (LV). After obtaining stable hemodynamic measurements, whole blood (1.44 g/kg) or PolyHeme solution (1.08 g/kg) was infused through the jugular vein, at a rate of 100 μl/min. PVAN software (AD Instruments, Inc., Colorado Springs, CO) was used to analyze LV pressure-volume loop measurements obtained before and 4 min after the end of infusion.
Measurement of vascular reactivity on isolated aortic rings
WT and db/db mice were euthanized with pentobarbital (200 mg/kg, i.p.). Krebs-Henseleit physiological salt solution was prepared as previously described.8 Two ml of ice-cold Krebs solution was injected retrograde into the left ventricle, and the thoracic aorta was dissected free of connective and adipose tissue and placed in ice-cold Krebs-Henseleit physiological salt solution pre-equilibrated with 95% O2-5% CO2 for 15 min. Two rings of 3–4 mm length were taken from the aorta and mounted between two tungsten wire hooks over wet blotting paper. Rings were suspended vertically in 10 ml organ baths of the myograph (TSE Systems, Bad Homburg, Germany) containing physiological salt solution and maintained at 37°C, pH 7.4, and continuously tonometered with a mixture of 95% O2-5% CO2. The rings were equilibrated for 90 min at a resting force of 1.0 g, with changes of the bathing solution at 15 min intervals. The viability of the vessel was checked by stable and reproducible responses to the addition of phenylephrine (10−6 M). The integrity of the endothelium was assessed by acetylcholine (10−6 M) challenge producing a reproducible and stable relaxation of phenylephrine-precontracted rings. Cumulative dose-tension response curves to sequential addition of murine tetrameric hemoglobin (6×10−9 to 1.8×10−5 M) or PolyHeme (6×10−9 to 1.8×10−5 M) were obtained. Tissue was washed with physiological salt solution (maintained at 37°C, pH 7.4) 4–5 times after each dose-response study until the aortic ring returned to a stable resting tension, before starting a new dose-response.
In an additional series of experiments, aortic rings from WT mice were pre-incubated with a submaximal dose (a dose which decreases the vasodilator response to acetylcholine but does not completely block the response) of NG-nitro-L-arginine methyl ester (L-NAME, 10−7 M) for 30 min and then we obtained cumulative dose responses to murine tetrameric hemoglobin (6×10−9 to 1.8×10−5 M) or PolyHeme (6×10−9 to 1.8×10−5 M). The net change in tension developed in the aortic ring was calculated from the baseline value obtained immediately before commencing the dose-response curve for each ring.
Statistical analysis
All values are expressed as mean±SEM. Data were analyzed by a repeated measures two-way ANOVA with interaction (SigmaStat 3.0.1 from Systat Software, Inc., San Jose, CA). A paired t test (two-tailed) with a Holm-Sidak adjustment was used to compare the changes in systolic blood pressure in WT and db/db mice. A repeated measures two-way ANOVA was used to assess the invasive hemodynamic measurements (anesthetized mice) and the changes of net tension of murine aortic rings. Probability values <0.05 were considered significant.
Results
Hemodynamic effect of infusion of PolyHeme in awake lambs
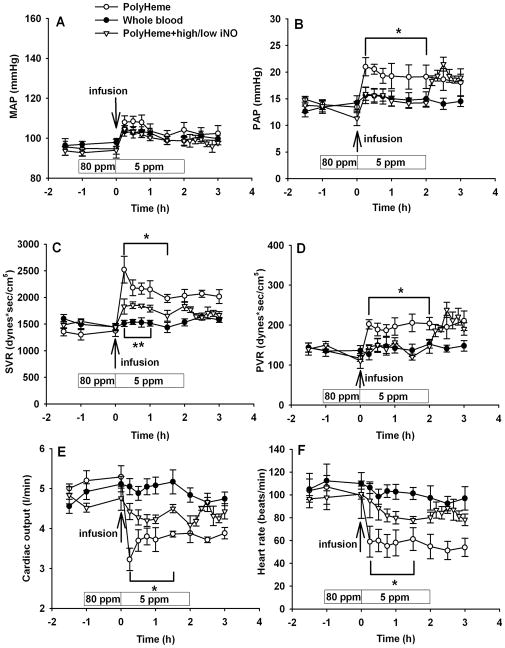
We compared the hemodynamic effects of infusing PolyHeme (1.08 g/kg) or a similar volume of whole blood in awake lambs (fig. 1). Infusion of whole blood did not alter mean arterial pressure, PAP, systemic vascular resistance, or pulmonary vascular resistance (PVR). PolyHeme infusion did not alter mean arterial pressure, but the PAP, PVR, and systemic vascular resistance increased and cardiac output and heart rate decreased.
Figure 1.
We previously reported that the effects of HBOC-201 on systemic and pulmonary vascular tone6 could be inhibited by breathing nitric oxide gas (80 ppm for 1 h before and 5 ppm for 2 h after HBOC administration) without oxidizing the extracellular hemoglobin. Similarly, we observed that this two-level nitric oxide breathing completely prevented the PolyHeme-induced changes in PVR, cardiac output, and heart rate but only attenuated the increase in systemic vascular resistance induced by PolyHeme infusion (P<0.05 differs from PolyHeme without added inhaled nitric oxide, fig. 1C–F). However, when breathing 5 ppm nitric oxide was acutely discontinued at 2 h, the PAP and PVR immediately increased (fig. 1B, D). After administration of PolyHeme, plasma methemoglobin levels in the group breathing 80 ppm and then 5 ppm nitric oxide did not increase above the low levels measured in the PolyHeme group breathing at FiO2=0.30 without added nitric oxide (data not shown).
Effects of PolyHeme infusion on SBP in WT, high-fat fed, and db/db mice
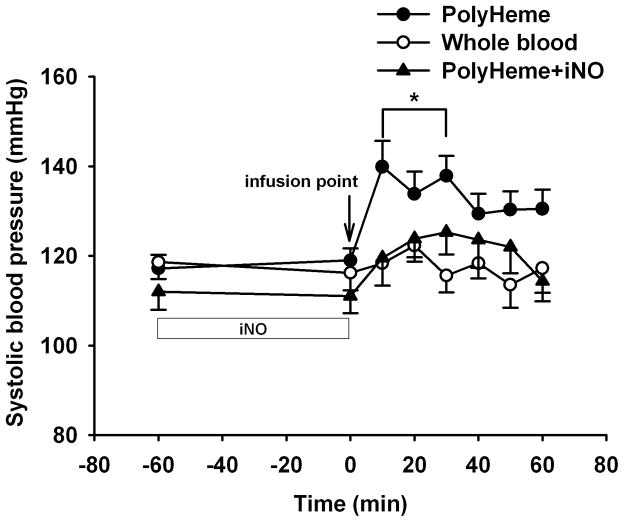
In contrast to HBOC-201, infusion of PolyHeme (1.08 g/kg) did not cause systemic hypertension in awake WT mice fed a standard diet (fig. 2).
Figure 2.
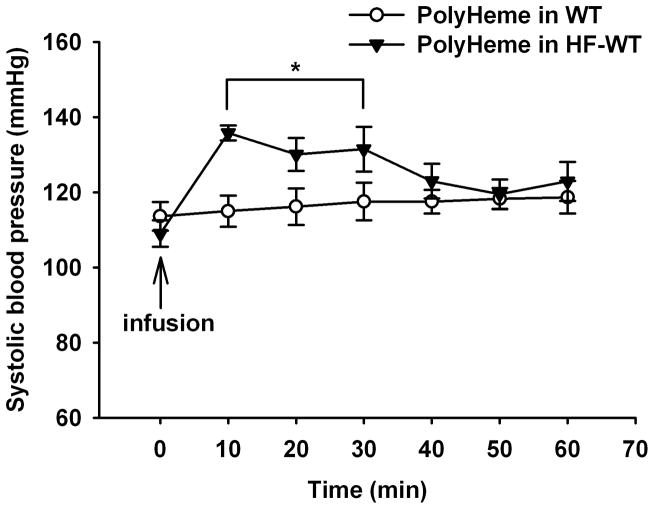
To determine whether reduced endothelial nitric oxide bioavailability associated with endothelial dysfunction alters the vascular response to HBOCs, we measured the change in SBP induced by iv PolyHeme in WT mice fed a high-fat diet for 4–6 weeks and in db/db mice. In WT mice fed a high-fat diet, PolyHeme increased the SBP from 109±4 (baseline) to 136±2 mmHg at 10 min after infusion of PolyHeme (P<0.05, fig. 2). Similarly in db/db mice, infusion of PolyHeme (1.08 g/kg) increased SBP from 117±3 at baseline to 140±6 mmHg at 10 min (P<0.05; fig. 3). In contrast, infusion of murine whole blood did not change SBP in db/db mice. Pretreatment by inhalation of nitric oxide (80 ppm, 1 h) prevented the systemic hypertension induced by subsequent infusion of PolyHeme in db/db mice.
Figure 3.
Invasive hemodynamic measurements in anesthetized mice
To confirm the findings obtained in awake mice, invasive hemodynamic measurements were performed in anesthetized WT (n=14) and db/db mice (n=13) both before and 4 min after infusion of PolyHeme or whole blood. At baseline, LV end-systolic pressure (LVESP), LV end-diastolic pressure (LVEDP), maximum rate of developed LV pressure (dP/dtmax), minimum rate of developed LV pressure (dP/dtmin), cardiac index, systemic vascular resistance index (SVRI), arterial elastance (Ea), time constant of isovolumic relaxation (τ), and central venous pressure did not differ in WT and db/db mice (table 1). Infusion of murine whole blood did not change the heart rate, LVESP, LVEDP, dP/dtmax, dP/dtmin, cardiac index, SVRI, Ea, τ, or central venous pressure in either genotype. Infusion of PolyHeme into WT mice did not alter heart rate, LVESP, LVEDP, dP/dtmax, dP/dtmin, cardiac index, SVRI, Ea, τ, or central venous pressure. In contrast, iv infusion of PolyHeme into db/db mice increased LVESP, LVEDP, SVRI, and Ea while decreasing heart rate and cardiac index without affecting dP/dtmax, dP/dtmin, or central venous pressure. The increase in SVRI and Ea strongly suggests that PolyHeme induces systemic vasoconstriction in db/db mice.
Table 1.
Comparison of cardiac function and systemic hemodynamic measurements in WT and db/db mice before and after infusion of whole blood or PolyHeme.
| WT | db/db | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Whole Blood | Baseline | PolyHeme | Baseline | Whole Blood | Baseline | PolyHeme | |
| HR (bpm) | 624±16 | 595±16 | 658±18 | 602±18 | 593±12 | 546±31 | 621±17 | 566±26* |
| LVESP (mmHg) | 106±2 | 115±3 | 111±1 | 123±3 | 109±1 | 117±3 | 112±3 | 143±5† |
| LVEDP (mmHg) | 3±1 | 4±1 | 4±1 | 5±1 | 4±1 | 4±0 | 4±0 | 6±1* |
| dP/dtmax (mmHg/s) | 12370±717 | 12295±1106 | 13385±644 | 12099±849 | 14629±1651 | 14310±1782 | 17903±1394 | 17040±1138 |
| dP/dtmin (mmHg/s) | −13821±625 | −14807±862 | −15442±590 | −15692±1082 | −12548±967 | −14018±474 | −13007±769 | −14158±924 |
| CI (ml/min/g) | 0.49±0.02 | 0.45±0.03 | 0.54±0.05 | 0.48±0.02 | 0.40±0.04 | 0.33±0.05 | 0.35±0.04 | 0.22±0.05* |
| SVRI (mmHg·min/ml·g) | 217±14 | 342±40 | 214±22 | 316±21 | 272±21 | 412±117 | 356±49 | 654±128* |
| Ea (mmHg/μl) | 5±0 | 6±0 | 5±1 | 6±0 | 4±0 | 5±1 | 5±1 | 8±2* |
| τ (msec) | 5±0 | 5±0 | 5±0 | 5±0 | 6±0 | 6±0 | 5±0 | 5±0 |
| CVP (mmHg) | 3±0 | 3±1 | 3±1 | 3±0 | 4±1 | 5±1 | 4±1 | 4±1 |
CI: cardiac index, which was derived from cardiac output divided bybody weight; CVP: central venous pressure; db/db: infusion of whole blood or PolyHeme (n=13) in db/db mice; dP/dtmax: maximum rate of developed left ventricular pressure; dP/dtmin: minimum rate of developed left ventricular pressure; Ea: arterial elastance; HR: heart rate; LVEDP: left ventricular end-diastolic pressure; LVESP: left ventricular end-systolic pressure; SVRI: systemic vascular resistance index; τ: time constant of isovolumic relaxation; WT: wild-type, infusion of whole blood or PolyHeme with breathing air (n=14) in WT mice.
Values are mean±SEM.
P<0.05 differs versus baseline in db/db mice.
P< 0.0002 differs versus baseline in db/db mice.
Dose-responses to infusion of murine tetrameric hemoglobin in WT or db/db mice
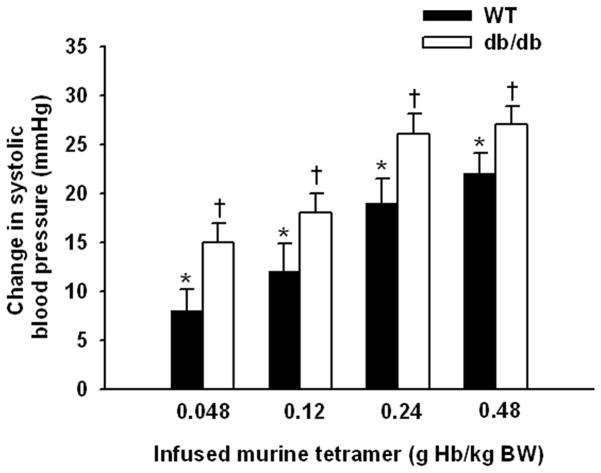
The observation that PolyHeme induced hypertension in db/db mice but not in WT mice fed a standard diet suggested the possibility that db/db mice were more sensitive to the vasoconstricting effects of HBOCs. To further examine this possibility, the change in SBP induced by infusing a range of doses of murine tetrameric hemoglobin (0.048, 0.12, 0.24, and 0.48 g/kg) was compared in awake WT mice fed standard chow and db/db mice. The vasoconstrictor effect of HBOC is dose-dependent in both genotypes. The infusion of tetrameric hemoglobin caused greater systemic hypertension in db/db mice than in WT mice at all of the doses we studied (fig. 4).
Figure 4.
Vascular reactivity to PolyHeme or tetrameric hemoglobin addition to isolated murine aortic rings
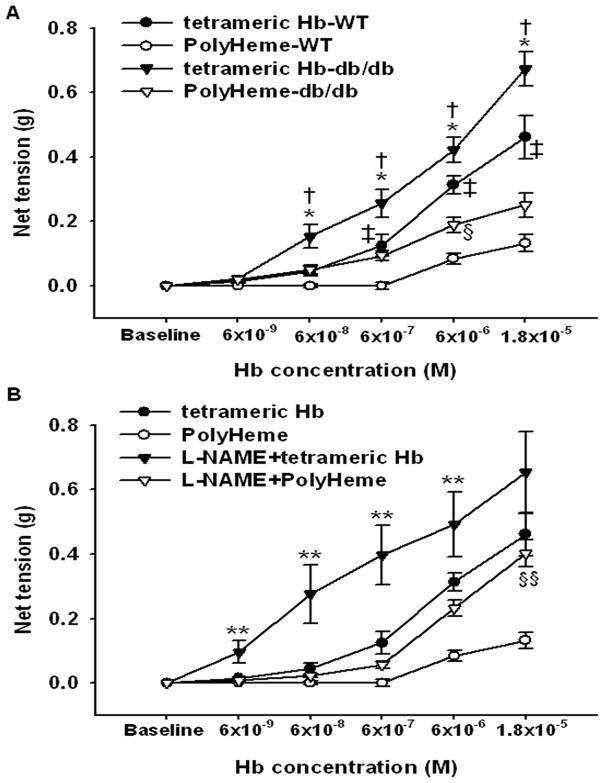
To determine whether the vasoconstricting effects of PolyHeme in db/db mice were attributable to a direct effect of the HBOC on the vasculature, we measured the development of isometric tension in response to cumulative concentration increments of PolyHeme or tetrameric hemoglobin in aortic rings isolated from db/db and WT mice (fig. 5). In both genotypes, PolyHeme produced a concentration-dependent increase of net tension in aortic rings, with a greater maximum response in db/db (0.25±0.04 g) than in WT mice (0.13±0.03 g). PolyHeme induced vasoconstriction in WT aortic rings at very high concentrations (6×10−6 M and 1.8×10−5 M). Similarly, tetrameric hemoglobin (6×10−9 M to 1.8×10−5 M) evoked a concentration-dependent increase in the net tension of the aortic rings of both genotypes (fig. 5A). The increment of net tension in response to addition of tetrameric hemoglobin was greater in db/db mice than in WT mice (P<0.05). The maximal tension responses achieved in db/db mice were greater than those in WT mice (0.67±0.05 vs 0.46±0.07 g, P<0.01). The maximum contraction responses produced by PolyHeme were less than those produced by tetrameric hemoglobin, in both db/db and WT mice (P<0.05 differs versus tetrameric hemoglobin for both).
Figure 5.
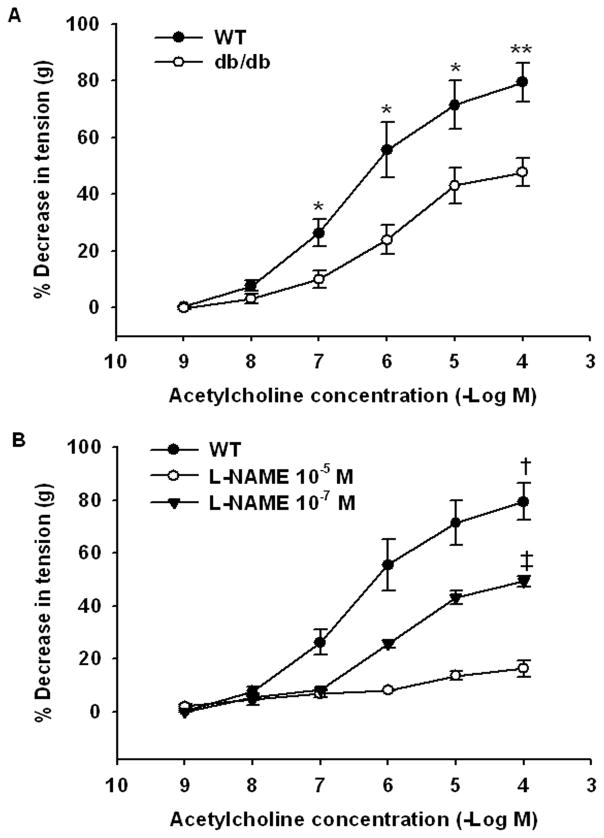
The observations that PolyHeme induced hypertension in both WT mice fed a high-fat diet and db/db mice but not in WT mice fed a standard diet, as well as the finding that db/db aortic rings were more sensitive to PolyHeme than were WT aortic rings, suggested the possibility that endothelial dysfunction sensitized blood vessels to PolyHeme-induced constriction. We previously reported that WT mice fed a high-fat diet have endothelial dysfunction.9 Moreover, we confirmed that aortic rings from db/db mice have an impaired vasodilator response to acetylcholine (fig. 6A). Then endothelial dysfunction seen in db/db mice10 and mice fed a high-fat diet11 has been attributed to reduced nitric oxide bioavailability. To test the hypothesis that reduced nitric oxide bioavailability associated with endothelial dysfunction enhanced the vasoconstrictor response to PolyHeme, we examined whether inhibition of nitric oxide synthase with NG-nitro-L-arginine methyl ester (L-NAME) could sensitize aortic rings from WT mice (fed a standard diet) to the vasoconstrictor effects of PolyHeme. We chose a concentration of L-NAME which only partially inhibited nitric oxide synthase 3 (submaximal dose: 10−7 M; fig. 6B), because complete congenital deficiency of nitric oxide synthase 3 completely blocked the vasoconstrictor effects of HBOCs.6 In the presence of L-NAME (10−7 M), the vasoconstrictor response of WT aortic rings to both tetrameric hemoglobin and PolyHeme was markedly augmented (fig. 5B). The maximum net tension developed with tetrameric hemoglobin in rings preincubated with or without L-NAME was 0.65±0.13 and 0.46±0.06 g, respectively. The increment of net tension in response to cumulative addition of PolyHeme was higher in L-NAME-preincubated aortic rings than in rings without L-NAME. Taken together, these findings suggest that reduced vascular nitric oxide levels (due to endothelial dysfunction or treatment with submaximal doses of L-NAME) markedly sensitizes vascular tissues to the constricting effects of HBOCs.
Figure 6.
Discussion
In the present study, we report that intravenous infusion of PolyHeme did not cause systemic hypertension in awake healthy lambs, but increased the pulmonary artery pressure and decreased cardiac output and heart rate; these effects were markedly attenuated by treatment with inhaled nitric oxide. Infusion of PolyHeme did not cause systemic hypertension or reduce cardiac output in WT mice. However, in mice with endothelial dysfunction (either WT mice fed a high-fat diet for 4–6 weeks or db/db mice), infusion of PolyHeme induced hypertension and reduced cardiac output. Pretreatment with inhaled nitric oxide prevented the hypertensive response to PolyHeme in db/db mice. Invasive hemodynamic measurements confirmed that infusion of PolyHeme into db/db mice increased LVESP, LVEDP, and SVRI and decreased heart rate and cardiac output; this did not occur in WT mice given a PolyHeme infusion. Both an in-vivo dose-response study and an in vitro study using murine aortic rings revealed that db/db mice were more sensitive than WT mice to the vasoconstrictor effects of infusing either murine tetrameric hemoglobin or PolyHeme.
Vasoconstriction, linked in part to the presence of low-molecular weight tetrameric hemoglobin, has hindered the development of HBOCs.12 Polymerized hemoglobins, such as HBOC-201 and PolyHeme, are designed to diminish Hb extravasation, vasoactivity, and adverse renal effects.13,14 Our previous study showed that infusion of HBOC-201 (containing 3% low molecular weight tetrameric Hb) caused systemic hypertension in awake lambs and WT mice.6 In the current study, PolyHeme (containing less than 1% tetrameric hemoglobin) did not cause systemic hypertension in awake, healthy lambs or in WT mice fed a standard diet. It is conceivable that the lower concentration of tetrameric Hb in PolyHeme than in HBOC-201 was responsible for the less marked systemic vasoconstrictor effects seen with the former.
Similar to our previous studies infusing HBOC-201,7 PolyHeme infusion caused systemic and pulmonary vasoconstriction in lambs, and treatment with inhaled nitric oxide (80 ppm for 1 h before and 5 ppm concurrently with HBOC administration) attenuated the PolyHeme-induced increase in systemic vascular resistance and PVR (fig. 1). Inhaled nitric oxide at these doses did not produce an increase in plasma methemoglobin levels preserving the oxygen-carrying capacity of PolyHeme. Acute discontinuation of nitric oxide breathing caused an immediate increase in the PAP and PVR. Catastrophic pulmonary vasoconstriction has been reported after acute withdrawal of nitric oxide inhalation in a patient with pulmonary hypertension.15
We previously observed that HBOC-201 caused sustained systemic hypertension in awake mice, but not in nitric oxide synthase 3-deficient mice.6 These findings demonstrated that HBOCs cause systemic vasoconstriction by scavenging nitric oxide produced by nitric oxide synthase 3. We hypothesized that, under conditions of decreased vascular nitric oxide levels, such as those associated with endothelial dysfunction, the vasoconstrictor response to HBOCs would be enhanced. Endothelial dysfunction is characterized by a reduced vasodilator response to acetylcholine in animal models and human beings and is associated with diabetes mellitus and hyperlipidemia. Endothelial dysfunction has been observed in WT mice fed a high-fat diet and in db/db mice.9,10 In db/db mice, endothelial dysfunction has been attributed to reduced vascular nitric oxide levels caused by increased superoxide production (superoxide reacts with nitric oxide to produce peroxynitrite)10 and/or reduced vascular nitric oxide synthase 3 protein levels.16 We found that infusion of PolyHeme into awake db/db or high-fat fed WT mice caused severe systemic hypertension (figs. 2 and 3), in marked contrast to WT mice. Again, pretreatment with inhaled nitric oxide prevented the systemic hypertension that induced by PolyHeme administration in awake db/db mice. Invasive hemodynamic studies demonstrated that the hypertension seen in db/db mice challenged with PolyHeme was attributable to systemic vasoconstriction (table 1). A dose-response study further revealed that db/db mice are more sensitive to the vasoconstricting effects of an infusion of murine tetrameric hemoglobin than are WT mice fed a standard diet (fig. 4).
To confirm that db/db mice are more sensitive to the vasoconstricting effects of HBOCs, we compared the vasomotor responses of aortic rings from db/db mice and WT mice fed a standard diet. As anticipated, the vasodilator effects of acetylcholine were less in db/db mice than in WT mice (acetylcholine, fig. 6). Moreover, PolyHeme and tetrameric hemoglobin induced greater vasoconstriction in aortic rings from db/db mice than in those from WT mice (fig. 5A). We hypothesized that if reduced vascular nitric oxide levels sensitized db/db mice to nitric oxide scavenging by HBOCs, then partial inhibition of nitric oxide synthesis in WT mice should also result in an enhanced vasoconstrictor response to HBOC infusion. We identified a dose of L-NAME (10−7 M), a NOS inhibitor, that attenuated acetylcholine-induced vasodilation mimicking the endothelial dysfunction seen in db/db mice and WT mice fed a high-fat diet. When WT aortic rings pretreated with this submaximal dose of L-NAME the vasoconstrictor effects of tetrameric hemoglobin and PolyHeme were markedly enhanced (fig. 5B). Taken together, these observations suggest that reduced vascular nitric oxide levels associated with endothelial dysfunction sensitize mice to the vasoconstrictor effects of HBOC administration.
Recently, Natanson and colleagues conducted a meta-analysis of clinical trials involving 5 HBOC products and reported that HBOC-receiving patients had a higher risk of death and MI.3 MI typically occurs when an occlusive thrombus develops in an atherosclerotic coronary artery. Nitric oxide scavenging by HBOCs may predispose individuals to coronary thrombosis, in part, by attenuating the platelet inhibitory effects of nitric oxide.17 Our findings that mice with endothelial dysfunction are more sensitive to the nitric oxide scavenging effects of HBOCs may provide an explanation of why HBOC infusion causes hypertension in some patients and not in others and provide a basis for understanding the higher mortality and increased rate of occurrence of MI and stroke in some HBOC recipients. It is conceivable that patients with endothelial dysfunction may be predisposed to experiencing a MI when receiving a HBOC. Of note, in our study, we did not investigate animal models of hemorrhagic shock, since the metabolic and hemodynamic effects of this complex syndrome would make it difficult to isolate the vascular effects of HBOCs and the influence of endothelial dysfunction on HBOC-induced vasoconstriction. Indeed, hemorrhagic shock alone produces endothelial dysfunction in animal models.18 In the future, HBOCs should be studied in animal models of endothelial dysfunction, since reduced vascular nitric oxide levels appear to sensitize these animals (and presumably humans) to the adverse effects of HBOCs. Conquering these adverse effects will be vital in producing a useful HBOC for safe transfusion into humans suffering from hemorrhagic shock.
In conclusion, we report that top-load infusion of PolyHeme did not cause systemic hypertension in awake healthy lambs and WT mice fed a standard diet but caused severe systemic vasoconstriction and hypertension in mice with endothelial dysfunction (db/db mice and WT mice fed a high-fat diet). HBOC-induced vasoconstriction and hypertension were prevented by treatment with inhaled nitric oxide. Dose-response trials in intact mice, as well as in vitro studies using murine aortic rings, further demonstrated that db/db mice are more vulnerable than healthy WT mice to the vasoconstrictor effects of HBOC administration. In the future, HBOCs should be routinely evaluated in animal models with reduced vascular nitric oxide bioavailability to ensure their safety when given to patients, who may have known or occult metabolic or vascular diseases associated with endothelial dysfunction.
Acknowledgments
Sources of Financial Support
This work was supported by US Public Health Service Grant HL-42397 to Warren M. Zapol from the National Institutes of Health, Bethesda, Maryland.
The authors thank and acknowledge the assistance provided in sheep experiments by Dr. Gian Paolo Volpato, M.D., Anesthesiologist in the Department of Anesthesia and Critical Care at Hospital Clinico Mutual de Seguridad and Clinica Las Condes, Santiago, Chile. The authors would like to acknowledge Hui Zheng, Ph.D., Assistant Professor in Medicine at Harvard Medical School, Massachusetts General Hospital Biostatistics Center, Boston, Massachusetts for statistical assistance and help with data analysis.
Footnotes
Conflicts of Interest
Dr. Zapol receives royalties on patents licensed by Massachusetts General Hospital to Linde Corporation, Munich, Germany, and Ikaria, Clinton, New Jersey, on inhaled nitric oxide. Dr. Bloch has received grants from Ikaria and Linde to study inhaled nitric oxide. Northfield Corp. (Evanston, IL) funded the animal charges and provided the PolyHeme at no cost. The remaining authors report no conflicts of interest.
References
- 1.Moore EE, Moore FA, Fabian TC, Bernard AC, Fulda GJ, Hoyt DB, Duane TM, Weireter LJ, Jr, Gomez GA, Cipolle MD, Rodman GH, Jr, Malangoni MA, Hides GA, Omert LA, Gould SA. PolyHeme Study Group: Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA multicenter trial. J Am Coll Surg. 2009;208:1–13. doi: 10.1016/j.jamcollsurg.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 2.Yu B, Bloch KD, Zapol WM. Hemoglobin based red blood cell substitutes and nitric oxide. Trends Cardiovasc Med. 2009;19:103–7. doi: 10.1016/j.tcm.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA. 2008;299:2304–12. doi: 10.1001/jama.299.19.jrv80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, III, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–9. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 5.Gladwin MT, Sachdev V, Jison MJ, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, Hunter LA, Blackwelder WC, Schechter AN, Rodgers GP, Castro O, Ognibene FP. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–95. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 6.Yu B, Raher MJ, Volpato GP, Bloch KD, Ichinose F, Zapol WM. Inhaled nitric oxide enables artificial blood transfusion without hypertension. Circulation. 2008;117:1982–90. doi: 10.1161/CIRCULATIONAHA.107.729137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu B, Volpato GP, Chang K, Bloch KD, Zapol MW. Prevention of the pulmonary vasoconstrictor effects of HBOC-201 in awake lambs by continuously breathing nitric oxide. Anesthesiology. 2009;110:113–22. doi: 10.1097/ALN.0b013e318190bc4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iaccarino G, Cipolletta E, Fiorillo A, Annecchiarico M, Ciccarelli M, Cimini V, Koch WJ, Trimarco B. Beta (2)-adrenergic receptor gene delivery to the endothelium corrects impaired adrenergic vasorelaxation in hypertension. Circulation. 2002;106:349–55. doi: 10.1161/01.cir.0000022690.55143.56. [DOI] [PubMed] [Google Scholar]
- 9.Raher MJ, Thibault HB, Buys ES, Kuruppu D, Shimizu N, Brownell AL, Blake SL, Rieusset J, Kaneki M, Derumeaux G, Picard MH, Bloch KD, Scherrer-Crosbie M. A short duration of high-fat diet induces insulin resistance and predisposes to adverse left ventricular remodeling after pressure overload. Am J Physiol Heart Circ Physiol. 2008;295:H2495–502. doi: 10.1152/ajpheart.00139.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pannirselvam M, Wiehler WB, Anderson T, Triggle CR. Enhanced vascular reactivity of small mesenteric arteries from diabetic mice is associated with enhanced oxidative stress and cyclooxygenase products. Br J Pharmacol. 2005;144:953–60. doi: 10.1038/sj.bjp.0706121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A, Vaziri ND. A high-fat, refined-carbohydrate diet induces endothelial dysfunction and oxidant/antioxidant imbalance and depresses NOS protein expression. J Appl Physiol. 2005;98:203–10. doi: 10.1152/japplphysiol.00463.2004. [DOI] [PubMed] [Google Scholar]
- 12.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, III, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–9. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 13.Jahr JS, Walker V, Manoochehri K. Blood substitutes as pharmacotherapies in clinical practice. Curr Opin Anaesthesiol. 2007;20:325–30. doi: 10.1097/ACO.0b013e328172225a. [DOI] [PubMed] [Google Scholar]
- 14.Rice J, Philbin N, Light R, Arnaud F, Setinbach T, McGwin G, Collier S, Malkevich N, Moon-Massatt P, Rentko V, Pearce LB, Ahlers S, McCarron R, Handrigan M, Freilich D. The effects of decreasing low-molecular weight hemoglobin components of hemoglobin-based oxygen carriers in swine with hemorrhagic shock. J Trauma. 2008;64:1240–57. doi: 10.1097/TA.0b013e318058245e. [DOI] [PubMed] [Google Scholar]
- 15.Atz AM, Wessel DL. Sildenafil ameliorates effects of inhaled nitric oxide withdrawal. Anesthesiology. 1999;91:307–10. doi: 10.1097/00000542-199907000-00041. [DOI] [PubMed] [Google Scholar]
- 16.Belmadani S, Palen DI, Gonzalez-Villalobos RA, Boulares HA, Martrougui K. Elevated epidermal growth factor receptor phosphorylation induces resistance artery dysfunction in diabetic db/db mice. Diabetes. 2008;57:1629–37. doi: 10.2337/db07-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653–62. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 18.Wang P, Ba ZF, Chaudry HI. Endothelial cell dysfunction occurs very early following trauma-hemorrhage and persists despite fluid resuscitation. Am J Physiol Heart Circ Physiol. 1993;265:H973–79. doi: 10.1152/ajpheart.1993.265.3.H973. [DOI] [PubMed] [Google Scholar]