SUMMARY
Leber’s congenital amaurosis (LCA) is a group of inherited blinding diseases with onset during childhood. One form of the disease, LCA2, is caused by mutations in the retinal pigment epithelium–specific 65-kDa protein gene (RPE65). We investigated the safety of subretinal delivery of a recombinant adeno-associated virus (AAV) carrying RPE65 complementary DNA (cDNA) (ClinicalTrials.gov number, NCT00516477). Three patients with LCA2 had an acceptable local and systemic adverse-event profile after delivery of AAV2.hRPE65v2. Each patient had a modest improvement in measures of retinal function on subjective tests of visual acuity. In one patient, an asymptomatic macular hole developed, and although the occurrence was considered to be an adverse event, the patient had some return of retinal function. Although the follow-up was very short and normal vision was not achieved, this study provides the basis for further gene therapy studies in patients with LCA.
Leber’s congenital amaurosis (LCA) is a group of inherited disorders involving retinal degeneration with severe vision loss noted in early infancy. The condition is usually identified through behaviors, including abnormal roving-eye movements (nystagmus). The diagnosis is confirmed by both abnormal electroretinographic responses and pupillary light reflexes.1–4 Most patients with LCA have severe visual impairment throughout childhood; vision deteriorates over time, and patients usually have total blindness by the third or fourth decade of life.4 There is no treatment for LCA.
The LCA2 form of the disease is associated with mutations in RPE65, which encodes a protein requisite for the isomerohydrolase activity of the retinal pigment epithelium. This activity produces 11-cis-retinal from all-trans-retinyl esters.5–8 In the absence of 11-cis-retinal, the natural ligand and chromophore of the opsins of rod and cone photoreceptors, the opsins cannot capture light and transduce it into electrical responses to initiate vision.4,7,9 Although this biochemical defect results in an immediate and profound impairment of visual function, histologic degeneration of retinal cells is delayed in patients with LCA2 and the relevant animal models, even at a time when there exist behaviors that indicate blindness and nearly absent electrophysiological responses.7,10–12
Recombinant AAV2.hRPE65v2 is a replication-deficient AAV vector containing RPE65 cDNA.13 In vitro, AAV2.hRPE65v2 induces the production of RPE65 protein in target cells. AAV2.hRPE65v2 that was injected behind the retina of animal models of LCA2 resulted in rapid development of visual function.13 We have documented long-term, sustained (>7.5 years, with ongoing observation) restoration of visual function in a canine model of LCA2 after a single subretinal injection of AAV2.RPE65.13–15 This and additional safety and efficacy data13 provide the basis for a phase 1 trial of gene therapy in human patients with LCA2.
METHODS
SURGICAL DELIVERY OF THE VECTOR
The transgene cassette in the AAV2.hRPE65v2 vector carries a chicken β actin (CBA) promoter that drives the expression of the human RPE65 cDNA.13 The excipient is supplemented with a surfactant to prevent the loss of the vector to surfaces in contact with the product (see the Methods section of the Supplementary Appendix, available with the full text of this article at www.nejm.org).13 The vector was manufactured by the Center for Cellular and Molecular Therapeutics at the Children’s Hospital of Philadelphia, and current Good Manufacturing Practices were used.
Surgery was performed under general anesthesia with the use of a standard three-port pars plana vitrectomy with removal of the posterior cortical vitreous (see the Methods section of the Supplementary Appendix).16 An injection of 1.5×1010 vector genome of AAV2.hRPE65v2 in a volume of 150 µl of phosphate-buffered saline supplemented with Pluronic F-68 NF Prill Poloxamer 188 was administered into the subretinal space, thereby creating a localized dome-shaped retinal detachment (Fig. 1 and Video 1).16
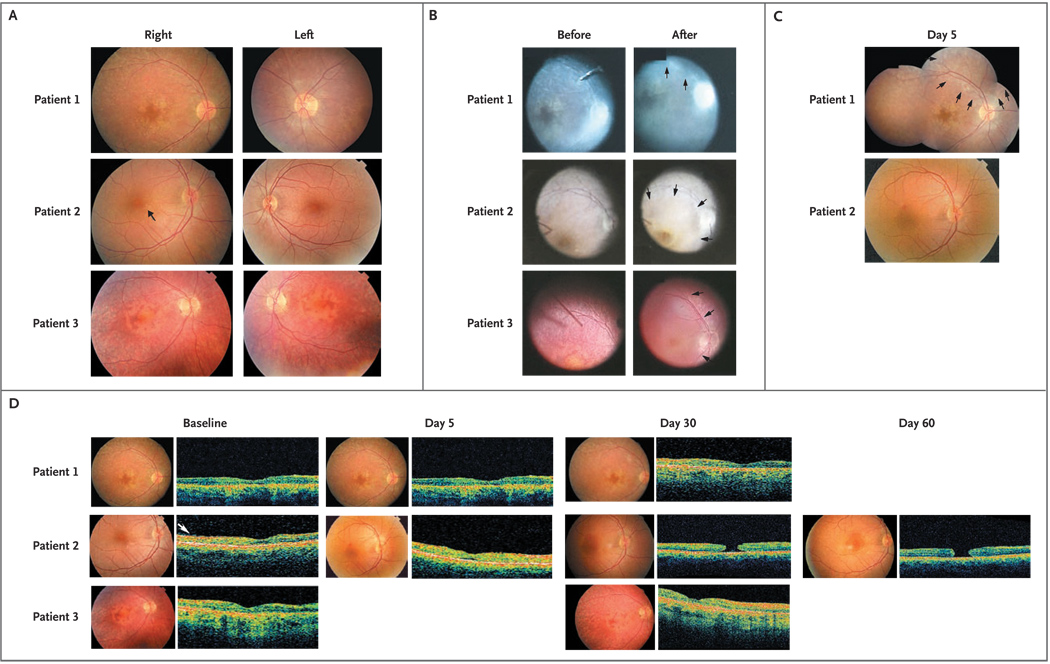
Figure 1. Retinal Appearance and Morphologic Features before and after Surgery in the Three Patients.
In Panel A, 30-degree fundus photographs taken with a Topcon camera show the disk and macula of the right and left eyes of Patients 1, 2, and 3 before surgery. In the right eye of Patient 2, the preretinal membrane and retinal striae are visible (arrow), and in Patient 3, pigmentary changes in the foveal region are apparent. In Panel B, still photographs from video footage taken through the operating microscope show the right eye of each patient during subretinal injection of AAV2.hRPE65v2. The “before” photographs show the injection cannula just before delivery of the vector, and the “after” photographs show the appearance of the bleb. In Patient 1, the raised edge of the superonasal bleb is visible (arrows). In Patients 2 and 3, the margins of the macular blebs can be seen (arrows). In Panel C, fundus photographs taken during the early postoperative period show that blebs have resolved, leaving the retinas flat and without hemorrhage or retinal opacification. On day 5, a montage of fundus photographs of Patient’s 1 retina shows both the macula and the injected region, with the extent of the original detachment indicated (arrows); in Patient 2, the fovea is visibly intact. (Fundus photographs were not taken of Patient 3’s retina on day 5, but there was no evidence of hemorrhage or retinal opacification.) Panel D shows the retinal structure measured by optical coherence tomography before and after surgery in all three patients, with the respective fundus photographs. Optical coherence–tomographic images through the fovea are shown for all three patients at baseline and on day 30, for Patients 1 and 2 on day 5, and for Patient 2 on day 60. In Patient 2, the epiretinal membrane is visible at baseline (arrow) and can be seen in several subsequent scans; also visible is the normal foveal depression despite mild thinning. A full-thickness macular hole is apparent on day 30 and has not enlarged on day 60, and the surrounding retina has not detached. There was no cystic macular edema at any point.
SAFETY AND EFFICACY
Patients were evaluated before surgery and at designated follow-up visits after surgery by complete ophthalmic examination, a general physical examination, and clinical and laboratory tests, including an assessment of vector biodistribution and immune response.
Efficacy for each patient was monitored with objective and subjective measures of vision by comparison of the average of at least four preoperative values with the average of at least four measurements taken at least 1 month after injection. Objective measures included evaluation of the pupillary light reflex and nystagmus testing. Subjective measures included standard tests of visual acuity, the Goldmann visual-field examination, and mobility testing to assess differences in the ability of the patients to navigate a standardized obstacle course (Table 1, and Fig. 3 and the Methods section of the Supplementary Appendix and Video 2A and 2B).
Table 1.
Characteristics of the Patients and Test Results before and after Injection.*
| Patient No. | Age | Sex | Mutation | Nystagmus Frequency† | Anomalies on OCT‡ | Visual Acuity | Visual Field | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ETDRS§ | LogMAR Score¶ | ||||||||||||||||||||||
| Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | ||||||||||||||
| Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | ||||
| yr | Hz | degree | |||||||||||||||||||||
| 1 | 26 | F | Homozygote p.Glu102Lys c.304G→A |
2.0 | 1.2 | 2.0 | 1.2 | ND | ND | ND | ND | Hand motion |
20/1050‖ | 20/1040 | 20/1100 | 2.0 | 1.72‖ | 1.72 | 1.74 | 41 | 177 | 36 | 26 |
| 2 | 26 | M | Homozygote p.Glu102Lys c.304G→A |
1.0 | 0.9 | 1.0 | 0.9 | EM | MH | ND | ND | Hand motion |
20/710‖ | 20/500 | 20/220 | 2.0 | 1.55‖ | 1.40 | 1.04 | 62 | 213 | 55 | 75 |
| 3 | 19 | F | Homozygote p.R234X c.700C→T |
1.5 | 1.4 | 1.37 | 1.1 | ND | ND | ND | ND | 20/640 | 20/290** | 20/220 | 20/210 | 1.50 | 1.16** | 1.05 | 1.03 | 147 | 210 | 203 | 160 |
EM denotes epiretinal membrane, ETDRS Early Treatment Diabetic Retinopathy Study, logMAR log10 of the minimum angle of resolution, MH macular hole, ND not detectable, and OCT optical coherence tomography.
The frequency of nystagmus was measured from short videotaped sequences, and the significance of these results cannot be calculated.
Measurements of precise retinal thicknesses by OCT varied because of the lack of fixation and the presence of nystagmus, so retinal thickness was estimated on some of the macular scans. However, OCT was useful in detecting structural anomalies, such as EM and MH.
The detection of hand motion corresponds to a visual acuity of a Snellen equivalent of <20/2000, according to ETDRS charts and letter counts.
The logMAR score ranges from 0.00 to 2.00, with higher values indicating poorer vision.
P<0.001.
P = 0.002.
Visual acuity was measured by trained vision examiners using a standard protocol involving Early Treatment Diabetic Retinopathy Study (ETDRS) charts and letter counts. Letter scores were converted to the log of the minimum angle of resolution (logMAR), on a scale ranging from 0.00 to 2.00, with higher values indicating poorer vision. Eyes that could detect hand motions were assigned a score that was one line worse than the largest printed line on the chart tested at a standardized distance of 4 m (<20/1600) to provide the most conservative evaluation in terms of underestimating the actual extent of visual impairment.17
RESULTS
CHARACTERISTICS OF THE PATIENTS
Three consecutive patients who had LCA2 and were between the ages of 19 and 26 years provided written informed consent (see the Supplementary Appendix). The patients were enrolled in the trial from September 2007 through January 2008. Eligibility was confirmed, and the eye with worse function was selected for delivery of AAV2.hRPE65v2 (Table 1 of the Supplementary Appendix). In all three patients, the right eye was selected for surgery (Table 1).
SAFETY OF SUBRETINAL INJECTION OF VECTOR
In Patient 1, the injection exposed the superonasal macula and the retina peripheral to the superonasal vascular arcade; the macula was exposed in Patients 2 and 3, with some extension beyond the temporal arcade in Patient 3 (Fig. 1B and 1C). An epiretinal membrane that had been noted during baseline studies in Patient 2’s right (injected) eye was not removed before injection (Fig. 1A and 1D). The localized retinal detachments resolved within 14 hours after surgery (Fig. 1C and 1D). All postoperative retinal examinations were unremarkable except for the formation of an outer lamellar cyst in the fovea of Patient 2 that was noted on day 5 after injection. The retina was imaged by optical coherence tomography (OCT) on day 5 (Fig. 1D). By day 14, a macular hole, detectable both on ophthalmoscopy and on OCT, had developed. The patient was unaware of this change. The hole had not expanded at the subsequent visits (Fig. 1D). There were no serious adverse events, as defined by the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use or the Food and Drug Administration, in any of the patients (Table 2 of the Supplementary Appendix).
Vector DNA sequences were found only in a tear sample from Patient 1 on day 1 after surgery. There was no evidence of systemic dissemination of vector sequences in any of the patients at any time. There was no evidence of humoral immune response to the RPE65 protein or of cell-mediated immune responses to AAV2 capsid or RPE65 protein. Neutralizing antibody titers to the AAV2 capsid increased in Patient 2 and diminished with time.
CHANGES IN VISION
Pupillometry
The normal pupillary light reflex is consensual — in other words, a stimulus delivered to either eye alone will cause both pupils to contract equally. When the right eye is stimulated briefly with light, both pupils constrict and then begin to dilate (Fig. 2A). Before the eyes have fully recovered, the left eye is then stimulated. Again, both pupils constrict simultaneously and to a similar degree. The pattern continues as stimuli alternate between the right eye and the left eye. The first stimulus in this protocol elicits the greatest response (constriction amplitude) because in subsequent stimuli, the timing is such that the pupils do not have time to return to their baseline diameter. The pattern is similar when the left eye is the first eye to be stimulated (Fig. 2B).
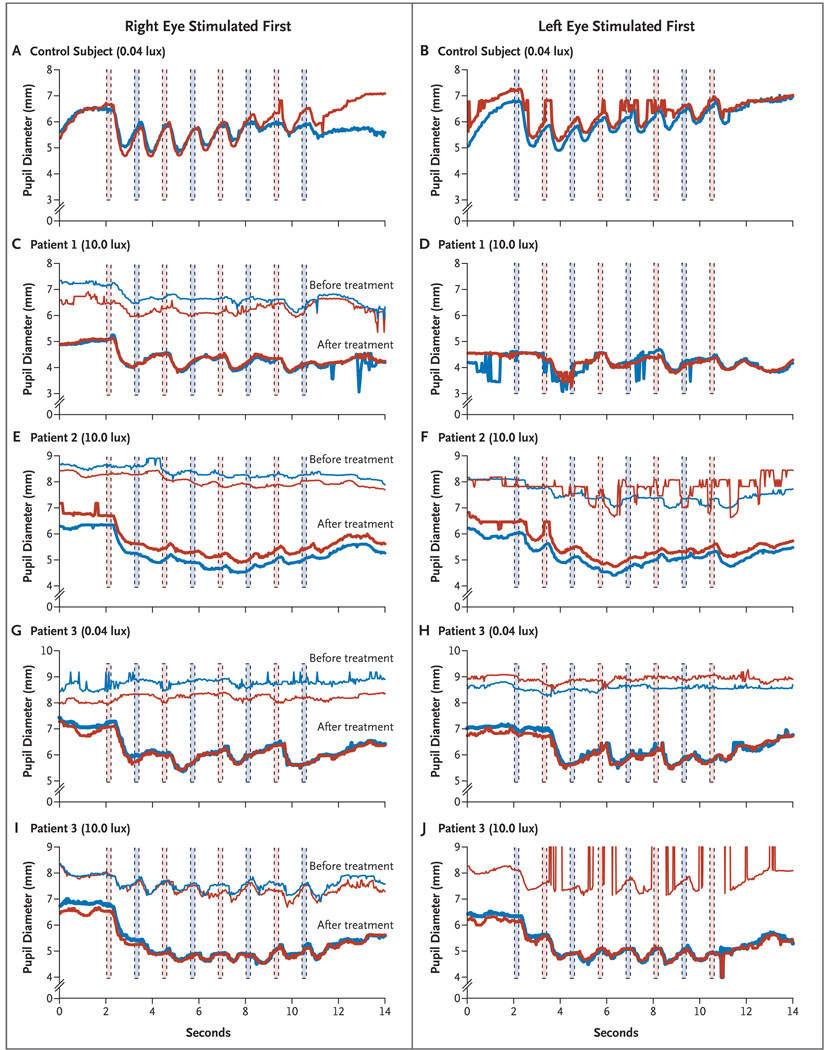
Figure 2. Representative Results of Pupillometry in a Control Subject and before and after Subretinal Injection in the Right Eye of the Three Patients.
Panels A and B show pupillary light reflexes in a control subject after dark adaptation and alternating stimulation with 0.04 lux, starting first in the right eye (red columns) and then in the left eye (blue columns), respectively. The red curves represent the diameter of the right pupil, and the blue curves represent the diameter of the left pupil. The pupillary light reflexes are shown after alternating stimulation with 10.0 lux, starting in the right and then in the left eye, respectively, for Patient 1 at baseline and 4.75 months after injection (Panels C and D) and for Patient 2 at baseline and 2.75 months after injection (Panels E and F). The pupillary light reflexes for Patient 3 at baseline and 1 month after injection are shown after alternating stimulation with 0.04 lux (Panels G and H) and with 10.0 lux (Panels I and J), first in the right eye and then in the left eye, respectively. To facilitate the comparison of overlapping curves in Panel C and Panels E through J, the baseline curves have been shifted up 2 mm with respect to the curves after treatment. The “before” curves were not captured in Panel D or for the left eye in Panel J because of interference from nystagmus.
Baseline testing showed that the pupillary light reflexes of the three patients were much less sensitive to light than those of control subjects. Thus, although the pupils of control subjects responded with a constriction of nearly 2 mm to a dim stimulus delivered to either eye (0.04 scoto lux, 200 msec) (Fig. 2A and 2B), baseline responses of the three patients to the same stimulus were negligible. Before injection, Patients 1 and 2 had a weak response even to 10.0 lux, which is more intense than 0.04 lux by a factor of 250 (Fig. 2C through 2F). Even Patient 3, whose right eye had a Snellen equivalent of 20/640 at baseline, had a weak pupillary response to both 0.04 lux and 10.0 lux (Fig. 2G through 2J).
After injection, the responsiveness of the patients’ right (injected) eyes was reliably greater than that of their left (uninjected) eyes. We observed a strong response in Patient 3, when the dimmest stimulus (0.04 lux, 200 msec) was initially delivered to the right eye 1 month after injection (Fig. 2G). In contrast, we observed minimal constriction when the left eye was stimulated with a “dim flashlight” (0.04 lux). In the subsequent trial, when the same dim stimulus was presented initially to the left eye, the pupillary light reflexes of both pupils were minimal, but when the stimulus was presented to the right eye, again a robust response was recorded (Fig. 2H), a pattern that was repeated with successive alternating flashes. The net result was the appearance of a relative afferent pupillary defect, in which the injected eye drives the pupillary light reflex, whereas the uninjected eye remains defective. Patients 1 and 2 showed improvement in the responsivity of the right (injected) eye to the 10.0-lux stimulus (Fig. 2C and 2E) relative to that of the left (uninjected) eye (Fig. 2D and 2F, shown at 4.75 months and 2.75 months after injection, respectively). Moreover, for both Patients 1 and 2, there was a clear difference in responsivity to the second stimulus in the trial, such that stimulation of the right eye produced the stronger response even if the left eye was stimulated first (Fig. 2D and 2F).
We performed statistical comparisons between the response of each pupil after initial stimulation of the right (injected) eye and the response of the same pupil after initial stimulation of the left (uninjected) eye. For Patient 3, in 16 of 18 such trials, stimulation of the right eye yielded a greater amplitude of response, and the null hypothesis of no difference was rejected (P<0.001). The initial stimulation of the right eye produced a greater response in 15 of 17 paired trials for Patient 1 (P = 0.003) and for 14 of 14 paired trials for Patient 2 (P<0.001). In a similar way, Student’s t-tests for the difference in magnitude of pupillary light reflexes resulting from stimulation of the right eye and the left eye were highly significant for Patient 1 (P<0.001), Patient 2 (P<0.02), and Patient 3 (P<0.001) (see the Results section of the Supplementary Appendix).
In summary, after injection of AAV2.hRPE65v2, each of the three eyes that received injection became more effective in driving the pupillary response. Each eye that received injection became approximately three times as sensitive to light as it had been at baseline, and the sensitivity of the eye that received injection surpassed that of the (previously better functioning) other eye.
EFFECTS ON VISION
Starting 2 weeks after surgery, all three patients reported having improved vision in dimly lit environments. Improvements in the patients’ pupillary light reflexes were accompanied by significant improvements in visual acuity. Testing showed that the postoperative average visual-acuity logMAR score improved by 0.28 for Patient 1, whose scores increased from hand-motion recognition (0 letters) to a Snellen equivalent of 20/1050 (approximately 3 lines on the eye chart) (P<0.001 by the Wilcoxon rank-sum test). The logMAR score for Patient 2 improved by 0.45, from hand-motion recognition (0 letters) to a Snellen equivalent of 20/710 (22.5 letters, or >4.5 lines on the chart) (P<0.001 by the Wilcoxon rank-sum test). Patient 2 also showed an increase in visual acuity in his left (uninjected) eye, with an increase in the Snellen equivalent from 20/500 to 20/220 and an improvement in the logMAR score of 0.36 (Table 1). For the right (injected) eye of Patient 3, the change in the logMAR score was 0.34, more than 3.5 lines of letters (P = 0.002 by the Wilcoxon rank-sum test).
We observed a trend toward improvement in the visual-field area of each of the three subjects (Table 1, and Fig. 3 of the Supplementary Appendix). Although visual-field testing in groups of patients with severe vision impairment shows substantial variability,18,19 the observed enlargements exceeded the variation in the eye that had not received injection.
All three patients had significant nystagmus at baseline (see the Results section of the Supplementary the Appendix). After injection of AAV2 hRPE65v2, the patients had a decrease in both monocular and binocular amplitude and frequency of nystagmus in primary position, with eccentric gaze, and with monocular cover.
In tests of ability to navigate an obstacle course before the injection of AAV2.hRPE65v2, Patients 1 and 2 had great difficulty and collided with the majority of the 14 obstacles and strayed off course many times (Fig. 2A of the Supplementary Appendix and Video 2A). Patient 3, who had more central vision than the other two patients, was challenged only by objects in her peripheral vision. After injection, Patient 2 was able to follow the arrows on the course (Video 2B).
DISCUSSION
All three patients with LCA2 who received AAV2.hRPE65v2 by subretinal injection showed evidence of improvement in retinal function. Improvement in the pupillary light reflex by objective physiological testing was accompanied by improved values in subjective psychophysical measures. Testing revealed gains in visual acuity at 6 weeks; thereafter, there was a slower rate of improvement. Reduction in nystagmus, such as the reduction we previously reported in canine studies,20 may account for the improved visual acuity in the left (uninjected) eye of Patient 2. The improvements in the eyes that received injection exceeded the limits of test–retest variability and were of a magnitude believed to be of functional importance.17 However, a placebo effect may have contributed to the improved measures and cannot be ruled out in this unmasked surgical trial.
In our study, testing of the pupillary light reflex not only confirmed increased retinal sensitivity in the eye that received injection but also showed better function after surgery, as compared with function in the other eye. Although in control subjects there is variability in the latency of pupil movement and in the amplitude of pupil constriction as a function of light intensity, latency and amplitude are normally well matched between the two eyes of individual subjects.21–23 Finally, since we did not inject empty vector, we cannot be sure that the improvement reflects expression of the protein encoded in the AAV vector.
There were no apparent local or systemic adverse events elicited by exposure to the AAV vector. The macular hole that developed in Patient 2’s right eye 2 weeks after subretinal injection did not appear to be related to AAV2.hRPE65v2, since we observed no signs of inflammation or acute retinal toxicity. We hypothesize that the macular hole was caused by contraction of a preexisting membrane stimulated by the surgical procedure, although it is possible that it was a direct result of the surgical procedure itself.24,25 Whereas the development of a macular hole would not be expected to affect retinal function in patients with a loss of central vision similar to that of our patients, it could critically affect the vision of those with a lesser degree of retinal degeneration.
The clinical benefit to the patients has been sustained during the 6 months since the experimental treatment of LCA2 in Patient 1. Longer follow-up and a larger number of patients will be needed to define measures of safety and efficacy and to identify factors influencing the extent and duration of visual recovery. It is possible that efficacy will be improved if treatment is applied before amblyopia and retinal degeneration are established (i.e., in a pediatric population). Our study provides the foundation for gene-therapy approaches to the treatment of LCA and possibly other forms of retinal degeneration.
Supplementary Material
Acknowledgments
Supported by the Center for Cellular and Molecular Therapeutics at the Children’s Hospital of Philadelphia, the Foundation Fighting Blindness sponsored by the Pediatric Center for Retinal Degenerations, Research to Prevent Blindness, a grant (EY-10820) from the National Institutes of Health, the Macula Vision Foundation, the Paul and Evanina Mackall Foundation Trust, the Scheie Eye Institute, the F.M. Kirby Foundation, grants (TIGEM-P21, to Drs. Auricchio, Surace, and Banfi, and GGP07180, to Dr. Simonelli) from the Italian Telethon Foundation, a grant (UL1-RR-024134) from the National Center for Research Resources, the Rosanne H. Silbermann Foundation, the Associazione Italiana Amaurosi Congenita di Leber, and the Howard Hughes Medical Institute (to Drs. Stone and High).
Drs. Maguire and Bennett report being coinventors on a pending patent for “a method of treating or retarding the development of blindness” and collaborating on studies involving novel AAV serotypes and retinal-cell tropism supported by GlaxoSmith-Kline. Dr. Bennett also reports serving on a scientific advisory board for Ceregene; Dr. Mingozzi, holding a patent on the imposition and methods for detection and modulation of T-cell responses to gene therapy vectors; Dr. Wright, receiving consulting fees from Genable, Tacere, and Genzyme and being an inventor on patents (two issued, one pending, and one provisional) relative to AVV vector development (but waiving any financial interest); and Dr. High, receiving consulting fees from Tacere and lecture fees from Genzyme and serving on an advisory board for Amsterdam Molecular Therapeutics.
We thank the patients and their families for their continuous support of the study; the medical, operating-room, anesthesia, and nursing staff at Children’s Hospital of Philadelphia and the physicians and staff in the Division of Ophthalmology; Douglas Anderson, David Cairns, and Optos for their partnership in evaluating the Optos P200; Drs. Leslie Raffini and Giovanni Cucchiaro for lending their clinical expertise; the members of the hospital’s institutional review board and its chair, Dr. Mark Schreiner, along with David Brint, Dr. Richard Hurwitz, Dr. Mark Blumenkranz, and David Birch, for their invaluable guidance; Drs. Robert Nelson, Andrea Ballabio, Alfredo Ciccodicola, and Ernesto Rinaldi for their helpful discussions; Drs. Stuart Fine and Monte Mills for their support; Daniel Chung, Fred Letterio, Randall Toy, Matthew Canver, Fiorella Saponara, Debra Cheng, Sohani Amarasekera, Katherine H. Maguire, Brandon Johnson, Mary Leonard, Carmela Ziviello, Armida Faella, Anna Nesti, Emilia Maggio, Angelo Torre, and Valentina Di Iorio for their technical and clinical assistance; the members of the Penn–Cornell–Florida consortium for carrying out the initial proof-of-concept studies with us in animal models; and Drs. Kristina Narfstrom and Gregory Acland for supplying additional dogs for study.
REFERENCES
- 1.Aleman TS, Jacobson SG, Chico JD, et al. Impairment of the transient pupillary light reflex in Rpe65(−/−) mice and humans with leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2004;45:1259–1271. doi: 10.1167/iovs.03-1230. [DOI] [PubMed] [Google Scholar]
- 2.Lorenz B, Gyürüs P, Preising M, et al. Early-onset severe rod-cone dystrophy in young children with RPE65 mutations. Invest Ophthalmol Vis Sci. 2000;41:2735–2742. [PubMed] [Google Scholar]
- 3.Simonelli F, Ziviello C, Testa F, et al. Clinical and molecular genetics of Leber’s congenital amaurosis: a multicenter study of Italian patients. Invest Ophthalmol Vis Sci. 2007;48:4284–4290. doi: 10.1167/iovs.07-0068. [DOI] [PubMed] [Google Scholar]
- 4.Perrault I, Rozet JM, Gerber S, et al. Leber congenital amaurosis. Mol Genet Metab. 1999;68:200–208. doi: 10.1006/mgme.1999.2906. [DOI] [PubMed] [Google Scholar]
- 5.Moiseyev G, Chen Y, Takahashi Y, Wu B, Ma J. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci U S A. 2005;102:12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122:449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redmond TM, Yu S, Lee E, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 8.Redmond TM, Poliakov E, Yu S, Tsai J, Lu Z, Gentleman S. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci U S A. 2005;102:13658–13663. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu SM, Thompson DA, Srikumari CR, et al. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet. 1997;17:194–197. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- 10.Aguirre GD, Baldwin V, Pearce-Kelling S, Narfstrom K, Ray K, Acland GM. Congenital stationary night blindness in the dog: common mutation in the RPE65 gene indicates founder effect. Mol Vis. 1998;4:23. [PubMed] [Google Scholar]
- 11.Narfström K, Wrigstad A, Nilsson S. The Briard dog: a new animal model of congenital stationary night blindness. Br J Ophthalmol. 1989;73:750–756. doi: 10.1136/bjo.73.9.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobson SG, Aleman TS, Cideciyan AV, et al. Identifying photoreceptors in blind eyes caused by RPE65 mutations: prerequisite for human gene therapy success. Proc Natl Acad Sci U S A. 2005;102:6177–6182. doi: 10.1073/pnas.0500646102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennicelli J, Wright JF, Komaromy A, et al. Reversal of blindness in animal models of Leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol Ther. 2008;16:458–465. doi: 10.1038/sj.mt.6300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acland GM, Aguirre GD, Bennett J, et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12:1072–1082. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acland GM, Aguirre GD, Ray J, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 16.Joseph D, Thomas M. In: Surgical removal of subretinal choroidal neovascular membranes. Wilkinson C, Retina 3rd, editors. Mosby: St. Louis; 2001. pp. 2562–2572. [Google Scholar]
- 17.Beck RW, Maguire MG, Bressler NM, Glassman AR, Lindblad AS, Ferris FL. Visual acuity as an outcome measure in clinical trials of retinal diseases. Ophthalmology. 2007;114:1804–1809. doi: 10.1016/j.ophtha.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 18.Berson EL, Sandberg MA, Rosner B, Birch DG, Hanson AH. Natural course of retinitis pigmentosa over a three-year interval. Am J Ophthalmol. 1985;99:240–251. doi: 10.1016/0002-9394(85)90351-4. [DOI] [PubMed] [Google Scholar]
- 19.Seiple W, Clemens C, Greenstein VC, Carr RE, Holopigian K. Test-retest reliability of the multifocal electroretinogram and Humphrey visual fields in patients with retinitis pigmentosa. Doc Ophthalmol. 2004;109:255–272. doi: 10.1007/s10633-005-0567-0. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs JB, Dell’Osso LF, Hertle RW, Acland GM, Bennett J. Eye movement recordings as an effectiveness indicator of gene therapy in RPE65-deficient canines: implications for the ocular motor system. Invest Ophthalmol Vis Sci. 2006;47:2865–2875. doi: 10.1167/iovs.05-1233. [DOI] [PubMed] [Google Scholar]
- 21.Birch EE, Birch DG. Pupillometric measures of retinal sensitivity in infants and adults with retinitis pigmentosa. Vision Res. 1987;27:499–505. doi: 10.1016/0042-6989(87)90034-4. [DOI] [PubMed] [Google Scholar]
- 22.Bergamin O, Kardon RH. Latency of the pupil light reflex: sample rate, stimulus intensity, and variation in normal subjects. Invest Ophthalmol Vis Sci. 2003;44:1546–1554. doi: 10.1167/iovs.02-0468. [DOI] [PubMed] [Google Scholar]
- 23.Fosnaugh JS, Bunker EB, Pickworth WB. Daily variation and effects of ambient light and circadian factors on the human light reflex. Methods Find Exp Clin Pharmacol. 1992;14:545–553. [PubMed] [Google Scholar]
- 24.Gass JD. Idiopathic senile macular hole: its early stages and pathogenesis. Arch Ophthalmol. 1988;106:629–639. doi: 10.1001/archopht.1988.01060130683026. [DOI] [PubMed] [Google Scholar]
- 25.Ho AC. Macular hole. In: Guyer D, Yannuzzi L, Chang S, Shields J, Green W, editors. Retina-vitreous-macula. Philadelphia: W.B. Saunders; 1999. pp. 217–229. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.