Abstract
Human T-cell lymphotropic virus type 1 (HTLV-1), causes adult T cell leukemia/lymphoma (ATLL), and initiates a variety of immune mediated disorders. The viral genome encodes common structural and enzymatic proteins characteristic of all retroviruses and utilizes alternative splicing and alternate codon usage to make several regulatory and accessory proteins encoded in the pX region (pX ORF I to IV). Recent studies indicate that the accessory proteins p12I, p27I, p13II, and p30II, encoded by pX ORF I and II, contribute to viral replication and the ability of the virus to maintain typical in vivo expression levels. Proviral clones that are mutated in either pX ORF I or II, while fully competent in cell culture, are severely limited in their replicative capacity in a rabbit model. These HTLV-1 accessory proteins are critical for establishment of viral infectivity, enhance T- lymphocyte activation and potentially alter gene transcription and mitochondrial function. HTLV-1 pX ORF I expression is critical to the viral infectivity in resting primary lymphocytes suggesting a role for the calcineurin-binding protein p12I in lymphocyte activation. The endoplasmic reticulum and cis-Golgi localizing p12I activates NFAT, a key T cell transcription factor, through calcium-mediated signaling pathways and may lower the threshold of lymphocyte activation via the JAK/STAT pathway. In contrast p30II localizes to the nucleus and represses viral promoter activity, but may regulate cellular gene expression through p300/CBP or related co-activators of transcription. The mitochondrial localizing p13II induces morphologic changes in the organelle and may influence energy metabolism infected cells. Future studies of the molecular details HTLV-1 “accessory” proteins interactions will provide important new directions for investigations of HTLV-1 and related viruses associated with lymphoproliferative diseases. Thus, the accessory proteins of HTLV-1, once thought to be dispensable for viral replication, have proven to be directly involved in viral spread in vivo and represent potential targets for therapeutic intervention against HTLV-1 infection and disease.
1. Introduction
Human T-cell lymphotropic virus type 1 (HTLV-1), the first described human retrovirus, causes adult T cell leukemia/lymphoma (ATLL), an aggressive CD4-T cell malignancy and initiates a variety of immune mediated disorders including HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP), a chronic neurodegenerative disease 1-3. This virus infects approximately 20 million people worldwide and is endemic to the Caribbean, Japan, Africa, and South America4-7. It is also a serious problem among at-risk groups in the United States7. The details of how HTLV-1 promotes the development of these diseases is not clear, but is likely related to the ability of the virus to evoke lymphocyte activation 8-12. The epidemiology and diseases associated with HTLV-1 are well known, however the molecular mechanisms used by the virus to establish persistent infection and subsequently facilitate lymphocyte proliferation while circumventing immune elimination, remains less well defined.
The genome of HTLV-1 encodes structural (e.g., group specific core antigens, Gag) and enzymatic proteins (e.g., reverse transcriptase, RT) characteristic of all retroviruses. Additionally, HTLV-1, a complex retrovirus, utilizes alternative splicing mechanism and internal initiator codons, to make several regulatory and accessory proteins encoded by four open reading frames (ORFs) of the pX region (pX ORF I to IV) between the env gene and the 3′ long terminal repeat1,13}. ORF IV of HTLV-1 encodes the well-characterized Tax transactivating protein, while the ORF III encodes Rex, a key regulator of viral RNA transport. Tax is a 40 kD nuclear- localizing phosphoprotein, which interacts with cellular transcription factors to activate transcription from the viral promoter (Tax responsive element, TRE), as well as the enhancer elements of various cellular genes associated with host cell proliferation14-18. Rex is a 27 kD, nucleolar-localizing phosphoprotein that functions to enhance nuclear export of unspliced or singly spliced viral RNA thus contributing to virus propagation19-23. Both Tax and Rex have been the subject of recent reviews15-17,19,24-27. In this review we focus on the important role that accessory proteins have in HTLV-1 replication and pathogenesis. Base on recent studies it has become clear that these proteins are, in fact, essential for the virus life cycle and may determine disease outcome associated with HTLV-1 infection.
2. HTLV-1 Accessory Proteins Encoded by the pX Region
The HTLV-1 pX genome region includes ORF I and II that produce alternatively spliced forms of mRNA, which encode four accessory proteins, p12I, p27I, p13II, and p30II (Fig. 1)28-31. pX ORF I mRNA is produced by alternative splicing event s that combine the second exon of Tax with additional downs tream sequences and encodes p27I (152 amino acids long) and p12I (99 amino acid long). p12I can be translated from a singly spliced message produced by direct splicing of nucleotide 119 to the splice acceptor at position 6383 or by initiation at an internal methionine codon in the p27I ORF I32. p12I is thought to be preferentially expressed from the p27I mRNA since transfection of expression plasmids containing HA1-tagged versions of either the full- length p27I cDNA or the cDNA for the singly spliced p12I produced only the smaller p12I protein30. However, using in vitro transcription-translation systems, Ciminale et al. 32 produced p27I from the doubly spliced mRNA. Therefore, removal of the internal p12I AUG start codon could yield detectable levels of p27I. Interestingly, Pique et al. 33 demonstrated production of cytotoxic T cells in HTLV-1-infected subjects that were reactive against peptides representing all putative pX accessory proteins, including p27I. The accessory proteins encoded by pX ORF II of HTLV-1 are produced from two alternatively spliced mRNAs. The larger protein, p30II, is encoded by a doubly spliced message including the first and second exon of Tax spliced to the splice acceptor site at position 6478 31. p13II, the smaller protein contains the C-terminal 87 amino acids of p30II and is produced from a singly spliced message by splicing of the first Tax exon directly to the splice acceptor at position 6875 or translated from an internal methionine codon within p30II30,31. HTLV-1 accessory proteins were originally thought to be dispensable for viral replication 34. However, recent investigations performed by our laboratory and others have shed light into the role of the HTLV-1 accessory proteins in viral infectivity, maintenance of high viral loads, host cell activation, and regulation of gene transcription 35-48. There is evidence that pX ORFs I and II mRNAs and proteins are expressed both in vitro and in vivo. Studies employing reverse transcription-PCR (RT-PCR) assays have detected the presence of these mRNAs in infected cell lines and freshly isolated cells from HTLV-1-infected subjects 30 while semi quantitative RNase protection assays have identified these mRNAs in ATL and HAM/TSP patients49. In HTLV-1 infected patients, and asymptomatic carriers, studies have determined the presence of antibodies 45,50 and cytotoxic T cells33 against recombinant proteins or peptides of the pX ORF I and II proteins. However, to date, there are no conclusive reports on the temporal expression of Tax, Rex and the four accessory proteins messages in terms of their specific quantities or relative levels. Chronically infected cell lines were found to have pX-tax/rex mRNA at 500- fold to 2500-fold higher levels than pX tax-ORF II mRNA and 1000-fold higher levels than pX-rex-ORF I mRNA (D. Derse personal communication).
Figure 1.
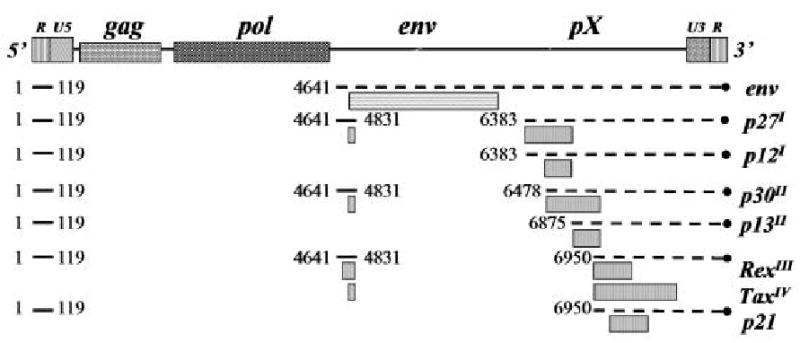
Diagrammatic illustration of HTLV-1 genome with nucleotide location (#) shown for spliced sites. Proteins produced or predicted from RNA shown on right with ORF origin shown as superscript.
Interestingly, analogous gene regions encoding the accessory proteins, especially the pX ORF I-encoded p12I, are highly conserved in the closely related virus HTLV-2 and the nonhuman primate counterpart, simian T-cell lymphotropic virus type 1 (STLV-1)13. Further illustration of the conserved nature of these gene regions comes from studies of another member of the deltaretroviruses, bovine leukemia virus (BLV). BLV, like HTLV-1, contains an X region between the env sequences and the 3′ long terminal repeat. Two proteins are expressed from this region of BLV: the protein R3, which shares a common nuclear localization signal (NLS) with the Rex protein of HTLV-1, and G4, an arginine rich protein that may exist as two isoforms following protease processing 51,52. Similar to HTLV-1, deletion of homologous sequences from BLV infectious molecular clones encoding these accessory proteins, R4 and G3, results in decreased viral loads in the experimental sheep model 51,53,54. Collectively these studies illustrate that these retroviruses, which are all associated with lymphoproliferative disorders, during the course of their evolution have retained conserved gene regions that apparently serve analogous functional roles.
3. pX ORF I p12I
3.1 Biochemical Characteristics of p12I: Features of a Signaling Molecule
HTLV-1 p12I is a highly hydrophobic protein, which contains a significant percentage of leucine (32%) and proline (17%) residues30. Hydropathy and immunogenicity plots demonstrate a minimal number of soluble regions and two putative transmembrane domains extending from amino acid 12 to 30 and amino acid 48 to 6748,55, which overlap with two predicted leucine zipper motifs that form alpha- helices. These distinct structural features could contribute to membrane localization or homo-oligomerization of the protein (Fig. 2). Immunoprecipitation and immunoblot analysis have demonstrated that p12I indeed forms at least dimers, if not oligomers48. However, helix-breaking proline residues within the predicted leucine zippers suggest that these may not be functional leucine zippers. Additionally, p12I contains four predicted SH3-binding motifs (Fig. 2). Typically, SH3-binding motifs in cellular signaling proteins are proline rich with a minimal core of PXXP and are often preceded by an arginine residue at +2 56. Interestingly, amino acids 8 to 11 and 70 to 74, encoding the first and third PXXP motifs of p12I are highly conserved among viral strains, suggesting a possible role for these domains in the function of p12I. We have described another conserved sequence (PSLP(I/L)T) extending from amino acid 48 to 99 in p12I, highly homologous to the PXIXIT calcineurin-binding motif of NFAT and found to be critical for the interaction between p12I and calcineurin35. In addition, p12I contains a dileucine motif (DXXXLL) at amino acid positions 26 to 31, with no functional role ascertained yet (Fig. 2). However, dileucine motifs have been described in viral proteins such as HIV Nef and are commonly involved in directing protein trafficking through endosomal compartments by mediating association of the protein with adapter protein 1 (AP-1) to AP-357. In addition to the motifs described above, sequence analysis of p12I suggests possible post-translational modifications of the protein. There is a ubiquitynation motif surrounding the lysine at position 88 of p12I. Although the functional significance of this motif remains unclear, arginine substitution at this position, commonly found among natural HTLV-1 strains, significantly enhances the half- life of the protein48. In addition to the ubiquitylation site, potential phosphorylation sites and glycosylation sites are also present in p12I. Sequence analysis reveals a putative phosphorylation site of protein kinase C (LTMR) at amino acid 75, a potential phosphorylation site of casin kinase 2 (SPGD) at amino acid 23, a potential N-linked glycosylation site at amino acid 51 (Asn) and multiple potential O-linked glycosylation sites (Ser and Thr) in HTLV-1 p12I. However, a deglycosylation study demonstrated that p12I is not a glycoprotein 55 while p12I does not appear to be a phosphoprotein (S. Kim, personal communication).
Figure 2.

Diagram of key HTLV-1 accessory proteins with known motifs. PxxP = proline motifs predicted to be SH3 binding domains, LZip = leucine zipper like motif, DxxLL = putative AP-1 adaptor binding motif, IL-2R = region of IL-2 receptor binding, PxIxIT = calcineurin binding motif, NLS = nuclear localization motif, MTS = mitochondrial targeting signal.
3.2 Subcellular Localization of p12I and Interactions with Cellular Proteins
HTLV-1 p12I was originally reported to localize in cellular endomembranes, which was suggested by a spider- like staining of cells expressing the viral protein 29. We characterized the subcellular localization of p12I in transfected 293T cells and Hela-Tat cells by multiple methods including immunofluorescent confocal microscopy, electron microscopy and subcelluar fractionation. Our findings indicated that p12I accumulates in the endoplasmic reticulum (ER) and cis-Golgi apparatus and remained unchanged following both cycloheximide (blocking de novo protein synthesis) and brefeldin A (disrupting ER-to-Golgi protein transport) treatments, indicating the protein is retained in the ER and cis-Golgi. Using coimmunoprecipitation assays, we identify the direct binding of p12I with both calreticulin and calnexin, resident ER proteins, which regulate calcium storage. These results indicated that p12I directly binds key regulatory proteins involved in calcium- mediated cell signaling and suggested a role of the viral protein in the establishment of HTLV-1 infection by activation of host cells 55.
HTLV-1 p12I shares sequence homology with the bovine papilloma virus (BPV) E5 protein and Epstein-Barr virus (EBV) LMP-1 protein 58. The region of highest homology starts after the first transmembrane domain and extends into the second. Like E5, HTLV-1 p12I also binds to the 16 kD subunit of the vacuolar H+-ATPase (16K). Although p12I itself cannot induce the transformation of mouse C127 fibroblasts, it cooperates with E5 and potentiates the transforming ability of E558. Further study to map the motif in p12I associated with 16K protein revealed that a central proline-rich region between aa 36-48 is required for the interaction, but this region alone is not sufficient for binding 59. Although this association appears to be required for the E5- mediated transformation of epithelial cells, there is no clear correlation with the weak transforming ability of p12I, the p12I-16K interaction and cooperative transformation with BPV E5. Thus, the functional significance of the p12I-16K interaction remains to be determined. Interestingly, Nef, a key accessory protein of simian immunodeficiency virus (SIV) and human immunodeficiency virus (HIV), binds the catalytic subunit NBP-1 of the ATPase60. NBP-1 association of Nef mediated by the Nef C-terminal flexible loop is critical for Nef-dependent internalization of CD4 and viral infectivity 61.
Analogous to E5, p12I also associates with immature form of IL-2 receptor β and γ chain when transiently co-expressed, resulting in reduced surface expression of the receptor chains62. The IL-2R binding region of p12I mapped to the central proline-rich region (amino acids 37 to 47), which lie directly in front of the C-terminal proposed transmembrane domain of the protein. The p12I-binding site on the IL-2R chain overlaps with the binding site for JAK kinases 1 and 3 and the adapter protein Shc. p12I does not influence JAK3 kinase activity directly, however it is considered to be accountable for the modest increase in STAT5 DNA binding activity in 293T cells co-transfected components of the IL-2 receptor signaling complex and in primary human lymphocytes transduced with a lentiviral p12I-expressing vector63. As a consequence, p12I-expressing cells displayed a decreased requirement for IL-2 to induce proliferation during suboptimal stimulation with anti-CD3 and anti-CD28 antibodies 63. However, peripheral blood derived lymphocyte cell lines immortalized by a HTLV-1 proviral clone ablated for pX ORF I expression, ACH.p12I, have intact IL-2 receptor signaling pathways 64. A possible explanation for these conflicting observations is that p12I may modestly activate IL-2 receptor pathways during the early stages of HTLV-1 infection before immortalization. Nevertheless, p12I does not appear to be necessary for the activation of the IL-2R-associated Janus kinases, JAK1 and JAK3, or their downstream effectors STAT3 and STAT5, after immortalization. Collectively, these studies indicate that p12I may induce STAT activity to confer a growth advantage to infected cells during the early stages of infection, before immortalization. Future studies are necessary to elucidate the JAK3-independent pathway p12I uses to induce STAT5 activation.
HTLV-1 p12I was reported to associate with immature forms of the major histocompatibility complex class I (MHC I), interfere with the interaction of MHC I with β2-microglobulin, decrease the surface expression of transfected MHC-I and direct its degradation in the proteasome while co-expressed in Hela-Tat cells 65,65. Additionally, the surface expression of endogenous MHC-I is decreased in Jurkat cells transduced with a p12I encoding lentiviral vector. These results suggest that p12I might help the virus escape immune surveillance by down regulating MHC-I surface expression. However, levels of MHC-I and II were similar between T-lymphocytes immortalized with the wild type and p12I- mutant HTLV-1 molecular clones (ACH and ACH.p12 respectively), indicating that p12I-mediated modulation of MHC-I surface expression likely occurs only during the early stages of infection64. Intriguingly, the accessory proteins p10I and p11V of HTLV-2 also associate with MHC-I, however these do not bind to either 16K or IL-2Rβ or γ66. Additionally, HIV-1 Nef also binds to and down regulates the cell surface expression of MHC-I and is believed to contribute to immune evasion by HIV-167. However, down regulation of MHC-I of virus-infected cells does not explain the early loss of infectivity of a molecular clone of HTLV-1 that lacks ORF I expression, as virus infection is blocked as early as 1 week post-inoculation, before a possible active immune response occurs43. Future studies of early virus replication immediately after inoculation of virus infected cells in animal models might provide evidence to whether p12I indeed down regulates MHC-I expression on infected PBMC in vivo and actively contributes to viral spread or persistence.
In addition, p12I associates with two ER-resident calcium-binding proteins, calreticulin and calnexin55 and the calcium/calmodulin-dependent serine/threonine phosphatase, calcineurin 35. Calreticulin, a highly conserved and ubiquitous protein, serves as one of the major calcium-binding proteins in the ER, participates in calcium signaling, and has been linked to activation of the transcription factor nuclear factor of activated T cells (NFAT)68. Through protein-protein interactions, calcineurin regulates transcriptional activation of NFAT by triggering the dephosphorylation and subsequent nuclear translocation of NFAT, which results in transactivation of NFAT-inducible cytokine genes including interleukin-269-71. It would be advantageous for a virus to target such conserved proteins to dysregulate calcium signaling pathways to activate and modulate NFAT in infected T lymphocytes.
3.3 Role of 12I in Regulation of Viral Infectivity in vitro and in vivo
Earlier reports showed that deletion of pX ORF I from HTLV-1 infectious molecular clones did not affect the viral infectivity and primary lymphocyte transformation mediated by HTLV-1 infection in vitro34,72. In contrast, our research group demonstrated that selective elimination of pX ORF I from the molecular clone ACH resulted in dramatically reduced viral infectivity in vivo 43. Rabbits inoculated with ACH.p12, harboring selective mutations that abolish the expression of pX ORF I mRNA, failed to establish persistent infection as indicated by reduced anti- HTLV-1 antibody responses, failure to demonstrate viral p19 antigen production in PBMC cultures, and only transient detection of provirus by PCR43. A major difference between these in vitro and in vivo studies is that standard in vitro co-culture techniques used to transmit virus to naive PBMC utilize target cells stimulated by IL-2 and mitogen. However, in vivo the majority of circulating and tissue-associated lymphocytes are nondividing.
In support of these findings, using co-culture assays that would allow transmission of the virus to resting primary lymphocytes, our group demonstrated that pX ORF I mRNA is critical for viral infectivity in non-activated/quiescent PBMCs in vitro42. In this study, HTLV-1 was transmitted by co-culturing naive PBMCs with three different virus producing cells, including HTLV-1 immortalized cell lines, ACH transfected 293 T cells and newly infected PBMCs, in the absence of exogenous stimuli to more accurately reflect the virus-cell interactions during the natural infection. Under these conditions, a significant decrease in viral infectivity of ACH.p12 producer cells was detected in primary lymphocytes. More importantly, viral infectivity was restored upon addition of IL-2 and mitogen to the co-cultured PBMCs42. These data provided the first evidence that HTLV-1 p12I is required for optimal viral infectivity in nondividing primary lymphocytes and suggested a role of p12I in T- lymphocyte activation and in the early stage of viral infection. Analogously, studies of HIV-1 Nef indicate that the accessory protein is dispensable for transmission of the virus to activated target cells in vitro but is required for viral infectivity in nondividing lymphocytes73-75.
3.4 Role of 12I in Calcium-Mediated T Cell Activation
Reports from our laboratory illustrated that p12I expression in Jurkat cells results in ∼ 20-fold activation of NFAT dependent gene expression, while AP-1 or NF-κB- mediated transcription remained unchanged36. p12I specifically activates NFAT in synergy with Ras/MAP kinase activation stimulated by the phorbol ester, PMA. By inhibition of proximal signaling molecules, such as phospholipase C- γ (PLC-γ) and LAT, as well as distal signals, including calcineurin and NFAT, the function of p12I was mapped to be between PLC-γ and calcineurin36. p12I mediated NFAT activation was dependent on cytosolic calcium since this function was abolished by BAPTA-AM treatment. Importantly, p12I functionally substituted for thapsigargin, which specifically depletes the ER calcium store by blocking the ER calcium ATPase. Therefore, HTLV-1 p12I was found to activate NFAT-mediated transcription in lymphoid cells in a calcium-dependent manner. Indeed, p12I expression increases the base- line cytoplasmic calcium concentration in Jurkat T cells. This basal elevated calcium is likely secondary to reduced ER stores calcium content and subsequently higher extracellular calcium entry37. Both the calcium channel on ER membranes, IP3R, and the calcium channels on plasma membranes, calcium release activated calcium channels (CRAC), contribute to the p12I mediated NFAT activation, strongly indicating the modulation role of p12I on calcium homeostasis.
The localization of p12I to the ER appears to be essential for NFAT activation, since diffusely localizing p12I truncation mutants were unable to activate NFAT, and partially restored the ability to activate NFAT when redirected to ER by addition of ER localization signal76. p12I colocalizes with the ER-resident, calcium-binding proteins calreticulin and calnexin, which are involved in multiple cellular functions including calcium homeostasis, protein folding, as well as integrin mediated signaling pathways55. The direct binding between calreticulin and p12I does not correlate with the NFAT activation since expression of calreticulin dramatically reduced p12I-induced NFAT activation independent of direct calreticulin-p12I interaction37. Future studies are necessary to identify the biological significance of this protein-protein interaction in HTLV-1 infection and pathogenesis.
Interestingly, expression of p12I increased cytosolic calcium, indicating that HTLV-1 p12I induces release of calcium from the ER to activate NFAT 37, which would be an advantage for the virus during the early stages of HTLV-1 infection. Interestingly, this is similar to the function of a cellular protein CAML (Ca2+-modulating cyclophilin ligand), which also contain two putative transmembrane domains like p12I, colocalizes with calreticulin in the ER, induces calcium release from the ER and leads to NFAT activation 77. In addition, p12I mediated increase in NFAT activity could cause complete activation of cellular stimuli that would normally induce only partial activation of T cells (e.g., through AP-1). These stimuli could be triggered by cytokines or chemokines released from infected neighboring cells or by direct contact between viral envelope proteins and certain cell surface receptors on newly targeted lymphocytes prior to viral entry 78.
Expression of NFAT induces a highly permissive state to overcome the blockade at reverse transcription and permitted HIV replication in primary CD4+ T cells, therefore it is possible that p12I causes T cells to be hypersensitive to T cell receptor and CD28 stimulation and thus highly permissive for subsequent viral infection. Interestingly, susceptibility of these cells to HIV infection could be restored by mitogen treatment, likely due to the phytohemagglutinin- induced upregulation of NFAT activity. This is similar to earlier reports from our laboratory that addition of mitogens can rescue the infectivity of a p12I mutant viral clone in resting PBMC42, likely by overriding the requirement for p12I-induced activation of NFAT. It will therefore be critical to examine the effect of cyclosporine in HTLV-1 replication in primary lymphocytes and compare the drug's capacity to affect replication of wild type and p12I mutant clones. Interestingly, cyclosporine reduces the infectivity of HIV and is strongly dependent on the presence of a functional nef gene79.
Other retroviruses also encode proteins regulating Ca2+- related signals by analogous or different mechanisms in T lymphocytes or other cell types. HIV accessory protein, Nef, also activates NFAT in synergy with Ras/MAPK pathway in a calcium dependent fashion73,80,81. Similar to p12I, Nef is dispensable for viral infection of activated target cells in vitro, but is required for viral infectivity in quiescent lymphocytes75,82-84. Nef contains SH3 domain binding motif responsible for the interaction of this protein with multiple cellular proteins and causes NFAT activation, which is dependent on its interaction with IP3R73.
Importantly p12I appears to enhance the production of a downstream gene of NFAT activation, interleukin-2 (IL-2) in Jurkat T cells and primary lymphocytes, which could be abrogated by calcium chelator BAPTA-AM and calcineurin inhibitor, cyclosporin A, suggesting the effect is calcium pathway-dependent76. This increase in IL-2 could account for the decrease in requirement for the cytokine in proliferation of human primary lymphocytes in the presence of p12I63. Overall, p12I expression promotes T cell activation and likely facilitates the viral replication and productive infection, which correlates with our previous finding that p12I is necessary for viral infectivity in a rabbit model of infection43.
Recent reports from our laboratory further characterized the p12I-mediated NFAT activation by identifying the two positive (aa 33-47, aa 87-99) and two negative (aa 1-14, aa 70-86) regions in p12I that regulate the NFAT activation, using truncation mutants76. Interestingly, these two positive and two negative regions contain individual SH3 binding domains (PXXP motif). SH3 binding domain has been demonstrated to be important for protein-protein interaction and the PXXP motif in HIV Nef was found to be necessary for Nef-mediated NFAT activation and viral infection85-87. In a recent report from our laboratory, proline residues in these motifs were mutated into alanine residues to test the role of PXXP motifs in p12I- mediated NFAT activation. Interestingly, the third SH3 binding domain (aa 70-73) was responsible for the negative effect of region aa 70-86 on NFAT activation 35,76. Besides, mutations in the first two PXXP motifs (aa 8-11 and 35-38) caused only minimal changes in NFAT activation, while mutations in the last PXXP motif (aa 90-93) in the second positive region (aa 86-99), enhanced the NFAT activation. Future studies are necessary to elucidate the functional significance of specific residues within the PXXP motifs of p12I in NFAT activation.
We have demonstrated the role of the highly conserved calcineurin binding motif PSLP(I/L)T of p12I in its binding to calcineurin35. Interestingly, p12I competes with NFAT for calcineurin binding as evidenced by the calmodulin bead pull-down experiments. More strikingly, alanine substitution mutations in PSLP(I/L) caused more NFAT nuclear translocation and increased NFAT transcriptional activity (∼2-fold) than wild type p12I in a reporter gene assay35. Interestingly, PSLP(I/L)T calcineurin binding site is within the aa 70–86 negative region and third PXXP motif which was responsible for the negative effect of region aa 70-86 on NFAT activation. Additionally, NFAT- inhibitory function of the PSLP(I/L)T motif in p12I was verified to be not from the inhibition of calcineurin phosphatase activity. PSLP(I/L)T is homologous to the conserved functionally critical calcineurin-binding motif (PXIXIT) in the N-terminal regulatory domain of NFAT, which binds both inactivated and activated calcineurin 88. Many calcineurin-binding proteins such as the anti-apoptotic protein Bcl-2, calcineurin B homologous protein, a kinase anchoring protein AKAP79, and myocyte-enriched calcineurin- interacting protein 1 inhibit either calcineurin phosphatase activity or its substrate NFAT transcriptional activity89-91. However, PXIXIT motif mediated binding itself does not inhibit the catalytic activity of calcineurin, because NFAT activation requires enzymatic activity of calcineurin as well as binding via this motif. Not surprisingly, p12I binding to calcineurin via a motif similar to PXIXIT did not inhibit calcineurin catalytic activity but instead influenced NFAT and calcineurin interaction by competing for binding with NFAT similar to artificial peptides representing this motif 88.
Due to the existence of a calcineurin-binding motif in p12I, p12I may have at least two regulatory actions to modulate NFAT activation: (1) positive modulation by increasing cytosolic calcium concentration from ER stores and (2) negative modulation by calcineurin binding. It is unclear why p12I has two regulatory functions for NFAT transcriptional activity. It is notable that Bcl-2 has these similar properties like p12I, however the functional relationship between calcium release from the ER and calcineurin binding of Bcl-2 is also still unresolved. Bcl-2 maintains calcium homeostasis and prevents apoptosis by localizing not only at the mitochondrial membrane but also in the ER membranes 92,93. p12I may function like Bcl-2 at the ER membrane, acting like an ion channel protein to increase ER calcium permeability. Like Bcl-2, p12I may affect apoptosis in HTLV-1-infected T cells. Another ER membrane protein, CAML that activates NFAT by increasing calcium flux binds with calcineurin indirectly, through its association with cyclophilin77. Overall, p12I interacts with calcineurin, an important regulator of NFAT signaling, via a highly conserved PSLP(I/L)T motif, to further T cell activation, an important antecedent to effective viral infection, via a calcium/calcineurin/NFAT pathway.
3.5 Putative Role of p12I Variants in Disease
Factors determining the progression from asymptomatic state to HAM/TSP and the contribution of viral factors are not fully understood. Recent studies aimed to identify sequence variation/ viral strains that are neuropathogenic suggest a possible role for p12I in the pathogenesis of HAM/TSP. The proteasome destabilization of viral proteins is thought to be an intracellular defense mechanism against viral infection 94,95. Lysine is a known target for covalent binding of ubiquitin 94-96 and the metabolic instability p12I is mediated in part by ubiquitylation at a single lysine residue at position 88 and subsequent proteasomal degradation, as well as by destabilizing residues at its amino terminus48. Interestingly, earlier analysis of p12I ORF in 21 HTLV-1 strains from different geographical areas97 had demonstrated that p12I amino acid sequence is highly conserved and that, arginine residue is found more frequently at position 88 while the less frequent lysine carrying allele was found only in some TSP-HAM cases. Trovato et al 48 extended this information by studying an additional 32 ex vivo samples from healthy carriers, TSP-HAM patients, ATLL patients and families in which both diseases occur, and confirmed that the lysine residue is found only in patients with TSP/HAM, irrespective of geographical locations, suggesting that a selective pressure over p12I might occur in the host. However, lysine residue at position 88 in p12I was not found in all HAM/TSP patients. It is hypothesized that the reduced stability of p12I in HAM/TSP patients due to this sequence variation may facilitate generation of a viral-specific CTL response, since degradation of p12I would alleviate the reduction of MHC class I molecules at the cell surface98.
Martins et al extended these observations further to verify whether the presence of lysine at position 88 could be used as a marker of progression to HAM/TSP, by analyzing 37 HAM/TSP patients and 40 asymptomatic carriers at different stages of infection 99. Interestingly, in this study, lysine residue at position 88 of p12I did not appear to be a universal diagnostic marker for HTLV-1-associated neurological disease, since this phenotype was found not only in HAM/TSP patient (1 out of 37), but also in asymptomatic HTLV-1 carrier (1 out of 40) who did not develop neurologic signs for 5 years99. Even though this study analyzed a larger number of samples, all individuals in this study were born in the same geographic region, and thus it might simply represent a particular HTLV-1 carrier population in which the selective pressure on the p12I sequence would not be occurring. Overall, the significance of natural p12I alleles is unclear, and it is possible that the lysine at position 88 of p12I might have a significant effect on the biological effects of the protein in the host, including giving a possible selective advantage in individuals with a certain MHC I. Future studies including screening of HTLV-1-infected individuals in other populations may elucidate this further.
4. pX ORF II p30II
Earlier studies suggested that ORF II was dispensable for expression of HTLV-1 proteins Tax, Rex, or Env, viral replication and immortalization of primary lymphocytes in vitro34,72. In addition, the isolation of a viral clone containing a premature stop codon in pX ORF II, from leukemic cells led to the conclusion that pX ORF II was not necessary for the outgrowth of leukemic clones in vivo 100. However, possible functional role of pX ORF II during early infection was not be ruled out by these initial studies. To specifically test the functional role of pX ORF II in viral replication in vivo, we inoculated rabbits with lethally irradiated cell lines expressing the wild-type molecular clone of HTLV-1 (ACH) and a clone containing selected mutations in pX ORF II (ACH.p30/13)39. While all ACH-inoculated rabbits became infected as early as 2 weeks postinoculation, ACH.p30/13- inoculated animals failed to become infected or maintained low proviral copy numbers in their blood leukocytes. These animals also had weak and transient ex vivo p19 antigen production from their PBMC cultures and anti-HTLV-1 antibody titers declined towards the end of the study. Most strikingly, using quantitative competitive PCR, we demonstrated a dramatically reduced (up to 100- fold) viral load in the ACH.p30/13- infected animals 39. Taken together, these data suggested that pX ORF II is in fact necessary for maintenance of high viral loads in vivo.
4.1 Biochemical Characteristics of p30II: Features of a Transcription modulator
Several lines of evidence indicate that p30II acts as a transcription factor. Importantly, the protein localizes to the nucleus, specifically the nucleolus of cells transiently transfected with a p30II expression vector29,41. p30II contains a highly conserved bipartite nuclear localization signal (NLS) between amino acids 71 to 98, which can be functionally substituted for the NLS of Rex101. In addition, p30II contains serine and threonine-rich regions that share distant homology to the activation domain of cellular transcription factors, such as Oct-1/2, Pit-1, and POU-1 32 (Fig. 2). Taken together, these characteristics suggest that p30II has a role in transcription of viral and cellular gene expression.
4.2 Role of p30II in Modulating HTLV-1 LTR-Mediated Transcription
We have reported that, when provided in limiting concentrations, p30II expression stimulates HTLV-1 LTR-driven reporter gene activity, even in the presence of Tax, whereas higher concentrations represses LTR and CRE-driven reporter gene activity41. These activities are analogous to herpes simplex virus type 1 (HSV-1) regulation of its immediate-early (IE) gene promoter. VP16, a potent transcription factor from HSV-1, binds the host cell protein HCF, which facilitates the stable complex formation of the viral protein with Oct-1102,103. The IE gene promoter contains an Oct-1-like motif (TAATGARAT) that is important for IE gene expression, with both positive and negative effects, depending on the context of these cellular transcription factors and VP16102.
We have also demonstrated that p30II co-localizes with p300 in the nucleus and physically interacts with CREB binding protein (CBP)/p300, at the highly conserved KIX domain, the domain HTLV-1 Tax also interacts with. In addition, p30II is able to disrupt CREB-Tax-CBP/p300 complexes bound to the viral 21-bp Tax Responsive Elements (TRE) repeats38. To recruit CBP/p300, Tax acts as a high-affinity binding site within this complex104-106. Once associated with the viral promoter, CBP/p300 is believed to remodel chromatin and/or make communication with the basal transcription machinery possible.
HTLV-1 Tax, a transactivator of LTR mediated transcription, is a key player in the activation of the HTLV-1 viral genes through its interaction with the p300 and CBP coactivators104,105,107,108. Tax is able to regulate LTR mediated transcription by the recruitment of p300/CBP and P/CAF to these specific sites in the HTLV-LTR promoter104,108. Since p30II and Tax interacts with CBP/p300 through the same KIX domain, it is possible that the competitive CBP/p300 binding between p30II and Tax might be the mechanism by which p30II attenuate the formation of these multiprotein complexes and thereby repress transcription on CREB-responsive promoters. Similarly, Tax expression has been demonstrated to interfere with the transcriptional activity of c-Myb and the binding of Tax and c-Myb to KIX domain of CBP was found to be mutually exclusive109-112.
The coactivators CBP and p300 mediate transcriptional control of various cellular and viral DNA binding transcription factors. Although these coactivators have divergent functions, they are similar in nucleotide sequence, are evolutionarily conserved, and are commonly referred to as CBP/p300113,114. CBP and p300 are highly related and share many functional properties, ho wever there is evidence that these factors are not interchangeable. Several cellular and viral proteins that interact with either CBP or p300 have been identified, including steroid and retinoid hormone receptors, CREB, c-Jun, c-Myb, Sap-1a, c-Fos, MyoD, p53, Stat-1/2, NF-?B, pp90rsk, TATA-binding protein, TFIIB, HTLV-1 Tax, adenovirus E1A, Kaposi's sarcoma-associated herpes virus viral interferon regulatory factor protein, and simian virus 40 large T antigen113-115. CBP/p300 protein is available only at limiting concentrations within the cell nucleus, causing an environment for competition between coactivators and transcription factors, thus providing an additional layer of regulated gene expression104,116,117. There is evidence of a functional antagonistic relationship between transcription factors, as a result of competition for binding to common regions of CBP/p300104,113,118. Under such a condition of tight competition, relative concentrations of Tax/ p30II at various stages of disease might be a critical factor in determining the levels of viral transcription. We hypothesize that, at higher concentrations, p30II may support viral persistence by reducing viral expression, and thus reducing immune elimination of HTLV-1 infected cells. Additionally, p30II might also repress cellular genes necessary to maintain viral persistence. However, at low concentrations, p30II enhances TRE/viral over CRE/cellular mediated transcription. p30II has the potential to play a role in promoting viral transcription, cell proliferation, competitively repressing CBP/p300-dependent cellular gene transcription (e.g., p53-dependent p21WAF1/CIP1 gene activity), and for promoting the spread of the virus in vivo. This is also consistent with our previous finding that an infectious HTLV-1 molecular clone failed to maintain viral loads in vivo when p30II and p13II expression was abolished 39.
Recently, it was reported that p30II modulates LTR mediated transcription, in the context of the entire provirus, by a post-transcriptional mechanism (V. Franchini personal communication). However, our recent evidence indicates that, the role of CBP/p300 cannot be ruled out in the modulation of LTR mediated transcription by p30II, in the context of the provirus (Michael et al., manuscript in preparation). In the presence of increasing concentrations of p300, we were able to rescue the p30II-mediated repression on LTR driven gene transcription, in a dose-dependent manner, irrespective of the presence or absence of the provirus (Michael et al, manuscript in preparation). CBP and p300 bridge transcription factors to relevant promoters, has intrinsic histone acetyltransferase (HAT) activity, and form complexes with proteins such as CBP/p300 binding protein-associated factor, which also exhibits HAT activity 113. Recently, there is increasing knowledge of the mechanism and functional significance of the interactions between many viral proteins and CBP/p300. In the case of adenovirus oncoprotein E1A, interaction with CBP/p300 is critical for regulation of transcription, suppression of differentiation, and immortalization of cells in culture 113,114,119. The T antigen of SV40 regulates the expression of a group of cellular genes by modifying the HAT activity of CBP/p300 or by bridging the gap between DNA binding transcription factors and components of the general transcription machinery113,114. Identifying the molecular mechanism and functional significance of the interaction between p30II and p300 is very crucial in understanding of the role of p30II in the pathogenesis and replication of this important human pathogen. Therefore to further understand the molecular mechanism and functional significance of the interaction between p30II and p300, using N terminal and C terminal deletion mutants of p30II, we have identified the motifs within p30II that are critical in binding CBP/p300 and in regulating LTR mediated transcription, in the presence/ absence of the provirus. Our recent study confirmed the role of p30II as a regulator viral gene transcription, in association with p300. In addition, we identified the amino acid sequence 100-179 of p30II to be the domain critical for its function as a repressor of LTR- mediated transcription, irrespective of the presence or absence of the HTLV-1 provirus (Michael et al, manuscript in preparation). This region contains various important features like DNA binding domain and serine and threonine rich residues with homology to Oct-1 and POU family of transcription factors. Interestingly, there are five lysine residues within this domain, all preceded by at least one serine residue (SK motif), the consensus acetylation sequence, thus representing potential acetylation sites for CBP/p300. Intriguingly, the motif found to be critical for binding p300 is amino acid sequence 1-132 (Michael et al, manuscript in preparation).
It will be important for future studies to define relevant p30II target genes and perhaps yet-unidentified direct p30II-responsive DNA elements. These may include promoters of genes critical for T-cell function, such as the IL-2 promoter, which contains Oct-1-responsive elements120-123. Further structure-function analyses of p30II will help define the roles of five lysine residues within the domain of p30II critical for repressing LTR mediated transcription. As CBP/p300-mediated acetylation has become a common theme for regulation of protein function, it will be interesting to test whether the intrinsic histone acetyltransferase activity of CBP/p300 can in fact function to acetylate and potentially regulate HTLV-1 p30II.
As of yet, there is little information regarding the relative levels of various HTLV-1 proteins during various stages of the infection. It is possible that differences in expression levels of various viral proteins leading to differences in transcriptional regulation, might be the mechanism by which HTLV-1 infection/ disease progresses though various stages. This is likely to be in synergy with differential regulation of cellular gene regulation by Tax and/or p30II and thus changing the immune responses in accordance with different stages of progression of HTLV-1 infection and disease.
4.3 Role of p30II in Modulating Cellular Gene Transcription
Recently, using recombinant lentivirus expressing p30II in Jurkat T lymphocytes and Affymterix U133A human gene chip representing ∼33000 genes, we demonstrated the role of p30II as a regulator of cellular gene expression. In addition, we identified several potential new functional roles for p30II, in T cell activation or cell signaling and in regulation of apoptosis and cell cycle. More importantly, we illustrated the role of p30II as an activator of many key transcription factors involved in T cell signaling/ activation, such as Nuclear Factor of Activated T cells (NFAT), Nuclear Factor-Kappa B (NF-κB) and Activator Protein-1 (AP-1). Consistent with our previous hypothesis that p30II would modulate immune response, recently we found altered expression of cellular genes involved in immune modulation such as CD46, CD43, CD58, IFNγ and CD72 when p30II was expressed (Michael et al, manuscript in preparation).
Our recent findings showed that expression of p30II was associated with altered expression of multiple genes associated with transcription and translation, including transcriptional control genes like TATA-binding protein associated factor 4 (TAF4), homeo box genes, T-box genes, proteins containing helix- loop- helix domain, zinc finger proteins, coiled coil proteins, histone deacetylase-6, nuclear receptor coactivator 3, GAS 7 and translation initiation / elongation factors (Michael et al, manuscript in preparation). Among these, histone deacetylase-6 124,125 and nuclear receptor coactivator 3 (CBP interacting protein)126-128 with histone acetyltransferase and pCAF/CBP recruiting abilities are particularly interesting, since p30II contains multiple highly conserved lysines, which could play a role in acetylation. Another interesting candidate deregulated by p30II was GAS 7, which has sequence homology to Oct and POU family of transcription factors129. Based on this, p30II appears to regulate transcription through various mechanisms and different levels, some of which could be attributed to the interaction between p30II and CBP/p300.
4.4 Potential Role p30II in T Cell Activation
Our recent findings showed that p30II expression enhanced NFAT, AP-1 and NF-κB mediated transcriptional activity. In addition, expression of p30II was associated with altered expression of multiple genes involved in various stages of T cell activation, including CD28, Vav-2, CD72, CD46, Lck tyrosine kinase, CHP (an endogenous calcineurin inhibitor), c-Jun and c-Fos, protein kinase D, epidermal growth factor, ETS domain transcription factor Elk-1, IKKγ, HPK-1, Ras GRP2 and NFAT (Michael et al, manuscript in preparation).
Expression from the IL-2 promoter requires binding of several transcription factors, including NFAT, AP-1 and NF-κB. NFAT is vital to proliferation of peripheral lymphocytes for HTLV-1 infection 13 while AP-1 is linked to the dysregulated phenotypes of HTLV-1 infected T cells130 and malignant transformation131. Activation of AP-1 occurs through Tax-dependent and independent mechanisms in HTLV-1- infected T cells in vitro and in leukemia cells in vivo130. NF-κB is highly activated in many hematopoietic malignancies, HTLV-1 infected T cell lines and in primary ATL cells, even when Tax expression levels are low131 and due to its anti-apoptotic activity, it is considered to be a key survival factor for several types of cancer. HTLV-1 p30II is so far the only retroviral accessory protein to have broad modulating activities on the transcriptional activity of NF-κB, NFAT and AP-1.
It will be important for future studies to elucidate the mechanisms employed by p30II to enhance NFAT, AP-1 and NF-κB mediated transcription. Interestingly, HTLV-1 p12I stimulates NFAT mediated transcription, when stimulated with PMA, indicating that p12I acts synergistically with Ras/ MAPK pathway to promote NFAT activation and thus may facilitate host cell activation and establishment of persistent HTLV-1 infection36. Since p30II enhances NFAT driven transcription significantly when stimulated with ionomycin or CD3 (Michael et al., manuscript submitted), it likely employs a different mechanism than p12I. It is possible that these two accessory proteins act synergistically to modulate NFAT driven transcription and subsequent T cell activation / signaling. AP-1 is able to interact with transcriptional coactivator CBP/ p300, as well as viral CREs and mediate HTLV-I gene expression. NF-κB and NFAT are also known to interact with transcriptional coactivator CBP/p300 115. Therefore it is possible that p30II regulates the transcriptional activity of NFAT, NF-κB and AP-1, at least in part, by its interaction with CBP/p300.
4.5 Genes Modulated p30II in Apoptosis, Cell Cycle and Cell Adhesion
HTLV-1 mediated interference with normal T-cell apoptosis is thought to be a mechanism of tumorigenicity, but specific mechanisms by which HTLV-1 infection or any particular HTLV-1 gene products influence on T-cell survival are not fully understood. Similar to the effect of HTLV-1 Tax on apoptosis related genes24,132,133, we have recently used gene array to demonstrate that p30II also deregulates multiple genes resulting in pro-apoptotic and anti-apoptotic effects including Bcl-2 related/interacting genes, the Fas mediated apoptosis pathway genes, caspases and genes associated with the DNA fragmentation pathway (Michael et al, manuscript in preparation). Since apoptosis is a well-known mechanism of cellular defense against viral infection, a possible role of p30II in lymphocyte apoptosis might correlate with the requirement of p30II in maintaining proviral loads in vivo39.
Previous studies indicate that several members of the cell cycle machinery have altered expression in HTLV-1 infected cells134-137. Several studies examined the aberrations in cell cycle caused by HTLV-1 Tax134; however, not much is known about the role of other HTLV-1 proteins in causing abnormalities in cell cycle. Our data indicated that p30II expression altered the expression of multiple genes involved in regulation of different stages of cell cycle, including checkpoint suppressor 1, histone deacetylase 6, cyclin B1, WEE1 kinase, CDC14A, Lck, JAK2, GAS7, JUN and MDM2 (Michael et al, manuscript in preparation). Some of these genes are particularly interesting, for example, MDM2 is overexpressed in certain leukemias 138 and capable of enhancing the tumorigenic potential of cells by inhibiting p300 / PCAF mediated p53 acetylation 139. We also found that p30II expression was associated with altered expression of several genes involved in cell adhesion, including integrins, immunoglobulin (MADCAM1), cadherin, protocadherin, liprin, KIT ligand, CD84 / Ly-9, CD58, CD43 / sialophorin and glycosyl-phosphatidyl- inositol phospholipase D1 (Michael et al, manuscript in preparation).
5. pX ORF II p13II
5.1 Biochemical Characteristics of p13II: Mitochondrial Targeting
Less is known about the function of the smaller protein, p13II, encoded by the 87 carboxy-terminal amino acids of the 241-residue Tof or p30II protein. Initial studies demonstrated p13II localization to the nucleus30, but more-recent reports show mitochondrial localization of the protein47,140. Recently, by extraction of mitochondria-enriched fractions, D'Agostino et al demonstrated that full- length p13II is mostly membrane-associated and partly soluble 140. Using electron microscopy, the sub-mitochondrial localization of p13II was further verified to be in the inner mitochondrial membrane and cristae140. This localization is mediated by an atypical mitochondrial targeting sequence (MTS) in the N terminus of p13II between amino acids 22-31 (Fig 2). The 10-amino-acid MTS also targets green fluorescent protein to mitochondria when fused to the N terminus of green fluorescent protein47. Importantly, a fusion protein of the p13II MTS with HIV Rev can localize to mitochondria, indicating that the p13II MTS is, at least in part, able to override the potent NLS of Rev101. While the p13II MTS is also present in p30II, p30II is not localized to the mitochondria, suggesting that the signal has to be near the amino-terminus to direct mitochondrial targeting141. Analysis of the amino acid sequence of p13II reveals no characteristic DNA-binding motifs and previous data show neither DNA binding nor transcriptional activity142. Since p30II and p13II are expressed from two different mRNAs and accumulate in separate cellular compartments, it is believed that the two proteins play distinct roles in the viral life cycle, possibly at the level of RNA processing (p30II) and mitochondrial function (p13II)141.
5.2 p13II Alteration of Mitochondrial Morphology and Inner Membrane Potential
Functionally, expression of p13II disrupts the mitochondrial inner membrane potential and alters mitochondrial morphology and architecture, leading to apparent mitochondrial swelling and fragmentation of their normal interconnected string- like network, suggesting a role for p13II in induction of apoptosis47. Additionally, it is thought that p13II might possibly cause changes in mitochondrial permeability and/or alter processes like calcium signaling that relies on the mitochondrial network and the endoplasmic reticulum. Intriguingly, proteins that localize to mitochondria have been described for other human viruses including Vpr and Tat of HIV, vMIA of human cytomegalovirus, X of hepatitis B virus and BHRF-1 of Epstein- Barr virus143-145. The retroviral proteins Vpr and Tat of HIV have been shown to disrupt mitochondrial inner membrane potential, resulting in rapid swelling of mitochondria and release of cytochrome c145. Lately, mitochondrial swelling and altered permeability due to opening of the permeability transition pore (PTP) have been reported to play a critical role in triggering apoptosis, as demonstrated in the case of HIV-1 Vpr and X of hepatitis B virus143,144,146. However, a study using the p13 II amino acid region 9–41 did not show any involvement of the PTP in driven cation fluxes140. Unlike protein X and Vpr, p13II does not cause causes substantial cytochrome c release47. The biological significance of p13II mitochondrial localization and disruption of membrane potentials remains unclear.
In cells expressing p13II-GFP fusion protein, only some cells showed apparent mitochondrial swelling and maintained their inner membrane potential, while others had a complete loss of inner membrane potential and marked perinuclear clustering, a characteristic of early apoptosis47. Based on this, D'Agostino et al46 consider that p13II might initially induce selective permeability changes causing swelling and subsequent de-energization and irreversible swelling, and suggested that p13II triggers apoptosis. Despite these observations, thus far, increased levels of apoptosis have never been demonstrated in p13II-expressing cells, leaving open the possibility for other mitochondrion-based functions of this viral protein. Such functions could simply include an increased respiratory activity of mitochondria, which is often accompanied by swelling or Ca2+ signaling and Ca2+ dependent regulation of the oxidative phosphorylation machinery. Thus, p13II may facilitate later stages of HTLV-1 infection such as assembly and release.
A computer prediction program indicated that the amino-terminal portion of p13II was predicted to contain a short hydrophobic leader (amino acids 1–5) followed by an α-helix (amino acids 21–30) that includes the MTS47. The α-helix includes 4 arginine residues at positions 22, 25, 29, and 30, which are predicted to form a positively charged patch within the putative α-helix, thereby imparting amphipathic properties to this region. D'Agostino et al 140 demonstrated that amino acids 9–41 of p13II which includes the MTS, folds into an α helix in the context of a membrane-like environment and has specific effects on the permeability of isolated mitochondria to small cations. Furthermore, the presence of the four arginines in the MTS is essential for the increase in mitochondrial ion conductance and in situ effects on mitochondrial morphology but not for mitochondrial targeting. In addition, circular dichroism analysis illustrated that efficient α-helical folding of amino acids 9–41 of p13II requires the presence of detergent or phospholipid micelles to mimic the membrane environment. This suggests that interaction with or embedding into membranes might be necessary for the correct folding of full- length p13II140. However, the observation that the p13II MTS is not cleaved upon import into the mitochondria and that it does not require positively charged residues distinguishes it from most MTS, and suggests that the protein might utilize a mitochondrial import pathway distinct from that described for most MTS, which involves binding to a series of acidic receptors followed by cleavage of the signal.
5.3 p13II Cellular Protein Interactions
While screening a cDNA library from an HTLV-1-infected rabbit cell line by Saccharomyces cerevisiae two- hybrid assay, Hou et al.,147 discovered the association of p13II with two novel cellular proteins designated C44 and C254. C254 appears to be rabbit actin binding protein 280 (ABP280) present in the cytoskeleton of many different cell types and functions in the modulation of cell shape and polarity and is essential for migration in melanocytes and other cultured cells. More importantly, ABP-280 is important in the insertion of adhesion molecules into the cell membrane. While the region of ABP-280 that interacts with p13II is also involved in interactions with integrin B1, tissue factor, and presenilin, other regions of ABP-280 binds to glycoprotein and the cytoplasmic domain of furin147. C44 shares homology with archeal adenylate kinases, the eukaryotic homologues of which localize to mitochondria and are involved in energy metabolism. Interestingly, the human homologue of C44 is expressed in the Jurkat T-cell line and proliferating, but not resting, PBMC 147. These studies were performed using two molecular clones of HTLV-I, K30p and K34p, which have amino acid differences in rex, p13II, and p30II. Interestingly, only p13II K34, but not p13II K30 allow the interaction with C44. The amino acid sequence of the p13II variant used by Ciminale et al 47 was most similar to p13II K30, as it had the 25- amino-acid carboxyl tail and that it would not bind C44. This might provide clues to the role of specific amino acids within p13II, which are critical in binding C44 and in causing apoptosis. Additionally, p13II binds to farnesyl pyrophosphate synthetase (FPPS), an enzyme involved in the mevalonate/squalene pathway, and in the synthesis of farnesyl pyrophosphate, a substrate required for prenylation of Ras53. Interestingly, G4, a mitochondrial targeting accessory protein of BLV also binds to FPPS. Future studies on the functional significance of the interaction between p13II and these cellular proteins will possibly elucidate the role of p13II in altering mitochondrial physiology and thus in HTLV-1 replication and pathogenesis.
Furthermore, Mahana et al., 148 reported an increase in Vav phosphorylation in rabbit cells transfected with an HTLV-1 molecular clone that contains two mutations in pX ORF II, resulting in expression of truncated p13II and p30II. Vav is a hematopoietically restricted guanine nucleotide exchange factor for the Rac/Rho family of GTPases and is necessary for T-cell activation149. These findings suggest that p13II may play a role in controlling the activation state of Vav, which may relate to viral infectivity and leukemogenesis.
6. Accessory Gene Products of Related Deltaretroviruses
Deltaretroviruses like HTLV-1 HTLV-2, STLV, and BLV have similar genomic structure, viral replication, conserved organizational structure and pathology 13,150,151. HTLV-1, HTLV-2 and BLV are complex pathogenic retroviruses of the oncovirinae family that cause lymphoproliferative diseases and encode conserved regulatory and accessory genes from pX region ORFs at the 3′ portion of the viral genome13. There is increasing knowledge about the role of the homologous gene products in the pathogenesis of HTLV-2 and BLV, after the advent of infectious molecular clones.
HTLV-1 and HTLV-2 are distinct but genetically related viruses that share 60% amino acid identity, yet they differ in their pathogenicity in vivo. HTLV-1 is found mainly in CD4+ T cells, whereas HTLV-2 is mainly in CD8+ T cells in vivo152. However, both viruses are capable of infecting and transforming T cells in vitro. More importantly, HTLV-2 is also associated with leukemia and neurologic disease, although less frequently than HTLV-17,153. Analyzing the viral determinants that contribute to the pathogenesis and identifying the similarities and differences between HTLV-1 and -2 may provide a better understanding of HTLV pathogenesis. In addition, HTLV-2 is thought to be an important model to study HTLV pathogenesis, due to the similarities in genome structures and in vitro biological properties. As with HTLV-1, rabbits can be successfully infected with molecular clone of HTLV-2 154 and although proteins encoded by the pX region between env and the last exon of tax/rex of HTLV-2 appear to be dispensable for viral replication and cellular transformation in vitro155, they are important for viral replication in the rabbit model156. Thus, like HTLV-1, proteins encoded in the pX region of HTLV-2 are likely to play an important role in viral life cycle during the natural infection. Further studies will be required to determine the role of these accessory genes in the disease syndromes associated with HTLV-2 infections. Based on initial studies, HTLV-2 ORF I protein p10I and ORF V protein p11V which overlaps the ORF I region appear to be analogous to HTLV-1 p12I in binding MHC-I molecules and perhaps able to down regulate this important surface protein on infected cells66. Interestingly, p10I localizes within the nucleus28. However, unlike p12I, p10I and p11V could not bind 16-kDa vacuolar H+ ATPase or the α, β or γc chains of the IL-2R, suggesting that the basic mechanisms of host-virus interaction may be different between HTLV-1 and -266. More strikingly, C-terminal portion of HTLV-1 p30II shares 77.5% sequence homology with the N-terminal 49 amino acids of HTLV-2 ORFII protein p28II28. There are recent findings indicating that p30II and p28II can inhibit Tax and Rex function by retaining tax/rex RNA in the nucleus, thus decreasing the doubly spliced RNA pool in the cytoplasm, leading to decrease in Tax and Rex production (V. Franchini and P. Green personal communications).
Bovine leukemia virus infection of sheep offers a reliable model of disease associated with deltaretrovirus infections. The BLV accessory proteins R3 and G4 share varying degrees of homology with the HTLV-1 accessory proteins p12I, p13II and p30II157. The mRNAs coding for both R3 and G4 were originally identified by RT-PCR in PBMCs from BLV-infected cattle. G4 message is expressed at the beginning of the lymphoproliferative stage of BLV-induced disease while the R3 message is produced during the period surrounding seroconversion 54. Similar to initial reports of HTLV-1 deletion mutants, BLV molecular clones that disrupted the expression of pX ORF genes, encoding the G4 and R3 accessory proteins, failed to influence virus replication and infectivity in cell culture systems but reduced the virus transmission and more importantly the provirus load, leading to a drastic decrease in virus propagation in sheep 54. Importantly, BLV is the closest model system to study apoptotic process since leukemia occurs in vivo in experimental sheep models. Role of HTLV-1 in apoptosis is not clear, since reports are contradictory depending on experimental conditions and an appropriate animal system to elucidate the role of HTLV-1 in the apoptosis is deficient. Interestingly, attenuated mutant proviruses that harbor deletions in the G4 and/or R3 genes decrease the susceptibility of the PBMC to apoptosis and prevent uninfected cells from undergoing programmed cell death at similar levels as that of the wild-type virus, indicating that R3 and G4 genes are not required to maintain both direct and indirect protection against apoptosis in vivo 158. In addition, a live attenuated BLV vaccine with deletions in R3 and G4 genes was found protect 8 out of 9 sheep against a challenge by either wildtype BLV or BLV from blood of infected animals159.
The BLV G4 protein shares structural features and cellular distribution patterns with HTLV-1 p13II, while BLV R3 appears to be functionally related more closely to HTLV-1 p12I. G4 has 105 amino acids, including an N-terminal stretch of hydrophobic residues (aa 1-24) followed by putative proteolytic cleavage sites and an arginine-rich region (aa 58-72) in the middle while R3 has 44 amino acids, including an N-terminal hydrophilic region followed by hydrophobic sequences. The hydrophilic portion of R3 corresponds to the first 17 amino acids of Rex, which include the domain responsible for accumulation of the protein in the nucleus and in binding to its RNA target, the Rex-responsive element 157. Like p12I, R3 accumulates in the cellular endomembranes. However, since it also accumulates in the nucleus consistent with the presence of the Rex NLS at the N-terminal, it is speculated to regulate post-transcriptional activity of Rex 52. On the other hand, like p13II, G4 also contain a mitochondrial targeting signal and amphipathic α helix. G4 contain 3 helical segments; a long helix lying in the hydrophobic region (aa 8 to 22), another long helix at the second lying near the C- terminus (aa 76 to 89) and an amphipathic short helix in the arginine rich region (aa 63 to 69), among which the first and the last are required for mitochondrial targeting. Importantly, like p13II, G4 also localize to the nucleus and mitochondria53. To test the functional properties of the viral proteins further, Kerkhofs et al.,51 tested the oncogenic potential of R3 and G4, by determining their ability to transform primary rat embryo fibroblasts. In this system, G4 (analogous to HTLV-1 p13II), but not R3 (analogous to HTLV-1 p12I) cooperated with the Ha-ras oncogene to induce tumors in nude mice. Using yeast two- hybrid system and confocal microscopy, G4 was demonstrated to interact with farnesyl pyrophosphate (FPP) synthetase, an enzyme in the mevalonate/squalene pathway that is critical for synthesis of FPP, a substrate required for prenylation of Ras52. Interestingly, the arginine rich domain of G4 was found to be important in its oncogenic potential and in interacting with FPPS. Analogously, HTLV-1 p13II was also found to specifically interact with FPP synthetase and to colocalize with G4 in mitochondria. Whether these observations explain the function of G4 and p13II is yet to be determined, however these findings provide new directions for research in the role of these accessory proteins in signal transduction pathways, leading to cell transformation and potential therapeutic approaches to eliminate virus replication. Interestingly, infectious molecular clones of BLV with mutations in gene regions encoding G4 and R3 were limited in their ability to maintain proviral loads in infected sheep51. More importantly, while wild-type BLV typically produces leukemias and/or lymphosarcomas in the majority of infected sheep during the course of the infection, none out of 13 sheep infected with viruses with mutations in G4 or in R3 and G4 developed disease, indicating that G4 is required for pathogenesis in vivo51. Whether this diminished pathogenic ability is specifically related to these gene products or a generalized attenuation of replication capacity by the virus has not been resolved. Despite this, the BLV model provides an important system to test the role of the regulatory and accessory genes in the pathogenesis of the deltaretroviruses and in exploring possible vaccine trials.
7. Perspective
The mechanism of HTLV-1 mediated T call activation and transformation has been extensively investigated. Most of these studies are focused on Tax, which is known to be a critical transcriptional activator and a key protein in cell transformation. The oncogenic potential of Tax has been demonstrated in animal models, as well as in vitro transformation assays 15,17,25. Therefore, Tax apparently may be responsible for many of the required events necessary for HTLV-1-mediated lymphocyte immortalization. Nonetheless, it is uncertain whether Tax aids the virus in establishing persistent infection, a prerequisite for basal transcription of Tax itself. Recent studies demonstrate that expression of the accessory proteins encoded by pX ORFs I and II is critical for efficient HTLV-1 infection in vivo, although potentially dispensable for viral replication under activation conditions in vitro.
Based on recent findings from our own laboratory and others, we propose molecular functions for pX ORF I-encoded p12I and ORF II encoded p30II in HTLV-1-induced T-cell activation (Table 1). While the mechanism of p30II function awaits additional research, p12I function has been established to be calcium-dependent, independent of Tax and possibly occurs before Tax is expressed during a natural infection. However highly activated T cells mediated by expression of p12I and/or p30II likely allow HTLV-1 provirus to integrate into host cell genome and permit the early viral infection. Since Tax is also able to cause T cell activation, it appears to be redundant for HTLV-1 to use multiple proteins to activate T lymphocytes. It is possible that these proteins act coordinately or synergistically. We propose that, by modulating the expression of various HTLV-1 proteins, the virus employs selective use of different viral proteins during different stages of the infection. However, since information on the expression profile of HTLV-1 proteins during different stages of the infection is limited, additional studies designed to test the temporal expression patterns of HTLV-1 regulatory and accessory proteins during viral infection are required to explore this possibility. Additionally, it will be crucial to determine if p12I is present in HTLV-1 viral particles or selectively expressed before viral integration. If p12I is expressed after viral integration and not early in viral infection, it is likely to influence late events, such as viral replication, particle assembly and release. Functional and biological significance of the interaction of p12I with calcineurin and the regulatory functions on NFAT transcriptional activity also await further investigation. Additionally, p12I may play a role in regulation of cell cycle and apoptosis, since it is able to increase the intracellular calcium like the cellular protein Bcl-2 and modulate NFAT activity. However, p12I activates NFAT while Bcl-2 blocks NFAT-induced FasL transcription and retards the G0 to S phase transition160. Therefore, the mechanism employed by p12I and Bcl-2 might be distinct and future studies are necessary to test the possible role of p12I in cell cycle progression and apoptosis in T lymphocytes. Understanding the role of p12I in HAM/TSP also awaits further investigation.
Table 1.
Summary of the functional role of HTLV-1 accessory proteins
| Protein | pX ORF | Subcellular localization | In vitro functional activity | In vivo effects |
|---|---|---|---|---|
| p12I | I | ER and cis-Golgi | Induces release of calcium from ER, interact with calcineurin as well as calreticulin and cause calcium- mediated NFAT activation; decreases IL-2 requirement for T-cell activation; enhances the production of IL-2; decreases MHC-1 surface expression | Abolished infectivity in rabbit model; reduced infectivity in nondividing primary human T-cells |
| p27I | I | ? | ? | ?, However, recognized by CTLs of infected subjects, |
| p30II | II | Nucleus | Binds p300/CBP; differentially modulates HTLV-1 LTR mediated transcription; enhances transcription NFAT, NF-κB and AP-1 mediated transcription. | Reduced viral load in rabbit model* |
| p13II | II | Mitochondria and nucleus | Mitochondrial swelling and disruption of ? ? | Reduced viral load in rabbit model* |
Based on the reduced viral load from ACH.30/13 double-knockout proviral clone in rabbit model of infection (Bartoe et al, 2000)
? Function or expression unknown-predicted product.
There is increasing understanding of the functional significance of p30II p13II. Emerging evidence suggests that these proteins may act during later stages of infection to promote viral persistence and potentially aid in virus assembly. Currently, we are evaluating the effect of single p13II and p30II knockout mutations on the infectivity of HTLV-1 viral clones in vivo. Importantly, recent findings based on microarrays from our laboratory shed light on the possible mechanisms by which p30II functions in HTLV-1 pathogenesis and in leukemogenesis. However, based on the clues available from this study, future studies are essential not only to verify the findings, but also to test their functional and biological significance. Additional research is also crucial in elucidating the mechanisms employed by p30II to enhance NFAT, AP-1 and NF-κB mediated transcription. Such studies may possibly define additional p30II target genes and p30II-responsive DNA elements. Interestingly, one of the lysine residues within p30II appears to be critical for its ability to inhibit the HTLV-1 LTR mediated transcription, irrespective of the presence or the absence of the provirus (B. Michael, unpublished data). On the light of this finding, our current research focus on the intrinsic histone acetyltransferase activity of CBP/p300, whether CBP/p300 acetylates and potentially regulates HTLV-1 p30II via acetylation. In addition, findings from the deletion and site directed mutational analysis are valuable in designing experiments to reintroduce these mutations into infectious molecular clones to test their functional significance in vivo.
Little is known about the function of p13II. Future studies on the p13II protein are essential to determine if the mitochondrial swelling induced by p13II is significant in apoptosis or viral assembly. Functional significance of the interaction between p13II and cellular proteins like FPPS also await additional research. Biological significance of p13II mitochondrial localization and disruption of membrane potentials is also uncertain. In vivo experiments using infectious molecular clones with mutations in the motifs critical for the functions in vitro are necessary to determine the biological significance of these findings. Such detailed mutational analyses with each of the accessory proteins will be central in understanding the effect of specific mutations on protein function in the context of the whole virus in vitro or in vivo. In addition to the HTLV-1 rabbit animal model, BLV sheep model can be used to to test specific mutations of analogous gene regions in a disease model.
In conclusion, emerging evidence indicates that the accessory proteins of HTLV-1 and other deltaretroviruses associated with lymphoproliferative diseases, though once thought to be dispensable for viral replication, are in fact multifunctional proteins, critically involved in viral transmission and propagation and may be of potential use in designing therapeutic measures and vaccines.
Acknowledgments
The authors thank P.Green, K.Boris-Lawrie, T. Rosol, B.Albrecht, and A.Montgomery for their support of the research. We thank T. Vojt for illustrations. This work was supported by National Institute of Health grants RR-14324 and CA100730 awarded to Dr. Michael Lairmore; CA-70529 and CA-09338 awarded through the Ohio State University Comprehensive Cancer Center.
References
- 1.Mortreux F, Gabet AS, Wattel E. Molecular and cellular aspects of HTLV-1 associated leukemogenesis in vivo. Leukemia. 2003;17:26–38. doi: 10.1038/sj.leu.2402777. [DOI] [PubMed] [Google Scholar]
- 2.Bangham CR. HTLV-1 infections. J Clin Pathol. 2000;53:581–586. doi: 10.1136/jcp.53.8.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchiyama T. Human T cell leukemia virus type I (HTLV-I) and human diseases. Annual Review of Immunology. 1997;15:15–37. doi: 10.1146/annurev.immunol.15.1.15. [Review] [134 refs] [DOI] [PubMed] [Google Scholar]
- 4.Kakuda K, Ikematsu H, Chong WL, Hayashi J, Kashiwagi S. Molecular epidemiology of human T lymphotropic virus type 1 transmission in Okinawa, Japan. Am J Trop Med Hyg. 2002;66:404–408. doi: 10.4269/ajtmh.2002.66.404. [DOI] [PubMed] [Google Scholar]
- 5.Gotuzzo E, Arango C, Queiroz-Campos A, Isturiz RE. Human T-cell lymphotropic virus-I in Latin America. Infect Dis Clin North Am. 2000;14:211–2xi. doi: 10.1016/s0891-5520(05)70225-7. [DOI] [PubMed] [Google Scholar]
- 6.Gessain A, De TG. Geographic and molecular epidemiology of primate T lymphotropic retroviruses: HTLV-I, HTLV-II, STLV-I, STLV-PP, and PTLV-L. Advances in Virus Research. 1996;47:377–426. doi: 10.1016/s0065-3527(08)60740-x. [Review] [198 refs] [DOI] [PubMed] [Google Scholar]
- 7.Khabbaz RF, Onorato IM, Cannon RO, Hartley TM, Roberts B, Hosein B, Kaplan JE. Seroprevalence of HTLV-1 and HTLV-2 among intravenous drug users and persons in clinics for sexually transmitted diseases. N Engl J Med. 1992;326:375–380. doi: 10.1056/NEJM199202063260604. [DOI] [PubMed] [Google Scholar]
- 8.Szynal M, Cleuter Y, Beskorwayne T, Bagnis C, Van Lint C, Kerkhofs P, Burny A, Martiat P, Griebel P, Van den BA. Disruption of B-cell homeostatic control mediated by the BLV-Tax oncoprotein: association with the upregulation of Bcl-2 and signaling through NF-kappaB. Oncogene. 2003;22:4531–4542. doi: 10.1038/sj.onc.1206546. [DOI] [PubMed] [Google Scholar]
- 9.Leclercq I, Cavrois M, Mortreux F, Hermine O, Gessain A, Morschhauser F, Wattel E. Oligoclonal proliferation of human T-cell leukaemia virus type 1 bearing T cells in adult T-cell leukaemia/lymphoma without deletion of the 3′ provirus integration sites. Br J Haematol. 1998;101:500–506. doi: 10.1046/j.1365-2141.1998.00743.x. [DOI] [PubMed] [Google Scholar]
- 10.Cavrois M, Gessain A, Wainhobson S, Wattel E. Proliferation of HTLV-1 infected circulating cells in vivo in all asymptomatic carriers and patients with TSP/HAM. Oncogene. 1996;12:2419–2423. [PubMed] [Google Scholar]
- 11.Xu X, Kang SH, Heidenreich O, Okerholm M, Oshea JJ, Nerenberg MI. Constitutive activation of different Jak tyrosine kinases in human T cell leukemia virus type 1 (HTLV-1) tax protein or virus- transformed cells. J Clin Invest. 1995;96:1548–1555. doi: 10.1172/JCI118193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marriott SJ, Lindholm PF, Reid RL, Brady JN. Soluble HTLV-1 Tax Protein Stimulates Proliferation of Human Peripheral Blood Lymphocytes. New Biol. 1991;7:678–686. [PubMed] [Google Scholar]
- 13.Albrecht B, Lairmore MD. Critical role of human T- lymphotropic virus type 1 accessory proteins in viral replication and pathogenesis. Microbiol Mol Biol Rev. 2002;66:396–406. doi: 10.1128/MMBR.66.3.396-406.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuoka M. Human T-cell leukemia virus type I and adult T-cell leukemia. Oncogene. 2003;22:5131–5140. doi: 10.1038/sj.onc.1206551. [DOI] [PubMed] [Google Scholar]
- 15.Neuveut C, Jeang KT. Cell cycle dysregulation by HTLV-I: role of the tax oncoprotein. Front Biosci. 2002;7:157–163. doi: 10.2741/neuveut. [DOI] [PubMed] [Google Scholar]
- 16.Marriott SJ, Lemoine FJ, Jeang KT. Damaged DNA and miscounted chromosomes: human T cell leukemia virus type I tax oncoprotein and genetic lesions in transformed cells. J Biomed Sci. 2002;9:292–298. doi: 10.1007/BF02256583. [DOI] [PubMed] [Google Scholar]
- 17.Jeang KT. Functional activities of the human T-cell leukemia virus type I Tax oncoprotein: cellular signaling through NF-kappa B. Cytokine Growth Factor Rev. 2001;12:207–217. doi: 10.1016/s1359-6101(00)00028-9. [DOI] [PubMed] [Google Scholar]
- 18.Bex F, Gaynor RB. Regulation of gene expression by HTLV-I Tax protein. Methods. 1998;16:83–94. doi: 10.1006/meth.1998.0646. [DOI] [PubMed] [Google Scholar]
- 19.Ye J, Silverman L, Lairmore MD, Green PL. HTLV-1 Rex is required for viral spread and persistence in vivo but is dispensable for cellular immortalization in vitro. Blood. 2003 doi: 10.1182/blood-2003-05-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narayan M, Kusuhara K, Green PL. Phosphorylation of two serine residues regulates human T-cell leukemia virus type 2 Rex function. J Virol. 2001;75:8440–8448. doi: 10.1128/JVI.75.18.8440-8448.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D'Agostino DM, Ciminale V, Zotti L, Chieco-Bianchi L. Influence of Rex and intronic sequences on expression of spliced mRNAs produced by human T cell leukemia virus type I. AIDS Res Hum Retroviruses. 1999;15:1351–1363. doi: 10.1089/088922299310061. [DOI] [PubMed] [Google Scholar]
- 22.Kusuhara K, Anderson M, Pettiford SM, Green PL. Human T-cell leukemia virus type 2 Rex protein increases stability and promotes nuclear to cytoplasmic transport of gag/pol and env RNAs. J Virol. 1999;73:8112–8119. doi: 10.1128/jvi.73.10.8112-8119.1999. published erratum appears. [DOI] [PMC free article] [PubMed] [Google Scholar]; J Virol. 1999 Dec;73(12):10556. [Google Scholar]
- 23.Kusuhara K, Anderson M, Pettiford SM, Green PL. Human T-cell leukemia virus type 2 Rex protein increases stability and promotes nuclear to cytoplasmic transport of gag/pol and env RNAs. J Virol. 1999;73:8112–8119. doi: 10.1128/jvi.73.10.8112-8119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schavinsky-Khrapunsky Y, Gold E, Ben Aroya Z, Torgeman A, Aboud M, Huleihel M. Activation of HTLV-I long terminal repeat by apoptosis inducing agents: mechanism and implications for HTLV-I pathogenicity (review) Int J Mol Med. 2003;11:3–11. [PubMed] [Google Scholar]
- 25.Gatza ML, Watt JC, Marriott SJ. Cellular transformation by the HTLV-I Tax protein, a jack-of-all-trades. Oncogene. 2003;22:5141–5149. doi: 10.1038/sj.onc.1206549. [DOI] [PubMed] [Google Scholar]
- 26.Narayan M, Younis I, D'Agostino DM, Green PL. Functional domain structure of human T-cell leukemia virus type 2 rex. J Virol. 2003;77:12829–12840. doi: 10.1128/JVI.77.23.12829-12840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narayan M, Kusuhara K, Green PL. Phosphorylation of two serine residues regulates human t-cell leukemia virus type 2 rex function. J Virol. 2001;75:8440–8448. doi: 10.1128/JVI.75.18.8440-8448.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciminale V, D'Agostino D, Zotti L, Franchini G, Felber BK, Chieco-Bianchi L. Expression and characterization of proteins produced by mRNAs spliced into the X region of the human T-cell leukemia/lymphotropic virus type II. Virology. 1995;209:445–456. doi: 10.1006/viro.1995.1277. [DOI] [PubMed] [Google Scholar]
- 29.Koralnik IJ, Fullen J, Franchini G. The p12I, p13II, and p30II proteins encoded by human T-cell leukemia/lymphotropic virus type I open reading frames I and II are localized in three different cellular compartments. J Virol. 1993;67:2360–2366. doi: 10.1128/jvi.67.4.2360-2366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koralnik IJ, Gessain A, Klotman ME, Lo MA, Berneman ZN, Franchini G. Protein isoforms encoded by the pX region of human T-cell leukemia/lymphotropic virus type I. Proc Natl Acad Sci U S A. 1992;89:8813–8817. doi: 10.1073/pnas.89.18.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berneman ZN, Gartenhaus RB, Reitz MS, Blattner WA, Manns A, Hanchard B, Ikehara O, Gallo RC, Klotman ME. Expression of alternatively spliced human T-lymphotropic virus type 1 pX mRNA in infected cell lines and in primary uncultured cells from patients with adult T-cell leukemia/lymphoma and healthy carriers. Proc Natl Acad Sci USA. 1992;89:3005–3009. doi: 10.1073/pnas.89.7.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ciminale V, Pavlakis GN, Derse D, Cunningham CP, Felber BK. Complex splicing in the human T-cell leukemia virus (HTLV) family of retroviruses: Novel mRNAs and proteins produced by HTLV type I. J Virol. 1992;66:1737–1745. doi: 10.1128/jvi.66.3.1737-1745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pique C, Uretavidal A, Gessain A, Chancerel B, Gout O, Tamouza R, Agis F, Dokhelar MC. Evidence for the chronic in vivo production of human T cell leukemia virus type I Rof and Tof proteins from cytotoxic T lymphocytes directed against viral peptides. J Exp Med. 2000;191:567–572. doi: 10.1084/jem.191.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Derse D, Mikovits J, Ruscetti F. X-I and X-II open reading frames of HTLV-I are not required for virus replication or for immortalization of primary T-cells in vitro. Virology. 1997;237:123–128. doi: 10.1006/viro.1997.8781. [DOI] [PubMed] [Google Scholar]
- 35.Kim SJ, Ding W, Albrecht B, Green PL, Lairmore MD. A conserved calcineurin-binding motif in human T lymphotropic virus type 1 p12I functions to modulate nuclear factor of activated T cell activation. J Biol Chem. 2003;278:15550–15557. doi: 10.1074/jbc.M210210200. [DOI] [PubMed] [Google Scholar]
- 36.Albrecht B, D'Souza CD, Ding W, Tridandapani S, Coggeshall KM, Lairmore MD. Activation of nuclear factor of activated T cells by human T-lymphotropic virus type 1 accessory protein p12(I) J Virol. 2002;76:3493–3501. doi: 10.1128/JVI.76.7.3493-3501.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding W, Albrecht B, Kelley RE, Muthusamy N, Kim S, Altschuld RA, Lairmore MD. Human T lymphotropic virus type 1 p12(I) expression increases cytoplasmic calcium to enhance the activation of nuclear factor of activated T cells. J Virol. 2002;76:10374–10382. doi: 10.1128/JVI.76.20.10374-10382.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang W, Nisbet JW, Albrecht B, Ding W, Kashanchi F, Bartoe JT, Lairmore MD. Human T- lymphotropic virus type 1 p30(II) regulates gene transcription by binding CREB binding protein/p300. J Virol. 2001;75:9885–9895. doi: 10.1128/JVI.75.20.9885-9895.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bartoe JT, Albrecht B, Collins ND, Robek MD, Ratner L, Green PL, Lairmore MD. Functional role of pX open reading frame II of human T- lymphotropic virus type 1 in maintenance of viral loads in vivo. J Virol. 2000;74:1094–1100. doi: 10.1128/jvi.74.3.1094-1100.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lairmore MD, Albrecht B, D'Souza C, Nisbet JW, Ding W, Bartoe JT, Green PL, Zhang W. In vitro and in vivo functional analysis of human T cell lymphotropic virus type 1 pX open reading frames I and II. AIDS Res Hum Retroviruses. 2000;16:1757–1764. doi: 10.1089/08892220050193272. [DOI] [PubMed] [Google Scholar]
- 41.Zhang W, Nisbet JW, Bartoe JT, Ding W, Lairmore MD. Human T-lymphotropic virus type 1 p30(II) functions as a transcription factor and differentially modulates CREB-responsive promoters. J Virol. 2000;74:11270–11277. doi: 10.1128/jvi.74.23.11270-11277.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albrecht B, Collins ND, Burniston MT, Nisbet JW, Ratner L, Green PL, Lairmore MD. Human T- lymphotropic virus type 1 open reading frame I p12(I) is required for efficient viral infectivity in primary lymphocytes. J Virol. 2000;74:9828–9835. doi: 10.1128/jvi.74.21.9828-9835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collins ND, Newbound GC, Albrecht B, Beard JL, Ratner L, Lairmore MD. Selective ablation of human T-cell lymphotropic virus type 1 p12I reduces viral infectivity in vivo. Blood. 1998;91:4701–4707. [PubMed] [Google Scholar]
- 44.Johnson JM, Franchini G. Retroviral proteins that target the major histocompatibility complex class I. Virus Res. 2002;88:119–127. doi: 10.1016/s0168-1702(02)00124-7. [DOI] [PubMed] [Google Scholar]
- 45.Dekaban GA, Peters AA, Mulloy JC, Johnson JM, Trovato R, Rivadeneira E, Franchini G. The HTLV-I orfI protein is recognized by serum antibodies from naturally infected humans and experimentally infected rabbits. Virology. 2000;274:86–93. doi: 10.1006/viro.2000.0406. [DOI] [PubMed] [Google Scholar]
- 46.D'Agostino DM, Zotti L, Ferro T, Franchini G, Chieco-Bianchi L, Ciminale V. The p13II protein of HTLV type 1: comparison with mitochondrial proteins coded by other human viruses. AIDS Res Hum Retroviruses. 2000;16:1765–1770. doi: 10.1089/08892220050193281. [DOI] [PubMed] [Google Scholar]
- 47.Ciminale V, Zotti L, Dagostino DM, Ferro T, Casareto L, Franchini G, Bernardi P, Chiecobianchi L. Mitochondrial targeting of the p13(II) protein coded by the x-II ORF of human T-cell leukemia/lymphotropic virus type I (HTLV-I) Oncogene. 1999;18:4505–4514. doi: 10.1038/sj.onc.1203047. [DOI] [PubMed] [Google Scholar]
- 48.Trovato R, Mulloy JC, Johnson JM, Takemoto S, de Oliveira MP, Franchini G. A lysine-to-arginine change found in natural alleles of the human T-cell lymphotropic/leukemia virus type 1 p12(I) protein greatly influences its stability. J Virol. 1999;73:6460–6467. doi: 10.1128/jvi.73.8.6460-6467.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cereseto A, Berneman Z, Koralnik I, Vaughn J, Franchini G, Klotman ME. Differential expression of alternatively spliced pX mRNAs in HTLV-I-infected cell lines. Leukemia. 1997;11:866–870. doi: 10.1038/sj.leu.2400665. [DOI] [PubMed] [Google Scholar]
- 50.Chen YMA, Chen SH, Fu CY, Chen JY, Osame M. Antibody reactivities to tumor-suppressor protein p53 and HTLV-I Tof Rex and Tax in HTLV-I-infected people with differing clinical status. Int J Cancer. 1997;71:196–202. doi: 10.1002/(sici)1097-0215(19970410)71:2<196::aid-ijc12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 51.Kerkhofs P, Heremans H, Burny A, Kettmann R, Willems L. In vitro and in vivo oncogenic potential of bovine leukemia virus G4 protein. J Virol. 1998;72:2554–2559. doi: 10.1128/jvi.72.3.2554-2559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lefebvre L, Vanderplasschen A, Ciminale V, Heremans H, Dangoisse O, Jauniaux JC, Toussaint JF, Zelnik V, Burny A, Kettmann R, Willems L. Oncoviral Bovine Leukemia Virus G4 and Human T-Cell Leukemia Virus Type 1 p13(II) Accessory Proteins Interact with Farnesyl Pyrophosphate Synthetase. J Virol. 2002;76:1400–1414. doi: 10.1128/JVI.76.3.1400-1414.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lefebvre L, Ciminale V, Vanderplasschen A, D'Agostino D, Burny A, Willems L, Kettmann R. Subcellular localization of the bovine leukemia virus R3 and G4 accessory proteins. J Virol. 2002;76:7843–7854. doi: 10.1128/JVI.76.15.7843-7854.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Willems L, Kerkhofs P, Dequiedt F, Portetelle D, Mammerickx M, Burny A, Kettmann R. Attenuation of bovine leukemia virus by deletion of R3 and G4 open reading frames. Proc Natl Acad Sci U S A. 1994;91:11532–11536. doi: 10.1073/pnas.91.24.11532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ding W, Albrecht B, Luo R, Zhang W, Stanley JR, Newbound GC, Lairmore MD. Endoplasmic Reticulum and cis-Golgi Localization of Human T-Lymphotropic Virus Type 1 p12(I): Association with Calreticulin and Calnexin. J Virol. 2001;75:7672–7682. doi: 10.1128/JVI.75.16.7672-7682.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cantrell D. T cell antigen receptor signal transduction pathways. Annu Rev Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 57.Craig HM, Reddy TR, Riggs NL, Dao PP, Guatelli JC. Interactions of HIV-1 nef with the mu subunits of adaptor protein complexes 1, 2, and 3: role of the dileucine-based sorting motif. Virology. 2000;271:9–17. doi: 10.1006/viro.2000.0277. [DOI] [PubMed] [Google Scholar]
- 58.Franchini G, Mulloy JC, Koralnik IJ, Lo MA, Sparkowski JJ, Andresson T, Goldstein DJ, Schlegel R. The human T-cell leukemia/lymphotropic virus type I p12I protein cooperates with the E5 oncoprotein of bovine papillomavirus in cell transformation and binds the 16-kilodalton subunit of the vacuolar H+ ATPase. J Virol. 1993;67:7701–7704. doi: 10.1128/jvi.67.12.7701-7704.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koralnik IJ, Mulloy JC, Andresson T, Fullen J, Franchini G. Mapping of the intermolecular association of human T cell leukaemia/lymphotropic virus type I p12I and the vacuolar H+-ATPase 16 kDa subunit protein. J Gen Virol. 1995;76(Pt 8):1909–1916. doi: 10.1099/0022-1317-76-8-1909. [DOI] [PubMed] [Google Scholar]
- 60.Lu X, Yu H, Liu SH, Brodsky FM, Peterlin BM. Interactions between HIV1 Nef and vacuolar ATPase facilitate the internalization of CD4. Immunity. 1998;8:647–656. doi: 10.1016/s1074-7613(00)80569-5. [DOI] [PubMed] [Google Scholar]
- 61.Mandic R, Fackler OT, Geyer M, Linnemann T, Zheng YH, Peterlin BM. Negative factor from SIV binds to the catalytic subunit of the V-ATPase to internalize CD4 and to increase viral infectivity. Mol Biol Cell. 2001;12:463–473. doi: 10.1091/mbc.12.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mulloy JC, Crownley RW, Fullen J, Leonard WJ, Franchini G. The human T-cell leukemia/lymphotropic virus type 1 p12I proteins bind the interleukin-2 receptor beta and gammac chains and affects their expression on the cell surface. J Virol. 1996;70:3599–3605. doi: 10.1128/jvi.70.6.3599-3605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nicot C, Mulloy JC, Ferrari MG, Johnson JM, Fu K, Fukumoto R, Trovato R, Fullen J, Leonard WJ, Franchini G. HTLV-1 p12(I) protein enhances STAT5 activation and decreases the interleukin-2 requirement for proliferation of primary human peripheral blood mono nuclear cells. Blood. 2001;98:823–829. doi: 10.1182/blood.v98.3.823. [DOI] [PubMed] [Google Scholar]
- 64.Collins ND, D'Souza C, Albrecht B, Robek MD, Ratner L, Ding W, Green PL, Lairmore MD. Proliferation response to interleukin-2 and Jak/Stat activation of T cells immortalized by human T-cell lymphotropic virus type 1 is independent of open reading frame I expression. J Virol. 1999;73:9642–9649. doi: 10.1128/jvi.73.11.9642-9649.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johnson JM, Nicot C, Fullen J, Ciminale V, Casareto L, Mulloy JC, Jacobson S, Franchini G. Free Major Histocompatibility Complex Class I Heavy Chain Is Preferentially Targeted for Degradation by Human T-Cell Leukemia/Lymphotropic Virus Type 1 p12(I) Protein. J Virol. 2001;75:6086–6094. doi: 10.1128/JVI.75.13.6086-6094.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnson JM, Mulloy JC, Ciminale V, Fullen J, Nicot C, Franchini G. The MHC class I heavy chain is a common target of the small proteins encoded by the 3′ end of HTLV type 1 and HTLV type 2. AIDS Res Hum Retroviruses. 2000;16:1777–1781. doi: 10.1089/08892220050193308. [DOI] [PubMed] [Google Scholar]
- 67.Piguet V, Schwartz O, Legall S, Trono D. The downregulation of CD4 and MHC-I by primate lentiviruses: a paradigm for the modulation of cell surface receptors. Immunol Rev. 1999;168:51–63. doi: 10.1111/j.1600-065x.1999.tb01282.x. [DOI] [PubMed] [Google Scholar]
- 68.Mesaeli N, Nakamura K, Zvaritch E, Dickie P, Dziak E, Krause KH, Opas M, MacLennan DH, Michalak M. Calreticulin is essential for cardiac development. J Cell Biol. 1999;144:857–868. doi: 10.1083/jcb.144.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mellstrom B, Naranjo JR. Mechanisms of Ca(2+)-dependent transcription. Curr Opin Neurobiol. 2001;11:312–319. doi: 10.1016/s0959-4388(00)00213-0. [DOI] [PubMed] [Google Scholar]
- 70.Kuklina EM, Shirshev SV. Role of transcription factor NFAT in the immune response. Biochemistry (Mosc) 2001;66:467–475. doi: 10.1023/a:1010238931555. [DOI] [PubMed] [Google Scholar]
- 71.Zhu J, Mckeon F. Nucleocytoplasmic shuttling and the control of NF-AT signaling. Cell Mol Life Sci. 2000;57:411–420. doi: 10.1007/PL00000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Robek MD, Wong FH, Ratner L. Human T-Cell leukemia virus type 1 pX-I and pX-II open reading frames are dispensable for the immortalization of primary lymphocytes. J Virol. 1998;72:4458–4462. doi: 10.1128/jvi.72.5.4458-4462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manninen A, Saksela K. HIV-1 Nef interacts with inositol trisphosphate receptor to activate calcium signaling in T cells. J Exp Med. 2002;195:1023–1032. doi: 10.1084/jem.20012039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Petit C, Buseyne F, Boccaccio C, Abastado JP, Heard JM, Schwartz O. Nef is required for efficient hiv-1 replication in cocultures of dendritic cells and lymphocytes. Virology. 2001;286:225–236. doi: 10.1006/viro.2001.0984. [DOI] [PubMed] [Google Scholar]
- 75.Wu Y, Marsh JW. Selective transcription and modulation of resting T cell activity by preintegrated HIV DNA. Science. 2001;293:1503–1506. doi: 10.1126/science.1061548. [DOI] [PubMed] [Google Scholar]
- 76.Ding W, Kim SJ, Nair AM, Michael B, Boris-Lawrie K, Tripp A, Feuer G, Lairmore MD. Human T-Cell Lymphotropic Virus Type 1 p12(I) Enhances Interleukin-2 Production during T-Cell Activation. J Virol. 2003;77:11027–11039. doi: 10.1128/JVI.77.20.11027-11039.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.von Bulow GU, Bram RJ. NF-AT activation induced by a CAML- interacting member of the tumor necrosis factor receptor superfamily. Science. 1997;278:138–141. doi: 10.1126/science.278.5335.138. [DOI] [PubMed] [Google Scholar]
- 78.Ballard DW. Molecular mechanisms in lymphocyte activation and growth. Immunol Res. 2001;23:157–166. doi: 10.1385/IR:23:2-3:157. [DOI] [PubMed] [Google Scholar]
- 79.Aiken C. Mechanistic independence of Nef and cyclophilin A enhancement of human immunodeficiency virus type 1 infectivity. Virology. 1998;248:139–147. doi: 10.1006/viro.1998.9254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Manninen A, Saksela K. HIV-1 Nef Interacts with Inositol Trisphosphate Receptor to Activate Calcium Signaling in T Cells. J Exp Med. 2002;195:1023–1032. doi: 10.1084/jem.20012039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Manninen A, Huotari P, Hiipakka M, Renkema GH, Saksela K. Activation of NFAT-dependent gene expression by Nef: conservation among divergent Nef alleles, dependence on SH3 binding and membrane association, and cooperation with protein kinase C-theta. J Virol. 2001;75:3034–3037. doi: 10.1128/JVI.75.6.3034-3037.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou J, Aiken C. Nef enhances human immunodeficiency virus type 1 infectivity resulting from intervirion fusion: evidence supporting a role for Nef at the virion envelope. J Virol. 2001;75:5851–5859. doi: 10.1128/JVI.75.13.5851-5859.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang JK, Kiyokawa E, Verdin E, Trono D. The Nef protein of HIV-1 associates with rafts and primes T cells for activation. Proc Natl Acad Sci U S A. 2000;97:394–399. doi: 10.1073/pnas.97.1.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Manninen A, Herma RG, Saksela K. Synergistic activation of NFAT by HIV-1 nef and the Ras/MAPK pathway. J Biol Chem. 2000;275:16513–16517. doi: 10.1074/jbc.M910032199. [DOI] [PubMed] [Google Scholar]
- 85.Tokunaga K, Ikuta K, Adachi A, Matsuda M, Kurata T, Kojima A. The cellular kinase binding motifs (PxxP and RR) in human immunodeficiency virus type 1 Nef protein are dispensable for producer-cell-dependent enhancement of viral entry. Virology. 1999;257:285–289. doi: 10.1006/viro.1999.9682. [DOI] [PubMed] [Google Scholar]
- 86.Asamitsu K, Morishima T, Tsuchie H, Kurimura T, Okamoto T. Conservation of the central proline-rich (PxxP) motifs of human immunodeficiency virus type 1 Nef protein during the disease progression in two hemophiliac patients. FEBS Lett. 1999;459:399–404. doi: 10.1016/s0014-5793(99)01288-0. [DOI] [PubMed] [Google Scholar]
- 87.Saksela K, Cheng G, Baltimore D. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO J. 1995;14:484–491. doi: 10.1002/j.1460-2075.1995.tb07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aramburu J, Yaffe MB, Lopez-Rodriguez C, Cantley LC, Hogan PG, Rao A. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285:2129–2133. doi: 10.1126/science.285.5436.2129. [DOI] [PubMed] [Google Scholar]
- 89.Vogel KW, Briesewitz R, Wandless TJ, Crabtree GR. Calcineurin inhibitors and the generalization of the presenting protein strategy. Adv Protein Chem. 2001;56:253–291. doi: 10.1016/s0065-3233(01)56008-8. [DOI] [PubMed] [Google Scholar]
- 90.Crabtree GR. Calcium, calcineurin, and the control of transcription. J Biol Chem. 2001;276:2313–2316. doi: 10.1074/jbc.R000024200. [DOI] [PubMed] [Google Scholar]
- 91.Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- 92.Abeele FV, Skryma R, Shuba Y, Van Coppenolle F, Slomianny C, Roudbaraki M, Mauroy B, Wuytack F, Prevarskaya N. Bcl-2-dependent modulation of Ca(2+) homeostasis and store-operated channels in prostate cancer cells. Cancer Cell. 2002;1:169–179. doi: 10.1016/s1535-6108(02)00034-x. [DOI] [PubMed] [Google Scholar]
- 93.Pinton P, Ferrari D, Rapizzi E, Di Virgilio FD, Pozzan T, Rizzuto R. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 2001;20:2690–2701. doi: 10.1093/emboj/20.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dantuma NP, Masucci MG. The ubiquitin/proteasome system in Epstein- Barr virus latency and associated malignancies. Semin Cancer Biol. 2003;13:69–76. doi: 10.1016/s1044-579x(02)00101-3. [DOI] [PubMed] [Google Scholar]
- 95.Scheffner M, Whitaker NJ. Human papillomavirus- induced carcinogenesis and the ubiquitin-proteasome system. Semin Cancer Biol. 2003;13:59–67. doi: 10.1016/s1044-579x(02)00100-1. [DOI] [PubMed] [Google Scholar]
- 96.Coscoy L, Ganem D. PHD domains and E3 ubiquitin ligases: viruses make the connection. Trends Cell Biol. 2003;13:7–12. doi: 10.1016/s0962-8924(02)00005-3. [DOI] [PubMed] [Google Scholar]
- 97.Franchini G. Molecular mechanisms of human T-cell leukemia/lymphotropic virus type I infection. Blood. 1995;86:3619–3639. [PubMed] [Google Scholar]
- 98.Barmak K, Harhaj E, Grant C, Alefantis T, Wigdahl B. Human T cell leukemia virus type I- induced disease: pathways to cancer and neurodegeneration. Virology. 2003;308:1–12. doi: 10.1016/s0042-6822(02)00091-0. [DOI] [PubMed] [Google Scholar]
- 99.Martins ML, Soares BC, Ribas JG, Thorun GW, Johnson J, Kroon EG, Carneiro-Prioetti AB, Bonjardim CA. Frequency of p12K and p12R Alleles of HTLV Type 1 in HAM/TSP Patients and in Asymptomatic HTLV Type 1 Carriers. AIDS Res Hum Retroviruses. 2002;18:899–902. doi: 10.1089/088922202760265560. [DOI] [PubMed] [Google Scholar]
- 100.Chou KS, Okayama A, Tachibana N, Lee TH, Essex M. Nucleotide sequence analysis of a full- length human T-cell leukemia virus type I from adult T-cell leukemia cells: A prematurely terminated pX open reading frame II. Int J Cancer. 1995;60:701–706. doi: 10.1002/ijc.2910600522. [DOI] [PubMed] [Google Scholar]
- 101.Dagostino DM, Ciminale V, Zotti L, Rosato A, Chiecobianchi L. The human T-cell lymphotropic virus type 1 Tof protein contains a bipartite nuclear localization signal that is able to functionally replace the amino-terminal domain of Rex. J Virol. 1997;71:75–83. doi: 10.1128/jvi.71.1.75-83.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wysocka J, Herr W. The herpes simplex virus VP16-induced complex: the makings of a regulatory switch. Trends Biochem Sci. 2003;28:294–304. doi: 10.1016/S0968-0004(03)00088-4. [DOI] [PubMed] [Google Scholar]
- 103.Latchman DS. Regulation of DNA virus transcription by cellular POU family transcription factors. Rev Med Virol. 1999;9:31–38. doi: 10.1002/(sici)1099-1654(199901/03)9:1<31::aid-rmv231>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 104.Georges SA, Giebler HA, Cole PA, Luger K, Laybourn PJ, Nyborg JK. Tax recruitment of CBP/p300, via the KIX domain, reveals a potent requirement for acetyltransferase activity that is chromatin dependent and histone tail independent. Mol Cell Biol. 2003;23:3392–3404. doi: 10.1128/MCB.23.10.3392-3404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Georges SA, Kraus WL, Luger K, Nyborg JK, Laybourn PJ. p300- mediated tax transactivation from recombinant chromatin: histone tail deletion mimics coactivator function. Mol Cell Biol. 2002;22:127–137. doi: 10.1128/MCB.22.1.127-137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lu H, Pise-Masison CA, Fletcher TM, Schiltz RL, Nagaich AK, Radonovich M, Hager G, Cole PA, Brady JN. Acetylation of nucleosomal histones by p300 facilitates transcription from tax-responsive human T-cell leukemia virus type 1 chromatin template. Mol Cell Biol. 2002;22:4450–4462. doi: 10.1128/MCB.22.13.4450-4462.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Okada M, Jeang KT. Differential requirements for activation of integrated and transiently transfected human T-cell leukemia virus type 1 long terminal repeat. J Virol. 2002;76:12564–12573. doi: 10.1128/JVI.76.24.12564-12573.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Harrod R, Kuo YL, Tang Y, Yao Y, Vassilev A, Nakatani Y, Giam CZ. p300 and p300/cAMP-responsive element-binding protein associated factor interact with human T-cell lymphotropic virus type-1 Tax in a multi- histone acetyltransferase/activator-enhancer complex. J Biol Chem. 2000;275:11852–11857. doi: 10.1074/jbc.275.16.11852. [DOI] [PubMed] [Google Scholar]
- 109.Campbell KM, Lumb KJ. Structurally distinct modes of recognition of the KIX domain of CBP by Jun and CREB. Biochemistry. 2002;41:13956–13964. doi: 10.1021/bi026222m. [DOI] [PubMed] [Google Scholar]
- 110.Nicot C, Mahieux R, Pise-Masison C, Brady J, Gessain A, Yamaoka S, Franchini G. Human T-Cell Lymphotropic Virus Type 1 Tax Represses c-Myb-Dependent Transcription through Activation of the NF-kappaB Pathway and Modulation of Coactivator Usage. Mol Cell Biol. 2001;21:7391–7402. doi: 10.1128/MCB.21.21.7391-7402.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nicot C, Mahieux R, Opavsky R, Cereseto A, Wolff L, Brady JN, Franchini G. HTLV-I Tax transrepresses the human c-Myb promoter independently of its interaction with CBP or p300. Oncogene. 2000;19:2155–2164. doi: 10.1038/sj.onc.1203536. [DOI] [PubMed] [Google Scholar]
- 112.Colgin MA, Nyborg JK. The human T-cell leukemia virus type 1 oncoprotein Tax inhibits the transcriptional activity of c-Myb through competition for the CREB binding protein. J Virol. 1998;72:9396–9399. doi: 10.1128/jvi.72.11.9396-9399.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Janknecht R. The versatile functions of the transcriptional coactivators p300 and CBP and their roles in disease. Histol Histopathol. 2002;17:657–668. doi: 10.14670/HH-17.657. [DOI] [PubMed] [Google Scholar]
- 114.Blobel GA. CBP and p300: versatile coregulators with important roles in hematopoietic gene expression. J Leukoc Biol. 2002;71:545–556. [PubMed] [Google Scholar]
- 115.Vo N, Goodman RH. CREB-binding protein and p300 in transcriptional regulation. J Biol Chem. 2001;276:13505–13508. doi: 10.1074/jbc.R000025200. [DOI] [PubMed] [Google Scholar]
- 116.Snowden AW, Perkins ND. Cell cycle regulation of the transcriptional coactivators p300 and CREB binding protein. Biochem Pharmacol. 1998;55:1947–1954. doi: 10.1016/s0006-2952(98)00020-3. [DOI] [PubMed] [Google Scholar]
- 117.Shikama N, Lyon J, Lathangue NB. The p300/CBP family: Integrating signals with transcription factors and chromatin. Tr Cell Biol. 1997;7:230–236. doi: 10.1016/S0962-8924(97)01048-9. [DOI] [PubMed] [Google Scholar]
- 118.Ego T, Ariumi Y, Shimotohno K. The interaction of HTLV-1 Tax with HDAC1 negatively regulates the viral gene expression. Oncogene. 2002;21:7241–7246. doi: 10.1038/sj.onc.1205701. [DOI] [PubMed] [Google Scholar]
- 119.Vandel L, Trouche D. Physical association between the histone acetyl transferase CBP and a histone methyl transferase. EMBO rep. 2001;2:21–26. doi: 10.1093/embo-reports/kve002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Satoh M, Toma H, Sugahara K, Etoh KK, Shiroma Y, Kiyuna S, Takara M, Matsuoka M, Yamaguchi K, Nakada K, Fujita K, Kojima S, Hori E, Tanaka Y, Kamihira S, Sato Y, Watanabe T. Involvement of IL-2/IL-2R system activation by parasite antigen in polyclonal expansion of CD4(+)25(+) HTLV-1- infected T-cells in human carriers of both HTLV-1 and S. stercoralis. Oncogene. 2002;21:2466–2475. doi: 10.1038/sj.onc.1205329. [DOI] [PubMed] [Google Scholar]
- 121.Butscher WG, Haggerty CM, Chaudhry S, Gardner K. Targeting of p300 to the Interleukin-2 promoter via CREB/Rel cross-talk during mitogen and oncogenic molecular signaling in activated T-cells. J Biol Chem. 2001 doi: 10.1074/jbc.M009614200. [DOI] [PubMed] [Google Scholar]
- 122.Boulougouris G, Mcleod JD, Patel YI, Ellwood CN, Walker LS, Sansom DM. IL-2-independent activation and proliferation in human T cells induced by CD28. J Immunol. 1999;163:1809–1816. [PubMed] [Google Scholar]
- 123.Varga G, Dreikhausen U, Kracht M, Appel A, Resch K, Szamel M. Molecular mechanisms of T lymphocyte activation: convergence of T cell antigen receptor and IL-1 receptor- induced signaling at the level of IL-2 gene transcription. Int Immunol. 1999;11:1851–1862. doi: 10.1093/intimm/11.11.1851. [DOI] [PubMed] [Google Scholar]
- 124.Voelter-Mahlknecht S, Mahlknecht U. Cloning and structural characterization of the human histone deacetylase 6 gene. Int J Mol Med. 2003;12:87–93. [PubMed] [Google Scholar]
- 125.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 126.Liao L, Kuang SQ, Yuan Y, Gonzalez SM, O'Malley BW, Xu J. Molecular structure and biological function of the cancer-amplified nuclear receptor coactivator SRC-3/AIB1. J Steroid Biochem Mol Biol. 2002;83:3–14. doi: 10.1016/s0960-0760(02)00254-6. [DOI] [PubMed] [Google Scholar]
- 127.Timmermann S, Lehrmann H, Polesskaya A, Harel-Bellan A. Histone acetylation and disease. Cell Mol Life Sci. 2001;58:728–736. doi: 10.1007/PL00000896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sterner DE, Berger SL. Acetylation of histones and transcription-related factors. Microbiol Mol Biol Rev. 2000;64:435–459. doi: 10.1128/mmbr.64.2.435-459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Latchman DS. Regulation of DNA virus transcription by cellular POU family transcription factors. Rev Med Virol. 1999;9:31–38. doi: 10.1002/(sici)1099-1654(199901/03)9:1<31::aid-rmv231>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 130.Fujii M, Iwai K, Oie M, Fukushi M, Yamamoto N, Kannagi M, Mori N. Activation of oncogenic transcription factor AP-1 in T cells infected with human T cell leukemia virus type 1. AIDS Res Hum Retroviruses. 2000;16:1603–1606. doi: 10.1089/08892220050193029. [DOI] [PubMed] [Google Scholar]
- 131.Mori N, Fujii M, Iwai K, Ikeda S, Yamasaki Y, Hata T, Yamada Y, Tanaka Y, Tomonaga M, Yamamoto N. Constitutive activation of transcription factor AP-1 in primary adult T- cell leukemia cells. Blood. 2000;95:3915–3921. [PubMed] [Google Scholar]
- 132.Kawata S, Ariumi Y, Shimotohno K. p21(Waf1/Cip1/Sdi1) prevents apoptosis as well as stimulates growth in cells transformed or immortalized by human T-cell leukemia virus type 1-encoded tax. J Virol. 2003;77:7291–7299. doi: 10.1128/JVI.77.13.7291-7299.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Haoudi A, Semmes OJ. The HTLV-1 tax oncoprotein attenuates DNA damage induced G1 arrest and enhances apoptosis in p53 null cells. Virology. 2003;305:229–239. doi: 10.1006/viro.2002.1642. [DOI] [PubMed] [Google Scholar]
- 134.Liu B, Liang MH, Kuo YL, Liao W, Boros I, Kleinberger T, Blancato J, Giam CZ. Human T-Lymphotropic Virus Type 1 Oncoprotein Tax Promotes Unscheduled Degradation of Pds1p/Securin and Clb2p/Cyclin B1 and Causes Chromosomal Instability. Mol Cell Biol. 2003;23:5269–5281. doi: 10.1128/MCB.23.15.5269-5281.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Haoudi A, Daniels RC, Wong E, Kupfer G, Semmes OJ. HTLV-1 tax oncoprotein functionally targets a subnuclear complex involved in cellular DNA damage-response. J Biol Chem. 2003 doi: 10.1074/jbc.M301649200. [DOI] [PubMed] [Google Scholar]
- 136.Wang L, Deng L, Wu K, de La FC, Wang D, Kehn K, Maddukuri A, Baylor S, Santiago F, Agbottah E, Trigon S, Morange M, Mahieux R, Kashanchi F. Inhibition of HTLV-1 transcription by cyclin dependent kinase inhibitors. Mol Cell Biochem. 2002;237:137–153. doi: 10.1023/a:1016555821581. [DOI] [PubMed] [Google Scholar]
- 137.Pise-Masison CA, Mahieux R, Radonovich M, Jiang H, Duvall J, Guillerm C, Brady JN. Insights into the molecular mechanism of p53 inhibition by HTLV type 1 Tax. AIDS Res Hum Retroviruses. 2000;16:1669–1675. doi: 10.1089/08892220050193128. [DOI] [PubMed] [Google Scholar]
- 138.Konikova E, Kusenda J. Altered expression of p53 and MDM2 proteins in hematological malignancies. Neoplasma. 2003;50:31–40. [PubMed] [Google Scholar]
- 139.McCoy MA, Gesell JJ, Senior MM, Wyss DF. Flexible lid to the p53-binding domain of human Mdm2: implications for p53 regulation. Proc Natl Acad Sci U S A. 2003;100:1645–1648. doi: 10.1073/pnas.0334477100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.D'Agostino DM, Ranzato L, Arrigoni G, Cavallari I, Belleudi F, Torrisi MR, Silic-Benussi M, Ferro T, Petronilli V, Marin O, Chieco-Bianchi L, Bernardi P, Ciminale V. Mitochondrial alterations induced by the p13II protein of human T-cell leukemia virus type 1. Critical role of arginine residues. J Biol Chem. 2002;277:34424–34433. doi: 10.1074/jbc.M203023200. [DOI] [PubMed] [Google Scholar]
- 141.D'Agostino DM, Zotti L, Ferro T, Cavallori I, Silic- Benussi M, Chieco-Bianchi L, Ciminale V. Expression and functional properties of proteins encoded in the x-II ORF of HTLV-I. Virus Res. 2001;78:35–43. doi: 10.1016/s0168-1702(01)00282-9. [DOI] [PubMed] [Google Scholar]
- 142.Roithmann S, Pique C, Le Cesne A, Delamarre L, Pham D, Tursz T, Dokhelar MC. The open reading frame I (ORF I)/ORF II part of the human T-cell leukemia virus type 1 X region is dispensable for p40tax, p27rex, or envelope expression. J Virol. 1994;68:3448–3451. doi: 10.1128/jvi.68.5.3448-3451.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Thomson BJ. Viruses and apoptosis. Int J Exp Pathol. 2001;82:65–76. doi: 10.1111/j.1365-2613.2001.iep0082-0065-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Barber GN. Host defense, viruses and apoptosis. Cell Death Differ. 2001;8:113–126. doi: 10.1038/sj.cdd.4400823. [DOI] [PubMed] [Google Scholar]
- 145.Everett H, McFadden G. Viruses and apoptosis: meddling with mitochondria. Virology. 2001;288:1–7. doi: 10.1006/viro.2001.1081. [DOI] [PubMed] [Google Scholar]
- 146.Everett H, McFadden G. Viral proteins and the mitochondrial apoptotic checkpoint. Cytokine Growth Factor Rev. 2001;12:181–188. doi: 10.1016/s1359-6101(00)00025-3. [DOI] [PubMed] [Google Scholar]
- 147.Hou X, Foley S, Cueto M, Robinson MA. The Human T-Cell Leukemia Virus Type I (HTLV-I) X Region Encoded Protein p13(II) Interacts with Cellular Proteins. Virology. 2000;277:127–135. doi: 10.1006/viro.2000.0604. [DOI] [PubMed] [Google Scholar]
- 148.Mahana W, Zhao TM, Teller R, Robinson MA, Kindt TJ. Genes in the pX region of human T cell leukemia virus I influence Vav phosphorylation in T cells. Proc Natl Acad Sci U S A. 1998;95:1782–1787. doi: 10.1073/pnas.95.4.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kaminuma O, Deckert M, Elly C, Liu YC, Altman A. Vav-Rac1- mediated activation of the c-Jun N-terminal kinase/c-Jun/AP-1 pathway plays a major role in stimulation of the distal NFAT site in the interleukin-2 gene promoter. Mol Cell Biol. 2001;21:3126–3136. doi: 10.1128/MCB.21.9.3126-3136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Slattery JP, Franchini G, Gessain A. Genomic evolution, patterns of global dissemination, and interspecies transmission of human and simian T-cell leukemia/lymphotropic viruses. Genome Res. 1999;9:525–540. [PubMed] [Google Scholar]
- 151.Franchini G, Streicher H. Human T-cell leukaemia virus. Baillieres Clin Haematol. 1995;8:131–148. doi: 10.1016/s0950-3536(05)80235-5. [DOI] [PubMed] [Google Scholar]
- 152.Ye J, Xie L, Green PL. Tax and overlapping rex sequences do not confer the distinct transformation tropisms of human T-cell leukemia virus types 1 and 2. J Virol. 2003;77:7728–7735. doi: 10.1128/JVI.77.14.7728-7735.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zehender G, Colasante C, Santambrogio S, De Maddalena C, Massetto B, Cavalli B, Jacchetti G, Fasan M, Adorni F, Osio M, Moroni M, Galli M. Increased risk of developing peripheral neuropathy in patients coinfected with HIV-1 and HTLV-2. J Acquir Immune Defic Syndr. 2002;31:440–447. doi: 10.1097/00126334-200212010-00011. [DOI] [PubMed] [Google Scholar]
- 154.Cockerell GL, Weiser MG, Rovnak J, Wicks-Beard B, Roberts B, Post A, Chen IS, Lairmore MD. Infectious transmission of human T-cell lymphotropic virus type II in rabbits. Blood. 1991;78:1532–1537. [PubMed] [Google Scholar]
- 155.Green PL, Ross TM, Chen ISY, Pettiford S. Human T-cell leukemia virus type II nucleotide sequences between env and the last exon of tax/rex are not required for viral replication or cellular transformation. J Virol. 1995;69:387–394. doi: 10.1128/jvi.69.1.387-394.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cockerell GL, Rovnak J, Green PL, Chen ISY. A deletion in the proximal untranslated pX region of human T- cell leukemia virus type II decreases viral replication but not infectivity in vivo. Blood. 1996;87:1030–1035. [PubMed] [Google Scholar]
- 157.Willems L, Burny A, Collete D, Dangoisse O, Dequiedt F, Gatot JS, Kerkhofs P, Lefebvre L, Merezak C, Peremans T, Portetelle D, Twizere JC, Kettmann R. Genetic determinants of bovine leukemia virus pathogenesis. AIDS Res Hum Retroviruses. 2000;16:1787–1795. doi: 10.1089/08892220050193326. [DOI] [PubMed] [Google Scholar]
- 158.Dequiedt F, Hanon E, Kerkhofs P, Pastoret PP, Portetelle D, Burny A, Kettmann R, Willems L. Both wild-type and strongly attenuated bovine leukemia viruses protect peripheral blood mononuclear cells from apoptosis. J Virol. 1997;71:630–639. doi: 10.1128/jvi.71.1.630-639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Reichert M, Winnicka A, Willems L, Kettmann R, Cantor GH. Role of the proline-rich motif of bovine leukemia virus transmembrane protein gp30 in viral load and pathogenicity in sheep. J Virol. 2001;75:8082–8089. doi: 10.1128/JVI.75.17.8082-8089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nutt LK, Pataer A, Pahler J, Fang B, Roth J, Mcconkey DJ, Swisher SG. Bax and Bak promote apoptosis by modulating endoplasmic reticular and mitochondrial Ca2+ stores. J Biol Chem. 2002;277:9219–9225. doi: 10.1074/jbc.M106817200. [DOI] [PubMed] [Google Scholar]


