Abstract
Background
Alterations in regional fat are often reported in HIV infection. Prior studies have not distinguished between normal changes in regional fat related to sexual maturation and those due to HIV. The study aim was to compare changes in regional fat distribution in HIV-infected (HIV+) and healthy (HIV−) children and adolescents living in the United States.
Methods
Serial dual energy X-ray absorptiometry was performed at baseline and two annual follow-up visits in 64 HIV+ and 147 HIV− participants aged 6–16 years. Total, leg, arm, and trunk fat masses (kg) and regional fat distribution as the percentage of total body fat (%) were compared.
Results
HIV+ and HIV− participants did not differ in total fat mass, but the HIV+ group had significantly lower leg and greater arm fat and trunk fat percentage at all time points. Over time, decreases in leg fat percentage and increases in arm fat percentage were more marked among the HIV+ group. Differences between HIV+ and HIV− groups in arm and leg fat percentage remained significant when age, sex, race, height, and pubertal stage were accounted for by mixed effect modeling. Apart from prior treatment with stavudine, no differences in fat distribution were observed according to treatment or degree of immunodeficiency or viremia.
Conclusion
Although no single pattern of change in regional fat distribution was uniquely associated with HIV, perinatally HIV-infected youth manifest significantly decreased leg fat and increased arm and trunk fat. These differences increase over time and may contribute to cardiovascular disease risk.
Keywords: adolescent HIV infection, dual X-ray absorptiometry, lipoatrophy, lipodystrophy, pediatric HIV infection, regional fat
Introduction
Changes in regional fat distribution including peripheral fat loss and central fat gain occur in HIV-infected adults, children, and adolescents [1–8]. These alterations variably referred to as ‘lipodystrophy’ or ‘fat redistribution syndrome’ can be stigmatizing and negatively affect adherence to HIV medications and the course of treatment [9–12]. HIV-associated lipodystrophy is also frequently encountered with hyperlipidemia, hypertriglyceridemia and insulin resistance, which are risk factors for cardiovascular disease [2,13–16].
Due to differences in measurement methods and case definitions, determining the true prevalence of lipodystrophy in children is difficult and estimates vary from 1 to 43% [14,15]. Prior descriptive studies have used a variety of methods and criteria to measure HIV-associated abnormalities in regional fat distribution including physical inspection [6,17,13], skin fold thickness and circumference-based case definitions normalized against age and sex standards [2,16] and laboratory methods that include dual energy X-ray absorptiometry (DXA), computed tomography (CT), and magnetic resonance imaging [4,5,8,14,15]. However, none of these studies compared results to rigorously assessed changes in regional fat stores that accompany normal growth and sexual maturation.
The objective of this study was to characterize and compare changes over 2 years in arm, leg and trunk fat in a sample of HIV-infected children and adolescents with healthy participants by means of whole body DXA scans.
Methods
Study population
Data for this study were obtained from two studies conducted at the St. Luke's Roosevelt Pediatric Body Composition Unit of the New York Obesity Research Center; in HIV-infected and healthy participants, entitled ‘The effects of vitamin D and calcium on bone in pediatric HIV’ (ClinicalTrials.gov protocol NCT00724178) [17] and ‘Bone mineral density in childhood study’, respectively [18]. HIV-infected participants aged 6–16 years were recruited from among children and adolescents enrolled in four hospital-based pediatric HIV treatment programs at St. Luke's-Roosevelt Hospital Center, Harlem Hospital Center, Bronx-Lebanon Hospital Center and Metropolitan Hospital Center, which are located in New York City, New York and received vitamin D and calcium supplementation or placebo during the 2-year study. Healthy volunteers aged 6–16 years were recruited by advertisements run in local newspapers, school newsletters, flyers in nearby hospital waiting rooms and word of mouth.
Informed consent
This study was approved by the Institutional Review Boards of all participating institutions. Informed consent from parents or guardians and assent of patients were obtained prior to enrollment of participants.
Anthropometric and body composition measures
All study measurements were performed at baseline and two annual follow-up visits to the St. Luke's-Roosevelt Pediatric Body Composition Unit. Identical procedures and DXA scanner were used for all participants throughout the study period. Total, leg, arm, and trunk fat masses were measured by DXA (Hologic Delphi, Waltham, Massachusetts, USA). Fat distribution as the percentage of total body fat (percentage of total) was calculated for each region. Standing height was measured with a Holtain Stadiometer and weight on a calibrated digital scale (Ohaus Corp., Florham Park, New Jersey, USA). Pubertal stage was determined by the stage of breast development in girls or testicular volume by orchidometer in boys and stage of pubic hair in both boys and girls using the criteria of Tanner by two trained investigators (S.A. and M.H.) [19,20]. Pubertal status of less than stage 3 or at least 3 based on stage of breast development for girls and pubic hair for boys was used in the analysis. Height-for-age, weight-for-age and BMI-for-age were determined based on Centers for Disease Control and Prevention (CDC) norms [21]. For participants with HIV disease classification was determined using CDC criteria [22].
Data analysis
We evaluated leg, arm, and trunk fat both as actual mass (kg) and as percentage of total fat (%). Similarly, we evaluated change in leg, arm, and trunk fat percentage as change from one time to another [percentage change = (later value – earlier value)/(earlier value)] and as change in percentage of total fat from one time to another [change = (later percentage) – (earlier percentage)] × 100. Baseline characteristics were compared using χ2 tests and Wilcoxon rank-sum tests. Total, leg, arm, and trunk fat masses (kg) and fat distribution (%) as the percentage of total body fat in each region were also compared using Wilcoxon rank-sum tests. Mixed effects, repeated measures models were used to compare arm, leg, and trunk fat percentage of total between HIV+ and healthy children over three time periods in both univariate and multivariate models. Multivariate mixed effects repeated measures models were developed by considering all possible subsets of variables as well as interactions between significant predictors. Categorical classification of changes in regional fat between visits (increase, no change, decrease) was determined based on whether the observed change exceeded the coefficient of variation of DXA measurement [23]. χ2 tests were used to test for differences in frequencies of types of changes between subgroups (e.g., HIV-infected versus healthy participants).
Differences in regional fat were evaluated among HIV-infected participants receiving different categories of antiretrovirals by comparing median arm, leg, and trunk fat percentage of total among HIV-infected participants receiving the following classes of medication at the time of enrollment in the study: nucleoside reverse transcriptase inhibitors (NRTIs), nonnucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors. NRTI were further classified as thymidine analogues (stavudine and zidovudine) and nonthymidine analogues (all other NRTIs). Analyses based on any past use of the above antiretroviral categories were also performed. The Wilcoxon rank-sum test was used to test for differences between medians. In addition, we used a mixed effects analysis to evaluate CD4 cell percentage and viral load as predictors of percentage arm, leg, and trunk fat. Multivariate models were developed in a multistage process. First, variables were tested as predictors of percentage body fat in each of three compartments, both alone and in conjunction with HIV infection status. Next, significant predictors for each compartment were combined into a single model. Third, interactions between HIV and other predictors were tested for significance. The final model includes variables that are significant either independently or through interactions.
Results
Table 1 presents the baseline characteristics of study participants. Sixty-four HIV+ and 147 healthy participants, aged 6–16 years, were enrolled. HIV+ and healthy participants were similar at baseline with respect to pubertal stage, sex, height-for-age, and weight-for-age. However, the HIV+ group was younger [10.3 (3.7) vs. 11.6 (2.8) years, mean (SD); P = 0.002] and had a greater proportion of African–Americans (69 vs. 48%, P = 0.006).
Table 1. Baseline characteristics of healthy (n = 157) and HIV-infected (n = 64) patients.
| Variables | Healthy | HIV-infected | P value (DF) |
|---|---|---|---|
| Age, mean (SD) | 11.6 (2.8) | 10.3 (2.7) | 0.002 |
| Sex, n (%) | |||
| Male participants | 73 (49.7) | 31 (48.4) | 0.870 (1) |
| Female participants | 74 (50.3 | 33 (51.6) | NA |
| Race/ethnicity, n (%) | |||
| Black | 71 (48.3) | 44 (68.8) | 0.006 (1) |
| Hispanic | 76 (51.7) | 20 (31.3) | NA |
| CDC HIV Clinical Classification, n (%) | |||
| A | NA | 25 (39.1) | NA |
| B | NA | 26 (40.6) | NA |
| C | NA | 13 (20.3) | NA |
| CD4+ cell count (cell/μl), mean (SD) | NA | 792 (384) | NA |
| HIV RNA (log 10 copies/ml), mean (SD)a | NA | 3.34 (0.79) | NA |
| Receiving antiretroviral medications, n (%) | NA | 60 (94) | NA |
| Pubertal stage: girls, n (%) | |||
| <3 | 31 (41.9) | 13 (39.4) | 0.808 (1) |
| ≥3 | 43 (58.1) | 20 (60.6) | NA |
| Pubertal stage: boys, n (%) | |||
| <3 | 45 (62.5) | 19 (61.3) | 0.908 (1) |
| ≥3 | 27 (37.5) | 12 (38.7) | NA |
| Height-for-age | |||
| Percentile, mean (SD) | 55.7 (25.9) | 50.4 (28.4) | 0.208 |
| Z-scores, mean (SD) | 0.2 (0.8) | −0.0 (1.1) | 0.159 |
| Minimum, percentile | 2.55 | 0.03 | ND |
| Maximum, percentile | 96.9 | 99.81 | ND |
| Range, percentile | 94.41 | 99.78 | ND |
| Weight-for-age | |||
| Percentile, mean (SD) | 60.8 (24.3) | 62.0 (29.1) | 0.770 |
| Z-scores, mean (SD) | 0.3 (0.8) | 0.4 (1.1) | 0.467 |
| Minimum, percentile | 5.87 | 0.47 | ND |
| Maximum, percentile | 97.09 | 99.62 | ND |
| Range, percentile | 91.22 | 99.15 | ND |
| BMI-for-age | |||
| Percentile, mean (SD) | 60.6 (23.9) | 65.7 (28.1) | 0.205 |
| Z-scores, mean (SD) | 0.3 (0.8) | 0.5 (1.2) | 0.130 |
| Total fat (kg), median (IQR) | 8.86 (6.62) | 8.43 (4.33) | 0.624 |
| Total fat (% of weight), median (IQR) | 22 (10) | 22 (9) | 0.944 |
| Arm fat (kg), median (IQR) | 0.92 (0.92) | 0.99 (0.78) | 0.372 |
| Leg fat (kg), median (IQR) | 4.26 (3.29) | 3.73 (2.10) | 0.049 |
| Trunk fat (kg), median (IQR) | 2.58 (2.47) | 2.52 (1.83) | 0.939 |
| Arm/total fat, (%) median (IQR) | 11 (2) | 13 (3) | <0.001 |
| Leg/total fat (%), median (IQR) | 47 (6) | 44 (9) | 0.002 |
| Trunk/total fat (%), median (IQR) | 31 (6) | 32 (10) | 0.368 |
DF, degrees of freedom; IQR, interquartile range; NA, not applicable; ND, not done; SD, standard deviation.
Among those (38%) with detectable viral load.
All HIV+ participants were infected perinatally and at baseline, 94% were receiving two or more antiretroviral drugs. The mean (SD) baseline CD4 lymphocyte count was 792 (384) cells/μl; among those (38%) with a HIV RNA viral load above the assay level of detection, the mean (SD) was 3.34 (0.79)Log10 copies/ml. Antiretroviral use was as follows: two RTIs + protease inhibitor, n = 9 (14.1%), two RTI + NNRTI, n = 7 (10.9%), RTI-only regimen, n = 11 (17.2%), more than two RTI + protease inhibitor, n = 14 (21.9%), RTIs + protease inhibitors, and NNRTI, n = 14 (21.9%), other combinations, n = 5 (7.8%) [>two RTIs + NRTI (one); RTI + protease inhibitor + entry inhibitor (two); one RTI + NRTI (one); one RTI + two protease inhibitors (one)], and none, n = 4 (6.2%).
Table 1 also shows baseline anthropometric and body composition measurements. HIV-infected and healthy groups did not differ in total fat mass or trunk fat as percentage of total fat, but the HIV+ group had significantly lower leg and greater arm fat as percentage of total at baseline compared with the healthy group. Figure 1 illustrates percentage change between baseline, year 1, and year 2 follow-up in fat in arm, leg, and trunk for HIV+ and healthy groups. Significant differences between the groups were detectable in the percentage change in arm fat mass between baseline and year 1 follow-up (median 5.5% among HIV-infected vs. −3.0% among healthy individuals, P < 0.0001) and in percentage change in leg fat mass (median 0.0% among HIV-infected patients vs. 2.0%, P = 0.021); the difference observed in percentage change in trunk fat was not significant (2.0 vs. 0.0%, P = 0.284). Changes between baseline and year 2 were similar for arm fat mass (median 6.0% among HIV-infected vs. −5.0% among healthy individuals, P < 0.0001) and leg fat mass (median 0.0% among HIV-infected patients vs. 2.0%, P = 0.074); differences between HIV-infected and healthy participants were not significant for changes between baseline and year 2.
Fig. 1. Percentage change from baseline to year 1 and year 2 follow-up in leg, arm, and trunk fat mass among HIV-infected and healthy children and adolescents.
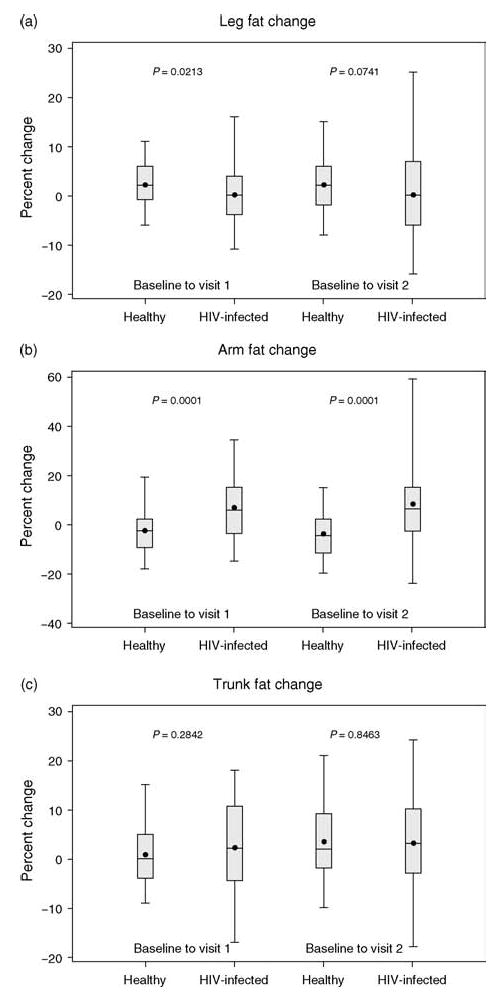
Percentage change from baseline to year 1 and year 2 follow-up in leg (a), arm (b), and trunk (c) fat mass. Top and bottom lines represent 95th and 5th percentiles. The upper and lower borders of the box represent 75th and 25th percentiles. The middle line represents the median and the dot represents the mean.
Table 2 shows the pattern of changes in regional fat for the HIV-infected and healthy participants between baseline and visit 1 and baseline and visit 2. There were significant differences in the patterns of change in leg and arm fat as a percentage of total fat (P = 0.005 and <0.001, respectively) in the HIV-infected versus healthy participants between baseline and the year 1 follow-up but not in changes in trunk fat percentage of total (P = 0.4) or total fat as percentage of body weight (P = 0.5). Specifically, a greater proportion of the HIV+ group had a decrease in leg fat percentage of total in comparison to healthy participants among whom an increase in leg fat percentage of total was more common (P = 0.005). Similarly, the pattern of change also differed for arm fat percentage of total, which was more likely to increase among HIV-infected participants, for whom an increase in arm fat percentage of total was the most common pattern seen between baseline and the 1-year follow-up, in comparison to healthy participants (P < 0.001). Similar patterns of change in arm, leg, and trunk fat percentage of total were observed in the interval between baseline and year 2.
Table 2. Change in fat distribution in HIV-infected and healthy participants from baseline to visit 1 (1 year) and visit 2 (2 years).
| Baseline to visit 1 | Baseline to visit 2 | |||||
|---|---|---|---|---|---|---|
| Direction of change | HIV-infected (n = 52) n (%) |
Healthy (n = 131) n (%) |
P value (DF) | HIV-infected (n = 49) n (%) |
Healthy (n = 117) n (%) |
P value (DF) |
| Leg fat/total fat (%) | ||||||
| Decrease | 19 (37) | 20 (15) | 0.005 (2) | 21 (43) | 28 (24) | 0.037 (2) |
| No change | 16 (31) | 46 (35) | NA | 7 (14) | 30 (26) | NA |
| Increase | 17 (33) | 65 (50) | NA | 21 (43) | 59 (50) | NA |
| Arm fat/total fat (%) | ||||||
| Decrease | 10 (19) | 55 (42) | <0.001 (2) | 13 (27) | 59 (50) | <0.001(2) |
| No change | 14 (27) | 47 (36) | NA | 10 (20) | 37 (32) | |
| Increase | 28 (54) | 29 (22) | NA | 26 (53) | 21 (18) | |
| Trunk fat/total fat (%) | ||||||
| Decrease | 14 (27) | 37 (28) | 0.406 (2) | 12 (24) | 21 (18) | 0.436 (2) |
| No change | 17 (33) | 54 (41) | NA | 15 (31) | 47 (40) | NA |
| Increase | 21 (40) | 40 (31) | NA | 22 (45) | 49 (42) | NA |
| Total fat (% of weight) | ||||||
| Decrease | 15 (29) | 37 (28) | 0.492 (2) | 16 (33) | 33 (28) | 0.660 (2) |
| No change | 9 (17) | 33 (25) | NA | 8 (16) | 26 (22) | NA |
| Increase | 28 (54) | 61 (47) | NA | 25 (51) | 58 (50) | NA |
DF, degrees of freedom; NA, not applicable.
Table 3 shows the results of univariate analysis with HIV status, age, sex, pubertal stage, race, and height as predictors of percentage fat of each compartment for each follow-up visit. Only HIV status and pubertal stage were significant predictors for percentage fat in all three compartments (e.g. arm/total, leg/total, and trunk/fat percentage). In addition, age, sex, race, and height are predictive of fat content in at least one compartment. Specifically, HIV infection is associated with increased arm fat, decreased leg fat, and increased trunk fat, as a proportion of total fat; advanced pubertal stage is associated with decreased arm fat and increased leg and trunk fat; older age is associated with increased leg and trunk fat; female sex is associated with increased arm and trunk fat; Hispanic race/ethnicity is associated with increased trunk fat; and height is associated with increased leg and trunk fat.
Table 3. Results of univariate analyses of predictors of percentage arm, leg, and trunk fat.
| Models for arm fat/total fat | Models for leg fat/total fat | Models for trunk fat/total fat | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictors | Coefficient | Standard error | P value | Coefficient | Standard error | P value | Coefficient | Standard error | P value |
| HIV infection | 0.025 | 0.003 | <0.0001 | −0.047 | 0.008 | <0.0001 | 0.019 | 0.008 | 0.0141 |
| Age | −0.001 | 0.000 | 0.0599 | 0.002 | 0.001 | 0.0044 | 0.005 | 0.001 | <0.0001 |
| Female sex | 0.007 | 0.003 | 0.0205 | 0.001 | 0.008 | 0.8567 | 0.020 | 0.007 | 0.0039 |
| Pubertal stage ≥ 3 | −0.006 | 0.002 | 0.0077 | 0.012 | 0.004 | 0.0047 | 0.016 | 0.004 | 0.0001 |
| Hispanic race/ethnicity | −0.002 | 0.003 | 0.5759 | −0.010 | 0.006 | 0.1098 | 0.021 | 0.006 | 0.0003 |
| Height | −0.012 | 0.007 | 0.0967 | 0.047 | 0.015 | 0.0024 | 0.083 | 0.015 | <0.0001 |
The results of analyses using multivariate models for predicting percentage fat in each compartment are presented in Table 4. In all cases, the associations between the predictors and fat distributions are similar to those described above for univariate models, though the effects of HIV infection are modified in two compartments by interactions with other variables. Variables that significantly accounted for variance in the changes in percentage arm fat are as follows: Female participants have about 0.8% more arm fat than male participants, and participants of pubertal stage 3 or higher have about 0.6% less arm fat that those in lower pubertal stages, both irrespective of HIV status. In addition, HIV-infected participants have increased percentage arm fat as compared with uninfected participants; the rate of increase accelerates by approximately 0.3% for each year of age.
Table 4. Models for predicting percentage arm, leg, and trunk fat using HIV status, age, sex, pubertal stage, race, and height.
| Model for arm fat/total fata | Model for leg fat/total fatb | Model for trunk fat/total fatc | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictors | Coefficient | SE | P value | Coefficient | SE | P value | Coefficient | SE | P value |
| HIV infection | −0.009 | 0.010 | 0.4116 | 0.150 | 0.049 | 0.0022 | 0.031 | 0.007 | <0.0001 |
| Female participants | 0.008 | 0.003 | 0.0036 | NA | NA | NA | 0.025 | 0.006 | 0.0001 |
| Pubertal stage ≥ 3 | −0.006 | 0.002 | 0.0139 | NA | NA | NA | NA | NA | NA |
| Hispanic race/ethnicity | NA | NA | NA | −0.016 | 0.006 | 0.0069 | 0.026 | 0.006 | <0.0001 |
| Age | −0.000 | 0.001 | 0.7536 | NA | NA | NA | 0.005 | 0.001 | <0.0001 |
| Age*HIV infection | 0.003 | 0.001 | 0.0007 | NA | NA | NA | NA | NA | NA |
| Height | NA | NA | NA | 0.073 | 0.017 | <0.0001 | NA | NA | NA |
| HIV infection*height | NA | NA | NA | −0.136 | 0.033 | <0.0001 | NA | NA | NA |
The model for predicting arm fat contains terms for HIV infection status, sex, pubertal status, age, and age–HIV infection interaction. Other variables were not significant in this model and thus not included, as indicated by ‘NA’ (not applicable).
The model for predicting leg fat contains terms for HIV infection status, Hispanic ethnicity, height, and height–HIV infection interaction. Other variables were not significant in this model and thus not included, as indicated by ‘NA’ (not applicable).
The model for predicting trunk fat contains terms for HIV infection status, sex, Hispanic ethnicity, and age. Other variables were not significant in this model and thus not included, as indicated by ‘NA’ (not applicable).
Results of the multivariate analyses also indicated that Blacks have a greater percentage of leg fat than Hispanics, irrespective of HIV status. HIV infection is also associated with decreased leg fat, though this relationship is modulated by height: children and adolescents with HIV have decreased percentage leg fat as compared with uninfected; this difference accelerates by approximately −0.14% for each meter of height.
Finally, we found that female participants with pubertal stage of 3 or greater, and male and female Hispanics at all pubertal stages, have higher trunk fat percentage of total, irrespective of HIV status. Age also significantly predicted trunk fat percentage of total increases by 0.1% for each year. In addition, HIV infection was also an independent predictor of the percentage of trunk fat. Trunk fat percentage of total is about 3.1% higher for those with HIV infection.
Thus, differences between the HIV-infected and healthy groups in arm and leg fat as percentage of total fat remained significant when age, sex, race, height, and pubertal stage were accounted for by multivariate modeling. Figure 2 illustrates the increase of differences in arm and leg fat as percentage of total fat with age.
Fig. 2. Adjusted arm, leg, and trunk fat, as a percentage (pct) of total body fat, by age for HIV-infected (HIV+) and healthy (HIV−) children and adolescents aged 6–16 years (HIV+, year 1, n = 52 and year 2, n = 49; HIV−, year 1, n = 131 and year 2, n = 117).
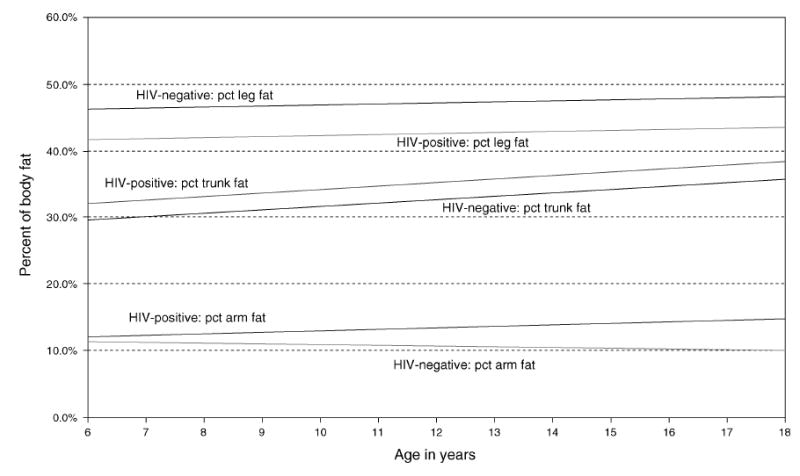
Additional analyses were performed among HIV-infected participants. No differences in fat distribution were observed by antiretroviral treatment (ART) category used either at the time of enrollment in the study or in the past. However, a univariate mixed model analysis revealed that prior treatment with stavudine was associated with an estimated increase of 4.1% in the trunk fat percentage of total fat compared with those never treated (P = 0.019, data not shown). No differences were detected in the frequency of the patterns of regional fat depot changes for those on thymidine analogues compared to those not receiving thymidine analogues. In addition, neither CD4 cell percentage nor HIV RNA viral load significantly predicted arm or leg fat. CD4 cell percentage was marginally significant for predicting trunk fat (model estimate −0.00105, P = 0.046).
Discussion
Serial measurements performed over 2 years confirm that despite having similar total body fat percentage, alterations in regional fat are detectable in perinatally HIV-infected compared with healthy youth. On average, trunk fat as a percentage of total is greater, and leg fat as a percentage of total is decreased compared with healthy participants. We also found that in contrast to prior reports, arm fat as a percentage of total is significantly increased. These findings persisted when other factors such as race/ethnicity, stage of sexual maturation, and somatic growth were taken into account.
These differences appear to accelerate over time: the disparity between HIV-infected and healthy participants in percentage arm fat increases with age, whereas the difference in percentage leg fat increases with greater height, which correlates with age. As all study participants were infected perinatally, it is not possible to distinguish effects of age from those related to duration of infection or length of treatment. Although the study methods differ considerably from those used in our study, the duration of infection and treatment as well as sexual maturation have been reported to be important risks for development of lipodystrophy in other longitudinal studies of perinatally HIV-infected children [16,24].
We did not find a pattern of change in regional fat that is unique to children and adolescents infected with HIV. Our findings indicate that some of the changes in regional fat, such as decrease in arm or leg fat that have been reported to accompany HIV infection and considered an indication of abnormal lipodystrophy, in fact, occur during growth in some healthy children and adolescents. Although there is considerable overlap in the patterns we observed, the frequency and degree of leg fat decrease, and arm fat increase is greater in the infected children. In our study, a decrease in leg fat of more than 6% in a 1-year period or an increase in arm fat in excess of 20% in a 1-year period was uncommon in healthy black and Hispanic children between the ages of 6 and 17 years included in our sample (e.g. frequency <5%).
There is no single widely accepted case definition of lipodystrophy for either children or adults with HIV. Valid criteria are important in order to evaluate the safety of therapies as well as optimally manage HIV infection and minimize morbidity and long-term mortality. Prior studies have used a number of different methods and criteria to detect disturbances in the regional distribution of adipose tissues including self or parental report, physical inspection, and anthropometric or laboratory-based measurements [1–3,10–16,25]. The current study demonstrates the importance of conducting large longitudinal studies that include healthy children and detailed assessments of fat depots in order to attain an understanding of abnormalities in regional fat, establish diagnostic thresholds for lipodystrophy, and distinguish these from normal changes in regional fat related to growth and sexual maturation.
The study has several limitations, including small sample size and baseline differences between healthy and HIV-infected patients. The study had 147 healthy participants and 64 HIV-infected patients available for analysis; larger sample sizes would be required for more definitive results. Furthermore, healthy participants differed significantly by age and ethnicity at baseline, both possible confounders. However, we stratified the data analysis by age and ethnicity, as well as controlling for these covariates in multivariate analysis. Our fundamental results showing increased arm fat and decreased leg fat still held after controlling for these characteristics.
Another limitation of this study is that DXA cannot detect changes in facial fat nor distinguish between intraabdominal or visceral adipose tissue and subcutaneous adipose tissue. Facial lipoatrophy is among the more distinctive and distressing changes in regional fat reported in HIV-infected individuals, and determining a valid means of detection is important. Methods such as ultrasound or infrared three-dimensional typology may prove useful in the detection of facial lipoatrophy. Future studies would also benefit from inclusion of methods to evaluate visceral adipose tissue, as abnormal accumulations of visceral adipose stores have been reported in HIV-infected children receiving ART [8,26,27].
We found that prior but not current treatment with stavudine was associated with differences in regional fat distribution or among HIV-infected participants. Treatment with thymidine analogues, especially stavudine, has been reported to be associated with alterations of subcutaneous fat, particularly lipoatrophy, in both children and adults [28,29]. We did not, however, find either active or past protease inhibitor exposure to be associated with differences in regional fat distribution. Protease inhibitor use is variably reported in association with changes in body fat distribution [1–8,13–16]. Our study has a number of important limitations with respect to evaluating possible associations between alterations in regional fat and specific medications or class of medication. Neither adherence to treatments was assessed nor was duration of prior drug exposure, which varies greatly among older children and adolescents with perinatally acquired HIV, considered in the analysis. Perhaps most important is that multiclass combinations is the standard recommended approach to HIV treatment, and antiretroviral drugs are virtually always used in combination with at least one and most often two or more drug classes. In addition, evaluating for effects by drug class categories that contain as many as nine different agents, rather than by individual drugs, may have masked an effect of a specific drug. Thus, isolating an effect is problematic with our small sample size, and larger cohorts are necessary to better assess the contribution of specific medications or drug classes to changes in body fat.
Accurately detecting abnormalities in regional fat depots remains an important area of clinical research. Regional fat distribution appears to have a substantial impact on lipid and glucose metabolism both of which are abnormal in children and adolescents with HIV [5,15,16,24]. The marked decreases in percentage leg fat among HIV-infected children and adolescents are of particular concern. The loss of subcutaneous leg fat is an independent risk factor for unfavorable glucose and lipid levels in both HIV-infected and healthy adults [30–32]. The changes in fat distribution are also of potential cosmetic concern especially during adolescence when body image awareness is high, adding to the many challenges HIV-infected adolescents confront [33]. Whether concern about fat changes affects adherence to medication has not been evaluated in this age group. Many children and adolescents have difficulty achieving the high levels of adherence that are required to achieve sustained suppression of HIV replication [34–36]. With survival of perinatally infected children with HIV routinely extending into adulthood, the long-term metabolic and cardiovascular consequences as well as the impact on adherence of these alterations in regional fat distribution are likely to be significant and warrant further investigation.
Acknowledgments
This study was funded in part by grants RO1-DK-63666, N01-HD-3-3345, NO1-HD-3332, and RR00645.
The authors acknowledge and thank Dr Jack Moye and the Bone Mineral Density Study of Childhood Study, including Margaret M. Frederick (PhD), Vicente Gilsanz (MD), Heidi J. Kalkwarf (PhD), Joan M. Lappe (PhD), Sharon Oberfield (MD), John A. Shepherd (PhD), Karen Winer (MD), Babette Zemel (PhD). We also thank Robert Warford (NP) and Emma Stuard (MD) for assistance in completing this study.
Footnotes
There was no conflict of interests.
All coauthors have significantly contributed to the study design, data analysis, and manuscript preparations. S.A, M.B., E.J.A., M.H., M.P. and E.S.E. also contributed to data collection.
References
- 1.Babl FE, Regan, Pelton SI. Abnormal body-fat distribution in HIV-1-infected children on antiretrovirals. Lancet. 1999;353:1243–1244. doi: 10.1016/S0140-6736(98)05754-7. [DOI] [PubMed] [Google Scholar]
- 2.Jaquet D, Levine M, Ortega-Rodriquez E, Faye A, Polak M, Vilmer E, Levy-Marchal C. Clinical and metabolic presentation of the lipodystrophic syndrome in HIV-infected children. AIDS. 2000;14:2123–2128. doi: 10.1097/00002030-200009290-00008. [DOI] [PubMed] [Google Scholar]
- 3.Wedekine CA, Pugatch D. Lipodystrophy syndrome in children infected with human immunodeficiency virus. Pharmocotherapy. 2001;21:861–866. doi: 10.1592/phco.21.9.861.34555. [DOI] [PubMed] [Google Scholar]
- 4.Arpadi SM, Cuff PA, Horlick M, Wang J, Kotler DP. Lipodystrophy in HIV-infected children is associated with high viral load and low CD4+-lymphocyte count and CD4+-lymphocyte percentage at baseline and use of protease inhibitors and stavudine. J Acq Immun Def Syndr. 2001;27:30–34. doi: 10.1097/00126334-200105010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Melvin AJ, Lennon S, Mohan KM, Purnell JQ. Metabolic abnormalities in HIV type-1 infected children treated and not treated with protease inhibitors. AIDS Res Hum Retroviruses. 2001;17:1117–1123. doi: 10.1089/088922201316912727. [DOI] [PubMed] [Google Scholar]
- 6.Amaya R, Kozinetz C, McMeans A, Schwarzwald H, Kline M. Lipodystrophy syndrome in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 2002;21:405–410. doi: 10.1097/00006454-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Brockhurst JL, Ksseiry I, Toye M, Chipkin SR, Stechenberg BW, Fisher DJ, Allen HF. Evidence of human immunodeficiency syndrome virus-associated lipodystrophy in children treated with protease inhibitors. Pediatr Infect Dis J. 2003;22:463–465. [PubMed] [Google Scholar]
- 8.Vigano A, Mora S, Testolin C, Beccio S, Schneider L, Bricalli D, et al. Increased lipodystrophy is associated with increased exposure to highly active antiretroviral therapy in HIV-infected children. J Acquir Immune Defic Syndr. 2003;32:482–489. doi: 10.1097/00126334-200304150-00003. [DOI] [PubMed] [Google Scholar]
- 9.Collins E, Wagner C, Walmsley S. Psychosocial impact of the lipodystrophy syndrome in HIV infection. AIDS Read. 2000;10:546–550. [PubMed] [Google Scholar]
- 10.Duran S, Saves M, Spire B, Cailleton V, Sobel A, Carrieri P, et al. APROCO Study Group Failure to maintain long-term adherence to highly active antiretroviral therapy: the role of lipodystrophy. AIDS. 2001;15:2441–2444. doi: 10.1097/00002030-200112070-00012. [DOI] [PubMed] [Google Scholar]
- 11.Ammassari A, Antinori A, Cozzi-Lepri A, Trotta MP, Nasti G, Ridolfo AL, et al. Relationship between HAART adherence and adipose tissue alterations. J Acquir Immune Defic Syndr. 2002;3(Suppl 3):S140–S144. doi: 10.1097/00126334-200212153-00011. [DOI] [PubMed] [Google Scholar]
- 12.Corless IB, Kirksey KM, Kemppainen J, Nicholas PK, McGibbon C, Davis SM, Dolan S. Lipodystrophy-associated symptoms and medication adherence in HIV/AIDS. AIDS Patient Care STDS. 2005;19:577–586. doi: 10.1089/apc.2005.19.577. [DOI] [PubMed] [Google Scholar]
- 13.European Paediatric Lipodystrophy Group. Antiretroviral therapy, fat redistribution and hyperlipidaemia in HIV-infected children in Europe. AIDS. 2004;18:1443–1451. doi: 10.1097/01.aids.0000131334.38172.01. [DOI] [PubMed] [Google Scholar]
- 14.Brambilla P, Sala N, Renzetti F, Manzoni P, Vazulli A, Chiumello G, et al. Highly active antiretroviral-treated HIV infected children show fat distribution changes even in absence of lipodystrophy. AIDS. 2001;15:2415–2422. doi: 10.1097/00002030-200112070-00009. [DOI] [PubMed] [Google Scholar]
- 15.Bitnun A, Sochett E, Babyn P, Holowka S, Stephens D, Read S, et al. Serum lipids, glucose homeostasis and abdominal adipose tissue distribution in protease inhibitor-treated and naïve HIV-infected children. AIDS. 2003;17:1319–1327. doi: 10.1097/00002030-200306130-00006. [DOI] [PubMed] [Google Scholar]
- 16.Beregszaszi M, Dollfus C, Levine M, Faye A, Deghmoun S, Bellal N, et al. Longitudinal evaluation and risk factors of lipodystophy and associated metabolic changes in HIV-infected children. J Acquir Immune Defic Syndr. 2005;40:161–168. doi: 10.1097/01.qai.0000178930.93033.f2. [DOI] [PubMed] [Google Scholar]
- 17.Arpadi S, McMahon D, Abrams EJ, Bamji M, Purswani M, Engelson E, et al. Effect of bimonthly supplementation with oral cholecalciferol on serum 25-hydroxyvitamin D concentrations in HIV-infected children and adolescents. Pediatrics. 2009;123:e121–e126. doi: 10.1542/peds.2008-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, et al. The Bone Mineral Density in Childhood Study (BMDCS): bone mineral content and density according to age, sex and race. J Clin Endocrinol Metab. 2007;92:2087–2099. doi: 10.1210/jc.2006-2553. [DOI] [PubMed] [Google Scholar]
- 19.Marshall WA, Tanner JM. Variations in patterns of pubertal changes in boys. Arch Dis Child. 1970;45:123. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall WA, Tanner JM. Variations in patterns of pubertal changes in girls. Arch Dis Child. 1971;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC growth charts for the United States: methods and development. National Center for Health Statistics. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. 1994 revised classification of system for human immunodeficiency virus infection in children less than 13 years of age. Morb Mortal Wkly Rep. 1994;43:1–10. [Google Scholar]
- 23.Marguiles L, Horlick M, Thornton J, Wang J, Ioannidou M, Heymsfeld S. Reproducibility of whole body bone and body composition measurements by dual x-ray absorptiometry using the GE lunar prodigy. J Clin Densiometry. 2005;8:298–304. doi: 10.1385/jcd:8:3:298. [DOI] [PubMed] [Google Scholar]
- 24.Taylor P, Worrel C, Steinberg SM, Hazra R, Jankelevich S, Wood LV, et al. Natural history of lipid abnormalities and fat redistribution among human immunodeficiency virus-infected children receiving long-term, protease inhibitor-containing, highly active antiretroviral therapy regimens. Pediatrics. 2004;114:e235–e242. doi: 10.1542/peds.114.2.e235. [DOI] [PubMed] [Google Scholar]
- 25.Haubrich RH. Metabolic outcomes of ACTG 5142: a prospective, randomized, phase III trial of NRTI-, PI-, and NNRTI-sparing regimens for initial treatment of HIV-1 infection (abstract 38). Presented at the 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles: California, USA; 2007. [Google Scholar]
- 26.Arpadi SM, Cuff PA, Horlick M, Kotler DP. Visceral obesity, hypertriglyceridemia, and hypercortisolism in a boy with perinatally acquired HIV infection receiving protease inhibitor-containing treatment. AIDS. 1999;13:2312–2313. doi: 10.1097/00002030-199911120-00020. [DOI] [PubMed] [Google Scholar]
- 27.Viganò A, Mora S, Manzoni P, Schneider L, Beretta S, Molinaro M, et al. Effects of recombinant growth hormone on visceral fat accumulation: pilot study in human immunodeficiency virus-infected adolescents. J Clin Endocrinol Metab. 2005;90:4075–4080. doi: 10.1210/jc.2004-2431. [DOI] [PubMed] [Google Scholar]
- 28.Mallal SA, John M, Moore CB, James IR, McKinnon EJ. Contribution of nucleoside analogue reverse transcriptase inhibitors to subcutaneous fat wasting in patients with HIV infection. AIDS. 2000;14:1309–1316. doi: 10.1097/00002030-200007070-00002. [DOI] [PubMed] [Google Scholar]
- 29.Carr A, Miller J, Law M, Cooper DA. A syndrome of lipoatrophy, lactic acidaemia and liver dysfunction associated with HIV nucleoside analogue therapy. AIDS. 2000;14:F25–F32. doi: 10.1097/00002030-200002180-00001. [DOI] [PubMed] [Google Scholar]
- 30.Albu JB, Kenya S, He Q, Wainwright M, Berk ES, Heshka S, et al. Independent associations of insulin resistance with high whole-body intermuscular and low leg subcutaneous adipose tissue distribution in obese HIV-infected women. Am J Clin Nutr. 2007;86:100–106. doi: 10.1093/ajcn/86.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Yudkin JS, et al. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care. 2004;27:372–377. doi: 10.2337/diacare.27.2.372. [DOI] [PubMed] [Google Scholar]
- 32.Snijder MB, Visser M, Dekker JM, Goodpaster BH, Harris TB, Kritchevsky SB, et al. Low subcutaneous thigh fat is a risk factor for unfavorable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia. 2005;48:301–308. doi: 10.1007/s00125-004-1637-7. [DOI] [PubMed] [Google Scholar]
- 33.Wedekine CA, Pugatch D. Lipodystrophy syndrome in children infected with human immunodeficiency virus. Pharmacotherapy. 2001;21:861–866. doi: 10.1592/phco.21.9.861.34555. [DOI] [PubMed] [Google Scholar]
- 34.Gifford AL, Bormann JE, Shively MJ, Wright BC, Richman DD, Bozzette SA. Predictors of self-reported adherence and plasma HIV concentrations in patients on multidrug antiretroviral regimens. J Acquir Immun Defic Syn. 2000;23:386–395. doi: 10.1097/00126334-200004150-00005. [DOI] [PubMed] [Google Scholar]
- 35.Watson DC, Farley JJ. Efficacy of and adherence to highly active antiretroviral therapy in children infected with human immunodeficiency virus type 1. Pediatr Infec Dis J. 1999;18:682–696. doi: 10.1097/00006454-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Steele RG, Grauere D. Adherence to antiretroviral therapy for HIV-infected children: review of the literature and recommendations for research. Clinic Child Family Psychol Rev. 2003;6:17–30. doi: 10.1023/a:1022261905640. [DOI] [PubMed] [Google Scholar]


