Abstract
Abnormal sphingolipid metabolism has been previously reported in Alzheimer's disease (AD). To extend these findings, several sphingolipids and sphingolipid hydrolases were analyzed in brain samples from AD patients and age-matched normal individuals. We found a pattern of elevated acid sphingomyelinase (ASM) and acid ceramidase (AC) expression in AD, leading to a reduction in sphingomyelin and elevation of ceramide. More sphingosine also was found in the AD brains, although sphingosine-1-phosphate (S1P) levels were reduced. Notably, significant correlations were observed between the brain ASM and S1P levels and the levels of amyloid beta peptide (Aβ) and phosphorylated tau protein. Based on these findings, neuronal cell cultures were treated with Aβ oligomers, which were found to activate ASM, increase ceramide, and induce apoptosis. Pre-treatment of the neurons with purified, recombinant AC prevented the cells from undergoing Aβ-induced apoptosis. We propose that ASM activation is an important pathological event leading to AD, perhaps due to Aβ deposition. The downstream consequences of ASM activation are elevated ceramide, activation of ceramidases, and production of sphingosine. The reduced levels of S1P in the AD brain, together with elevated ceramide, likely contribute to the disease pathogenesis.
Keywords: Alzheimer's disease, Human brain, Neurons, Sphingomyelinases, Ceramidases, Sphingomyelin, Ceramide, Sphingosine-1-phosphate
1. Introduction
Alzheimer's disease (AD) is the most common form of dementia in adults. It affects ~10% of the population over 65 years of age, and approaches 50% by age 85. There are about 15 million individuals with AD worldwide. AD is characterized clinically by progressive loss of memory, pathologically by the presence of neuritic plaques and neurofibrillary tangles, and biochemically by the accumulation of amyloid beta peptides (Aβ) and hyperphosphorylated tau proteins (Morishima-Kawashima and Ihara, 2002; Yankner, 1996; Yankner et al., 2007).
Numerous hypotheses have been put forth to describe the molecular mechanisms leading to AD. The five most common hypotheses include: excessive Aβ production, tau protein abnormalities, genetic predisposition (including mutations or polymorphisms in the presenilin 1 and 2, Aβ peptide precursor [APP], and/or apolipoprotein E genes), oxidative stress, and lipid alterations (phospholipids and neutral lipids) (Farooqui et al., 2007; Hartmann et al., 2007; Yankner et al., 2007).
A large body of evidence supports the fact that Aβ plays an important role in AD. In vitro, Aβ has been shown to induce apoptosis via the sphingomyelin/ceramide pathway in various brain cells, including human and rat primary neurons (Jana and Pahan, 2004; Ju et al., 2005; Malaplate-Armand et al., 2006), rat oligodendrocytes (Cheng et al., 2003; Lee et al., 2004; Malaplate-Armand et al., 2006; Zeng et al., 2005), rat astrocytes and glial cells (Ayasolla et al., 2004), and murine neuroblastoma cells (Satoi et al., 2005). Calcium-dependent phospholipase A (cPLA) (Malaplate-Armand et al., 2006), inducible nitric oxide synthase (iNOS) (Ayasolla et al., 2004; Zeng et al., 2005), the p75 neurotrophin receptor (p75NTR) (Costantini et al., 2005), and NADPH oxidase (Jana and Pahan, 2004) have each been shown to be involved in the Aβ-related activation of the sphingomyelin/ceramide pathway. Tumor necrosis factor-alpha (TNF-α) (Ayasolla et al., 2004; Zeng et al., 2005) and interleukin-6 (Fiebich et al., 1995) also are involved in Aβ-induced apoptosis. In vivo, Alessenko and colleagues found that the activation of the sphingomyelin/ceramide pathway lies downstream of the oxidative stress that follows Aβ administration (Alessenko et al., 2004).
Ceramide is the core constituent of most sphingolipids. It can be produced by hydrolysis of sphingomyelin via sphingomyelinases, or synthesized de novo from fatty acyl CoA and sphingosine. Sphingomyelin degradation is the probable source of most ceramide in cells (Goni and Alonso, 2002). Ceramide is an important second messenger molecule that regulates diverse cellular processes including cell growth, differentiation, and apoptosis. Ceramide levels also increase in response to aging and various age-related stress factors (e.g., oxidative stress), and are directly involved in apoptotic signaling in various cell types, including neurons (Costantini et al., 2005; Cutler et al., 2004; Kolesnick and Kronke, 1998; Perez et al., 2005). Furthermore, ceramide stabilizes the APP cleaving enzyme 1 (BACE1), promoting Aβ biogenesis (Patil et al., 2007; Puglielli et al., 2003), and reduction of ceramide levels leads to reduced secretion of APP and Aβ in human neuroblastoma cells (Tamboli et al., 2005). Thus, it has been suggested that ceramide and Aβ may synergize to induce neuronal death in AD.
Several studies have examined the lipid abnormalities in AD brain. For example, the total phospholipid and sulfatide content in AD was decreased as compared to normal (Cheng et al., 2003; Gottfries et al., 1996; Han et al., 2002; Pettegrew et al., 2001; Soderberg et al., 1992), while the ceramide and cholesterol levels were elevated (Cutler et al., 2004; Han et al., 2002). Satoi et al. found that the ceramide levels in the cerebrospinal fluid (CSF) also were increased in patients with AD (Satoi et al., 2005), and we recently reported that the level and activity of acid ceramidase (AC) was elevated as well, perhaps in response to the elevated ceramide (Huang et al., 2004). Herein we investigated the levels of sphingomyelin and several other sphingolipid metabolites in the brains of AD patients, as well as the levels and activities of several lipid-related enzymes. We report for the first time activation of acid sphingomyelinase (ASM), elevation of sphingosine, and reduction of sphingosine-1-phosphate (S1P). The elevated ASM and reduced S1P levels in AD were highly correlated with the levels of Aβ and phosphorylated tau protein. We also found that treatment of neuronal cell cultures with Aβ mimicked these sphingolipid changes and induced apoptosis, which was prevented by pretreatment with recombinant human AC (rhAC). The therapeutic implications of these findings and the role of ceramide and its metabolites in AD are discussed.
2. Materials and Methods
2.1 Chemicals and reagents
Sphingomyelin and ceramide were from Matreya (Pleasant Gap, PA, USA). Sphingosine and S1P were from Avanti Polar Lipids (Alabaster, AL, USA). Bodipy-C12 sphingomyelin, Amplex Red, naphthalene-2,3-dialdehyde (NDA), and a human Aβ42 ELISA (HS) kit were from Invitrogen (Carlsbad, CA, USA). Aβ (40-1) and Aβ (1-42) were obtained from the American Peptide Company (Sunnyvale, CA, USA). Bodipy-C12 ceramide was a gift from Professor Shimon Gatt (Hebrew University-Hadassah School of Medicine, Jerusalem, Israel). High performance liquid chromatography (HPLC)-grade solvents and cell culture materials were from Fisher Scientific (Pittsburgh, PA, USA). A protein assay kit was purchased from Bio-Rad (Hercules, CA, USA). All other biochemical reagents were from the Sigma Chemical Co. (St. Louis, MO, USA).
2.2 Human brain tissue
All of the human brain tissues were obtained from the Harvard Brain Tissue Resource Center (Belmont, MA). Postmortem tissues from 9 AD patients (mean age 73.2 ± 10.1 years) and 6 control individuals (mean age 73.6 ± 8.2 years) were included in this study. The average postmortem delay before tissue collection was 4.7 ± 3.2 hrs for AD and 5.0 ± 1.2 hrs for controls. All of the AD brain samples were analyzed by histopathology to confirm the diagnosis of AD. This was further confirmed by determining the levels of Aβ and phosphorylated tau proteins (see below). The use of frozen human brain tissue was in accordance with the National Institutes of Health guidelines and approved by our Institutional Review Boards.
2.3 Cell culture
Neuronal progenitor cells were isolated from the adult rat hippocampus and cultured in neurobasal A medium consisting of 2% B27, 0.5 mM glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, and 10 ng/ml FGF at 37°C in a humidified 5% CO2 atmosphere (Chen et al., 2007). The media was routinely changed every 2-3 days. When the cells reached ~80% confluency, they were differentiated by replacing FGF with 5 μM retinoic acid and 10% fetal calf serum. The neuronal cultures were used for experiments after 5-7 days of growth in the differentiation medium. At this stage, ~80% of the cells expressed the neuronal markers βIII-tubulin and microtubule-associated protein 2, and less than 5% expressed the astroglial marker GFAP or the oligodendrocyte marker O4. For Aβ treatment of the neuronal cells, stocks of soluble Aβ in distilled water were incubated at room temperature for 5 days and allowed to form oligomers before addition to the cell media. The presence of Aβ oligomers was confirmed by SDS-PAGE and immunoblot analysis.
2.4 Preparation of human brain homogenates
Gray matter was dissected from the frontotemporal area of the human brain samples and homogenized at 4°C in 9 volumes of homogenization buffer containing 50 mM Tris, pH 7.6, 150 mM NaCl, 1.0 mM CaCl2, 1.0 mM MnCl2, 1.0 mM MgCl2, 0.5 mM PMSF, and 2.0 μg/mL each of leupeptin, aprotinin and pepstatin A. After brief centrifugation at 270×g for 10 min to remove large debris, the supernatant was collected and further centrifuged at 100,000×g at 4°C for 30 min. This second supernatant (from the 100,000g spin) was referred to as the “soluble (cytosolic) fraction”. The 100,000g pellet, which mainly consisted of cytoplasmic membranes and subcellular organelles other than nuclei, was extracted in the homogenizing buffer plus 0.5% Triton X-100 and 0.05% SDS, followed by centrifugation again at 100,000×g at 4°C for 30 min. The resulting supernatant containing extracted membrane components was defined as the “membrane fraction”.
2.5 Lipid extraction
Twenty-five μl of each fraction (soluble or membrane) was mixed with 150 μl of chloroform:methanol (1:2, v/v) and sonicated for 5 min. One hundred μl each of 1 M NaCl and additional chloroform, and 10 μl of 3N NaOH were then added. After thorough vortexing and centrifugation (13,000g for 2 min), the upper aqueous phase containing S1P was transferred to a new tube, and the lower organic phase (containing sphingomyelin, ceramide and sphingosine) was dried using a SpeedVac Concentrator (Thermo Electron Corporation, Milford, MA, USA). To quantify the sphingomyelin, ceramide and sphingosine levels, the dried lipid extract was resuspended in 25 μl of 0.2% Igepal CA-630 and the levels of each lipid were determined as described below. For S1P quantification, 150 μl of chloroform and 10 μl of HCl (concentrated) were added to the aqueous phase and vigorously mixed. After centrifugation (13,000×g) for 2 min, the lower phase was transferred to a new tube, dried with a SpeedVac Concentrator, and resuspended in 25 μl of ethanol.
2.6 S1P and sphingosine quantification
Ten μl of the lipid extracts (see above) were added into 20 μl of NDA derivatization reaction mixture (25 mM borate buffer, pH 9.0, containing 2.5 mM each of NDA and NaCN). The reaction mixture was diluted 1:3 with ethanol, incubated at 50°C for 10 min, and centrifuged (13,000×g for 5 min). An aliquot (30 μl) of the supernatant was then transferred to a sampling glass vial and 5 μl was applied onto an HPLC system (Waters, Milford, MA, USA) for analysis. The fluorescent sphingosine or S1P derivatives were monitored using a model 474 scanning fluorescence detector (Waters, Milford, MA, USA) at excitation and emission wavelengths of 252 and 483 nm, respectively. Quantification of the S1P and sphingosine peaks were calculated from S1P and sphingosine standard calibration curves using the Waters Millennium software. More detailed information regarding this method is currently being prepared for publication.
2.7 Ceramide quantification
To quantify ceramide, human recombinant acid ceramidase (rhAC) was added to the lipid extracts, and the ceramide was fully hydrolyzed to sphingosine. This was then quantified using the procedure described above (He et al., 2005). Briefly, 3 μl of the lipid extracts in 0.2% Igepal CA-630 was mixed with 3 μl of an AC assay solution (0.2 M citrate-phosphate buffer, pH 4.5, 0.3 M NaCl, 0.2% Igepal CA-630, 10% fetal bovine serum, 50 ng/μl rhAC) and incubated at 37°C for 1 hr. The reaction was stopped by adding ethanol (1:5) and centrifugation for 5 min at 13,000×g. Ten μl of the supernatant was transferred into 20 μl of 25 mM sodium borate buffer (pH 9.0) containing 1.25 mM sodium cyanide and 1.25 mM NDA. The reaction mixture was incubated at 50°C for 10 min, diluted with ethanol (1:3), and centrifuged for 5 min at 13,000×g. Fifty μl of the supernatant was then transferred to a sampling glass vial and 5 μl was applied onto an HPLC system for analysis (He et al., 2005). To calculate the final ceramide content of the samples, the background of the endogenous sphingosine (determined as above using a reaction mixture lacking rhAC) was subtracted from the signal obtained in the presence of rhAC.
2.8 Sphingomyelin quantification
Sphingomyelin was quantified as described previously (He et al., 2005). Briefly, 3 μl of the lipid extracts was mixed with 3 μl of an enzyme assay solution (0.2 M sodium acetate buffer, pH 5.0, 0.3 M NaCl, 0.2 mM ZnCl2, 0.05% BSA, 100 ng/μl rhAC, 10 ng/μl recombinant human acid sphingomyelinase [rhASM]) and incubated at 37°C for 1 hr. The reaction was stopped by adding ethanol (1:5), and then centrifuged for 5 min at 13,000×g. Ten μl of the supernatant was then used to quantify the ceramide produced by enzymatic hydrolysis of sphingomyelin (see above). To calculate the final sphingomyelin content of the samples, the background of the endogenous ceramide (using the reaction mixture lacking rhASM) was subtracted from the signal obtained in the presence of rhAC and rhASM.
2.9 ASM and neutral sphingomyelinase (NSM) assays
Three μl of the human brain homogenates were mixed with 3 μl of ASM (200 μM Bodipy-C12-sphingomyelin, 0.2 M of sodium acetate buffer, pH 5.0, 0.2 mM ZnCl2, and 0.2% Igepal CA-630) or NSM (200 μM Bodipy-C12-sphingomyelin, 50 mM of HEPS, pH 7.2, 5 mM MgCl2, and 0.2% Igepal CA-630) assay buffer and incubated at 37°C for 1 h. The hydrolysis reactions were stopped by adding 24 μl of ethanol, and centrifuged (13,000×g) for 5 min. Thirty μl of the supernatant was then transferred to a sampling glass vial and 5 μl was applied onto an HPLC system (Waters, Milford, MA, USA) for analysis (He et al., 2003). Quantification was achieved by comparison to Bodipy-C12 sphingomyelin and ceramide standards.
2.10 AC and neutral ceramidase (NC) assays
Three μl of the human brain homogenates were mixed with 3 μl of AC (200 μM Bodipy-C12-ceramide, 0.2 M of citrate/phosphate, pH 4.5, 10% FBS, 0.3 M NaCl, and 0.2% Igepal CA-630) or NC (200 μM Bodipy-C12-ceramide, 0.1 M of Tris-HCl, pH 7.2, and 0.2% Igepal CA-630) assay buffer and incubated at 37°C. The hydrolysis reactions were stopped by adding 24 μl of ethanol and centrifuged (13,000×g) for 5 min. Thirty μl of the supernatant was then transferred to a sampling glass vial and 5 μl was applied onto an HPLC system (Waters, Milford, MA, USA) for analysis (He et al., 1999).
2.11 SDS-PAGE and Western blot analyses
Samples were boiled in loading buffer (12 mM Tris-HCl, 0.5% SDS, 5 mM β-mercaptoethanol, 5% glycerol, 0.02% bromophenol blue, pH 6.8) for 10 min, applied onto 4~20% gradient polyacrylamide gels, and electrophoresed in an XCELL II mini-cell apparatus (Novex, San Diego, CA) for 1 h. The voltage was maintained at 150 voltage and the running buffer contained 0.1% SDS. Following electrophoresis, proteins in the gel were electrotransferred to a nitrocellulose membrane (45 volts for 1h) using a semi-dry transfer cell (Bio-Rad, Hercules, CA). The nitrocellulose membrane was blocked with 5% nonfat dry milk in PBST buffer (PBS with 0.1% Tween 20) at room temperature for 2 hrs, and then incubated with either rabbit anti-human ASM (1:5000), rabbit anti-human AC (1:2000) IgG, or mouse anti-human hyperphosphorylated tau in the form of paired helical filaments (PHF-1) (1:500) in PBST buffer containing 3% nonfat dry milk and 0.1% bovine serum albumin at 4°C overnight. After washing four times with PBS, the blot was incubated with horseradish peroxidase conjugated goat anti-rabbit IgG (1:5000) for ASM and AC, or sheep anti-mouse IgG (1:5000) for PHF-1, for 1 hr in PBST buffer at room temperature.
The membrane was subsequently washed twice with 0.3% Tween-20 in PBS and then twice more with 0.1% Tween-20 in PBS. Finally, the blot was soaked in an enhanced chemiluminescence reagent (DuPont NEN) for 1 min and exposed to X-ray film. Bands corresponding to the proteins of interest were quantified using the ImageJ1.38x software (National Institutes of Health, USA).
2.12 Aβ quantification
Brain supernatant fractions were used for Aβ quantification using a human Aβ42 ELISA kit from Invitrogen according to the manufacturer's instructions (Carlsbad, CA, USA). Briefly, samples were diluted with standard dilution buffer (supplied with the kit) to keep the concentration within the detectable range (1-100 pg/ml). After the addition of stop solution, the 96-well plate was read at 450 nm, and the sample concentration was calculated from a standard curve prepared using purified Aβ □□ various concentrations.
3. Results
3.1 ASM and AC levels in normal and AD brain
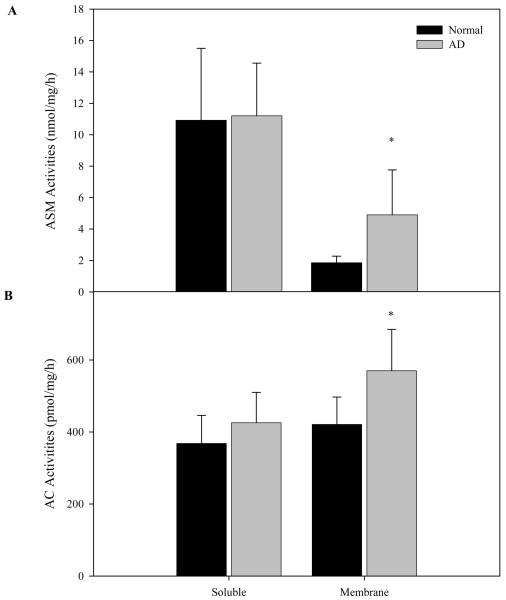
ASM and AC activities were determined in AD (N=9) and age-matched normal (N=6) brain samples. As shown in Fig. 1, no significant differences in the soluble enzyme activities were found between the two groups. In contrast, the membrane-associated activities were significantly increased in the AD brain. Neutral sphingomyelinase and ceramidase activities were also determined in the two fractions. No significant differences were found for neutral sphingomyelinase, while neutral ceramidase activities were modestly increased (on average about 20%; data not shown).
Fig. 1. ASM and AC activities in normal and AD brain.
Soluble (cytosolic) and membrane fractions from normal (N=6) and AD (N=9) brains were prepared as described in the “Materials and Methods”. ASM and AC activities were measured after 1 hr and overnight incubations at 37°C, respectively. *p<0.05, compared to normal brains. Values are expressed as the mean ± S.D.
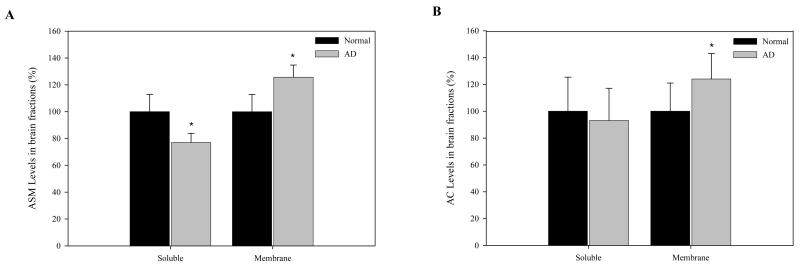
We next examined expression of the ASM and AC proteins by western blot analyses. As shown in Fig. 2A, consistent with the pattern of ASM activity, expression of the ASM protein was elevated in the AD membrane fractions. A significant increase in AC protein expression also was detected (Fig. 2B), consistent with the increased AC activity. Overall, these data revealed a pattern of elevated ASM and AC in the brains of AD patients, and a possible shift in the distribution of the enzymes from the cytosol to the membrane.
Fig. 2. ASM and AC protein levels in normal and AD brain.
Soluble (cytosolic) and membrane fractions from normal (N=6) and AD (N=9) brains were prepared as described in the “Materials and Methods”. Rabbit polyclonal antibodies against human ASM and AC were used for western blotting as described. The ASM and AC-specific protein bands were scanned and the signals were normalized to β-actin signals from the same blot (see “Materials and Methods”). *p<0.05, compared to normal brains. Values are expressed as the percentage of control.
3.2 Sphingomyelin, ceramide, sphingosine, and S1P levels in normal and AD brain
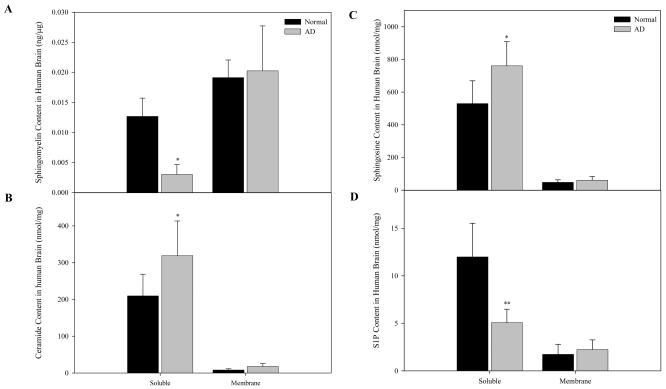
To investigate whether elevated ASM and AC expression in the AD brain caused an imbalance in the sphingolipid content, we determined the levels of sphingomyelin, ceramide, sphingosine and S1P. As previously described, the majority of sphingomyelin was found in the membrane fractions of both normal and AD brain (Fig. 3A). In contrast, the majority of ceramide, sphingosine, and S1P were in the soluble (cytosolic) fractions (Figs. 3B-D). Notably, significant sphingomyelin reductions and ceramide elevations were detected in the AD samples as compared to the age-matched controls (Figs. 3A & 3B). Sphingosine levels were similarly elevated in AD brain (Fig. 3C), although the S1P content was markedly reduced (Fig. 3D). Together, the elevation of ceramide and sphingosine, and reduction of S1P, may be synergistically contributing to the pathologic state of the AD brain.
Fig. 3. Sphingomyelin, ceramide, sphingosine, and S1P contents of normal and AD brain.
Soluble (cytosolic) and membrane fractions from normal (N=6) and AD (N=9) brains were prepared as described in the “Materials and Methods”. For each fraction two lipid extracts were prepared, one containing S1P and the other containing sphingomyelin, ceramide and sphingosine. Sphingomyelin, ceramide, sphingosine, and S1P were determined using HPLC based methods as described in the “Materials and Methods”. *p<0.05, **p<0.01, compared to normal brains. Values are expressed as the mean ± S.D.
3.3 Aβ and PHF-1 levels in normal and AD brain
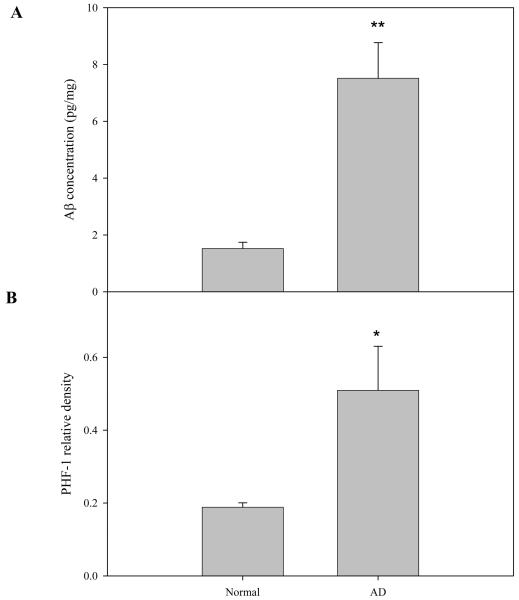
Next, the levels of Aβ and hyperphosphorylated tau protein (in the form of PHF-1) were determined in the normal and AD brain samples. As shown in Fig. 4, the Aβ concentration in the supernatant fraction of AD brains was 4 times higher than that from normal brain (P<0.01). Similarly, the PHF-1 levels in the AD brain samples were significantly elevated compared with normal individuals. This data confirmed the fact that the samples analyzed from the AD patients were pathologic sites, and had Aβ enrichment in plagues and PHF-1 elevation in fibrillar tangles.
Fig. 4. Aβ and PHF-1 levels in normal and AD brain.
Soluble (cytosolic) fractions from normal (N=6) and AD (N=9) brains were prepared as described in the “Materials and Methods”. Aβ levels in these fractions were determined using a human Aβ42 ELISA kit (see “Materials and Methods”). Mouse monoclonal antibody against human hyperphosphorylated tan protein, PHF-1, was used for western blotting as described. The PHF-1 protein bands were scanned and the signals were normalized to β-actin signals from the same blot. *p<0.05, **p<0.01, compared to normal brains. Values are expressed as the mean ± S.D.
3.4 Correlations between Aβ, PHF-1, ASM, and S1P levels in normal and AD brain
To correlate the above findings on Aβ and PHF-1 with the sphingolipid and/or sphingolipid hydrolase results, Pearson correlation analyses were carried out. As can be seen in Table 1, ASM activity had a significant, positive correlation with the Aβ □□ PHF-1 levels in the normal and AD brain samples (P<0.005). In addition, there was a strong negative correlation between the S1P content and Aβ or PHF-1 levels (P<0.05). Surprisingly, no correlations were observed between these two AD biomarkers and the ceramide or sphingosine levels (data not shown). Possible reasons for this are discussed below.
Table 1.
Correlations between Aβ, PHF-1, ASM, and S1P levels in normal and AD individuals
| PHF-1 | ASM | S1P | |
|---|---|---|---|
| Aβ | 0.733(0.0019)* | 0.746(0.00141) | −0.635(0.011) |
| PHF-1 | - | 0.892(0.0000078) | −0.521(0.0467) |
Aβ and PHF-1 determinations were carried out as described in the “Materials and Methods”.
Correlation coefficients were calculated using the SigmaStat 3.11 Software (Systat Software Inc, San Joe, CA, USA), and P values are indicated in parentheses (sample number=15, 6 normal and 9 AD).
3.5 Effect of Aβ on the sphingomyelinase and ceramidase activities in neuronal cell cultures
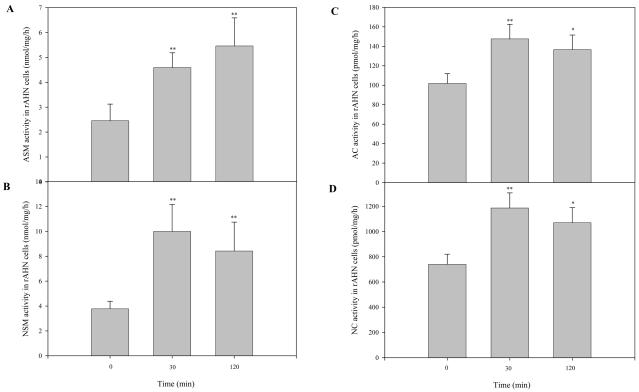
To further examine our findings in the AD brain, neuronal cultures were treated with Aβ oligomers for varying lengths of time, and the sphingomyelinase and ceramidase activities were determined. As shown in Figs. 5A & B, following Aβ treatment ASM and NSM activities were rapidly elevated in the neuronal cells (within 30 min). Similarly, AC and NC activities were increased after 30 min of Aβ treatment (Fig. 5C & D). Overall, these in vitro findings were consistent with the changes observed in the AD brain, and support the notion that Aβ is an important pathogenic factor stimulating sphingolipid hydrolases and affecting the sphingomyelin/ceramide signaling pathway.
Fig. 5. Sphingomyelinase and ceramidase activities in neuronal cultures after Aβ treatment.
After 5-7 days of growth in differentiation media, neuronal cultures were treated with 1 μM of Aβ o1□□□□ers for the indicated times. Cell lysates were then prepared and sphingomyelinase and ceramidase activities were measured after incubation at 37°C for 1 hr and overnight, respectively. *p<0.05, **p<0.01, compared to normal brains. Values are expressed as the mean ± S.D (N=3). A, ASM and NSM activities. B, AC and NC activities.
3.6 Effect of Aβ on ceramide levels and apoptosis in neuronal cell cultures
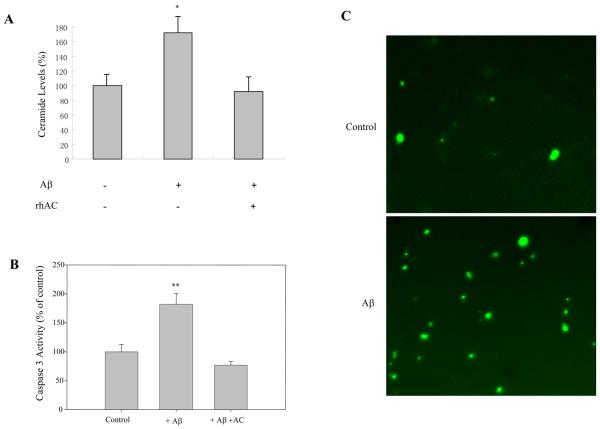
As described above, ASM activity was elevated in the AD brain and after Aβ treatment of neuronal cultures. Consistent with these findings, ceramide levels were also significantly elevated after treating neuronal cultures with Aβ (Fig. 6A). More apoptotic cells were also found as determined by TUNEL staining (Fig. 6B) and caspase 3 activity (Fig. 6C). Importantly, however, ceramide levels and caspase 3 activity did not increase in response to Aβ when purified, rhAC was included in the culture media (Fig. 6C), suggesting that the ceramide increase in the AD brain is directly responsible for the apoptotic effects of Aβ.
Fig. 6. The effect of Aβ on ceramide levels and apoptosis in neuronal cultures.
After 3-5 days of growth in differentiation media, neuronal cultures were treated with 1 μM of Aβ for 30 min with or without 1 hr of rhAC pre-treatment (1 μg/ml). Ceramide levels (A) were verified using the DAG kinase method (He et al., 2001). Caspase 3 activity (B) was measured using EnzCheck Caspase-3 assay kit. *p<0.05, **p<0.01, compared to normal brains. Values are expressed as the mean ± S.D (N=3). C, Neuronal cultures were treated with 1 μM of Aβ for 17 hr, and then processed with the DeadEnd Fluorometric TUNEL system and analyzed by microscopy. Pictures were captured in the green channel (20x magnification).
4. Discussion
Aging results in the accumulation of various stress factors, including pro-inflammatory and oxidative stress molecules that can stimulate sphingomyelinase activities, leading to the production of the pro-apoptotic lipid, ceramide (Cutler et al., 2004; Joseph et al., 2000). Alzheimer's disease is the most common form of age-related dementia, characterized by the accumulation of Aβ peptides in the brain. Aβ accumulation has been shown to cause neuronal death in cell culture, although the precise mechanisms by which this occurs remain largely unknown. The relationship between Aβ-induced neuronal apoptosis and sphingolipids has been studied in cell culture, although few studies have systemically addressed changes in the sphingomyelin/ceramide pathway in vivo using brain samples from AD patients. We therefore examined the sphingomyelinase and ceramidase activities in several AD and age-matched normal brain samples, as well as the levels of several biologically relevant sphingolipids.
Surprisingly, ASM activity was elevated in membrane fractions prepared from the AD brains. Although ASM is a lysosomal enzyme with important housekeeping functions, under stress conditions it may translocate from intracellular compartments to the extracellular leaflet of the cell membrane, where it hydrolyzes sphingomyelin into ceramide to form signaling platforms (Cifone et al., 1994). The ceramide-enriched platforms cluster receptor molecules, transmit apoptotic stimuli into the cell, and eventually cause cell death (Bollinger et al., 2005; Gulbins, 2003). ASM is most active in an acidic environment (pH 4.5), but it can also function at neutral pH (7.0) (Schissel et al., 1998), consistent with its movement to the plasma membrane.
In addition to ASM, acid and netural ceramidase (AC and NC) activities were also elevated in the AD brains. This is likely a response to the increased ceramide content, i.e., a “protective” response on the part of cells to remove ceramide and prevent cell death. Elevated ceramidase activities, in turn, led to an increase in sphingosine, but surprisingly, there was a marked reduction in the levels of S1P. This may be due to reduced sphingosine kinase activity, or enhanced S1P phosphatase and/or lyase activities (see below). Together, the elevated ceramide and sphingosine, as well as low S1P, likely creates a “pro-apoptotic” environment in the AD brain that leads to neuronal death.
Our findings are consistent with previous observations of increased ceramide, TUNEL staining and caspase activation in postmortem AD brain (Gervais et al., 1999; Smale et al., 1995), and decreased sphingomyelin (Gottfries et al., 1996; Soderberg et al., 1992). In addition, we have demonstrated a new possible mechanism by which accumulation of Aβ, which is believed to be one of the key pathologic mechanisms leading to AD, contributes to neuronal death via activation of the sphingomyelin/ceramide pathway.
Extracellular neuritic plaques and intracellular neurofibrillary tangles are hallmarks of AD. As expected, the levels of Aβ and hyperphosphorylated tau protein (PHF-1) were significantly elevated in the AD brain samples compared with the age-matched normal samples. This (together with histological analysis) further confirmed that the AD brain regions we studied had pathological lesions. Importantly, we also found that the levels of ASM activity were positively correlated with the Aβ and PHF-1 levels in the normal and AD brains (P<0.05). Similarly, there was a significant (negative) correlation of S1P levels with Aβ and PHF-1 in the normal and AD brains (Table 1).
As noted above, several groups have reported that ceramide is elevated in AD brain and CSF. We have confirmed these findings, and suggest that elevated ASM activity is one novel mechanism by which this occurs. Interestingly, we did not observe significant correlations between the levels of Aβ or PHF-1 with ceramide or ceramidase activities in our AD and normal brain samples. It is important to recognize, however, that once formed ceramide can rapidly enter several metabolic pathways, and used for either the biosynthesis of complex lipids or broken down into sphingosine, which itself is rapidly converted to S1P. The fact that we did not see correlations between Aβ, PHF-1, and ceramide (or sphingosine) likely reflects the complex mechanisms that exist to metabolize these lipids and shuttle them into different pathways.
In addition to these in vivo findings, we also studied Aβ-induced sphingolipid metabolism in neuronal cell cultures. Two forms of Aβ are generally used for in vitro studies, fibrillar and oligomer. Several studies suggest that fibrillar Aβ induces primary neuron apoptosis via the activation of NSM (Ayasolla et al., 2004; Chen et al., 2006; Jana and Pahan, 2004; Lee et al., 2004; Satoi et al., 2005; Zeng et al., 2005). However, our data using postmortem AD brain revealed that ASM, not NSM, activity was elevated. Although ASM and NSM share the same substrate, they are encoded by different genes, and have different pH optima, subcellular location, and cation dependency. To further confirm our in vivo findings, neuronal cultures were treated with Aβ that was primarily oligomeric. In this case, both ASM and NSM activities were significantly increased in the cells after 30 min. Very recently, Pillot and colleagues have also demonstrated that ASM was activated when rat cortical neurons were incubated with Aβ oligomers (Malaplate-Armand et al., 2006). The conflicting data with ASM and NSM implys that different molecular mechanisms and cellular targets may be involved in the neuronal apoptosis and cell death induced by soluble oligomers versus fibrillar Aβ (Sponne et al., 2004; White et al., 2005). These differences also may be due to the different cell types and culture conditions used.
Ceramide may induce apoptosis by reorganization of the plasma membrane, but others have shown that ceramide may also promote the production of Aβ by stabilizing the APP-cleaving enzyme 1 (BACE1) (Buchet and Pikula, 2000; Patil et al., 2007; Puglielli et al., 2003; Sawamura et al., 2004). Thus, under normal circumstances the levels of Aβ and ceramide may be balanced to maintain neuronal cell homeostasis, but upon aging, various stress factors become elevated that may activate sphingomyelinases and produce ceramide. Ceramide, in turn, may further enhance Aβ production by promoting APP processing, leading to a positive feedback regulatory pathway that promotes cell death.
Although increasing evidence supports a role for ceramide in the pathogenesis of the AD brain, the role of ceramidases, enzymes responsible for degrading ceramide, remain unclear. There are at least three distinct ceramidases (acid, neutral, and alkaline) encoded by individual genes and distinguished by their pH optimum and subcellular location (He et al., 2003; Okino et al., 1999; Tani et al., 2000). Ceramide is hydrolyzed into sphingosine and fatty acid by the action of these enzymes. Presumably, one or more ceramidase activities could be up-regulated in response to the accumulation of ceramide in the AD brain. Supporting this notion, we have previously reported that the level and activity of AC was elevated in postmortem AD brain (Huang et al., 2004), a finding that was confirmed by the data presented in the present manuscript. In addition, we found that NC activity was also increased in the AD brain. In vitro studies further confirmed that Aβ treatment of neuronal cultures enhanced both AC and NC activities.
An important product of ceramide hydrolysis by ceramidases is sphingosine. Accumulating evidence also supports a role for sphingosine in apoptosis, cooperatively or independently from ceramide signaling (Hung et al., 1999; Lepine et al., 2004; Sweeney et al., 1998). Sphingosine, in turn, can be phosphorylated by sphingosine kinase to form S1P. In most cases, S1P is rapidly metabolized either by a lyase to hexadecenal and phosphoethanolamine, or by S1P phosphatase to sphingosine (Spiegel and Kolesnick, 2002). In contrast to ceramide and sphingosine, S1P can enhance cell proliferation and antagonize apoptosis (Hla, 2003; Spiegel and Kolesnick, 2002). Notably, it has been suggested that S1P may be a potent neuroprotective factor against Aβ-induced neuronal apoptosis, and that it that acts by inhibiting ASM activation (Gomez-Munoz et al., 2003; Malaplate-Armand et al., 2006).
Thus, during the past decade a model has been proposed in which the balance between the levels of ceramide and S1P, the ‘ceramide/S1P rheostat’, contributes to the fate of cells (Cuvillier et al., 1996). Based on this model, the regulation of sphingomyelinases and ceramidases, as well as sphingosine kinase, S1P phosphatase and lysase may play pivotal roles in the apoptotic signaling of cells by regulating the ratio between sphingomyelin, ceramide, sphingosine, and S1P. To our knowledge, our study is the first to describe the sphingosine and S1P levels in the brains of AD patients. The sphingosine content was significantly elevated in the AD brain, consistent with the accumulation of ceramide and elevation of AC and NC activities. Surprisingly, however, the S1P levels in the AD brain were significantly reduced compared with the age-matched normal controls. Although the mechanism underlying this observation remains unknown, three enzyme that metabolize S1P (sphingosine kinase, S1P phosphatase, and lyase) might be involved. Of direct relevance to this observation, a recent report showed that expression of the S1P phosphatase gene was markedly up-regulated in AD brain using microarray technology (Katsel et al., 2007). Decreased S1P may further contribute to the elevation of ASM, leading to more ceramide in AD brain (Gomez-Munoz et al., 2003). This severe disruption of the balance between ceramide, sphingosine and S1P may eventually result in neuronal apoptosis and cell death in the AD brain. In vitro, neurons quickly underwent apoptosis when they were treated with Aβ, and adding pure rhAC to the culture media prevented this from occurring. This provides the first direct experimental evidence that Aβ-induced ceramide production is a major pathogenic event leading to neuronal cell death, and suggests that manipulation of the sphingolipid signaling pathway, either by removing ceramide or perhaps enhancing S1P production, may be a potential therapeutic approach.
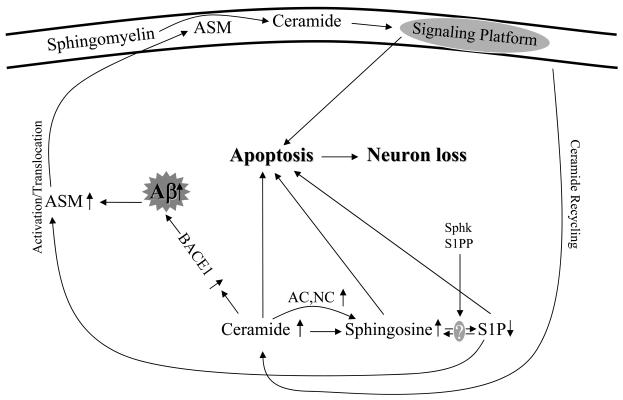
In conclusion, this study extends previous observations on sphingolipid metabolism in the AD brain, and reports for the first time elevation of ASM, elevation of sphingosine, and reduction in S1P. Cell culture studies were also used to demonstrate that removal of ceramide by AC protects neurons from Aβ-induced apoptosis, suggesting new therapeutic approaches for the amelioration of this complex disorder. Taken together, our results support the notion that the ‘ceramide/S1P rheostat’ is severely impaired in the AD brain, contributing to neuronal cell death. A summary of these changes is shown in Fig. 7. In the future, it will be interesting to breed ASM knock-out mice to AD mice that accumulate Aβ, and evaluate the effects on AD brain pathology. In addition, S1P treatment may be evaluated in AD mouse models, as well as other therapeutic modalities that are targeted towards the sphingomyelin/ceramide signaling pathway.
Fig. 7. Schematic pathway proposed for the Aβ-induced sphingolipid changes in the AD brain.
Sphk, sphingosine kinase, S1PP, sphingosine-1-phosphate phosphatase, Lyase, sphingosine-1-phosphate lyase.
Acknowledgments
This work was supported by grants from the National Institutes of Health to EHS (DK54830 and HD28607) and CXG (AG027429), and a U.S. Alzheimer's Association grant to CXG (IIRG-05-13095). The authors would like to thank Zhenxiang Zu, Michelle Ku, Mu He, and Chien-Ling Huang for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The manuscript has been approved by all of the authors, and there are no conflicts of interest regarding it.
References
- Alessenko AV, Bugrova AE, Dudnik LB. Connection of lipid peroxide oxidation with the sphingomyelin pathway in the development of Alzheimer's disease. Biochem. Soc. Trans. 2004;32:144–146. doi: 10.1042/bst0320144. [DOI] [PubMed] [Google Scholar]
- Ayasolla K, Khan M, Singh AK, Singh I. Inflammatory mediator and beta-amyloid (25-35)-induced ceramide generation and iNOS expression are inhibited by vitamin E. Free Radic. Biol. Med. 2004;37:325–338. doi: 10.1016/j.freeradbiomed.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Bollinger CR, Teichgraber V, Gulbins E. Ceramide-enriched membrane domains. Biochim. Biophys. Acta. 2005;1746:284–294. doi: 10.1016/j.bbamcr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Buchet R, Pikula S. Alzheimer's disease: its origin at the membrane, evidence and questions. Acta Biochim. Pol. 2000;47:725–733. [PubMed] [Google Scholar]
- Chen H, Tung YC, Li B, Iqbal K, Grundke-Iqbal I. Trophic factors counteract elevated FGF-2-induced inhibition of adult neurogenesis. Neurobiol. Aging. 2007;28:1148–1162. doi: 10.1016/j.neurobiolaging.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Chen S, Lee JM, Zeng C, Chen H, Hsu CY, Xu J. Amyloid beta peptide increases DP5 expression via activation of neutral sphingomyelinase and JNK in oligodendrocytes. J. Neurochem. 2006;97:631–640. doi: 10.1111/j.1471-4159.2006.03774.x. [DOI] [PubMed] [Google Scholar]
- Cheng H, Xu J, McKeel DW, Jr., Han X. Specificity and potential mechanism of sulfatide deficiency in Alzheimer's disease: an electrospray ionization mass spectrometric study. Cell. Mol. Biol. (Noisy-le-grand) 2003;49:809–818. [PubMed] [Google Scholar]
- Cifone MG, De Maria R, Roncaioli P, Rippo MR, Azuma M, Lanier LL, Santoni A, Testi R. Apoptotic signaling through CD95 (Fas/Apo-1) activates an acidic sphingomyelinase. J. Exp. Med. 1994;180:1547–1552. doi: 10.1084/jem.180.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini C, Weindruch R, Della Valle G, Puglielli L. A TrkA-to-p75NTR molecular switch activates amyloid beta-peptide generation during aging. Biochem. J. 2005;391:59–67. doi: 10.1042/BJ20050700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proc. Natl. Acad. Sci. USA. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Horrocks LA, Farooqui T. Interactions between neural membrane glycerophospholipid and sphingolipid mediators: a recipe for neural cell survival or suicide. J. Neurosci. Res. 2007;85:1834–1850. doi: 10.1002/jnr.21268. [DOI] [PubMed] [Google Scholar]
- Fiebich BL, Lieb K, Berger M, Bauer J. Stimulation of the sphingomyelin pathway induces interleukin-6 gene expression in human astrocytoma cells. J. Neuroimmunol. 1995;63:207–211. doi: 10.1016/0165-5728(95)00145-x. [DOI] [PubMed] [Google Scholar]
- Gervais FG, Xu D, Robertson GS, Vaillancourt JP, Zhu Y, Huang J, LeBlanc A, Smith D, Rigby M, Shearman MS, Clarke EE, Zheng H, Van Der Ploeg LH, Ruffolo SC, Thornberry NA, Xanthoudakis S, Zamboni RJ, Roy S, Nicholson DW. Involvement of caspases in proteolytic cleavage of Alzheimer's amyloid-beta precursor protein and amyloidogenic A beta peptide formation. Cell. 1999;97:395–406. doi: 10.1016/s0092-8674(00)80748-5. [DOI] [PubMed] [Google Scholar]
- Gomez-Munoz A, Kong J, Salh B, Steinbrecher UP. Sphingosine-1-phosphate inhibits acid sphingomyelinase and blocks apoptosis in macrophages. FEBS letters. 2003;539:56–60. doi: 10.1016/s0014-5793(03)00197-2. [DOI] [PubMed] [Google Scholar]
- Goni FM, Alonso A. Sphingomyelinases: enzymology and membrane activity. FEBS letters. 2002;531:38–46. doi: 10.1016/s0014-5793(02)03482-8. [DOI] [PubMed] [Google Scholar]
- Gottfries CG, Karlsson I, Svennerholm L. Membrane components separate early-onset Alzheimer's disease from senile dementia of the Alzheimer type. Int. Psychogeriatr. 1996;8:365–372. doi: 10.1017/s1041610296002736. [DOI] [PubMed] [Google Scholar]
- Gulbins E. Regulation of death receptor signaling and apoptosis by ceramide. Pharmacol. Res. 2003;47:393–399. doi: 10.1016/s1043-6618(03)00052-5. [DOI] [PubMed] [Google Scholar]
- Han X, D MH, McKeel DW, Jr., Kelley J, Morris JC. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer's disease: potential role in disease pathogenesis. J. Neurochem. 2002;82:809–818. doi: 10.1046/j.1471-4159.2002.00997.x. [DOI] [PubMed] [Google Scholar]
- Hartmann T, Kuchenbecker J, Grimm MO. Alzheimer's disease: the lipid connection. J. Neurochem. 2007;103(Suppl 1):159–170. doi: 10.1111/j.1471-4159.2007.04715.x. [DOI] [PubMed] [Google Scholar]
- He X, Chen F, Dagan A, Gatt S, Schuchman EH. A fluorescence-based, high-performance liquid chromatographic assay to determine acid sphingomyelinase activity and diagnose types A and B Niemann-Pick disease. Anal. Biochem. 2003;314:116–120. doi: 10.1016/s0003-2697(02)00629-2. [DOI] [PubMed] [Google Scholar]
- He X, Chen F, Gatt S, Schuchman EH. An enzymatic assay for quantifying sphingomyelin in tissues and plasma from humans and mice with Niemann-Pick disease. Anal. Biochem. 2001;293:204–211. doi: 10.1006/abio.2001.5108. [DOI] [PubMed] [Google Scholar]
- He X, Dagan A, Gatt S, Schuchman EH. Simultaneous quantitative analysis of ceramide and sphingosine in mouse blood by naphthalene-2,3-dicarboxyaldehyde derivatization after hydrolysis with ceramidase. Anal. Biochem. 2005;340:113–122. doi: 10.1016/j.ab.2005.01.058. [DOI] [PubMed] [Google Scholar]
- He X, Li CM, Park JH, Dagan A, Gatt S, Schuchman EH. A fluorescence-based high-performance liquid chromatographic assay to determine acid ceramidase activity. Anal. Biochem. 1999;274:264–269. doi: 10.1006/abio.1999.4284. [DOI] [PubMed] [Google Scholar]
- He X, Okino N, Dhami R, Dagan A, Gatt S, Schulze H, Sandhoff K, Schuchman EH. Purification and characterization of recombinant, human acid ceramidase. Catalytic reactions and interactions with acid sphingomyelinase. J. Biol. Chem. 2003;278:32978–32986. doi: 10.1074/jbc.M301936200. [DOI] [PubMed] [Google Scholar]
- Hla T. Signaling and biological actions of sphingosine 1-phosphate. Pharmacol. Res. 2003;47:401–407. doi: 10.1016/s1043-6618(03)00046-x. [DOI] [PubMed] [Google Scholar]
- Huang Y, Tanimukai H, Liu F, Iqbal K, Grundke-Iqbal I, Gong CX. Elevation of the level and activity of acid ceramidase in Alzheimer's disease brain. Eur. J. Neurosci. 2004;20:3489–3497. doi: 10.1111/j.1460-9568.2004.03852.x. [DOI] [PubMed] [Google Scholar]
- Hung WC, Chang HC, Chuang LY. Activation of caspase-3-like proteases in apoptosis induced by sphingosine and other long-chain bases in Hep3B hepatoma cells. Biochem. J. 1999;338(Pt 1):161–166. [PMC free article] [PubMed] [Google Scholar]
- Jana A, Pahan K. Fibrillar amyloid-beta peptides kill human primary neurons via NADPH oxidase-mediated activation of neutral sphingomyelinase. Implications for Alzheimer's disease. J. Biol. Chem. 2004;279:51451–51459. doi: 10.1074/jbc.M404635200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JA, Denisova NA, Bielinski D, Fisher DR, Shukitt-Hale B. Oxidative stress protection and vulnerability in aging: putative nutritional implications for intervention. Mech. Ageing Dev. 2000;116:141–153. doi: 10.1016/s0047-6374(00)00128-7. [DOI] [PubMed] [Google Scholar]
- Ju TC, Chen SD, Liu CC, Yang DI. Protective effects of S-nitrosoglutathione against amyloid beta-peptide neurotoxicity. Free Radic. Biol. Med. 2005;38:938–949. doi: 10.1016/j.freeradbiomed.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Katsel P, Li C, Haroutunian V. Gene expression alterations in the sphingolipid metabolism pathways during progression of dementia and Alzheimer's disease: a shift toward ceramide accumulation at the earliest recognizable stages of Alzheimer's disease? Neurochem. Res. 2007;32:845–856. doi: 10.1007/s11064-007-9297-x. [DOI] [PubMed] [Google Scholar]
- Kolesnick RN, Kronke M. Regulation of ceramide production and apoptosis. Annu. Rev. Physiol. 1998;60:643–665. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- Lee JT, Xu J, Lee JM, Ku G, Han X, Yang DI, Chen S, Hsu CY. Amyloid-beta peptide induces oligodendrocyte death by activating the neutral sphingomyelinaseceramide pathway. J. Cell Biol. 2004;164:123–131. doi: 10.1083/jcb.200307017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepine S, Lakatos B, Courageot MP, Le Stunff H, Sulpice JC, Giraud F. Sphingosine contributes to glucocorticoid-induced apoptosis of thymocytes independently of the mitochondrial pathway. J. Immunol. 2004;173:3783–3790. doi: 10.4049/jimmunol.173.6.3783. [DOI] [PubMed] [Google Scholar]
- Malaplate-Armand C, Florent-Bechard S, Youssef I, Koziel V, Sponne I, Kriem B, Leininger-Muller B, Olivier JL, Oster T, Pillot T. Soluble oligomers of amyloid-beta peptide induce neuronal apoptosis by activating a cPLA(2)-dependent sphingomyelinase-ceramide pathway. Neurobiol. Dis. 2006;23:178–189. doi: 10.1016/j.nbd.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Morishima-Kawashima M, Ihara Y. Alzheimer's disease: beta-Amyloid protein and tau. J. Neurosci. Res. 2002;70:392–401. doi: 10.1002/jnr.10355. [DOI] [PubMed] [Google Scholar]
- Okino N, Ichinose S, Omori A, Imayama S, Nakamura T, Ito M. Molecular cloning, sequencing, and expression of the gene encoding alkaline ceramidase from Pseudomonas aeruginosa. Cloning of a ceramidase homologue from Mycobacterium tuberculosis. J. Biol. Chem. 1999;274:36616–36622. doi: 10.1074/jbc.274.51.36616. [DOI] [PubMed] [Google Scholar]
- Patil S, Melrose J, Chan C. Involvement of astroglial ceramide in palmitic acid-induced Alzheimer-like changes in primary neurons. Eur. J. Neurosci. 2007;26:2131–2141. doi: 10.1111/j.1460-9568.2007.05797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez GI, Jurisicova A, Matikainen T, Moriyama T, Kim MR, Takai Y, Pru JK, Kolesnick RN, Tilly JL. A central role for ceramide in the age-related acceleration of apoptosis in the female germline. Faseb J. 2005;19:860–862. doi: 10.1096/fj.04-2903fje. [DOI] [PubMed] [Google Scholar]
- Pettegrew JW, Panchalingam K, Hamilton RL, McClure RJ. Brain membrane phospholipid alterations in Alzheimer's disease. Neurochem. Res. 2001;26:771–782. doi: 10.1023/a:1011603916962. [DOI] [PubMed] [Google Scholar]
- Puglielli L, Ellis BC, Saunders AJ, Kovacs DM. Ceramide stabilizes beta-site amyloid precursor protein-cleaving enzyme 1 and promotes amyloid beta-peptide biogenesis. J. Biol. Chem. 2003;278:19777–19783. doi: 10.1074/jbc.M300466200. [DOI] [PubMed] [Google Scholar]
- Satoi H, Tomimoto H, Ohtani R, Kitano T, Kondo T, Watanabe M, Oka N, Akiguchi I, Furuya S, Hirabayashi Y, Okazaki T. Astroglial expression of ceramide in Alzheimer's disease brains: a role during neuronal apoptosis. Neuroscience. 2005;130:657–666. doi: 10.1016/j.neuroscience.2004.08.056. [DOI] [PubMed] [Google Scholar]
- Sawamura N, Ko M, Yu W, Zou K, Hanada K, Suzuki T, Gong JS, Yanagisawa K, Michikawa M. Modulation of amyloid precursor protein cleavage by cellular sphingolipids. J. Biol. Chem. 2004;279:11984–11991. doi: 10.1074/jbc.M309832200. [DOI] [PubMed] [Google Scholar]
- Schissel SL, Jiang X, Tweedie-Hardman J, Jeong T, Camejo EH, Najib J, Rapp JH, Williams KJ, Tabas I. Secretory sphingomyelinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH. Implications for atherosclerotic lesion development. J. Biol. Chem. 1998;273:2738–2746. doi: 10.1074/jbc.273.5.2738. [DOI] [PubMed] [Google Scholar]
- Smale G, Nichols NR, Brady DR, Finch CE, Horton WE., Jr. Evidence for apoptotic cell death in Alzheimer's disease. Exp. Neurol. 1995;133:225–230. doi: 10.1006/exnr.1995.1025. [DOI] [PubMed] [Google Scholar]
- Soderberg M, Edlund C, Alafuzoff I, Kristensson K, Dallner G. Lipid composition in different regions of the brain in Alzheimer's disease/senile dementia of Alzheimer's type. J. Neurochem. 1992;59:1646–1653. doi: 10.1111/j.1471-4159.1992.tb10994.x. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Kolesnick R. Sphingosine 1-phosphate as a therapeutic agent. Leukemia. 2002;16:1596–1602. doi: 10.1038/sj.leu.2402611. [DOI] [PubMed] [Google Scholar]
- Sponne I, Fifre A, Koziel V, Oster T, Olivier JL, Pillot T. Membrane cholesterol interferes with neuronal apoptosis induced by soluble oligomers but not fibrils of amyloid-beta peptide. Faseb J. 2004;18:836–838. doi: 10.1096/fj.03-0372fje. [DOI] [PubMed] [Google Scholar]
- Sweeney EA, Inokuchi J, Igarashi Y. Inhibition of sphingolipid induced apoptosis by caspase inhibitors indicates that sphingosine acts in an earlier part of the apoptotic pathway than ceramide. FEBS letters. 1998;425:61–65. doi: 10.1016/s0014-5793(98)00198-7. [DOI] [PubMed] [Google Scholar]
- Tamboli IY, Prager K, Barth E, Heneka M, Sandhoff K, Walter J. Inhibition of glycosphingolipid biosynthesis reduces secretion of the beta-amyloid precursor protein and amyloid beta-peptide. J. Biol. Chem. 2005;280:28110–28117. doi: 10.1074/jbc.M414525200. [DOI] [PubMed] [Google Scholar]
- Tani M, Okino N, Mitsutake S, Tanigawa T, Izu H, Ito M. Purification and characterization of a neutral ceramidase from mouse liver. A single protein catalyzes the reversible reaction in which ceramide is both hydrolyzed and synthesized. J. Biol. Chem. 2000;275:3462–3468. doi: 10.1074/jbc.275.5.3462. [DOI] [PubMed] [Google Scholar]
- White JA, Manelli AM, Holmberg KH, Van Eldik LJ, Ladu MJ. Differential effects of oligomeric and fibrillar amyloid-beta 1-42 on astrocyte-mediated inflammation. Neurobiol. Dis. 2005;18:459–465. doi: 10.1016/j.nbd.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Yankner BA. New clues to Alzheimer's disease: unraveling the roles of amyloid and tau. Nat. Med. 1996;2:850–852. doi: 10.1038/nm0896-850. [DOI] [PubMed] [Google Scholar]
- Yankner BA, Lu T, Loerch P. The Aging Brain. Annu. Rev. Pathol. 2007;27:41–66. doi: 10.1146/annurev.pathmechdis.2.010506.092044. [DOI] [PubMed] [Google Scholar]
- Zeng C, Lee JT, Chen H, Chen S, Hsu CY, Xu J. Amyloid-beta peptide enhances tumor necrosis factor-alpha-induced iNOS through neutral sphingomyelinase/ceramide pathway in oligodendrocytes. J. Neurochem. 2005;94:703–712. doi: 10.1111/j.1471-4159.2005.03217.x. [DOI] [PubMed] [Google Scholar]