Abstract
Background
Defects in the development or activation of T cells result in immunodeficiency associated with severe infections early in life. T cell activation requires Ca2+ influx through Ca2+-release activated Ca2+ (CRAC) channels encoded by the gene ORAI1.
Objective
Investigation of the genetic causes and the clinical phenotype of immunodeficiency in patients with impaired Ca2+ influx and CRAC channel function.
Methods
DNA sequence analysis for mutations in the genes ORAI1, ORAI2, ORAI3, stromal interaction molecules (STIM) 1 and 2 as well as mRNA and protein expression analysis of ORAI1 in immunodeficient patients. Immunohistochemical analysis of ORAI1 tissue distribution in healthy human donors.
Results
We identified mutations in ORAI1 in patients from two unrelated families. One patient is homozygous for a nonsense mutation in ORAI1 (ORAI1-A88SfsX25) and a second patient is compound heterozygous for two missense mutations in ORAI1 (ORAI1-A103E/L194P). All three mutations abolish ORAI1 expression and impair Ca2+ influx and CRAC channel function. The clinical syndrome associated with ORAI1 deficiency is characterized by immunodeficiency with a defect in the function but not the development of lymphocytes, congenital myopathy and anhydrotic ectodermal dysplasia (EDA) with a defect in dental enamel calcification. In contrast to the limited clinical phenotype, we found ORAI1 protein expression in a wide variety of cell types and organs.
Conclusion
Ca2+ influx through ORAI1 is crucial for lymphocyte function in vivo. Despite almost ubiquitous ORAI1 expression, the channel has a non-redundant role in only a few cell-types judging from the limited clinical phenotype in ORAI1 deficient patients.
Keywords: ORAI1, STIM1, CRAC, calcium channel, Ca2+, store-operated Ca2+ entry, T cells, immunodeficiency, signal transduction, congenital myopathy, anhydrotic ectodermal dysplasia, dental enamel, amelogenesis imperfecta
Key messages
Nonsense and missense mutations in the Ca2+ channel gene ORAI1 abolish ORAI1 protein expression and Ca2+ channel function.
ORAI1 deficiency is defined clinically by immunodeficiency, myopathy and anhydrotic ectodermal dysplasia with a defect in dental enamel calcification.
ORAI1 is almost ubiquitously expressed in human tissues despite the limited clinical phenotype of ORAI1 deficiency indicating a non-redundant role for ORAI1 in store-operated Ca2+ influx in T cells, skeletal muscle and some ectodermal derived tissues.
Introduction
Severe combined immunodeficiency (SCID) is characterized by the absence or significant functional impairment of T, B and/or NK cells 1, 2. Lymphocyte activation follows immunoreceptor engagement which results in Ca2+ signaling, proliferation and cytokine gene expression 3. In T cells, Ca2+ influx occurs following activation of phospholipase C (PLC) γ1 and release of Ca2+ from intracellular ER stores. Release of stored Ca2+ results in a transient increase in [Ca2+]i and subsequently activation of the Ca2+ release activated Ca2+ (CRAC) channel in the plasma membrane 4. The Ca2+ influx resulting from CRAC channel activation is called store-operated Ca2+ entry (SOCE) because it depends on the depletion of ER Ca2+ stores.
The CRAC channel constitutes the major Ca2+ influx channel in T cells and is encoded by ORAI1 3, 4, a tetraspanning plasmamembrane protein that is structurally unrelated to other ion channels except its two paralogues ORAI2 and ORAI3. ORAI1 functions as the pore forming subunit of the CRAC channel 5–7. A missense mutation in ORAI1 (R91W) abolishes ORAI1 and CRAC channel function and causes SCID characterized by a severe defect in T cell activation 8, 9. ORAI1-CRAC channels are activated by the ER protein stromal interaction molecule (STIM) 1 which senses the ER Ca2+ concentration and, upon release of Ca2+ from ER stores, multimerizes and binds to ORAI1 4. Lack of STIM1 expression in human patients due to a nonsense mutation in STIM1 (E136X) severely impairs SOCE and causes immunodeficiency and autoimmunity associated with myopathy and abnormal enamel dentition 10.
In addition to patients with ORAI1-R91W and STIM1-E136X mutations 8, 10, a defect in SOCE and CRAC channel function has been described in patients from two kindreds in which the underlying gene defect remained undefined 11, 12. We here report three new mutations in ORAI1 in patients from two of the original kindreds that abolish ORAI1 protein expression and SOCE 11, 12. These ORAI1 mutations and those in ORAI1 and STIM1 reported before 8, 10 collectively define the clinical phenotype associated with defects in CRAC channel function.
Materials and Methods
Case reports
Case reports of patients P1 – P6 have been published 11–15. Follow-up data on all patients and clinical descriptions are provided in Table 1 and the online repository.
TABLE 1.
Clinical phenotypes of patients with ORAI1 mutations
| Patient | P1 (A-IV-1)* | P2 (A-IV-2) | P3 (B-V-1) | P4 (B-V-3) | P5 (C-II-2) | P6 (C-II-3) |
|---|---|---|---|---|---|---|
| ORAI1 mutation | R91W | R91W | nt | A88SfsX25 | nt | A103E/L194P |
| SOCE/ICRAC | undetectable | undetectable | nt | undetectable | nt | undetectable |
| Infections | BCGitis, meningitis, rotavirus enteritis, interstitial pneumonia, sepsis | No | Chronic diarrhea and candidiasis, pneumonia, pyelonephritis | Chronic diarrhea, chronic candidiasis, pneumonia, pyelonephritis, otitis. | Diarrhea, chlamydia pneumonia, toxoplasma encephalitis | Pneumonia, chronic diarrhea, CMV infection |
| Autoimmunity | No | No | No | Neutropenia and thrombocytopenia | No | No |
| Developmental features |
|
|
|
|
|
|
| Other manifestations |
|
|
|
|
|
|
| Outcome | Death at 11m from pneumonia and sepsis | Alive (16y) after HSCT with persisting myo- pathy and EDA | Death at 5m from pneumonia | Death at 11m from progressive encephalo- pathy, fever, seizures | Death at 8m | Alive (16y) after HSCT with persisting myopathy and EDA |
| References | (8–9, 13–15) | (8–9, 13–14) | (12) | (12) | (11) | (11) |
See pedigrees in Figure E1. Abbreviations: BCG, Bacille Calmette-Guérin; HSCT, hematopoietic stem cell transplantation; nt, not tested; m, months; P, patient; y, years.
Cells
SV40-transformed fibroblasts from P4, P6 and a healthy control and Ficoll-Paque (GE Healthcare, Piscataway, NJ) isolated peripheral blood mononuclear cells from P6’s parents and controls were grown in RPMI 1640 (Mediatech, Manassas, VA).
Plasmids and transfections
IRES-GFP containing bicistronic vectors for expression of myc-epitope tagged ORAI1, ORAI2, ORAI3 or STIM1 have been described 8, 16. ORAI1 A88SfsX25, A103E and L194P mutant plasmids were generated by overlap mutagenesis and used for retroviral transduction as described8. Transduction efficiencies were evaluated by GFP expression and immunoblotting using anti-myc antibody (clone 9E10, Santa Cruz Biotechnology, Santa Cruz, CA).
Genomic DNA sequencing
Genomic DNA was isolated from cells using standard methods. PCR was conducted using primers flanking exons and splice sites of ORAI1, ORAI2, ORAI3, STIM1 and STIM2 (Table E2). PCR products were sequenced directly (Genewiz Inc., South Plainfield, NJ). Sequence alignments were performed using TCoffee software (Swiss Institute of Bioinformatics) and sequence traces visualized using Xplorer software v1.0 (dnaTools). Single nucleotide polymorphism (SNP) searches were performed using dbSNP database (build 129; http://www.ncbi.nlm.nih.gov/SNP/).
Immunohistochemistry and antibodies
For detection of ORAI1 in patient fibroblasts, cell pellets were fixed in 3% phosphate-buffered paraformaldehyde, permeabilized with 1x PBS, 0.5% NP-40, 0.02% sodium azide and incubated with affinity-purified anti- ORAI1 antibodies raised against aa 275–291 of human ORAI1. For immunofluorescence, a muscle biopsy sample of P2 was co-incubated with antibodies to anti-ORAI1 and anti-myosin heavy chain fast (clone WB-MHCf, Novocastra, Newcastle upon Tyne, UK) at 1:50 dilution; MHCf was detected by Alexa Fluor 488 goat anti-mouse IgG staining (Invitrogen, Carlsbad, CA). For detection of ORAI1 in tissues from healthy donors, 5-μm sections of paraffin-embedded normal human tissue microarrays (FDA 801, US Biomax Inc., Rockville, MD) were incubated with anti-ORAI1 antibodies and prepared as described 17.
Muscle biopsy
A biopsy of P2’s vastus lateralis muscle was frozen in isopentane cooled in liquid nitrogen and 10 μm cryostat sections were stained with standard histological and histochemical techniques 18.
Ca2+ measurements
Single-cell Ca2+ imaging was performed as described 9. Traces in figures represent mean [Ca2+]i of one representative experiment; ~ 30–80 GFP+ cells per experiment were analyzed. Error bars represent SEM.
Additional methods are available in the online repository accompanying this article.
Results
Homozygous A88SfsX25 ORAI1 nonsense mutation abolishes ORAI1 expression
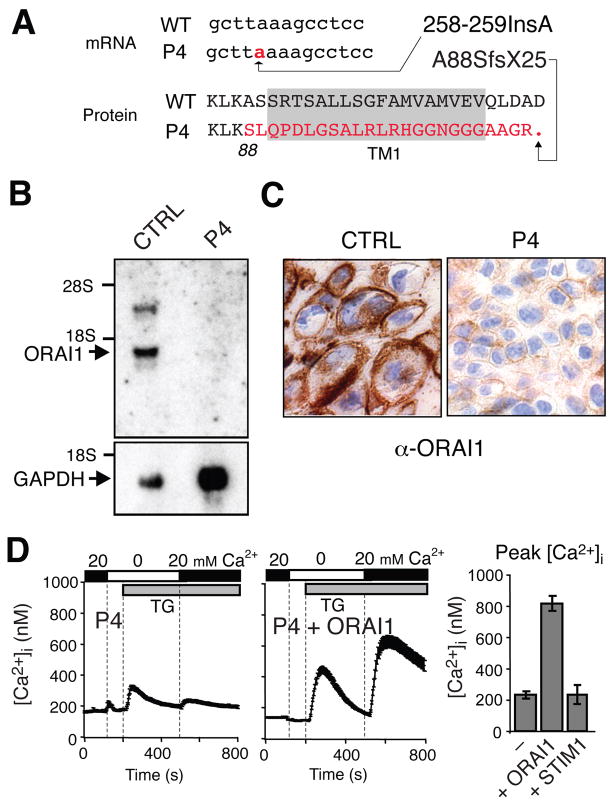
Ca2+ influx and CRAC channel currents were reported to be undetectable in T cells from immunodeficient patient 4 (P4) resulting in severely impaired T cell activation (Table E1) 12. Genomic DNA sequence analysis revealed that P4 is homozygous for a nonsense mutation in exon 1 of ORAI1 resulting from the insertion of a single adenine between positions 258 and 259 (258–259insA) of the ORAI1 coding sequence (NM_32790) (Fig 1,A). The mutation is not a known SNP and was not observed in two healthy siblings of P4 (B-V-4 and B-V-5 in Fig E1) and DNA from 50 control individuals (100 chromosomes). DNA from his parents and his older brother (P3) was not available for analysis. The insertion causes a frame shift starting at amino acid residue 88 and premature termination at position 112 of ORAI1 protein (ORAI1-A88SfsX25) at the end of the first transmembrane (TM) domain (Fig 1, A). No mutations in ORAI2, ORAI3, STIM1 and STIM2 were found in P4.
Figure 1. ORAI1 A88SfsX25 nonsense mutation abolishes ORAI1 expression in P4.
A, Adenosine insertion (258–259insA) results in frame shift, premature termination (A88SfsX25) and altered amino acid sequence (red) of TM1 in ORAI1 (shaded). B–C, Non-detectable ORAI1 mRNA (B) and protein (C) expression in P4 compared to control. D, Impaired Ca2+ influx in fibroblasts from P4 (left) is restored by expression of ORAI1 (middle). Bar graphs represent averages of peak [Ca2+]i from 4–6 experiments. TG, thapsigargin.
Northern blot analysis showed that ORAI1 mRNA transcripts were undetectable in P4 compared to cells from a healthy control (Fig 1, B) most likely due to nonsense-mediated mRNA decay. Fibroblasts from P4 also showed strongly reduced ORAI1 protein expression when cells were analyzed by immunohistochemistry using an anti-ORAI1 antibody (Fig 1, C). Since the antibody is directed against the C-terminus of ORAI1 we tested the possibility that a truncated ORAI1 fragment lacking the C terminus could be expressed. Ectopic expression of an N-terminally myc-tagged version of mutant ORAI1-A88SfsX25 in HEK293 cells, however, only yielded a weak single band observed at ~15 kD in SDS-PAGE, corresponding to the predicted ORAI1 fragment (Fig E2, A). This fragment, if expressed at all endogenously, is very unlikely to be functional as an ion channel because it lacks transmembrane domains required for Ca2+ conductance. Finally, reconstitution of patient fibroblasts with wild-type ORAI1, but not empty vector or STIM1, rescued Ca2+ influx upon stimulation with thapsigargin, an inhibitor of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) that induces passive Ca2+ store depletion and CRAC channel activation (Fig 1, D). Failure of STIM1 expression to rescue SOCE also argues against residual ORAI1 expression in the cells of P4 because co-expression of STIM1 and ORAI1 was shown to dramatically enhance CRAC channel function 19, 20. Collectively, these data show that the nonsense mutation in ORAI1 is responsible for the lack of Ca2+ influx in P4.
ORAI1 missense mutations A103E and L194P abolish ORAI1 protein expression
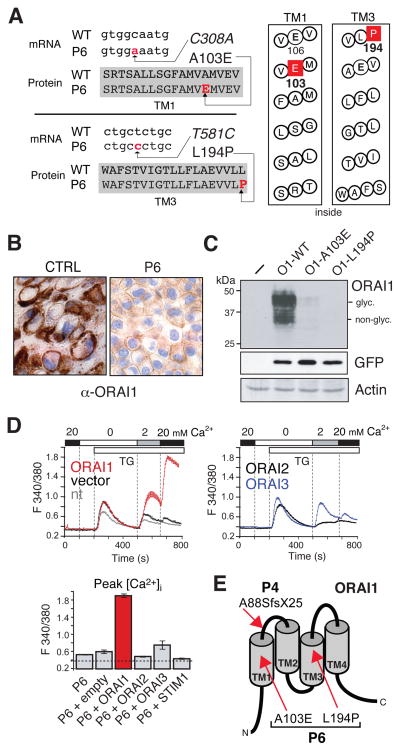
T cells from patient 6 (P6) and his deceased brother P5 displayed a severe proliferation defect (Table E1) 11. Ca2+ influx in T cells, B cells, platelets and fibroblasts from P6 was reported to be profoundly impaired (Fig E2, B) 11. Sequence analysis of genomic DNA from P6 demonstrated that he is compound heterozygous for two independent missense mutations in exon 2 of ORAI1. The C→A and T→C mutations at positions 308 and 581 of the ORAI1 coding sequence, respectively, are not known SNPs and were not detected in 50 healthy controls (100 chromosomes) (Fig 2, A). The missense mutations result in the substitution of an alanine with glutamate (A103E) and a leucine with proline (L194P) in the first and third TM domains of ORAI1, respectively (Fig 2, A, E). P6 inherited the mutated alleles from his father (C-I-1 in Fig E1: A103E) and his mother (C-I-2 in Fig E1: L194P) who are heterozygous for one but not the other mutation. Mutations in other genes including ORAI2, ORAI3, STIM1 and STIM2 were not observed in P6.
Figure 2. Two missense mutations abolish ORAI1 protein expression in P6.
A, ORAI1 C308A and T581C mutations result in single amino acid substitutions A103E and L194P in TM1 and TM3 (shaded or boxed) of ORAI1, respectively. B–C, Undetectable endogenous (B) and ectopic (C) expression of ORAI1 protein in fibroblasts from P6 (B) and HEK293 cells transfected with ORAI1 mutants (C). WT, wild-type; glyc., glycosylated. D, Impaired Ca2+ influx in nontransfected (nt) fibroblasts from P6 is restored by expression of ORAI1. Bar graphs represent averages of peak Ca2+ influx from 4–6 experiments. E, Location of ORAI1 mutations in P4 and P6.
The mutations had no effect on ORAI1 mRNA transcription (not shown), whereas ORAI1 protein expression was undetectable in fibroblasts from P6 by immunohistochemistry (Fig 2, B) and flow cytometry (not shown) suggesting that both ORAI1 mutations interfere with stable protein expression. To test this hypothesis, we ectopically expressed ORAI1-A103E and ORAI1-L194P separately in HEK293 cells using bicistronic IRES-GFP vectors. Expression of either mutant protein was undetectable compared to wild-type ORAI1 despite normal IRES-mediated GFP protein expression from the same mRNA transcript (Fig 2, C). A dominant negative effect of the mutations on CRAC channel function was ruled out because Ca2+ influx in T cells from P6’s parents, each heterozygous for one of the mutations, was normal (Fig E2, C) and ectopic expression of ORAI1-A103E or ORAI1-L194P in HEK293 cells had no effect on Ca2+ influx (Fig E2, D).
Reconstitution of P6’s fibroblasts with wild-type ORAI1, but not STIM1, ORAI2 or empty vector rescued Ca2+ influx upon stimulation with thapsigargin (Fig 2, D). Ectopic expression of ORAI3 partially restored SOCE consistent with similar observations in cells of P2 16. Taken together, these findings show that two independent missense mutations in ORAI1 severely compromise stable ORAI1 protein expression abolishing Ca2+ influx and causing immunodeficiency.
Myopathy and anhydrotic ectodermal dysplasia (EDA) in ORAI1 deficient patients
The clinical immunodeficiency in patients lacking ORAI1 expression (P3– P6) is very similar to that observed in patients (P1, P2) homozygous for an ORAI1-R91W missense mutation that abolishes ORAI1 function but not its expression (Table 1, Table E1) 8, 14.
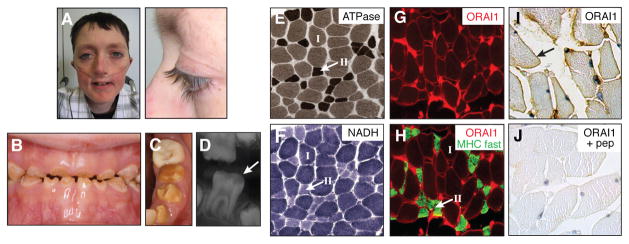
All six ORAI1-deficient patients showed non-immunological symptoms as well. Both patients surviving after HSCT (P2, P6) presented with EDA characterized by severely dysplastic dental enamel and pronounced anhydrosis resulting in dry skin and heat intolerance; no hair abnormalities or pigmentation defects were observed (Fig 3, A–D). Hypocalcified amelogenesis imperfecta (type III) in P2 and P6 is due to a failure of dental enamel matrix calcification resulting in use-dependent loss of the unusually soft dental enamel of both deciduous and permanent teeth. All patients (P1–P6) suffered from global muscular hypotonia from birth. In P2 and P6, the myopathy compromises the patients’ mobility and results in chronic pulmonary disease due to respiratory muscle insufficiency. Histologically, the myopathy in P2 is characterized by a variation in muscle fiber size with a predominance of type I fibers and atrophic type II fibers (Fig 3, E–F). Other histological abnormalities commonly found in myopathies were not observed in P2. Consistent with a role for ORAI1 in myocyte function 21–23, we found sarcolemmal expression of ORAI1 in muscle fibers of wild-type controls and both type I and type II muscle fibers of P2 (Fig 3, G–J).
Figure 3. EDA and myopathy in ORAI1 deficient patients.
A, Normal hair in P6 (age 16y). B–D, Hypocalcified amelogenesis imperfecta with significant loss of enamel substance in deciduous (B, P2 at age 6y) and permanent (C, P2 at age 10.5y; D, P6 at age 9.5y) teeth. E–H, Atrophy of type II muscle fibers in P2 (age 5y) by ATPase (E), NADH (F) and αORAI1(red)/αMHC fast (green) staining (G-H). I–J, ORAI1 expression in normal human skeletal muscle. Pep, blocking peptide.
Almost ubiquitous ORAI1 protein expression
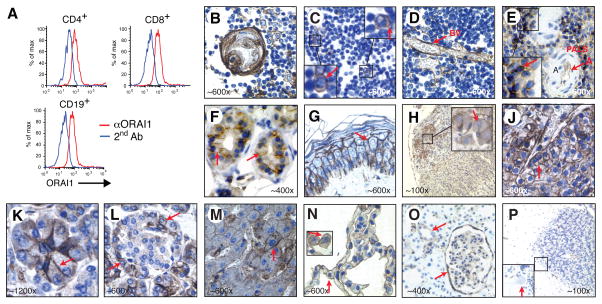
The clinical syndrome associated with ORAI1 deficiency indicates that ORAI1-dependent SOCE is essential for the function and/or development of lymphocytes, skeletal muscle and ectodermal derived tissues. ORAI1 mRNA expression and SOCE, however, have also been demonstrated in many other organs and cell types 16, 24. No data on the cellular distribution pattern of ORAI1 have been reported. We detected ORAI1 expression in CD4+ and CD8+ T cells and CD19+ B cells consistent with the known role of ORAI1 in lymphocyte function (Fig 4, A). Immunhistochemical analysis showed that ORAI1 is expressed in a subset of cells in primary and secondary lymphoid organs such as thymus, spleen and tonsils (Fig 4, B–E and data not shown). The strongest expression was observed in lymphoid cells in the periarterial lymphoid sheath (PALS) of spleen (Fig 4, E) and the paracortical zone in tonsils (data not shown) consistent with ORAI1 expression in T cells. Outside the immune system, ORAI1 expression was found in a wide variety of cell types and organs (Fig 4) including those affected in the patients such as eccrine sweat glands (Fig 4, F) and other tissues not apparently affected in the patients such as skin, vascular endothelium, mucosal epithelial cells of the gastrointestinal tract, several endocrine and exocrine glands, hepatocytes, pneumocytes in the lung and kidney tubules (Fig 4). Of note is the almost complete absence of ORAI1 staining in the central nervous system consistent with previously reported low ORAI1 mRNA levels in the brain (http://www.brain-map.org) 16, 25.
Figure 4. Almost ubiquitous ORAI1 tissue expression.
A, ORAI1 expression in human CD4+, CD8+ and CD19+ T and B cells analyzed by flow cytometry. B–P, ORAI1 expression in tissues from healthy donors incubated with αORAI1 antibody. Shown are thymus (B–D), spleen (E), eccrine sweat glands (F), skin (G), adrenal gland (H), parathyroid gland (J), exocrine pancreas (K), pancreatic islet (L), liver (M), lung (N), kidney (O), cerebellum (P). Specificity controls are shown in Figure E3. A, artery; BV, blood vessel; PALS, periarterial lymphoid sheath.
Discussion
The ORAI1 deficient patients described here represent all patients known to date with CRAC channel dysfunction due to mutations in ORAI1. Their clinical phenotypes illustrate the in vivo role of ORAI1 in human and together with patients lacking STIM1 expression 10 define a new disease syndrome resulting from a defect in SOCE and CRAC channel function 11, 12. The mutations described in this study cause a human null-phenotype for ORAI1 by interfering with mRNA expression as in the case of the A88SfsX25 nonsense mutation in P4 or protein stability and expression as in the case of the ORAI1-A103E and ORAI1-L194P missense mutations in P6. The A103E substitution introduces a negative charge in close proximity to E106 – a Ca2+ binding site in the CRAC channel pore 5–7 – likely resulting in electrostatic repulsion and destabilization of the first transmembrane alpha helix (Fig 2, A). Substitution of L194 with proline at the end of the third TM domain is likely to break or kink the TM alpha helix resulting in the destabilization of protein expression 26, 27.
Clinically, ORAI1 deficiency is characterized by a severe defect in adaptive immune responses resulting in life-threatening infections with viral, bacterial and fungal pathogens, congenital myopathy and EDA with defects in enamel dentition and sweat production (Table 1). The immunodeficiency is very similar to SCID but– unlike in the majority of SCID cases – lymphocyte development is unperturbed. Numbers of T cells and T cell subsets, B cells and NK cells in peripheral blood of patients were normal suggesting that lymphocyte differentiation including the selection and maturation of T cells occurs independently of ORAI1 (Table E1) 11, 12, 14. These findings are consistent with normal lymphocyte development observed in STIM1 deficient patients 10 and Orai1 and Stim1 deficient mice 28–31. By contrast, T cell activation is severely compromised in all six patients as skin delayed-type hypersensitivity reactions in vivo and proliferative responses to a variety of stimuli in vitro are reduced or absent consistent with the severe defect in cytokine production in T cells of P1 and P2 reported previously 13, 14. T cells from P4 and P6, but not those of P1 and P2, proliferated normally following PMA and ionomycin treatment. The discrepancy may be due to a potential inhibitory effect of mutant ORAI1-R91W on residual ORAI2 Ca2+ channels which have been implicated in Ca2+ influx in mouse T cells 31. Given the important role of Ca2+ influx for B and NK cell function 32–35 and ORAI1 expression in both cell types at least in mice 36, the SOCE defect documented in B cells from P2 37 and P6 (Fig E2) 11 and a potential SOCE defect in NK cells may contribute to the patients’ immunodeficiency.
Autoimmunity was observed in only one (P4) out of six ORAI1 deficient patients who presented with neutropenia and thrombocytopenia. By contrast, all STIM1 deficient patients showed autoimmune symptoms and one patient was observed to have reduced numbers of CD4+ Foxp3+ regulatory T cells (Treg) 10. Numbers of Treg cells in ORAI1 deficient patients could not be evaluated because blood samples preceding HSCT were not available. A plausible explanation for the absence of autoimmunity in most ORAI1 deficient patients is that four of the six patients (P1, P3-P5) died in their first year of life, presumably before the onset of autoimmune disease, and the two surviving patients (P2, P6) received HSCT at 4 months of age preventing autoimmunity. Taken together, our findings demonstrate that ORAI1, SOCE and CRAC channel function are required for T cell activation but largely dispensable for T cell development.
The non-immunological symptoms observed in ORAI1 deficient patients overlap with those found in patients lacking STIM1 strongly indicating that the phenotype is due to a defect in SOCE and CRAC channel function. All ORAI1 deficient patients suffer from congenital myopathy characterized by global muscular hypotonia and, in one patient, atrophy of type II muscle fibers. These observations suggest that SOCE is required for skeletal muscle function and/or differentiation. SOCE in murine skeletal myotubes is mediated by ORAI1 and STIM1 22, 23 and results in the refilling of sarcoplasmic reticulum Ca2+ stores 38, 39 thus ensuring the muscle’s ability to undergo repeated cycles of excitation-contraction coupling mediated by Ca2+ release from the SR. In addition, SOCE may be critical for skeletal muscle differentiation as it is involved in the expression of two early markers of myoblast differentiation, MEF2 and myogenin 21 and presumably the activation of the Ca2+ dependent transcription factor NFAT 40. Myoblasts of Stim1 and SOCE deficient mice not only fatigued rapidly but also showed severe morphological abnormalities consistent with a developmental defect 23.
EDA was observed in the two surviving ORAI1-deficient patients (P2, P6) and all STIM1-deficient patients indicating that SOCE is important for eccrine sweat gland function and/or development (skin biopsies from ORAI1 deficient patients to distinguish between these possibilities could not be obtained) and ameloblast function during calcification of the enamel matrix. Encephalopathy was observed only in P3 and P4 but not in the other ORAI1- (and STIM1-) deficient patients. It is therefore unlikely to be a common feature of ORAI1 deficiency but is potentially due to an additional genetic defect in a notably inbred kindred or infection with a neurotrophic pathogen, a relatively common complication in patients with defects in T cell mediated immunity. EDA with immunodeficiency (EDA-ID) caused by mutations in ORAI1 (and STIM1)10 differs from EDA-ID due to mutations in IKKγ (NEMO)41–43 and IκBα44 in that the latter are both characterized by hypodontia, conical teeth in all and sparse scalp hair in some of the patients. In addition, patients with mutations in IκBα and IKKγ, but not ORAI1 (and STIM1), show a hyper-IgM phenotype with decreased serum IgG and increased IgM. While the hypermorphic IκBα mutation impairs T cell proliferation similar to ORAI1 (and STIM1) deficiency, only IκBα but not ORAI1 deficient patients lack γδ and memory αβT cells44.
The limited clinical spectrum of ORAI1 deficiency contrasts with the almost ubiquitous expression of ORAI1 (Fig. 4) 16 and reports describing SOCE and CRAC channel function in many cell types outside the immune system 24, 45–48. Explanations for the absence of more extensive disease in the ORAI1-deficient patients include that ORAI1 plays a redundant role for SOCE in many tissues and can be functionally replaced by, for instance, ORAI2 or ORAI3 and that SOCE co-exists with non-store operated Ca2+ influx. ORAI2 and ORAI3 can form Ca2+ channels when co-expressed ectopically with STIM149, 50 but direct evidence for a physiological role of endogenous ORAI2 and ORAI3 in vivo is still missing.
In summary, the ORAI1 mutations described in this study together with the phenotypes of ORAI1- and STIM1-deficient patients reported earlier8, 10 define the clinical syndrome associated with defects in CRAC channel function and provide valuable insight into the role of ORAI1 and SOCE in human in vivo.
Acknowledgments
Supported by National Institutes of Health grants (SF; AR), a March of Dimes Foundation grant (SF) and an INSERM grant (AF).
We thank Dr. R. Hirschhorn for healthy control DNA samples, Dr. A. Freundorfer for images of P6’s teeth, Dr. M. Schlesier for reviewing patient data and C. Jacques and C. Harre for their technical help with culturing cells.
Abbreviations used in this paper
- CRAC
Ca2+ release activated Ca2+ (channel)
- EDA
ectodermal dysplasia with anhydrosis
- SCID
severe combined immunodefiency
- SOCE
store-operated Ca2+ entry
- STIM1
stromal interaction molecule 1
- TM
transmembrane
Footnotes
Disclosure of potential conflict of interest: A. Rao and S. Feske are scientific co-founders and members of the scientific advisory board of CalciMedica. No other potential conflict of interest relevant to this article was reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fischer A. Human primary immunodeficiency diseases. Immunity. 2007;27:835–45. doi: 10.1016/j.immuni.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Geha RS, Notarangelo LD, Casanova JL, Chapel H, Conley ME, Fischer A, et al. Primary immunodeficiency diseases: an update from the International Union of Immunological Societies Primary Immunodeficiency Diseases Classification Committee. J Allergy Clin Immunol. 2007;120:776–94. doi: 10.1016/j.jaci.2007.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 4.Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–7. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 5.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–3. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 6.Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, et al. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006;16:2073–9. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–9. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–85. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 9.Feske S, Prakriya M, Rao A, Lewis RS. A severe defect in CRAC Ca2+ channel activation and altered K+ channel gating in T cells from immunodeficient patients. J Exp Med. 2005;202:651–62. doi: 10.1084/jem.20050687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Picard C, McCarl CA, Papolos A, Khalil S, Lüthy K, Hivroz C, et al. STIM1 Mutation Associated with a Syndrome of Immunodeficiency and Autoimmunity. N Engl J Med. 2009;360:1971–80. doi: 10.1056/NEJMoa0900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Deist F, Hivroz C, Partiseti M, Thomas C, Buc HA, Oleastro M, et al. A primary T-cell immunodeficiency associated with defective transmembrane calcium influx. Blood. 1995;85:1053–62. [PubMed] [Google Scholar]
- 12.Partiseti M, Le Deist F, Hivroz C, Fischer A, Korn H, Choquet D. The calcium current activated by T cell receptor and store depletion in human lymphocytes is absent in a primary immunodeficiency. J Biol Chem. 1994;269:32327–35. [PubMed] [Google Scholar]
- 13.Feske S, Draeger R, Peter HH, Eichmann K, Rao A. The duration of nuclear residence of NFAT determines the pattern of cytokine expression in human SCID T cells. J Immunol. 2000;165:297–305. doi: 10.4049/jimmunol.165.1.297. [DOI] [PubMed] [Google Scholar]
- 14.Feske S, Muller JM, Graf D, Kroczek RA, Drager R, Niemeyer C, et al. Severe combined immunodeficiency due to defective binding of the nuclear factor of activated T cells in T lymphocytes of two male siblings. Eur J Immunol. 1996;26:2119–26. doi: 10.1002/eji.1830260924. [DOI] [PubMed] [Google Scholar]
- 15.Schlesier M, Niemeyer C, Duffner U, Henschen M, Tanzi-Fetta R, Wolff-Vorbeck G, et al. Primary severe immunodeficiency due to impaired signal transduction in T cells. Immunodeficiency. 1993;4:133–6. [PubMed] [Google Scholar]
- 16.Gwack Y, Srikanth S, Feske S, Cruz-Guilloty F, Oh-hora M, Neems DS, et al. Biochemical and functional characterization of Orai proteins. J Biol Chem. 2007;282:16232–43. doi: 10.1074/jbc.M609630200. [DOI] [PubMed] [Google Scholar]
- 17.Rodig SJ, Savage KJ, Nguyen V, Pinkus GS, Shipp MA, Aster JC, et al. TRAF1 expression and c-Rel activation are useful adjuncts in distinguishing classical Hodgkin lymphoma from a subset of morphologically or immunophenotypically similar lymphomas. Am J Surg Pathol. 2005;29:196–203. doi: 10.1097/01.pas.0000149689.75462.ff. [DOI] [PubMed] [Google Scholar]
- 18.Dubowitz V. A Practical Approach. London: Bailliere Tindall; 1985. Muscle Biopsy. [Google Scholar]
- 19.Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, et al. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–3. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281:20661–5. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- 21.Darbellay B, Arnaudeau S, Konig S, Jousset H, Bader C, Demaurex N, et al. STIM1- and Orai1-dependent store-operated calcium entry regulates human myoblast differentiation. J Biol Chem. 2009;284:5370–80. doi: 10.1074/jbc.M806726200. [DOI] [PubMed] [Google Scholar]
- 22.Lyfenko AD, Dirksen RT. Differential dependence of store-operated and excitation-coupled Ca2+ entry in skeletal muscle on STIM1 and Orai1. J Physiol. 2008;586:4815–24. doi: 10.1113/jphysiol.2008.160481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stiber J, Hawkins A, Zhang ZS, Wang S, Burch J, Graham V, et al. STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nat Cell Biol. 2008;10:688–97. doi: 10.1038/ncb1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 25.Huang YH, Hoebe K, Sauer K. New therapeutic targets in immune disorders: ItpkB, Orai1 and UNC93B. Expert Opin Ther Targets. 2008;12:391–413. doi: 10.1517/14728222.12.4.391. [DOI] [PubMed] [Google Scholar]
- 26.Orzaez M, Salgado J, Gimenez-Giner A, Perez-Paya E, Mingarro I. Influence of proline residues in transmembrane helix packing. J Mol Biol. 2004;335:631–40. doi: 10.1016/j.jmb.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 27.Yohannan S, Yang D, Faham S, Boulting G, Whitelegge J, Bowie JU. Proline substitutions are not easily accommodated in a membrane protein. J Mol Biol. 2004;341:1–6. doi: 10.1016/j.jmb.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 28.Beyersdorf N, Braun A, Vogtle T, Varga-Szabo D, Galdos RR, Kissler S, et al. STIM1-independent T cell development and effector function in vivo. J Immunol. 2009;182:3390–7. doi: 10.4049/jimmunol.0802888. [DOI] [PubMed] [Google Scholar]
- 29.Gwack Y, Srikanth S, Oh-Hora M, Hogan PG, Lamperti ED, Yamashita M, et al. Hair loss and defective T- and B-cell function in mice lacking ORAI1. Mol Cell Biol. 2008;28:5209–22. doi: 10.1128/MCB.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, et al. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–43. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vig M, DeHaven WI, Bird GS, Billingsley JM, Wang H, Rao PE, et al. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caraux A, Kim N, Bell SE, Zompi S, Ranson T, Lesjean-Pottier S, et al. Phospholipase C-gamma2 is essential for NK cell cytotoxicity and innate immunity to malignant and virally infected cells. Blood. 2006;107:994–1002. doi: 10.1182/blood-2005-06-2428. [DOI] [PubMed] [Google Scholar]
- 33.Cassatella MA, Anegon I, Cuturi MC, Griskey P, Trinchieri G, Perussia B. Fc gamma R(CD16) interaction with ligand induces Ca2+ mobilization and phosphoinositide turnover in human natural killer cells. Role of Ca2+ in Fc gamma R(CD16)-induced transcription and expression of lymphokine genes. J Exp Med. 1989;169:549–67. doi: 10.1084/jem.169.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King LB, Freedman BD. B-lymphocyte calcium influx. Immunol Rev. 2009;231:265–77. doi: 10.1111/j.1600-065X.2009.00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scharenberg AM, Humphries LA, Rawlings DJ. Calcium signalling and cell-fate choice in B cells. Nat Rev Immunol. 2007;7:778–89. doi: 10.1038/nri2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feske S. ORAI1 and STIM1 deficiency in human and mice: roles of store-operated Ca2+ entry in the immune system and beyond. Immunol Rev. 2009;231:189–209. doi: 10.1111/j.1600-065X.2009.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feske S, Giltnane J, Dolmetsch R, Staudt LM, Rao A. Gene regulation mediated by calcium signals in T lymphocytes. Nat Immunol. 2001;2:316–24. doi: 10.1038/86318. [DOI] [PubMed] [Google Scholar]
- 38.Kurebayashi N, Ogawa Y. Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J Physiol. 2001;533:185–99. doi: 10.1111/j.1469-7793.2001.0185b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Launikonis BS, Rios E. Store-operated Ca2+ entry during intracellular Ca2+ release in mammalian skeletal muscle. J Physiol. 2007;583:81–97. doi: 10.1113/jphysiol.2007.135046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–32. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 41.Doffinger R, Smahi A, Bessia C, Geissmann F, Feinberg J, Durandy A, et al. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat Genet. 2001;27:277–85. doi: 10.1038/85837. [DOI] [PubMed] [Google Scholar]
- 42.Jain A, Ma CA, Liu S, Brown M, Cohen J, Strober W. Specific missense mutations in NEMO result in hyper-IgM syndrome with hypohydrotic ectodermal dysplasia. Nat Immunol. 2001;2:223–8. doi: 10.1038/85277. [DOI] [PubMed] [Google Scholar]
- 43.Zonana J, Elder ME, Schneider LC, Orlow SJ, Moss C, Golabi M, et al. A novel X-linked disorder of immune deficiency and hypohidrotic ectodermal dysplasia is allelic to incontinentia pigmenti and due to mutations in IKK-gamma (NEMO) Am J Hum Genet. 2000;67:1555–62. doi: 10.1086/316914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Courtois G, Smahi A, Reichenbach J, Doffinger R, Cancrini C, Bonnet M, et al. A hypermorphic IkappaBalpha mutation is associated with autosomal dominant anhidrotic ectodermal dysplasia and T cell immunodeficiency. J Clin Invest. 2003;112:1108–15. doi: 10.1172/JCI18714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M. Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ Res. 2008;103:1289–99. doi: 10.1161/01.RES.0000338496.95579.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bergmeier W, Oh-Hora M, McCarl CA, Roden RC, Bray PF, Feske S. R93W mutation in Orai1 causes impaired calcium influx in platelets. Blood. 2009;113:675–8. doi: 10.1182/blood-2008-08-174516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braun A, Varga-Szabo D, Kleinschnitz C, Pleines I, Bender M, Austinat M, et al. Orai1 (CRACM1) is the platelet SOC channel and essential for pathological thrombus formation. Blood. 2009;113:2056–63. doi: 10.1182/blood-2008-07-171611. [DOI] [PubMed] [Google Scholar]
- 48.Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, et al. Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. FASEB J. 2009 doi: 10.1096/fj.09-131128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeHaven WI, Smyth JT, Boyles RR, Putney JW., Jr Calcium inhibition and calcium potentiation of Orai1, Orai2, and Orai3 calcium release-activated calcium channels. J Biol Chem. 2007;282:17548–56. doi: 10.1074/jbc.M611374200. [DOI] [PubMed] [Google Scholar]
- 50.Lis A, Peinelt C, Beck A, Parvez S, Monteilh-Zoller M, Fleig A, et al. CRACM1, CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties. Curr Biol. 2007;17:794–800. doi: 10.1016/j.cub.2007.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]