Abstract
The the influence of temperature on taste cues and the ability to discriminate and learn about different temperatures of foods are important factors regulating ingestion. The goal of this research was to demonstrate that thermal orosensory input can serve as a salient stimulus to guide ingestive behavior in the rat, and also that it interacts with gustatory input during choice and conditioned aversion experiments. A novel apparatus with Peltier refrigerators was used to control the temperature of solutions in 10-minute, 2-bottle tests. It was determined that naive rats preferred cold water (10°C) to warm water (40°). When cold water was paired with a toxic LiCl injection, rats avoided cold water and drank warm water, thus demonstrating that cold water could serve as the conditioned stimulus in a conditioned temperature aversion. Rats conditioned against cold water could discriminate 10° C water from 16° C water, but not from 13° C water, thus showing an ability to discriminate orosensory thermal cues to within 3–6° C. Rats also generalized conditioned aversions from cold water to cold saccharin and cold sucrose solutions. However, if rats were conditioned against a compound taste and thermal stimulus (10° C, 0.125% saccharin), the rats could distinguish and avoid each component individually, i.e., by avoiding cold water or warm saccharin. Finally, daily 2-bottle extinction tests were used to assess the strength of aversions conditioned against a taste cue (0.25 M sucrose), a thermal cue (10°C water), or the combination. Aversions to taste or temperature alone persisted for 7–14 days of extinction testing, but the combined taste-temperature aversion was stronger and did not extinguish after 20 days of extinction testing. These results demonstrate that temperature can serve as a salient cue in conditioned aversions that affect ingestion independent of taste cues or by potentiating taste cues.
Keywords: conditioned taste aversion, conditioned temperature aversion, lithium, sucrose, saccharin
Introduction
The influence of temperature on taste cues and the ability to discriminate and learn about different temperatures of foods are important factors regulating ingestion. While there is electrophysiological evidence that supports an interaction between taste and temperature, there have been few behavioral studies exploring the interaction. The goal of this research was to demonstrate that thermal orosensory input can serve as a salient stimulus to guide ingestive behavior, and also that it interacts with gustatory input during choice and conditioned aversion experiments.
There is electrophysiological evidence supporting a gustatory-temperature interaction at the peripheral level of the tongue in the taste bud [1], in the chorda tympani [2] [3], the geniculate ganglion [4, 5], and in the somatosensory lingual branch of the trigeminal nerve [6].
Human psychophysical work further supports an interaction of taste and temperature, such that the detection threshold or perceived intensity of tastants varies with the temperature of the taste solution [7–9]. Conversely, warming or cooling discrete patches of the tongue with a Peltier thermode can generate perceptions of taste qualities [10].
Despite the rat electrophysiological data and human psychophysical studies, there is little behavioral evidence of taste and temperature interactions in the rat. Some studies have determined the rat's preference and avoidance of water at different temperatures but with little or no gustatory properties. For example, Gold and Prowse [11] showed that rats in short-term 2-bottle tests initially drank more cold water than warm water, but showed a shift in preference to warm water after 6–8 min. Carlisle and Laudenslager [12] more broadly manipulated water temperature during short-term 1-bottle tests, and found that rats most prefer water at room temperature or slightly higher (up to 30°C) over water temperatures that fall above or below this range. The shape of the preference curves was asymmetrical for cold and warm water: intake gradually decreased as the temperature of the water decreased from room temperature, but intake sharply declined as the water temperature increased above room temperature. These studies demonstrated that rats can discriminate between different temperatures of water, and that rats have a range of preferences for water at different temperatures.
Conditioned Temperature Aversion
In addition to expressing unconditioned preference for water at different temperatures, rats can be given conditioned aversions to orosensory cues. Conditioned taste aversion is a particularly robust form of associative learning which is dependent on gustatory cues. When a animal is given access to a novel tastant (the conditioned stimulus, or CS) that is paired with an toxic agent (the unconditioned stimulus, or US), the animal will subsequently avoid that tastant. A similar avoidance response to a thermal orosensory cue can be conditioned, e.g. by pairing warm or cold water with toxic LiCl injection [13, 14]. Rats can also acquire conditioned aversions to capsaicin solutions [15]; because capsaicin is an agonist of the heat-sensitive TRPV1 channel on trigeminal fibers, a conditioned aversion to capsaicin may be functionally equivalent to a conditioned temperature aversion.
Conditioned aversions can also be used to establish other parameters of sensory detection. Discrimination can be more effectively measured when a specific cue has been paired with toxic effects (e.g., a LiCl injection). When given a 2-bottle choice between the conditioned thermal cue and a cue of a different temperature, rats would be more motivated to avoid the conditioned cue and consume the “safe” cue, thus demonstrating discrimination. A lack of avoidance under these conditions would more precisely define an inability to discriminate [16, 17].
Conversely, generalization behavior can also be shown with a conditioned aversion paradigm. When a taste stimulus is paired with a LiCl injection, the rat demonstrates a generalized aversion to other mixtures containing the conditioned taste stimulus [18]. If a compound CS is used (e.g. a mixture of thermal and gustatory cues), then relative saliency of the individual cues can be determined by measuring generalization to solutions containing the components in isolation [19].
Finally, the relative strength of a conditioned aversion can be measured by quantifying the rate of extinction of the aversion. In general, the stronger the conditioned aversion, the slower the rate of extinction. Additive interactions between conditioned stimuli can be assessed in this way. There is evidence that shows one form of orosensory cue can enhance the expression of an aversion to another sensory cue. This has been well studied with gustatory and olfactory cues in the rat [20, 21].
Thermal Stimulus Control
An important limitation of these earlier behavioral studies was the method of stimulus control. In the electrophysiological literature, the delivery of a thermal stimulus was brief in duration, and the control of temperature was usually maintained by either a bath set to a certain temperature or by Peltier units that electrically produce a desired temperature. In a behavioral paradigm with ad libitum drinking, a constant thermal stimulus is not so easily obtained within the constraints of the test chamber. In earlier reports, water temperature has been handled rather crudely, e.g. with water baths to heat or cool the water more or less distal from the point of delivery to the rat. In addition, earlier behavioral studies usually presented only one temperature during a 1-bottle test, so that comparisons were made across test sessions to determine preference (e.g., [11, 12]). Simultaneous access to two bottles at different temperatures would be more accurate for calculating short-term temperature preferences.
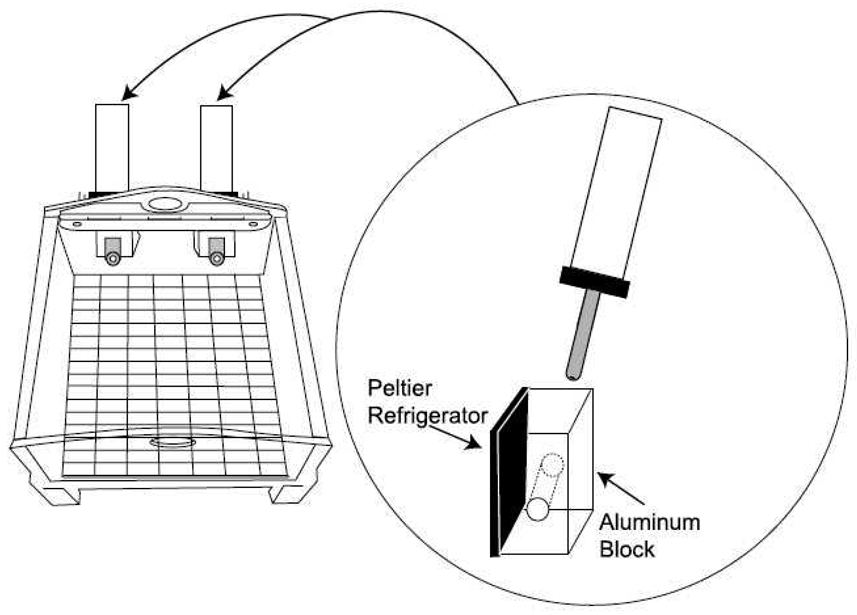
To address these issues, we have developed a novel apparatus that allowed precise control of fluid temperature (see General Methods, Figure 1). Peltier refrigerators were mounted directly to aluminum blocks that enclosed the stainless steel lick spouts of the water bottles. The temperature of the fluid could be adjusted to within 1°C of a desired set point immediately prior to access by the rat. When the level of current was held constant, the temperature of a fluid was also held constant without continual manipulations to a warm or cold bath. Furthermore, the apparatus could independently warm or cool 2 lick spouts simultaneously, thus allowing immediate comparison by the rat.
Figure 1.
Schematic of the temperature apparatus. Electrical current is sent from the controller to Peltier refrigerators, which convert electrical energy to thermal energy. For each bottle, a Peltier refrigerator chills or heats an aluminum block, which conducts heat to or from a sipper tube which passes through the block.
The goal of the present study was to measure the rat's response to water at different temperatures, and to measure the interaction of gustatory and thermal orosensory stimuli in short-term intake tests. In particular, we conducted 6 experiments to examine:
the unconditioned preference for cold vs. warm water;
acquisition and expression of a conditioned aversion to water using a thermal cue as the conditioned stimulus paired with toxic LiCl injection;
discrimination of water at different temperatures after acquisition of a conditioned aversion to cold water;
generalization of a conditioned temperature aversion against cold water to other cold solutions in the presence of normally-prefered tastants;
the relative salience of thermal and gustatory cues when rats were conditioned against a compound conditioned stimulus of a cold solution of saccharin;
the relative strength of conditioned temperature aversions and conditioned taste aversion, and the combination, as measured during 2-bottle extinction tests.
Methods
Subjects
All subjects were naïve, male Sprague-Dawley rats (300–400 g) that were maintained on a 12:12 light dark cycle, with the lights on at 0700 h. Rats were individually housed in Plexiglas shoebox cages and were given ad libitum access to Purina standard rodent chow and distilled water. Ambient temperature of the room was approximately 25°C.
Apparatus
The apparatus that was used as the testing chamber for all of these experiments was a Plexiglas chamber with dimensions of 60 cm (length) by 60 cm (width) by 60 cm (height). The bottom of the chamber was a wire-meshed floor (1 cm2 grid). To one side of the cage, there were two small openings where two fluid bottles could be placed onto the cage.. Stainless steel shutters controlled access to the sipper tubes of the bottles. In addition to passing into the Plexiglas cage, the sipper tubes also passed snugly through aluminum blocks. Both blocks were attached to individual Peltier units, which were controlled by a connection to a central processor that determined the polarity (making the temperature either hot or cold) and magnitude of the current (changing the temperature by × degrees). The temperature of the aluminum blocks, sipper tubes, and the fluid in the tubes could be raised to a maximum 50°C or lowered to a minimum 5°C by means of adjusting this controller, which could be set in increments of 0.1°C. The temperature of water was calibrated by using a temperature probe that was placed approximately 5 cm from the opening of each sipper tube. From such a calibration, the time required to go from room temperature to either extreme required at least one minute, and a temperature change from one extreme to the other required approximately two minutes. During these calibrations, the differences between the temperature probe and the controller reading never exceeded 0.1°C. A schematic of this apparatus can be seen in Figure 1.
Water Training
Rats in each experiment were water deprived for 24 h that started at approximately 0900 h (i.e., 2 h into the light cycle). After this initial deprivation period, all subjects received six days of water training. Each day of water training consisted of 10-min access to water in the testing apparatus, during which the temperature of the water was maintained at 25° C by the Peltier units. During each session, water was made available in one of the two tubes. The position of available water was alternated on each consecutive training day. After each daily training session, rats were returned to their home cages. Rats were then given a 1-h water supplement at approximately 1600 h. Thus, rats were deprived of water for 23-h each day, with an additional 10-min access to test stimuli. After this period of water training, rats were either used as subjects in a preference testing experiment (Experiment 1) or in a conditioned aversion experiment (Experiments 2–6). In conditioned aversion experiments, rats were divided into groups with equivalent mean water intakes on the last two days of the training period.
Conditioned Aversion Testing
Prior to conditioning, rats were divided into two groups with equivalent mean water intake for the last two days of the training period. Rats were given 10-min access to a conditioned stimulus (CS) which varied with the experiment. Five minutes after the CS access, rats were given an intraperitoneal injection (5 ml/kg) of either LiCl (0.6 M), acting as the unconditioned stimulus (US), or saline (0.15 M) as a control. This procedure was conducted in all conditioning experiments except where noted.
Preference and Extinction Testing
The post-conditioning period of each experiment consisted of daily 10-min, two-choice intake tests between two fluid sources: the CS and an alternative fluid for comparison. The positions of the two bottles on the cage were alternated on subsequent testing days. To measure the strength of aversions post-conditioning, rats were given daily 10-min 2-bottle preference tests between the CS and an alternative fluid source. Preference was calculated as the proportion of CS intake out of total fluid intake. Total fluid intakes (from both bottles) were also measured to show that LiCl-injected rats suppressed only their CS intake rather than their total water intake.
Experiment 1: Short-term preference for water at different temperatures
Previous studies [11, 12] have shown a change in water intake by rats in a 1-bottle test when the water temperature varied from room temperature. However, it is unknown as to whether rats can show a preference between two water sources at different temperatures in a 2- bottle test. Thus, 2-choice tests between different temperatures were studied in this experiment by giving pairwise combinations of warm water (40°C), cold water (10°C), and room temperature water (25°C). Since the discrimination of temperature in the oral cavity has not been determined for the rat, the most conservative way to test for short-term preference was to use thermal cues that were relatively far apart from each other. By doing so, this experiment had two purposes. First, it was important to show that rats show differences in water intake in a 2-bottle test when the contents of the two bottles were different in temperature. The second purpose of this experiment was to determine a water temperature that was preferred in a 2-bottle test for use as a novel and preferred thermal CS in subsequent conditioned aversion experiments.
Twenty rats were trained with water as above. After the water training period, rats were given 10-min access to two bottles containing room temperature (25°C) vs. warm (40°C) water. After this preference test, each rat was returned to its home cage. This preference test was repeated for two additional sessions, during which the positions of the two temperature-controlled water sources were alternated on each day. This 3-day preference testing sequence was repeated for two more conditions: room temperature (25°C) water vs. cold (10°C) water; and cold (10°C) water vs. warm (40°C) water.
Experiment 1 Results
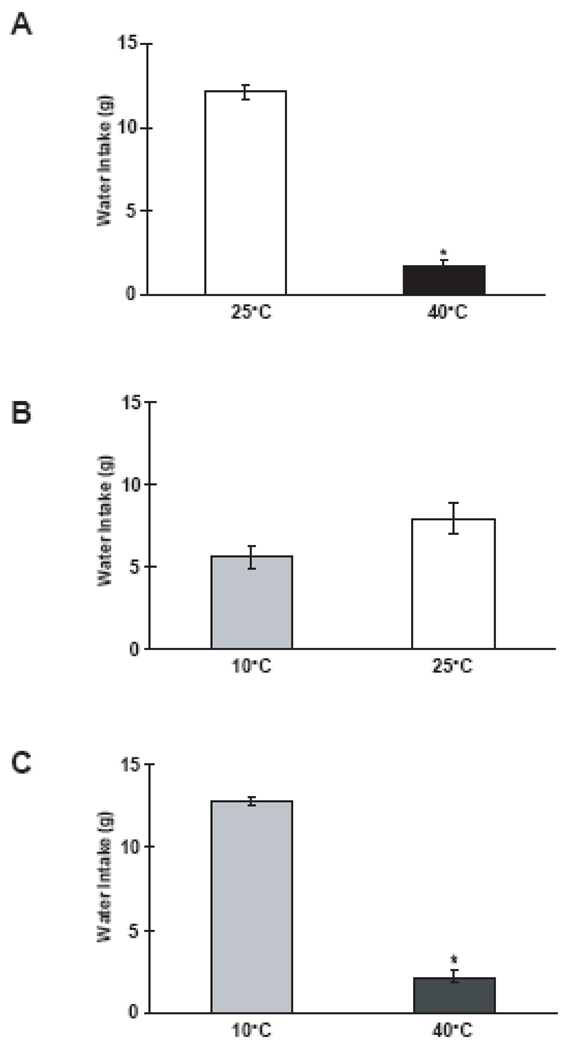
For each preference sequence, a one-way ANOVA revealed that intakes did not vary across the three days of testing (p's > 0.05), so intake for each of these temperatures was averaged (see Figure 2). A comparison by a matched t-test for dependent samples between the mean intake of room temperature (25°C) water vs. warm 40°C water showed that rats significantly preferred room temperature water over warm water (see Figure 2A; t = −22.57, df = 12, p < 0.001), showed no significant preference for cold water over room temperature water (Figure 2B) , and showed a significant preference for cold water over warm water (see Figure 2C), t=22.76, df=12, p < 0.001. On the basis of these preference sequences, the data show that rats can discriminate warm water from room temperature or cold water, and that rats prefer cold or room temperature water to warm water in two-choice tests.
Figure 2.
A. Mean (± SE) intake during 10-min 2-bottle test of (A) room temperature (25 °C) water vs. warm (40 °C) water; (B) room temperature water vs. cold (10 °C) water; and (C) cold water vs. warm water. *p < 0.05 vs. 40 °C water.
Experiment 1 Discussion
By comparing intake levels of water at different temperatures, preference for water at different temperatures has been shown. This experiment demonstrated that rats can discriminate between between 25° and 40°C water, but did not determine if rats can discriminate between 10°C and 25°C water, as the rats did not show a preference between these two temperatures. This issue was addressed in a subsequent experiment. Because rats clearly preferred water at 10°C over water at 40°C, cold water may be used as a novel yet preferred thermal cue in the acquisition of a conditioned temperature aversion.
Experiment 2: Expression of a Conditioned Temperature Aversion
The goal of Experiment 2 was to determine whether rats could be conditioned to avoid a thermal cue that is normally preferred. Since Experiment 1 showed that rats preferred cold water (10°C) over warm water (40° C), cold water was used as the CS in a conditioned aversion protocol. This experiment provided a replication of Nachman's work [14], but using a more effective cold stimulus than the hot stimulus (45°C) used by Nachman to illustrate conditioned temperature aversions.
Sixteen rats underwent water training as above. Rats were divided into two groups (a saline-injected group and a LiCl-injected group, n=8 per group), then given 10-min access to water at 10°C (CS) followed by an intraperitoneal injection of either saline or LiCl (US).
To measure the strength of the conditioned temperature aversion, rats were given daily 10-min 2-bottle preference tests between cold water (10° C) and warm water (40° C) for 11 days post-conditioning.
Experiment 2 Results
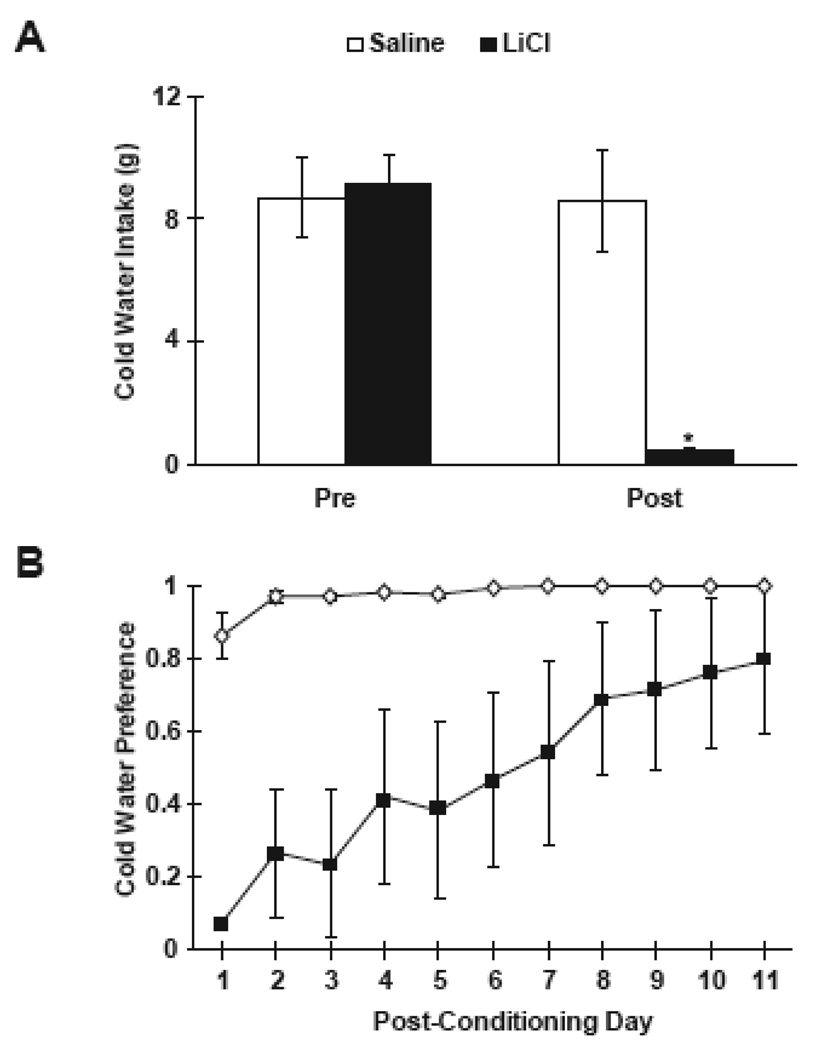
A t-test for independent samples revealed that there was no significant difference in the CS intake of cold water between the saline- and LiCl-injected rats (p > 0.05) on conditioning day. To determine whether there was a reduction of intake after conditioning, a 2×2 factorial ANOVA for repeated measures was conducted on conditioning day and the first day of post-conditioning (see Figure 3A). There were significant main effects for both injection group (F (1,14) = 12.58, p < 0.01), and day, (F (1,14) = 117.87, p < 0.0001), and there was a significant Group × Day interaction, F (1,14) = 118.55, p < 0.0001). Tukey's Honest Significant Difference (HSD) revealed that saline-injected controls did not significantly reduce their intake of the cold water from conditioning day to the first day of post-conditioning testing. However, the LiCl-injected rats significantly avoided the cold water on the first day of post-conditioning testing (p < 0.01).
Figure 3.
A. Mean (± SE) intake of cold water during 10-min access prior to saline (white bars) or LiCl (black bars) injection, and on the first day of post-conditioning access. * p < 0.05 vs. saline controls. B. Mean (± SE) preference scores for cold water vs. warm water between saline-injected (white diamonds) and LiCl-injected (black squares) rats across the post-conditioning period. Preference scores were calculated by dividing the intake of cold water by the total water intake.
Based on preference scores for the cold water CS, saline-injected rats showed a high preference for the cold water CS across all 11 post-conditioning tests (see Figure 3B) while LiCl-injected rats initially showed a clear aversion to the cold water CS. The aversion shown by LiCl-injected rats gradually extinguished across this post-conditioning period. A 2 × 11 (Group by Days) factorial ANOVA for repeated measures on the preferences scores showed significant main effects for both injection group (F (1,14) = 11.98, p < 0.01 ) and days (F (10,140) = 6.91, p < 0.01) and a significant Group × Day interaction (F (10,140) = 4.11, p < 0.01). Tukey's HSD test revealed that LiCl-injected rats demonstrated a significantly lower preference for the cold water CS compared to saline-injected controls for the first 8 days of post-conditioning (p's < 0.05).
In order to show that the avoidance of cold water by LiCl-injected rats was specific to the CS and not reflective of non-specific decrease in water intake, total intakes were compared across the post-conditioning period by using a 2 × 11 factorial ANOVA (Group by Days) for repeated measures. There were neither significant main effects nor a significant interaction (p's > 0.10; data not shown).
Experiment 2 Discussion
This result showed that rats could avoid a thermal stimulus after it was paired with a toxic injection of LiCl. Distilled, deionized water has minimal gustatory and olfactory cues, and a change in temperature of the water should not alter these cues. Thus, it is assumed that the conditioned aversion was due to an association between LiCl and the thermal properties of the CS. This experiment supports earlier work by Nachman, who showed a similar aversion in rats conditioned to avoid warm water paired with LiCl [14]. In Nachman's experiment, however, it was not clear whether the 45°C water was itself partially aversive to these rats, even prior to conditioning. These results are also similar to the work of Sako et al. [13], who produced conditioned aversions to cold (5° C) or warm (40°) water. In the present study, LiCl-injected animals reduced intake of a preferred cold water CS. Moreover, these animals showed a significant aversion to the cold water that persisted across 8 days of post-conditioning extinction tests, which suggests that this aversion to a thermal cue was relatively strong. This strength of a thermal aversion relative to a taste aversion was addressed in further experiments below.
Experiment 3: Discrimination between Thermal Stimuli
Experiment 2 showed that rats could discriminate cold (10°C) water from warm (40°C) water when the former was paired with a LiCl injection. To determine a least difference in temperature of drinking water that rats could discriminate, experiment 3 employed a conditioned temperature aversion followed by preference testing between the thermal CS and water at variable temperatures. Preference for an alternative water temperature over the cold water CS would imply the ability to discriminate between the two temperatures; equal intake of the cold water CS and the alternative water would show an inability to distinguish the two temperatures.
Forty rats underwent water training as above. Rats were assigned to one of four groups (two saline-injected control groups and two LiCl-injected groups, n=10 per group). Because the rats in this experiment were to be tested for several days after conditioning, conditioning was strengthened by giving two CS-US pairings. On the two consecutive conditioning days, each rat was given 10 minutes access to 10°C water (CS), followed by either a saline injection or a LiCl injection (US).
Post-conditioning testing consisted of daily 10-min, 2-bottle preference tests between the 10°C water CS and water at five different temperatures in either an descending or ascending sequence. One saline- and LiCl-injected group (each n =10) were given a descending temperature sequence, and the other saline and LiCl groups were given an ascending temperature sequence (see Table 1).
Table 1.
Order of presentation of water of different temperatures in Experiment 3; Rats were conditioned against 10 °C water, and on all days the 10° C water was presented in the second bottle.
| Day | Ascending Group |
Descending Group |
|---|---|---|
| 1 | 25° C | 13° C |
| 2 | 22 °C | 16° C |
| 3 | 19 ° C | 19 ° C |
| 4 | 16° C | 22 °C |
| 5 | 13° C | 25° C |
Experiment 3 Results
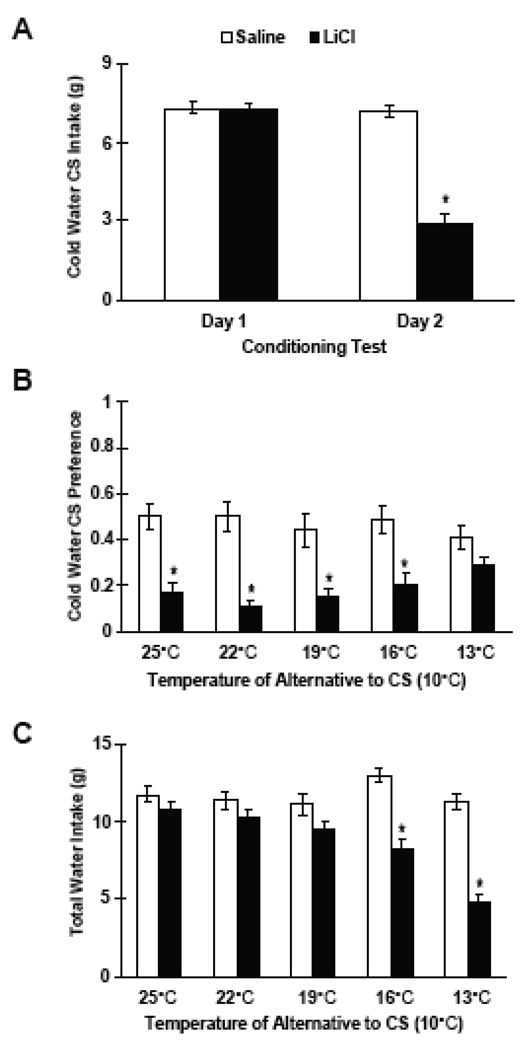
A 2 × 2 (Group × Day) factorial ANOVA for repeated measures was used to compare intakes of the 10°C water CS consumed during the two conditioning days (see Figure 4). There was a significant Group by Day interaction, (F (1,38) = 60.36, p < 0.001). Tukey's HSD test showed that LiCl-injected rats consumed significantly less of the 10°C water CS on the second conditioning day (p < 0.001; Figure 4A).
Figure 4.
A. Mean (± SE) intake of cold water (CS) of saline- (white bars) and LiCl-injected (black bars) rats across the two days of conditioning. * p < 0.05 vs. saline controls. B. Mean preference scores for cold (10°C) vs warmer (25° – 13° C) water in 10-min 2-bottle tests of saline- and LiCl-injected rats, in which the alternative water temperature differed from the CS at increments ranging from +15° C to +3° C. C. Mean total water intakes of saline- and LiCl-injected rats across the post-conditioning period. * p < 0.05 vs. saline controls
CS preference scores and total intakes of all four groups were compared across the post-conditioning days. Because a Group by Sequence by Day factorial ANOVA showed no main effect between ascending and descending sequences (and no interaction of sequence and group or day), rats were combined into a saline-injected group (n=20) and a LiCl-injected group (n=20). A 2 × 5 (Group by Day) factorial ANOVA for repeated measures revealed a significant Group by Day interaction, F (4,152) = 2.76, p < 0.04. Saline-injected rats showed no preference between 10°C water and warmer water (13°C to 25°C). Tukey's HSD test showed that LiCl-injected controls had significantly lower preference scores for the cold water CS than saline-injected rats across all temperatures except for the comparison between 10°C water and 13°C water (p's < 0.05; Figure 4B).
Total water intakes during the 2-bottle tests were also compared between saline- and LiCl-injected rats. A 2 × 5 (Group by Day) factorial ANOVA for repeated measures showed a significant Group × Day interaction, F(4,144) = 15.70, p<0.0001. Post hoc assessment using Tukey’s HSD revealed that LiCl-injected rats consumed significantly less overall water than the saline-injected rats when the alternative water temperature was at either 16°C or 13°C (p's < 0.05; Figure 4C).
Experiment 3 Discussion
The results suggest that rats were able to discriminate between different water temperatures in a 2-bottle choice test. LiCl-injected rats were able to show a significant aversion (decreased preference) to the 10°C water CS vs. an alternative water temperature with a difference in temperature as low as 6°C. Because LiCl-injected rats did not show a reduced preference for 10°C water vs. 13° C water, rats may not be able to discriminate a 3° C temperature difference. Because the LiCl-injected rats reduced intake for 16 °C water and 13° C water, rats apparently generalize from the 10°C water CS across a 3–6° C temperature difference. Thus the range of temperature discrimination for an oral water stimulus appears to be between 3 and 6° C. Only 3°C differences were tested in this experiment, and rats may be able to discriminate smaller differences temperature (e.g., as with their snout [22]).
Experiment 4: Generalization of an Taste-Temperature Aversion to a Thermal Stimulus
The first three experiments employed hot or cold water in the absence of strong gustatory stimuli. Experiment 4 tested whether an aversion to a thermal stimulus can be generalized to a solution having distinctive taste properties as well. In particular, a normally-prefered substance (e.g., sucrose or saccharin) was added to the water, which would be predicted to increases the rat's preference for the solution, and perhaps override a conditioned temperature aversion. Rats were conditioned against cold water (10°C) paired with LiCl, and then subsequently tested in 2-bottle preference tests with the addition of sucrose or saccharin to the original thermal CS, vs. a hot water alternative (40°C).
Sixteen rats underwent water training as above. Rats were divided into two groups (one saline-injected group and one LiCl-injected group, n=8 per group) and then given 10-min access to water at 10°C, followed by either a saline or LiCl injection. Four daily, 10-min post-conditioning tests were conducted, during which all rats received access to one bottle with a particular solution at 10°C (water, 0.125% saccharin, or 0.25 M sucrose), and an alternative bottle of 40°C water (see Table 2).
Table 2.
Solutions used in post-conditioning 2-bottle tests in Experiment 4. Rats were conditioned against 10° C water.
| Day | Bottle 1 | Bottle 2 |
|---|---|---|
| 1 | 10° C, water | 40° C, water |
| 2 | 10° C, 0.125% saccharin | 40° C, water |
| 3 | 10° C, 0.25 M sucrose | 40° C, water |
| 4 | 10° C, water | 40° C, water |
Experiment 4 Results
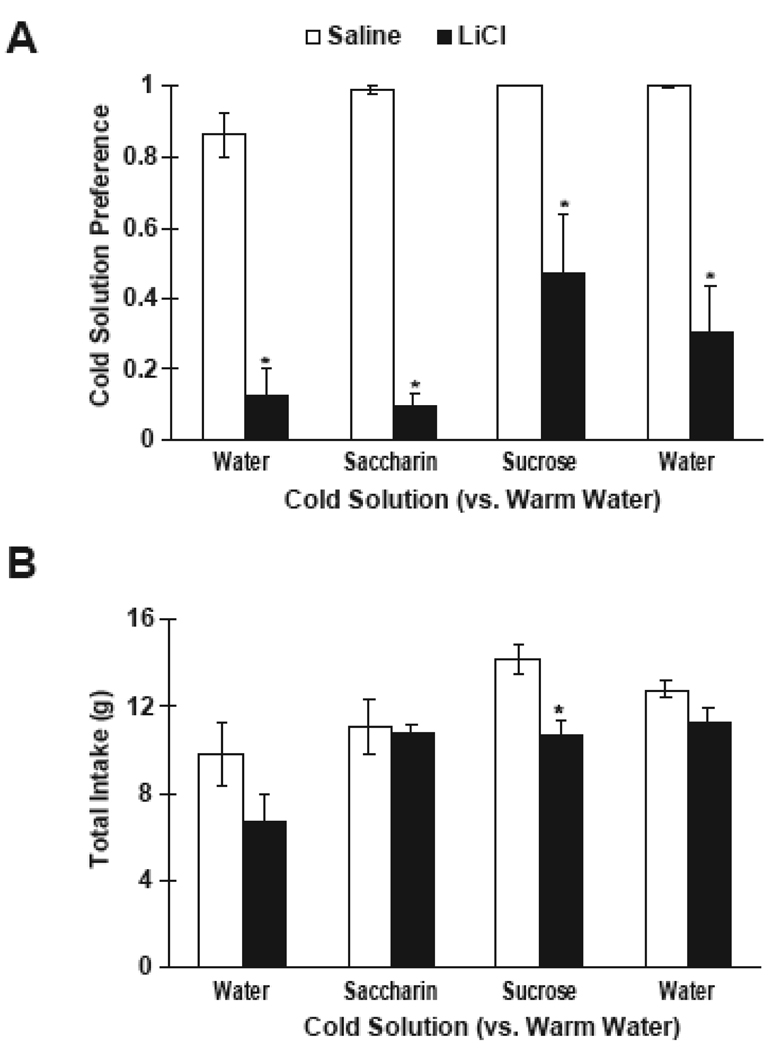
A t-test for independent samples showed that there were no differences between saline-and LiCl-injected groups in intake of the 10°C water on conditioning day. For each post-conditioning test day, a separate t-test for independent samples was used to compare preference scores for the cold solution vs. warm water between saline- and LiCl-injected rats (see Figure 5A). In all comparisons, saline-injected rats showed a large preference for the cold solution to the warm water. LiCl-injected rats showed a significantly lower preference for the cold solutions, even when saccharin or sucrose was present.
Figure 5.
A. Mean (± SE) preference scores for cold (10°) water, cold saccharin (0.125%) or cold sucrose (0.25M) vs. warm (40°) water in 10-min, 2-bottle tests after pairing cold water with saline- (white bars) or LiCl injection (black bars). B. Mean total intakes of saline- and LiCl-injected rats across the post-conditioning period. * p < 0.05 vs saline controls.
Independent t-tests were also used to compare total intakes between saline- and LiCl-injected rats across the post-conditioning tests. There were no significant differences in total intake, with the exception of the third post-conditioning test (cold sucrose vs. warm water) in which saline-injected rats consumed more total fluid (Figure 5B).
Experiment 4 Discussion
The results demonstrate that the addition of normally-prefered tastants was not sufficient to reverse a conditioned aversion to a cold thermal cue. These data suggest that an aversive thermal cue, in some cases, may override (or generalize to) a normally-prefered taste cue. However, in the case of 0.25M sucrose, LiCl-injected rats consumed equal amounts of the cold sucrose as warm water. This 50% preference for the CS may be considered to be a weak generalized aversion. The aversion to cold water may have only weakly generalized to cold sucrose, either because the sucrose solution was more prefered than the saccharin solution and water alone, or because the initial aversion was partially extinguished after 3 post-conditioning test days. Sako et al. [13] also found that aversions to warm or cold water did not generalize to tastant solutions of the same temperature, so the degree of generalization may depend on the exact concentrations and conditioning protocol used.
Thus, at least in some cases, a temperature aversion can generalize to taste mixtures. These results are similar to those of Smith and Theodore (1984), who showed generalized aversions to different conditioned tastants when in mixture with unconditioned tastants. In their experiment, it was shown that rats conditioned to avoid a NaCl, HCl, or sucrose CS would also avoid any mixture containing the CS. Moreover, the strength of the generalized aversion was proportional to concentration of the CS in the mixture. Although the present study did not look at the relative intensity of the thermal CS, it does provide evidence that thermal cues can drive generalization behavior.
Experiment 5: Relative Saliency of Gustatory and Temperature Cues
Normal ingestion usually entails exposure to foods with multimodal sensory characteristics, e.g. providing gustatory and somatosensory stimulation. For example, previous work [19] has shown that when a sucrose/corn oil emulsion was used as a multimodal CS, a series of post-conditioning tests showed aversions specific to the individual components of the emulsion (i.e., sucrose or corn oil). In particular, a more profound aversion to corn oil and little aversion to the sucrose was found after the rats were conditioned with a sucrose/corn oil emulsion, which suggested that corn oil was a more salient stimulus than the sucrose.
Although there are many possible reasons as to why the corn oil was the more salient substance from the original multimodal mixture (e.g., possible differences in gustatory, olfactory, or somatosensory input between corn oil and sucrose components), this experiment provides an excellent paradigm for comparing the relative saliencies of mixture components using conditioned aversions. Therefore, the present experiment used a cold saccharin solution as a multimodal CS (having both gustatory and thermal cues) in a conditioned aversion paradigm and assessed an animal’s tendency to avoid either or both sensory cues in subsequent post-conditioning tests.
Sixteen rats underwent water training as above. Before conditioning, rats were divided into two groups (one saline-injected group and one LiCl-injected group, n=8 per group) and then given 10-min access to 0.125% saccharin at 10°C, followed by either a saline or LiCl injection.
Rats then underwent daily, 10-min 2-bottle post-conditioning tests for 5 days, during which the gustatory and thermal properties of the original cold saccharin CS were present or absent (see Table 3). A temperature aversion was tested in the absence of taste cues (cold vs. warm water) or with taste cues constant in both bottles (cold saccharin vs. warm saccharin). A taste aversion was tested in the absence of a distinct temperature cue (room temperature saccharin vs. room temperature water), or with the temperature cues held constant in both bottles (cold saccharin vs. cold water; warm saccharin vs. warm water.) Together, this series of 2-bottle tests assessed the individual saliency of the gustatory and temperature components after conditioning against the mixed CS. Also, to test the relative saliency of each component of the original CS, the gustatory and temperature components were pitted against each other (day 5).
Table 3.
Solutions used in post-conditioning 2-bottle tests in Experiment 5. Rats were conditioned against 0.125% saccharin at 10°C.
| Day | Bottle 1 | Bottle 2 | Assessment |
|---|---|---|---|
| 1 | 10° C, saccharin | 40° C, water | verification of conditioned aversion |
| 2 | 10° C, water | 40° C, water | temperature aversion without taste cue |
| 3 | 10° C, saccharin | 40° C, saccharin | temperature aversion with taste constant |
| 4 | 25° C, saccharin | 25° C, water | taste aversion without temperature cue |
| 5 | 10° C, water | 40° C, saccharin | temperature cue vs. taste cue |
| 6 | 10° C, saccharin | 10° C, water | taste aversion with temperature constant |
| 7 | 40° C, saccharin | 40° C, saccharin | taste aversion with temperature constant |
Experiment 5 Results
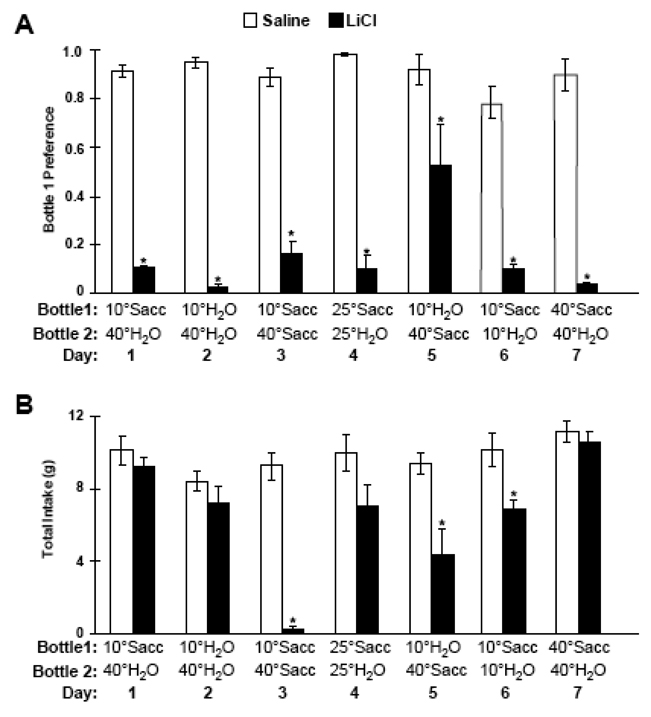
A t-test for independent samples showed that there were no significant differences in intake of the cold saccharin on conditioning day (p > 0.05). Across the post-conditioning test days, independent t-tests were used to compare preference scores between the saline- and LiCl-injected rats. Preference was calculated as the intake of the solution preferred by the saline-injected rats (bottle 1) over total intake from both bottles 1 and 2 in the 2-bottle test (Figure 6A).
Figure 6.
A. Mean (±SE) preference scores for Bottle 1 vs. Bottle 2 in 10-min, 2-bottle tests after pairing cold saccharin with saline (white bars) or LiCl injection (black bars). For each post-conditioning day, the contents and temperature of the bottles is shown below the x-axis. B. Mean total intakes of saline- and LiCl-injected rats across the post-conditioning period. * p < 0.05 vs saline controls.
Saline-injected rats (white bars in Figure 6A) showed a strong preference for cold solutions over warm solutions (days 1,2,3 and 5), and a strong preference for saccharin solution over plain water (days 4,6, and 7). Cold preference apparently predominated over saccharin preference, as saline-injected rats preferred 10° C water over 40° C saccharin (day 5).
LiCl-injected rats (black bars in Figure 6A) showed a strong aversion to cold saccharin vs. warm saccharin (day 3) and cold saccharin vs. cold water (day 6). Thus, the mixed CS (cold saccharin) was more salient than the individual components (cold alone or saccharin alone). The conditioned rats also showed a strong aversion to cold solutions vs. warm solutions (days 2 and 3), and an aversion to saccharin solutions at any temperature (days 4,6, and 7); thus, aversion to the mixed CS generalized to the individual components of the CS. Finally, when the rats were given the choice of cold water vs. warm saccharin, the LiCl-injected rats showed equal preference for each solution (day 5). This suggests that the temperature cue and the gustatory cue were of relatively equal salience.
Across the 7 different post-conditioning tests days, the LiCl-injected rats consumed significantly less total fluid than the saline-injected rats on days when both bottles contained at least one component of the original mixed CS of cold saccharin (Figure 6B). In other words, if both bottles were at 10° C, or both contained saccharin, or one bottle of each was presented, then LiCl-injected rats consumed less total fluid. This is consistent with generalization from the mixed CS to either temperature or taste qualities.
Experiment 5 Discussion
These results demonstrated that the gustatory and thermal components of a cold saccharin CS can be separated, and still serve as stimuli for generalization of a mixed temperature-taste aversion. Within the confines of individual 2-bottle tests, unconditioned rats showed a preference for cold solutions that predominated over saccharin solutions. Thus thermal cues may override gustatory cues even without aversive conditioning. Conditioned rats showed a greater aversion towards the original mixed CS vs. the individual components. Both gustatory and thermal cues appeared to be of relatively equal salience when tested individually, at least at the specific concentration and intensity used in this experiment.
This study did not determine, however, whether one sensory cue was more readily associated with the LiCl injection than the other sensory cue. It is possible that both cues together in a mixture may act as a relatively stronger CS than its individual components, but intakes from a 10-min post-conditioning test using a single mixed CS is not an effective way to measure such an interaction. The final experiment attempted to assess the relative saliency of a thermal-gustatory compound CS by comparing relative extinction rates across repeated 2-bottle tests.
Experiment 6: Relative Strength of Thermal and Gustatory Aversions
It has been demonstrated that both gustatory and thermal cues are sufficient conditioned stimuli for aversive conditioning, but the relative saliency of taste and temperature as conditioned stimuli has not been determined. To address taste-temperature interactions, this experiment compared the relative strength of aversions to a gustatory cue alone, a thermal cue alone, or the combination. The strength of the aversion was measured from the relative times of extinction for each aversion across daily 10-min, 2-bottle preference tests.
Forty rats underwent water training as above. After the water training period, rats were divided into four groups (a saline-injected control group and 3 LiCl-injected experimental groups, n=10 per group). On conditioning day, three groups were given 10-min access to a different conditioned stimulus that contained either 1) a distinctive thermal property (distilled water at 10°C; group "T"); 2) a distinctive gustatory property (0.25 M sucrose at room temperature; group "G"); or 3) or both properties (0.25 M sucrose at 10°C; group "T+G"). The conditioned stimulus was followed by a LiCl injection. The control group was given 10-min access to a 0.25 M sucrose at 10°C followed by a saline injection. Sucrose was used as the gustatory stimulus in this case in order to reduce the variability of intake between rats as well as across the days of post-conditioning [19, 23].
Beginning the day after conditioning, rats were tested in daily 10-min 2-bottle preference tests for 20 consecutive days. In the 2-bottle test, rats in each group were given 10-min access to their respective CS vs. warm water at 40°C. The positions of the two bottles were alternated on each test day.
Experiment 6 Results
A one-way ANOVA revealed that there were no significant differences in the intake of the CS between any of the groups on conditioning day.
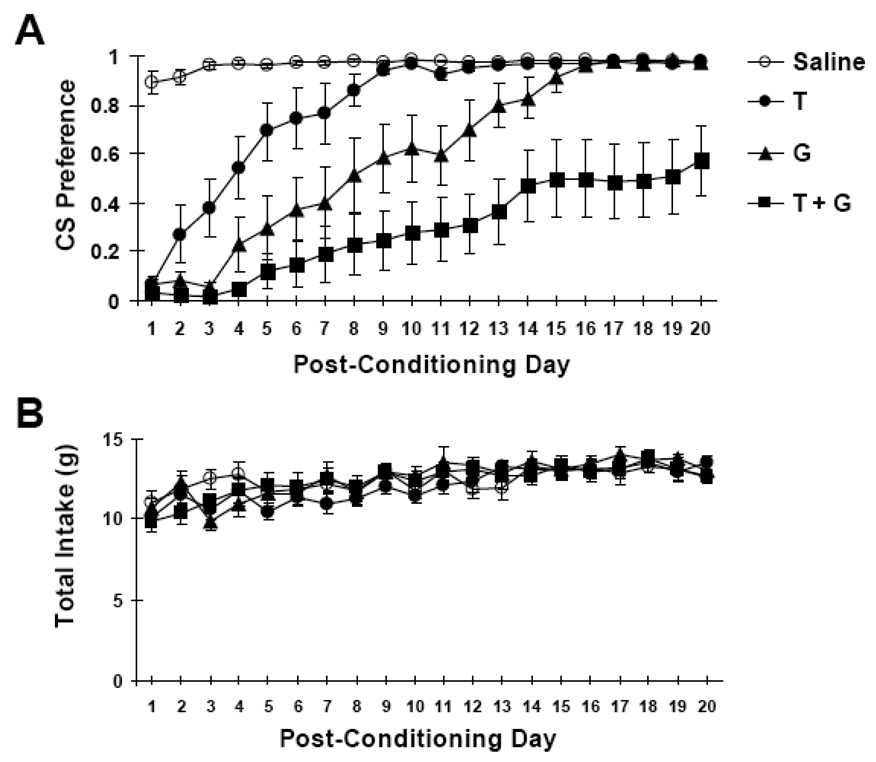
A one-way ANOVA across the four groups on the preference scores of the first post-conditioning day revealed a significant main effect (F (3,36) = 175.19, p < 0.0001). All LiCl-injected groups showed a profound aversion to their respective CS on the first post-conditioning day (Figure 7A). While the control group displayed a high preference for the cold sucrose, the LiCl-injected groups displayed preferences scores that barely exceeded 0.
Figure 7.
A. Mean (± SE) preference scores for CS vs. warm water in 10-min, 2-bottle tests after 4 treatments: 10°C sucrose paired with saline injection (white circles); 10°C water paired with LiCl injection ("T", black circles); 25°C sucrose paired with LiCl ("G", black triangles); and 10°C sucrose paired with LiCl ("T+G", black squares). B. Mean total intake of all groups across the post-conditioning period.
Across the 20 days of post-conditioning tests, a 4 × 20 factorial ANOVA (4 groups by 20 days) for repeated measures was conducted on preferences scores for the respective CS’s. Significant main effects for both group and days were found (F (3,36) = 23.75, p < 0.0001 and F (19,684) = 41.01, p < 0.0001, respectively). A significant drug × day interaction was also found (F (57,684) = 5.88, p < 0.001). Tukey's HSD was used for post hoc analysis. A group was considered to have extinguished their aversion when their mean preference for the CS was no longer statistically different from the preference of the saline-injected controls. By this definition of extinction, the aversion of group "T" rats for cold water extinguished on day 7; the aversion of group "G" rats for room temperature sucrose extinguished on day 14. The aversion of group "T+G" for cold sucrose did not extinguish by day 20, the last day of testing, when their mean preference for cold sucrose was still significantly lower than that of the saline-injected controls.
All groups had similar total intakes from both bottles across the entire post-conditioning period (Figure 7B). The total intakes across the post-conditioning periods were compared by using a 4 × 20 factorial ANOVA (Group by Days) for repeated measures. There was no significant main effect of Group (F (3,36) = 0.73, p > 0.50), but there was a significant main effect across Days (F (57,684) = 9.68, p < 0.01) such that total fluid intake increased gradually during the 20-day post-conditioning period.
Experiment 6 Discussion
Each LiCl-injected group showed profound aversions to its respective CS on the first day of post-conditioning. However, there were differences in the relative times of extinction between the LiCl-injected groups in the post-conditioning period. The data suggest that a thermal stimulus, although an effective stimulus in a conditioned aversion paradigm, does not produce as long-lasting an association with LiCl as a gustatory stimulus. However, the presence of both thermal and gustatory cues simultaneously produced an even more persistent aversion that took a longer time to extinguish. This enhanced aversion provides evidence for an interaction of temperature and taste observable at the behavioral level, resulting in apparent summation of sensory cues in conditioned aversion acquisition and expression.
There is a similar literature on the potentiation of conditioned aversions combining taste and smell as stimuli. In this literature, the addition of taste in the conditioning procedure enhances the aversive behavior to an odor that normally is not sufficient for acquiring a conditioned aversion (e.g. [20]). And in some cases, the presence of a pre-conditioned odor enhances the acquisition of a taste aversion (e.g. [21]). With regards to taste and temperature, it is possible that a.) the presence of a taste cue may potentiate a thermal aversion, or b.) the presence of a thermal cue may potentiate a taste aversion. Future studies using strong thermal cues with weak gustatory cues and vice versa will be required to determine the quantitative values for such studies.
General Discussion
Our findings provides evidence to support an influential role of thermal orosensory stimulation in short-term feeding behavior. The present series of experiments showed that the presence of distinct thermal cues in drinking water or tastant solutions can elicit general preferences and serve as the CS in conditioned aversions. Furthermore, rats have the ability to discriminate and generalize across temperatures with some precision, i.e. within 3–6° C. After an aversion was conditioned, rats were also able to avoid the temperature and taste cues of a compound CS both together and in isolation, suggesting that rats can respond distinctly to each sensory quality. Although both qualities were salient, the combination of taste and temperature cues potentiated the acquisition of a conditioned aversion, leading to a more persistent aversion than was acquired to either cue alone.
Previous work by others has demonstrated preferences by rats for different temperatures of drinking water[11, 12, 14], and that rats can acquire conditioned aversions to water of a specific temperature[13, 14]. The current results expand on this prior work by using an apparatus with more accurate temperature control, so that limits of thermal discrimination were established. Furthermore, by testing compound mixtures of water with thermal and taste cues, the relative saliency of temperature and gustatory inputs was assessed.
The experiments in the current study demonstrate an interaction of orosensory taste and temperature stimuli that is observable at the behavioral level, and specifically in unconditioned preference and conditioned aversion behavior during ingestion. Only a limited set of temperatures and only water or sweet tastants were presented, so the interactions of temperature and other taste qualities remain to be explored. Because the stimuli were presented to the rats via sipper tube, the sites and physiological mechanisms of gustatory and thermal interaction could not be specified. However, there is significant evidence that temperature and taste are integrated both in the periphery and within central relays of orosensation.
Peripheral Interactions
The effects of temperature on taste have been shown at the level of gustatory nerve fibers that innervate the tongue (and presumably, taste receptor cells) of the rat. The earliest work to show this was conducted by Fishman [2], who showed in rats that low temperature water alone (3–5°C) evoked an initial response in the chorda tympani similar to a 0.1 M salt solution at room temperature, but this response gradually decreased as the temperature of water was raised. He also demonstrated that chorda tympani responses to various concentrations of cold salt solutions gradually increased as the temperature was raised, with maximal sensitivity occurring around room temperature (25°C).
Similar modulation by temperature changes of gustatory responses from the chorda tympani have been shown in the rat [3] and cat [24, 25]. In these studies, it was shown that maximum responses to different tastants were found at 30°C, and that deviations from this temperature resulted in decreased responses to the tastants, independent of preadaptation to the thermal input. The substrate for these temperature effects may be within the taste receptor cells themselves. Taste buds express high levels of the temperature-sensitive cation channel TRPM5 which is required for temperature-dependent changes in gustatory nerve responses to tastants [1].
There has also been evidence to support these temperature effects on peripheral taste responses at the level of the geniculate ganglion [4, 5] which is broadly consistent with previous work using single- and whole- nerve recordings of the chorda tympani (for review, see [26]). Furthermore, it was shown that the only cell type in the geniculate ganglion that that specifically responded to discrete changes in temperature were those that also responded maximally to HCl (sour, or "acid-best" cells). Decreasing the temperature of a stimulus reduced sensitivity in all neuron types except NaCl-specialist neurons, which maintained a constant response rate. Thus, at the level of the chorda tympani or geniculate ganglion, the primary consequence of temperature sensitivity is to modulate responses to taste qualities, rather than to allow discrimination among thermal stimuli per se.
Lundy and Contreras [6] also provided a unique perspective on the relationship between taste and temperature input by examining responses of the rat lingual nerve to thermal and gustatory stimuli. Although the lingual nerve is a branch of the somatosensory trigeminal nerve, they found individual nerve fibers that responded to a thermal stimulus also had some sensitivity to certain tastants, such that dilute solutions of quinine, citric acid, and NaCl inhibited responses to warm thermal stimuli. Thus, in reversal of the chorda tympani, gustatory stimulation modulates the temperature responses of the lingual nerve.
Central Integration
It is notable that thermal orosensory cues when acting as a CS in a conditioned temperature aversion have many of the same properties as a gustatory CS in a conditioned taste aversion. Whether mediated by the trigeminal or by gustatory nerves, temperature information from the mouth can apparently be integrated with toxic chemical or visceral information similar to the integration of taste information during acquisition of conditioned aversions. It is possible that the same central circuitry that mediates conditioned taste aversions can also mediate conditioned temperature aversions. In fact, there is evidence for convergence of taste and temperature information at multiple central sites. The trigeminal nerve provides substantial innervation of the rostral (gustatory) nucleus of the solitary tract [27]. Neurons that respond to both gustatory and thermal stimulation can be found throughout the central gustatory pathways, including the rostral nucleus of the solitary tract [28], the thalamic taste area [29] , the amygdala [30], and the gustatory cortex [31]. There is also evidence that conditioned temperature and taste aversions are mediated by distinct pathways, however. In particular, rats with lesions of the medial parabrachial nucleus cannot acquire conditioned taste aversions, but can acquire conditioned aversions to capsaicin, an agonist of temperature-sensitive afferents [15]. The critical areas of the brain required for the acquisition of conditioned temperature aversions remain to be identified.
Acknowledgements
The work described was conducted in fulfillment of a doctoral dissertation (PLS). Supported by National Institute on Deafness and Other Communication Disorders Grants T32DC0044 (PLS) and R01DC03198 (TAH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, Margolskee RF, Nilius B. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature. 2005;438:1022–1025. doi: 10.1038/nature04248. [DOI] [PubMed] [Google Scholar]
- 2.Fishman IY. Single fiber gustatory impulses in rat and hamster. J. Cell. Comp. Physiol. 1957:49. doi: 10.1002/jcp.1030490213. [DOI] [PubMed] [Google Scholar]
- 3.Yamashita S, Sato M. The effects of temperature on gustatory response of rats. J. Cell. Comp. Physiol. 1965;66:1–18. doi: 10.1002/jcp.1030660102. [DOI] [PubMed] [Google Scholar]
- 4.Lundy RF, Jr, Contreras RJ. Gustatory neuron types in the rat geniculate ganglion. J. Neurophysiol. 1999;82:2970–2988. doi: 10.1152/jn.1999.82.6.2970. [DOI] [PubMed] [Google Scholar]
- 5.Breza JM, Curtis KS, Contreras RJ. Temperature modulates taste responsiveness and stimulates gustatory neurons in the rat geniculate ganglion. J. Neurophysiol. 2006;95 doi: 10.1152/jn.00793.2005. [DOI] [PubMed] [Google Scholar]
- 6.Lundy RF, Jr, Contreras RJ. Neural responses of thermal-sensitive lingual fibers to brief menthol stimulation. Brain Res. 1994;641:208–216. doi: 10.1016/0006-8993(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 7.McBurney DH, Collings VB, Glanz LM. Temperature dependence of human taste responses. Physiol. Behav. 1973;11:89–94. doi: 10.1016/0031-9384(73)90127-3. [DOI] [PubMed] [Google Scholar]
- 8.Pangborn RM, Chrisp RB, Bertolero II. Gustatory, salivary, and oral thermal responses to solutions of sodium chloride at four temperatures. Perceptual Psychophysics. 1970;8:69–75. [Google Scholar]
- 9.Bartoshuk LM, Rennert K, Rodin J, Stevens JC. Effects of temperature on the perceived sweetness of sucrose. Physiol. Behav. 1982;28:905–910. doi: 10.1016/0031-9384(82)90212-8. [DOI] [PubMed] [Google Scholar]
- 10.Cruz A, Green BG. Thermal stimulation of taste. Nature. 2000;403:889–892. doi: 10.1038/35002581. [DOI] [PubMed] [Google Scholar]
- 11.Gold RM, Prowse J. Water temperature preference shifts during hydration. Physiol. Behav. 1974;13:291–296. doi: 10.1016/0031-9384(74)90047-x. [DOI] [PubMed] [Google Scholar]
- 12.Carlisle HJ, Laudenslager ML. Separation of water and ambient temperature effects on polydipsia. Physiol. Behav. 1976;16:121–124. doi: 10.1016/0031-9384(76)90293-6. [DOI] [PubMed] [Google Scholar]
- 13.Sako N, Ohashi R, Sakai N, Katsukawa H, Sugimura T. Conditioned food aversion elicited by the temperature of drinking water as a conditioned stimulus in rats. Physiol. Behav. 2004;83:93–98. doi: 10.1016/j.physbeh.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Nachman M. Learned taste and temperature aversions due to lithium chloride sickness after temporal delays. J. Comp. Physiol. Psychol. 1970;73:22–30. doi: 10.1037/h0029807. [DOI] [PubMed] [Google Scholar]
- 15.Grigson PS, Reilly S, Shimura T, Norgren R. Ibotenic acid lesions of the parabrachial nucleus and conditioned taste aversion: further evidence for an associative deficit in rats. Behav. Neurosci. 1998;112:160–171. [PubMed] [Google Scholar]
- 16.Nowlis GH. Conditioned stimulus intensity in acquired alimentary aversions in the rat. J. Comp. Exper. Psychol. 1974;86:1173–1184. doi: 10.1037/h0037644. [DOI] [PubMed] [Google Scholar]
- 17.Scott TR, Giza BK. A measure of taste intensity discrimination in the rat through conditioned taste aversions. Physiol. Behav. 1987;41:315–320. doi: 10.1016/0031-9384(87)90394-5. [DOI] [PubMed] [Google Scholar]
- 18.Smith DV, Theodore RM. Conditioned taste aversions: Generalizations to taste mixtures. Physiol. Behav. 1984;32:983–989. doi: 10.1016/0031-9384(84)90289-0. [DOI] [PubMed] [Google Scholar]
- 19.Smith JC, Fisher EM, Maleszewski V, McClain B. Orosensory factors in the ingestion of corn oil/sucrose mixtures by the rat. Physiol. Behav. 2000;69:135–146. doi: 10.1016/s0031-9384(00)00197-9. [DOI] [PubMed] [Google Scholar]
- 20.Palmerino CC, Rusiniak KW, Garcia J. Flavor-illness aversions: the peculiar role of odor and taste in memory for poison. Science. 1980;208:753–755. doi: 10.1126/science.7367891. [DOI] [PubMed] [Google Scholar]
- 21.Batson JD, Batsell WR., Jr Augmentation, not blocking, in an A+/AX+ flavor-conditioning procedure. Psychonomic Bulletin and Review. 2000;7:466–471. doi: 10.3758/bf03214358. [DOI] [PubMed] [Google Scholar]
- 22.Porter LH, Hecht GS, Sheaffer R. Disturbances in the performance of thermal discrimination tasks following cortical ablations in rats. Brain Res. 1993;621:319–330. doi: 10.1016/0006-8993(93)90122-4. [DOI] [PubMed] [Google Scholar]
- 23.Spector AC, Smith JC, Hollander GR. A comparison of dependent measures used to quantify radiation-induced taste aversion. Physiol. Behav. 1981;27:887–901. doi: 10.1016/0031-9384(81)90059-7. [DOI] [PubMed] [Google Scholar]
- 24.Yamashita S, Yamada K, Sato M. The effect of temperature on neural taste response of cats. Japan. J. Physiol. 1964;14:505–514. doi: 10.2170/jjphysiol.14.505. [DOI] [PubMed] [Google Scholar]
- 25.Nagaki J, Yamashita S, Sato M. Neural response of cat to taste stimuli of varying temperatures. Japan. J. Physiol. 1964;14:67–89. doi: 10.2170/jjphysiol.14.67. [DOI] [PubMed] [Google Scholar]
- 26.Contreras RJ, Lundy RF., Jr Gustatory neuron types in the periphery: a functional perspective. Physiol. Behav. 2000;69:41–52. doi: 10.1016/s0031-9384(00)00187-6. [DOI] [PubMed] [Google Scholar]
- 27.Contreras RJ, Beckstead RM, Norgren R. The central projections of the trigeminal, facial, glossopharyngeal and vagus nerves: an autoradiographic study in the rat. J. Auton. Nerv. Sys. 1982;6:303–322. doi: 10.1016/0165-1838(82)90003-0. [DOI] [PubMed] [Google Scholar]
- 28.Ogawa H, Hayama T, Yamashita Y. Thermal sensitivity of neurons in a rostral part of the rat solitary tract nucleus. Brain Res. 1988;454:321–331. doi: 10.1016/0006-8993(88)90833-5. [DOI] [PubMed] [Google Scholar]
- 29.Verhagen JV, Scott TR, Giza BK. Responses to taste stimulation in the ventroposteromedial nucleus of the thalamus in rats. J. Neurophysiol. 2003;89:265–275. doi: 10.1152/jn.00870.2001. [DOI] [PubMed] [Google Scholar]
- 30.Azuma S, Yamamoto T, Kawamura Y. Studies on gustatory responses of amygdaloid neurons in rats. Exp. Brain Res. 1984;56:12–22. doi: 10.1007/BF00237437. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto T, Matsuo R, Kiyomitsu Y, Kitamura R. Taste responses of cortical neurons in freely ingesting rats. J. Neurophysiol. 1989;61:1244–1258. doi: 10.1152/jn.1989.61.6.1244. [DOI] [PubMed] [Google Scholar]