Abstract
Nucleophilic cationization reagents fitted with aminooxy groups are described. Practical syntheses of mono- and bis-aminooxy tetraalkylammonium iodides including N-hydroxyethyl-functionalized analogs are reported. An oximation example using one of the reagents is presented to illustrate their use in synthesis of cationic materials.
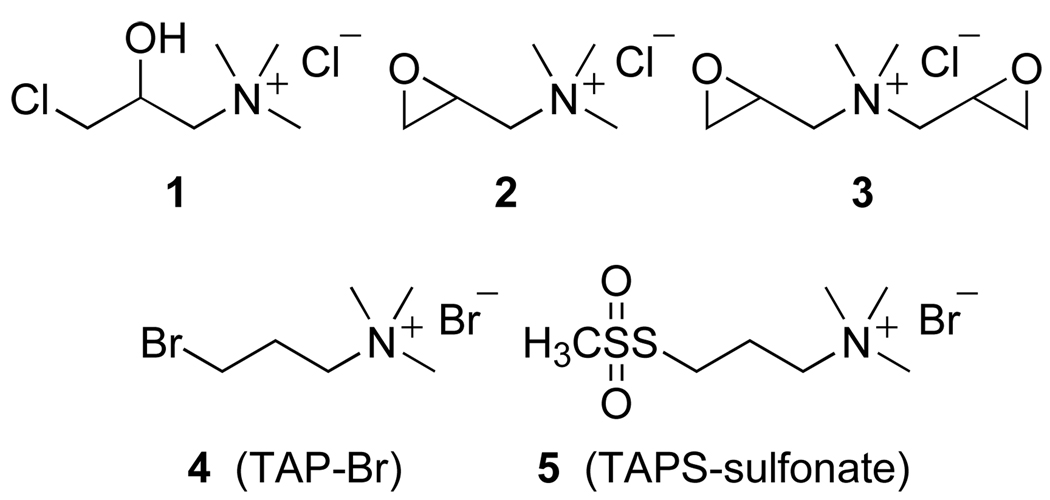
A wide variety of materials have been modified by the covalent attachment of quaternary ammonium functionality to furnish derivatives with overall positive charge.1 This process, a derivatization known as cationization, typically proceeds via reaction of the substrate with an electrophilic reagent containing a quaternary ammonium salt. For example, the cationization of proteins is performed to enhance their intracellular delivery via adsorptive-mediated endocytosis.2 The cationization of cellulose fibers (e.g., cotton) can improve the uptake of dyes in subsequent coloring operations.3 These applications and others principally rely on cationization reagents of the type 1–5 depicted in Figure 1. Indeed, chlorohydrin 14 and epoxide reagents 25 and 36 have been particularly useful for the cationization of carbohydrate domains in reactions with bases at elevated temperatures. The reagents 47 and 58 illustrate other permutations of reactive electrophilic groups used in reactions to derivatize materials with ammonium salts.
Figure 1.
Common cationization reagents.
Of particular interest to us9 is the use of quaternary ammonium salts attached to lipid- or polymer-frameworks as vehicles for the intracellular delivery of polynucleotides to mammalian cells.10 We felt that a mild, more convenient procedure for direct attachment of ammonium ions to substrates would greatly improve our abilities to access new cationic materials. On considering alternative strategies, the chemospecific reaction between aminooxy and ketone or aldehyde carbonyl groups appeared to be an ideal, nucleo-philic counterpart to current methods.11 Given the ease of oximation and the robust nature of the oxime ether linkage, we targeted aminooxy reagents 6.1 and 6.2 (Figure 2) for synthesis. Our interest in gene transfer materials also led us to prepare hydroxyethyl functionalized analogs 7.1 and 7.2. The benefit of hydroxyethylated polar domains in gene delivery is well documented.12 Consequently, we disclose herein a general synthesis of the novel nucleophilic cationization reagents 6 and 7.
Figure 2.
Cationic aminooxy reagents.
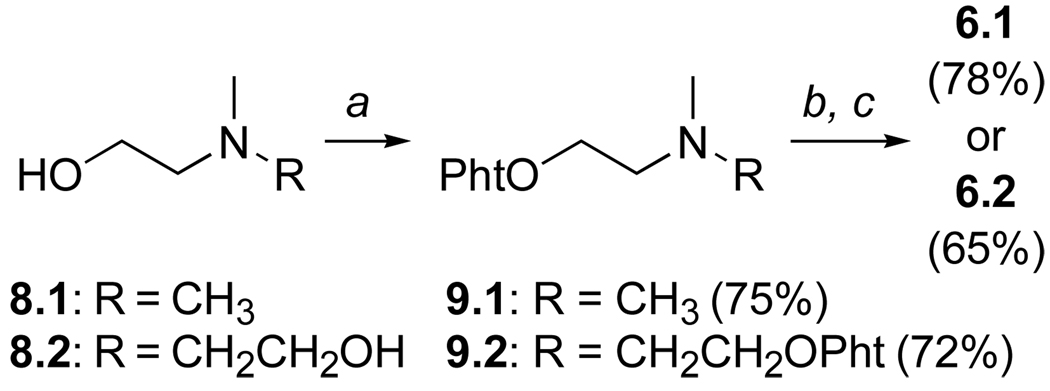
Reaction of the commercially available ethanolamines 8.1 and 8.2 (Scheme 1) with N-hydroxypthalimide (NHP) under Mitsunobu conditions13 (equimolar amounts of NHP:PPh3: DIAD) furnished phthaloyloxy amines 9.1 and 9.2, respectively. Amine quaternization was best accomplished by gently warming the amines in methyl iodide (ca. 0.2 M). The resultant, crude ammonium iodides were treated directly with hydrazine in ethanol to cleave the phthaloyl groups. After work-up, the water-soluble aminooxy reagents 6.1 and 6.2 were isolated by lyophilization of the aqueous layer and then purified using reverse phase HPLC.
Scheme 1.
Reagents and conditions: Pht = phthalimidoyl; (a) N-hydroxyphthalimide, PPh3, DIAD, THF, 0 °C – rt, 12 h; (b) CH3I, sealed tube, 45 °C, 2 h; (c) H2NNH2•H2O, EtOH, H2O, rt, 12 h.
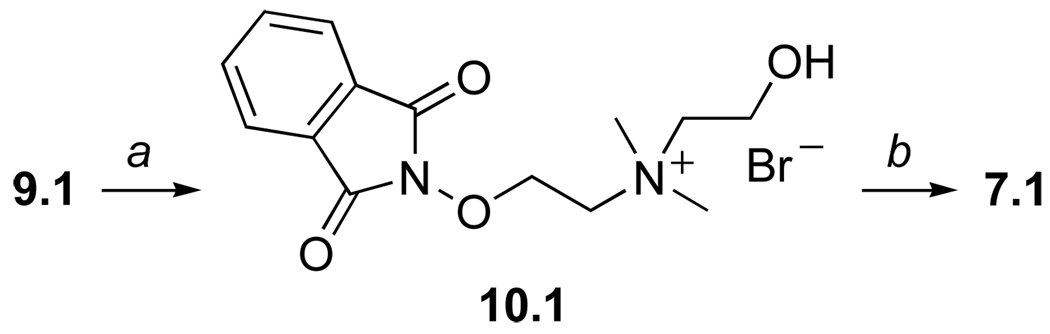
By analogy to previous syntheses of N-(2-hydroxyethyl) ammonium salts,14 we expected the N-alkylation of 9.1 using 2-bromoethanol to be a convenient route to hydroxyethyl-functionalized reagent 7.1 (Scheme 2). However, the N-alkylation required heating the reactants at 60 °C, and this resulted in a complex mixture of products containing ammonium bromide 10.1. Subsequent hydrazinolysis failed to deliver a product mixture that was more amenable to purification. Consequently, 7.1 was obtained in only poor yields (ca. <20%). Due to these complications, we opted to rely again on N-methylation as the penultimate, ammonium salt-forming step.
Scheme 2.
Reagents and conditions: (a) 2-bromoethanol, CH3CN, 60 °C; (b) H2NNH2•H2O, EtOH, rt, 12 h.
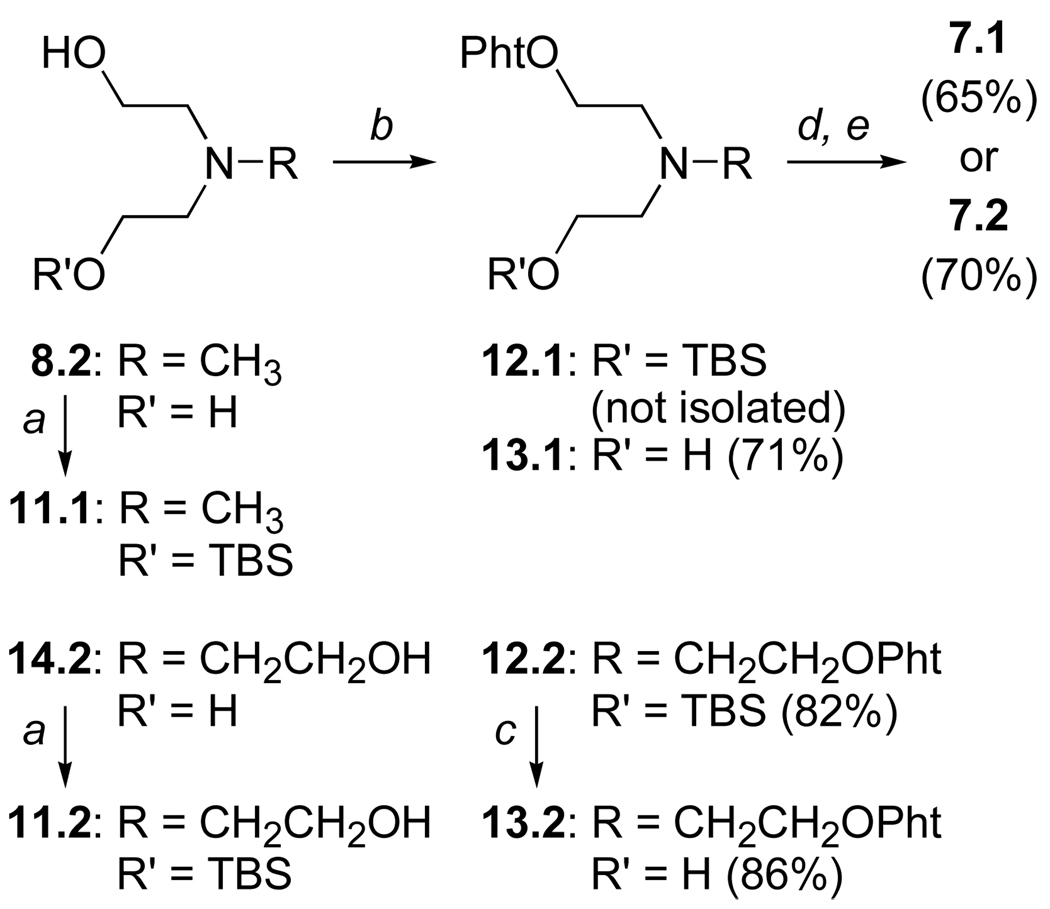
Monosilylation of di- (8.2) and triethanolamine (14.2, Scheme 3) was accomplished by reacting an excess of each ethanolamine with TBSCl as the limiting reagent. The resultant, mono-protected ethanolamines 11.1 and 11.2 were then transformed to the corresponding N-(2-hydroxyethyl)-functionalized aminooxy reagents using the path established for synthesis of reagents 6. While desilylation of the more polar phthaloyloxy amine 12.1 proceeded smoothly on work-up by stirring with aq. HCl, this approach did not work for phthaloyloxy amine 12.2. Furthermore, standard TBAF-mediated deprotection of 12.2 resulted in double N–O cleavage, giving 14.2 as the principal product. Other attempts (e.g., AcOH- or TsOH-mediated deprotections) were equally disappointing. We were gratified to find, however, that prolonged reaction with aqueous HF furnished the desired product 13.2. The amine quaternizations, this time using methyl iodide, and subsequent hydrazinolyses proceeded without incident, and the cationic aminooxy reagents 7.1 and 7.2 were isolated in good overall yield.
Scheme 3.
Reagents and conditions: TBS = t-BuMe2Si; (a) TBSCl (0.2 eq), Et3N (1 eq), CH2Cl2; (b) N-hydroxyphthalimide, PPh3, DIAD, THF, 0 °C – rt, 12h; (c) 48% aq. HF, THF, 0 °C – rt, 12h; (d) CH3I, sealed tube, 60 °C, 2h; (e) H2NNH2•H2O, EtOH, rt, 12h.
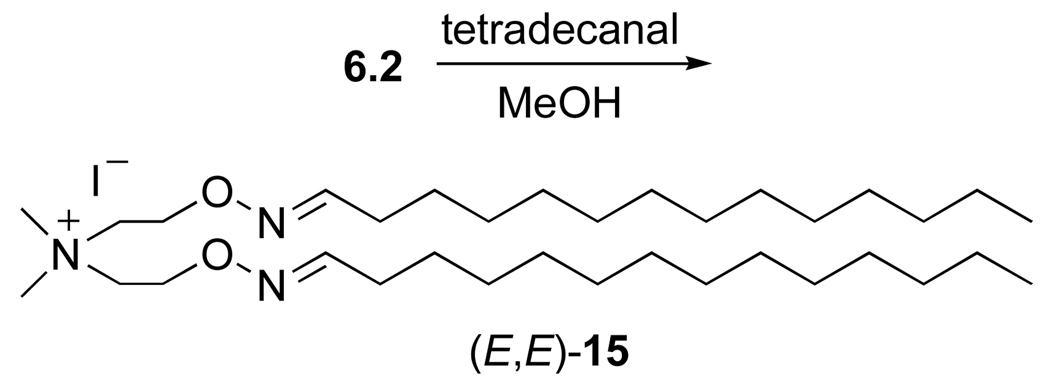
To demonstrate the synthetic utility of the aminooxy cationization reagents in a representative oximation reaction, we used bis(aminooxy) ammonium salt 6.2 to prepare a new cationic lipid. Simple mixing of 6.2 with tetradecanal in methanol furnished the corresponding bis(oxime ether) lipid 15 (Scheme 4) as a 2.7:1 mixture of diastereomers in 82% yield. By comparison to literature reports15 on oximyl proton shifts in 1H NMR, we assigned the major isomer the (E, E)-configuration of oxime ether stereochemistry as depicted in Scheme 4 and the (E, Z)-configuration to the minor isomer.16 The utility of the prototypic oxime ether lipid 15 as an agent for gene delivery is ongoing and will be reported elsewhere.
Scheme 4.
Cationic lipid synthesis.
In summary, we report a general synthesis of novel ammonium ion-based aminooxy reagents that complement existing reagents for cationization applications. The unfunctionalized and hydroxyethyl-functionalized aminooxy reagents as well as the bis(aminooxy) analogs prepared herein can serve as nucleophilic counterparts for reaction with aldehydes and ketones in oximation reactions designed to modify carbonyl surfaces, such as those formed on partial periodate oxidation of carbohydrate domains. In contrast to the harsh conditions typically employed for reaction with the electrophilic cationization reagents, the mild conditions of oxime ether formation should make the present aminooxy approach an attractive alternative. In one demonstration, we prepared a representative member of a new class of cationic lipids that features an oxime ether as the tethering moiety between hydrophobic and hydrophilic domains.
Supplementary Material
Acknowledgments
We thank the NIH (5R21DE019271) for support of our work. XH thanks the Department of Chemistry for a predoctoral fellowship award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data (experimental details for all reactions and spectral data for new compounds) associated with this Letter can be found at doi:
References and notes
- 1.(a) Antal M, Simkovic I, Ebringerová A, Micko MM. J. Appl. Polym. Sci. 1986;31:621–625. [Google Scholar]; (b) Haack V, Heinze R, Oelmeyer G, Kulicke W-M. Macromol. Mater. Eng. 2002;287:495–502. [Google Scholar]; (c) Hebeish A, Hashem M, Abdel-Rahman A, El-Hilw ZH. J. Appl. Polym. Sci. 2006;100:2697–2704. [Google Scholar]; (d) Wang C, Fang K, Ji W. Fibers Polym. 2007;8:225–229. [Google Scholar]
- 2.Futami J, Kitazoe M, Murata H, Yamada H. Expert Opin. Drug Discov. 2007;2:261–269. doi: 10.1517/17460441.2.2.261. [DOI] [PubMed] [Google Scholar]
- 3.(a) Grooby P, Lewis DM, Clark M. Adv. Color Sci. Tech. 2003;6:39–42. [Google Scholar]; (b) Hashem MM. Color. Technol. 2006;122:135–144. [Google Scholar]
- 4.(a) Hashem M, Hauser P, Smith B. Text. Res. J. 2003;73:1017–1023. [Google Scholar]; (b) Hyde K, Dong H, Hinestroza JP. Cellulose. 2007;14:615–623. [Google Scholar]
- 5.(a) Ott G, Schempp W, Krause T. Angew. Makromol. Chem. 1989;173:213–218. [Google Scholar]; (b) Fenart L, Casanova A, Dehouck B, Duhem C, Slupek S, Cecchelli R, Betbeder D. J. Pharmacol. Exp. Ther. 1999;291:1017–1022. [PubMed] [Google Scholar]; (c) Zhang M, Ju B-Z, Zhang S-F, Ma W, Yang J-Z. Carbohydrate Polymers. 2006;69:123–129. [Google Scholar]; (d) Bendoraitiene J, Kavaliauskaite R, Klimaviciute R, Zemaitaitis A. Starch. 2006;58:623–631. [Google Scholar]
- 6.El-Sakhawy M, Milichovsky M. Polym. Int. 2000;49:839–844. [Google Scholar]
- 7.Okazaki K, Imoto T, Yamada H. Anal. Biochem. 1985;145:87–90. doi: 10.1016/0003-2697(85)90330-6. [DOI] [PubMed] [Google Scholar]
- 8.Inoue M, Akimaru J, Nishikawa T, Seki N, Yamada H. Biotechnol. Appl. Biochem. 1998;28:207–213. [PubMed] [Google Scholar]
- 9.(a) Liu L, Zern MA, Lizarzaburu ME, Nantz MH, Wu J. Gene Therapy. 2003;10:180–187. doi: 10.1038/sj.gt.3301861. [DOI] [PubMed] [Google Scholar]; (b) Hauck ES, Zou S, Scarfo K, Nantz MH, Hecker JG. Molec. Therapy. 2008;16:1857–1864. doi: 10.1038/mt.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.For reviews on non-viral gene transfer, see: Karmali PP, Chaudhuri A. Med. Res. Rev. 2007;27:696–722. doi: 10.1002/med.20090. Flotte TR. J. Cell. Physiol. 2007;213:301–305. doi: 10.1002/jcp.21173.
- 11.(a) Kolb HC, Finn MG, Sharpless KB. Angew. Chem. Int. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]; (b) Marcaurelle LA, Shin Y, Goon S, Bertozzi CR. Org. Lett. 2001;3:3691–3694. doi: 10.1021/ol0166247. [DOI] [PubMed] [Google Scholar]
- 12.(a) Felgner JH, Kumar R, Sridhar CN, Wheeler CJ, Tsai Y-J, Border R, Ramsey P, Martin M, Felgner PL. J. Biol. Chem. 1994;269:2550–2561. [PubMed] [Google Scholar]; (b) Bennett MJ, Aberle AM, Balasubramaniam RP, Malone JG, Malone RW, Nantz MH. J. Med. Chem. 1997;40:4069–4078. doi: 10.1021/jm970155q. [DOI] [PubMed] [Google Scholar]; (c) Hattori Y, Ding W-X, Maitani Y. J. Control. Rel. 2007;120:1221–1230. doi: 10.1016/j.jconrel.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Grochowski E, Jurczak J. Synthesis. 1976:682–684. [Google Scholar]
- 14.(a) Rosenthal AF, Geyer RP. J. Biol. Chem. 1960;235:2202. [PubMed] [Google Scholar]; (b) Wheeler CJ, Sukhu L, Yang G, Tsai Y, Bustamente C, Felgner P, Norman J, Manthorpe M. Biochim. Biophys. Acta. 1996;1280:1–11. doi: 10.1016/0005-2736(95)00256-1. [DOI] [PubMed] [Google Scholar]
- 15.Oximyl proton shifts for (E)-oxime ethers are deshielded relative to the (Z)-counterparts; see: Pejkovic-Tadic I, Hranisavljevic-Jakovljevic M, Nesic S, Pascual C, Simon W. Helv. Chim. Acta. 1965;48:1157–1160. Sun R, Lü M, Chen L, Li Q, Song H, Bi F, Huang R, Wang Q. J. Agric. Food Chem. 2008;56:11376–11391. doi: 10.1021/jf801901h.
- 16.The oximyl proton shift for the (E)-oxime ether sidechain in 15 occurs at δ 7.58, (Z)-isomer at δ 6.91.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.