Abstract
We sought to evaluate the impact of C-reactive protein (CRP) levels on in-stent restenosis after percutaneous coronary intervention.
The plasma level of CRP is considered a risk predictor for cardiovascular diseases. However, the relationship between CRP and in-stent restenosis has been a matter of controversy. Meta-analysis reduces variability and better evaluates the correlation.
We performed a systemic search for literature published in March 2008 and earlier, using MEDLINE®, the Cochrane clinical trials database, and EMBASE®. We also scanned relevant reference lists and hand-searched all review articles or abstracts from conference reports on this topic. Of the 245 studies that we initially searched, we chose 9 prospective observational studies (1,062 patients).
Overall, CRP concentration was higher in patients who experienced in-stent restenosis. The weighted mean difference in CRP levels between the patients with in-stent restenosis and those without was 1.67, and the Z-score for overall effect was 2.12 (P=0.03). Our subgroup analysis that compared patients with stable and unstable angina showed a weighted mean difference in the CRP levels of 2.22 between the patients with and without in-stent restenosis, and the Z-score for overall effect was 2.23 (P=0.03) in 5 studies of unstable-angina patients. There was no significance in 4 studies of stable-angina patients.
In spite of significant heterogeneity across the studies, our meta-analysis suggests that preprocedurally elevated levels of CRP are associated with greater in-stent restenosis after stenting and that this impact appears more prominent in unstable-angina patients.
Key words: Angina pectoris/epidemiology; angioplasty, transluminal, percutaneous coronary; biological markers/blood; clinical trials as topic; coronary artery disease/etiology/physiopathology; coronary restenosis/etiology/pathology/prevention & control; C-reactive protein/analysis/metabolism; inflammation/complications; risk factors; stents/adverse effects
Coronary artery diseases remain the major cause of death in the Western world. Inflammation plays an important role in atherosclerotic disorders.1–3 Modest elevation of plasma inflammatory markers, such as C-reactive protein (CRP), is considered a risk predictor for cardiovascular disease and is thought to reflect inflammation in atherosclerosis.4,5
The development of coronary stents has revolutionized the field of interventional cardiology by reducing the incidence of restenosis after balloon angioplasty.6 Intracoronary stents improve procedural success rates and increase the safety and effectiveness of procedures by decreasing the number of cardiovascular events. However, coronary stenting is still associated with a serious complication—in-stent restenosis (ISR).7
Systemic inflammation characterizes the response to vascular injury after percutaneous coronary intervention (PCI).8–10 Stent implantation, in particular, precipitates arterial intimal cellular proliferation and extracellular matrix synthesis that is mediated largely by inflammatory processes.11 However, controversy exists regarding the clinical impact of early inflammatory response on ISR after coronary stent implantation. Previous studies have suggested that the magnitude of the systemic inflammation is linked to adverse late clinical outcomes after PCI.12–14 In contrast, other studies have shown that levels of inflammatory markers after PCI appear similar and that reduction in restenosis after stenting is likely not mediated by the attenuation of systemic markers, such as CRP.15,16 In view of these conflicting reports, we conducted a systematic review of evidence from observational studies, in order to evaluate the association between CRP levels and ISR rates after successful coronary stent implantation in patients with stable angina and unstable angina.
Methods
Meta-analyses of observational studies present particular challenges because of inherent biases and differences in study designs. Consequently, we performed this analysis in accordance with the guidelines of the Meta--analysis of Observational Studies in Epidemiology (MOOSE) group.17
Literature Search. A systemic search for all literature that was published in March 2008 or earlier was performed using MEDLINE®, the Cochrane clinical trials database (2008, issue 1), and EMBASE® (January 1990—March 2008) in order to evaluate the value of CRP in the prediction of ISR after successful coronary stent implantation. Searches combined free-text and MeSH terms relating to “CRP” or “c-reactive protein” or “c reactive protein,” “inflammation,” and “in-stent restenosis” or “restenosis.”
Inclusion Criteria. Studies were considered eligible for this review if they were of a prospective observational design, if they evaluated the potential association between CRP levels before coronary stent implantation and ISR after successful implantation, if they clearly used ISR rates as an outcome index, and if the period of follow-up was 6 months or longer.18
We excluded retrospective studies, laboratory studies, review articles, animal studies, and studies that were irrelevant to the current analysis; studies that lacked preprocedural CRP-level data or that did not reflect stent implantation in all patients; studies lacking definite ISR evaluation by quantitative coronary analysis—for example, if clinical outcomes were expressed as major adverse cardiac events (MACEs); or if follow-up periods were shorter than 6 months.
Identification of Studies. We considered studies in any language. We supplemented electronic searches by hand-searching reference lists of relevant articles and reviews and by contacting experts and manufacturers involved with CRP studies. Abstracts and titles of related articles were initially scanned by a reviewer. Potentially relevant articles were then considered by at least 2 independent reviewers. Disagreements were resolved by discussion or upon consensus from a 3rd or 4th reviewer. Two reviewers agreed on the inclusionary or exclusionary status of 90% of the reviewed studies. In addition, a manual search was conducted for all relevant review articles, bibliographies of original papers, and abstracts of the scientific sessions of the American College of Cardiology, the American Heart Association, and the European Society of Cardiology, for the past 20 years.
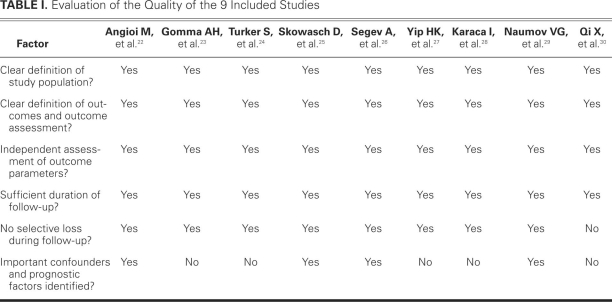
Quality Determination and Data Extraction. Because quality-scoring varies in meta-analyses of observational studies, we systematically evaluated several key points of study quality in accordance with a previous study.19 Two reviewers independently appraised each article included in our analysis with use of a checklist from the Dutch Cochrane Centre, which was proposed by MOOSE17: clear definition of study population, clear definition of outcomes and outcome assessment, independent assessment of outcome parameters, sufficient duration of follow-up, no selective loss during follow-up, and identification of important confounders and prognostic factors. If studies did not clearly mention one of these points, we concluded that it had not been performed and, consequently, that there was possible underestimation of the reported characteristics.
Two blinded reviewers independently used a standardized data-extraction form to determine eligibility for inclusion and to extract data.19,20 The extracted data included the lead author's last name, the publication year, and the origin of the studied population; the study design; the characteristics of the studied population (sample size, age, sex, diagnoses, drug therapies, methods of CRP measurement, types of stents, durations of follow-up, and withdrawals and dropouts of patients); endpoint evaluations (definitions of ISR and methods of ISR detection); rates of ISR; and means and SDs of CRP in each group. Disagreements were resolved by consensus from a 3rd or 4th reviewer.
If the study provided medians and interquartile ranges instead of means and SDs, we imputed the means and SDs as described by Hozo and colleagues.18 We calculated the lower and upper ends of the range by multiplying the difference between the median and ends of the interquartile range by 2 and adding or subtracting the product from the median, respectively, according to previous studies.18,19
Statistical Analysis
In order to accommodate differences in the ways in which CRP was measured and reported in various studies, the absolute CRP levels were converted into a common unit by calculating weighted-effect sizes. These sizes were derived by dividing the mean difference of CRP levels in ISR and no-ISR groups of each study by its SD. We used the I2 statistic to measure the extent of inconsistency among the results, and we tested heterogeneity by using the Cochran Q test.19,20 Because this test has poor power in the event of few studies, we considered both the presence of significant heterogeneity at the 10% level of significance and values of I2 exceeding 56% as an indicator of significant heterogeneity,21 so that a pooled effect could be calculated with a random-effects model that was used to take into account within-study and between-study variance, or otherwise, with a fixed-effects model. To explore sources of heterogeneity, we performed several sensitivity and subgroup analyses. Publication bias was also evaluated by use of a funnel plot. All analyses were conducted with the use of Review Manager, version 4.2 (Revman, The Cochrane Collaboration; Oxford, UK). The data conformed to each test that was used to analyze them.
Results
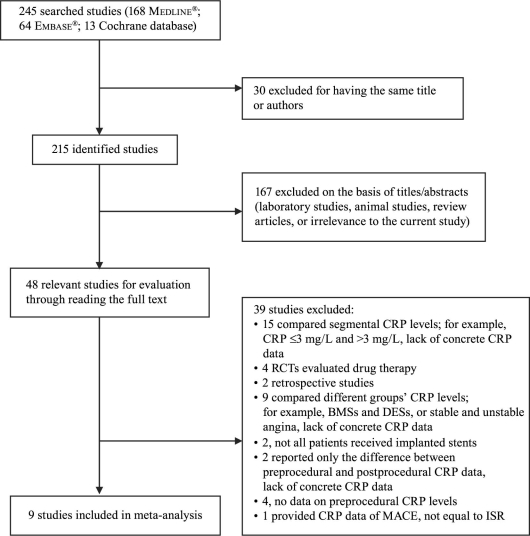
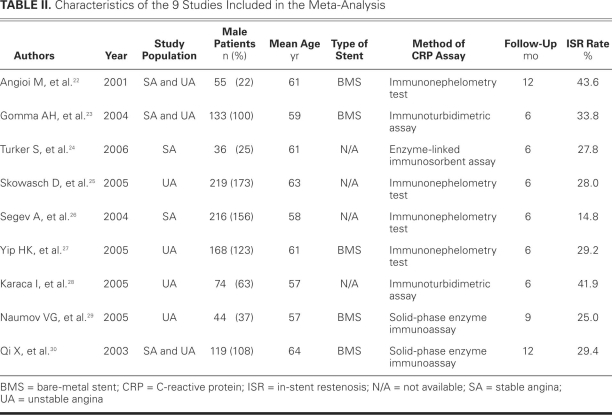
The search yielded 245 research reports, of which 30 were excluded for having the same title or authors; 167 were excluded because they were laboratory studies, review articles, animal studies, or irrelevant to the current analysis. Of the remaining 48 studies, 15 compared segmental CRP levels (for example, CRP ≤3 mg/L and >3 mg/L), and provided inadequate preprocedural CRP data. Four studies were randomized controlled trials that evaluated drug interventions on ISR after successful coronary stent implantation. Two studies were retrospective. Nine studies compared different groups' CRP levels (for example, bare-metal versus drug-eluting stents, or stable versus unstable angina), and lacked concrete preprocedural CRP data. In 2 studies, not all patients were implanted with a stent. Two studies reported only the difference between preprocedural and postprocedural CRP data and presented no concrete preprocedural CRP data. Four other studies had no data on preprocedural CRP levels. One study provided CRP data and MACE, but no exact information regarding ISR. The foregoing studies were all excluded, and 9 prospective observational cohort studies22–30 were included in our meta-analysis (Fig. 1). As a result, a total of 1,062 patients were involved in our review: 298 in the ISR group, and 764 in the no-ISR group. All follow-up periods were longer than 6 months. Table I22–30 shows the qualitative evaluation of the 9 studies; Table II22–30 presents the characteristics of each study.
Fig. 1 Flow diagram of the trial-selection process. BMSs = bare-metal stents; CRP = C-reactive protein; DESs = drug-eluting stents; ISR = in-stent restenosis; MACE = major adverse coronary event; RCTs = randomized controlled trials
TABLE I. Evaluation of the Quality of the 9 Included Studies
TABLE II. Characteristics of the 9 Studies Included in the Meta-Analysis
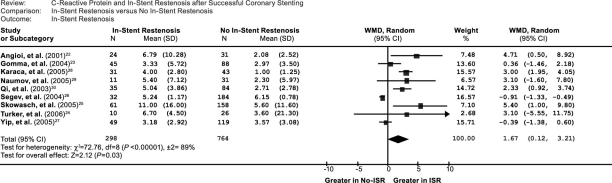
Seven studies22–25,28–30 showed that patients with ISR had higher CRP levels than did patients without ISR, whereas CRP levels did not significantly differ between those groups in 2 other studies.26,27 Overall, CRP concentration was greater in patients with ISR. The weighted mean difference in the CRP levels between patients with and patients without ISR was 1.67 (95% confidence interval [CI], 0.12–3.21) (Fig. 2), and the Z-score for overall effect was 2.12 (P=0.03).
Fig. 2 Comparison of CRP levels between ISR and no-ISR groups in the 9 included studies. CI = confidence interval; ISR = in-stent restenosis; WMD = weighted mean difference
The heterogeneity test showed significant differences among individual studies (P <0.01; I2=89%). We subsequently performed sensitivity and subgroup analyses in order to identify the origin of this heterogeneity.19 After the removal of 3 studies that had follow-up periods longer than 6 months, the analysis showed no significant influence on the results, and therefore long-er follow-up did not explain the cause of heterogeneity. We also evaluated the influence of 3 CRP assays on the results. In contrast with a previous study,19 the heterogeneity test showed no significant effects on the results. In addition, after the removal of 3 studies that presented no clear information regarding types of stents, the test showed no significant effects on the results. Therefore, the differences in follow-up period, method of CRP assay, and type of stent were not possible sources of heterogeneity.
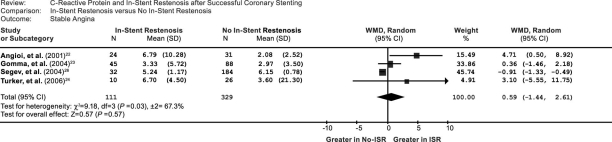
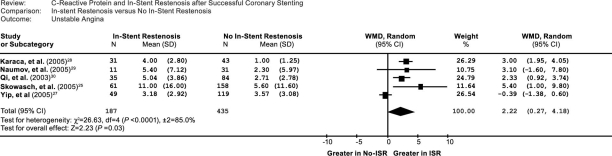
We performed a subgroup analysis of studies that were associated with stable angina and unstable angina. In the 5 unstable-angina studies, the weighted mean difference in the CRP levels between the patients with ISR and without ISR was 2.22 (95% CI, 0.27–4.18) (Fig. 3), and the Z-score for overall effect was 2.23 (P=0.03). In contrast, in the 4 stable-angina studies, the weighted mean difference in the CRP levels between the patients with and without ISR was 0.59 (95% CI, –1.44 to 2.61) (Fig. 4), and the Z-score for overall effect was 0.57 (P=0.57), although an increasing trend of CRP was found in the stable-angina patients. The data suggested that the impact of CRP on ISR is more prominent in patients who have unstable angina than in those who have stable angina.
Fig. 3 Comparison of C-reactive protein levels between ISR and no-ISR groups in the 4 included stable-angina studies. CI = confidence interval; ISR = in-stent restenosis; WMD = weighted mean difference
Fig. 4 Comparison of C-reactive protein levels between ISR and no-ISR groups in the 5 included unstable-angina studies. CI = confidence interval; ISR = in-stent restenosis; WMD = weighted mean difference
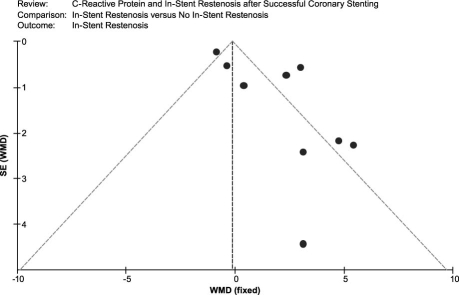
An asymmetric funnel plot shows the possible existence of publication bias (Fig. 5). Because of small sample size, we cannot explain the exact cause of heterogeneity in our meta-analysis.
Fig. 5 Funnel plot of the meta-analysis. Comparison: ISR versus no-ISR. Outcome: ISR. ISR = in-stent restenosis; SE = standard error; WMD = weighted mean difference
Discussion
The results of our meta-analysis clearly revealed a strong relationship between preprocedural CRP levels and a subsequently greater risk of ISR after successful coronary stenting in patients with coronary artery disease, although heterogeneity testing showed significant differences among individual studies (P <0.01; I2=89%). After the exclusion of 2 studies, the overall impact of CRP elevation on rates of ISR after stenting was much greater in patients with ISR. The weighted mean difference in CRP levels between the patients with and the patients without ISR was 1.67 (95% CI, 0.12–3.21), and the Z-score for overall effect was 2.12 (P=0.03) (Fig. 2). Thus, the information provided by our meta-analysis regarding the impact of inflammation on ISR is important because of the clinical significance of ISR after successful coronary stenting and because of the inconsistency of the published results.
Coronary Stent Implantation and Inflammation. Despite intensive studies that have been performed regarding ISR, the factors attributed to ISR have not been fully elucidated, and ISR remains a challenge for the interventional cardiologist.7,9 Most previous studies have shown that restenosis after coronary stenting is attributable to elastic recoil immediately after balloon deflation, neointimal proliferation triggered by injury to the vascular wall, and late negative remodeling.31 Research interest is increasing regarding the role of inflammation in the pathophysiology of ISR, and a few data from prospective observational studies have suggested that inflammation contributes to ISR.32,33 Coronary stenting is a strong inflammatory stimulus, and the acute systemic response to local inflammation that is produced by coronary stenting is a feature of PCI.34 An experimental study showed that leukocyte recruitment could be detected within 15 minutes after stent deployment, at the level of the coronary segment that had been injured by the stent.8 In addition, several clinical investigators who focused on early markers or initiators of the inflammatory response after coronary stenting35,36 found that soluble CD40 ligand exhibited the greatest relative rise during the first 10 minutes after coronary stenting. Moreover, the intensity of such a reaction as measured by high-sensitivity CRP has proved to be correlated with recurrent ischemic events and to be associated with restenosis.14,37 In patients with stable angina and normal baseline CRP plasma levels, successful stent implantation is followed by a rapid increase of CRP, with peak levels occurring 48 hours after stenting.10 Finally, local inflammation caused by stent deployment also elicits a systemic inflammatory response that is initially mediated by inflammatory leukins, such as interleukin-1 (IL-1), IL-6, and tumor necrosis factor-α (TNF-α). These molecules cause the liver to produce acute-phase reactants (such as CRP) that rapidly increase in the blood and may directly amplify the inflammatory stimulus.8
Inflammation and In-Stent Restenosis. The clinical impact of early inflammatory response on ISR after coronary stent implantation is a matter of controversy. Some data suggest that persistently increased plasma levels of CRP (for longer than 48 hours after coronary stenting) are associated with a higher incidence of cardiovascular events during follow-up.38 Similarly, Gottsauner-Wolf and colleagues39 found that increased CRP levels that persisted for longer than 48 hours were associated with a greater incidence of ISR. Kim and associates13 reported that drug-eluting stent implantation induced a significantly lower increase in plasma CRP levels at 48 and 72 hours after coronary stenting. This early inflammatory response pattern was found to be related to diameter stenosis and late loss at follow-up (6 months after stenting), as evaluated upon quantitative coronary analysis. Over time, patients with higher CRP levels after PCI experienced higher rates of death, up to 5 years after the procedure. Previous data indicated the possibility that an excessive increase in inflammatory mediators after PCI is associated with increased cardiovascular risk because of a short hyperreactivity of coagulation.40 Yazdani and co-authors41 studied the effect of PCI on levels of inflammatory markers. They found that IL-6 was significantly elevated after coronary stenting in unstable-angina patients in comparison with stable-angina patients. However, 1 month after stenting, there were no differences in comparative levels of IL-6 in the patients, which suggested that IL-6 levels correlate with instability of atheromatous plaque and that the decrease of IL-6 levels after stenting signifies plaque re-endothelialization and stabilization. Similarly, Saleh and coworkers42 showed that coronary stent implantation, but not pathogen burden (including cytomegalovirus, Chlamydia pneumoniae, Epstein-Barr virus, Helicobacter pylori, and herpes simplex virus), is associated with plasma CRP and IL-6 response to PCI. Some data have shown that events after PCI are associated both with restenosis and with new plaque formation.43 Therefore, further investigation into the impact of inflammatory response on ISR after PCI appears to be warranted.
Heterogeneity of C-Reactive Protein Across Studies. When between-study variation cannot be explained by chance, exploration of the reasons for heterogeneity rather than deviation of a single summary estimate emerges as the major goal of meta-analysis.19,44 Our heterogeneity testing showed significant differences among individual studies (P <0.01; I2=89%). In our sensitivity and subgroup analyses to find the origin of this heterogeneity, we focused on the potential effect of follow-up periods. The duration of follow-up did not explain the cause of heterogeneity in our results. We subsequently considered and eliminated the method of CRP assay and the type of stent as potential causes of heterogeneity.
According to our subgroup analysis of patients with stable angina and unstable angina, the weighted mean difference in CRP levels between the patients with and without ISR was 2.22 (95% CI, 0.27–4.18), and the Z-score for overall effect was 2.23 (P=0.03) in the 5 unstable-angina studies. However, the weighted mean difference in the CRP levels between the patients with and without ISR was 0.59 (95% CI, –1.44 to 2.61), and the Z-score for overall effect was 0.57 (P=0.57) in the 4 stable-angina studies. These data are inconsistent with the results of previous studies, which showed that CRP is a predictor of coronary disease severity as well as future cardiovascular events.4,45 In other words, results of our meta-analysis of the impact of CRP on ISR are similar to those previous observations concerning the impact of CRP on cardiovascular events.
Limitations of the Study
Our meta-analysis may provide novel information regarding the relationship between inflammation and rates of ISR. However, potential limitations include our small sample size. The numbers of studies and patients are rather limited, and the possibility of publication bias cannot be excluded.19,46 More important, our analysis is founded upon potential observational studies: no randomized studies are covered, which may increase potential bias. Therefore, the results of our analysis should be interpreted cautiously. In addition, converting non-normally distributed statistics (median and range) to normally distributed statistics (mean and SD) may be a cause of bias in our analysis.19 Finally, although most of the studies attempted to control potential confounders, the degree to which this was accomplished varied among them.19
Conclusion
Our meta-analysis shows that elevated preprocedural CRP is correlated with subsequent ISR after stenting in patients who have coronary artery disease. Although there is significant heterogeneity across the enrolled studies, ISR appears to be more prominent in patients who have unstable angina than in patients who have stable angina. The data suggest that inflammatory processes play an important role in the formation of ISR after coronary stent implantation, especially in patients who have unstable coronary disease. The clinical application of CRP levels in predicting ISR after stenting appears promising but warrants confirmation by larger, well-designed prospective and randomized studies.
Acknowledgments
In reporting our methods and results, we relied heavily upon a template that was kindly provided by the authors and publishers of Liu T, Li G, Li L, Korantzopoulos P. Association between C-reactive protein and recurrence of atrial fibrillation after successful electrical cardioversion: a meta-analysis. J Am Coll Cardiol 2007;49(15):1642–8.19 We used the framework of their excellent Methods and Results sections as a model for the reporting of our own data. We thank Dr. Liu and his co-authors and also the American College of Cardiology (the copyright holder) for their cooperation.
Footnotes
Address for reprints: Jian-Jun Li, MD, PhD, Department of Cardiology, Fu Wai Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing 100037, PRC
E-mail: lijnjn@yahoo.com.cn
The first 2 authors contributed equally to this study.
Source of support: This ar t icle is partly supported by a Fu Wai Hospital Grant (2004190), Na t ional Natural Scien t i f ic Founda t ion (30670861), Beijing Natural Scien t i f ic Founda t ion (7082081), Na t ional Project in the Five-Year-Period Grant, and Specialized Research Fund for the Doctoral Program of Higher Educa t ion of China (20060023044, 20070023047) awarded to Jian-Jun Li, MD, PhD.
References
- 1.Li JJ. Inflammation: an important mechanism for different clinical entities of coronary artery diseases. Chin Med J (Engl) 2005;118(21):1817–26. [PubMed]
- 2.Maseri A. Inflammation, atherosclerosis, and ischemic events –exploring the hidden side of the moon. N Engl J Med 1997; 336(14):1014–6. [DOI] [PubMed]
- 3.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med 1999;340(2):115–26. [DOI] [PubMed]
- 4.Li JJ, Fang CH. C-reactive protein is not only an inflammatory marker but also a direct cause of cardiovascular diseases. Med Hypotheses 2004;62(4):499–506. [DOI] [PubMed]
- 5.van der Meer IM, de Maat MP, Kiliaan AJ, van der Kuip DA, Hofman A, Witteman JC. The value of C-reactive protein in cardiovascular risk prediction: the Rotterdam Study. Arch Intern Med 2003;163(11):1323–8. [DOI] [PubMed]
- 6.Al Suwaidi J, Berger PB, Holmes DR Jr. Coronary artery stents. JAMA 2000;284(14):1828–36. [DOI] [PubMed]
- 7.Li JJ, Xu B, Yang YJ, Ma WH, Chen JL, Qiao SB, et al. A comparison of angiographic and clinical outcomes after sirolimus-eluting versus paclitaxel-eluting stents for the treatment of in-stent restenosis. Chin Med J (Engl) 2006;119(13):1059–64. [PubMed]
- 8.Versaci F, Gaspardone A. Prevention of restenosis after stenting: the emerging role of inflammation. Coron Artery Dis 2004;15(6):307–11. [DOI] [PubMed]
- 9.Li JJ, Nie SP, Zhang CY, Gao Z, Zheng X, Guo YL. Is inflammation a contributor for coronary stent restenosis? Med Hypotheses 2007;68(5):945–51. [DOI] [PubMed]
- 10.Gaspardone A, Versaci F. Coronary stenting and inflammation. Am J Cardiol 2005;96(12A):65L–70L. [DOI] [PubMed]
- 11.Welt FG, Rogers C. Inflammation and restenosis in the stent era. Arterioscler Thromb Vasc Biol 2002;22(11):1769–76. [DOI] [PubMed]
- 12.Gibson CM, Karmpaliotis D, Kosmidou I, Murphy SA, Kirtane AJ, Budiu D, et al. Comparison of effects of bare metal versus drug-eluting stent implantation on biomarker levels following percutaneous coronary intervention for non-ST-elevation acute coronary syndrome. Am J Cardiol 2006;97 (10):1473–7. [DOI] [PubMed]
- 13.Kim JY, Ko YG, Shim CY, Park S, Hwang KC, Choi D, et al. Comparison of effects of drug-eluting stents versus bare metal stents on plasma C-reactive protein levels. Am J Cardiol 2005; 96(10):1384–8. [DOI] [PubMed]
- 14.Hojo Y, Ikeda U, Katsuki T, Mizuno O, Fukazawa H, Kurosaki K, et al. Interleukin 6 expression in coronary circulation after coronary angioplasty as a risk factor for restenosis. Heart 2000;84(1):83–7. [DOI] [PMC free article] [PubMed]
- 15.Bhatt DL. Inflammation and restenosis: is there a link? Am Heart J 2004;147(6):945–7. [DOI] [PubMed]
- 16.Schneider DJ, Watkins MW, Terrien EF, Sobel BE, Dauerman HL. Systemic inflammation after drug-eluting stent placement. J Thromb Thrombolysis 2005;19(2):87–92. [DOI] [PubMed]
- 17.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283(15):2008–12. [DOI] [PubMed]
- 18.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5(1):13. [DOI] [PMC free article] [PubMed]
- 19.Liu T, Li G, Li L, Korantzopoulos P. Association between C-reactive protein and recurrence of atrial fibrillation after successful electrical cardioversion: a meta-analysis. J Am Coll Cardiol 2007;49(15):1642–8. [DOI] [PubMed]
- 20.Kinlay S. Low-density lipoprotein-dependent and -independent effects of cholesterol-lowering therapies on C-reactive protein: a meta-analysis. J Am Coll Cardiol 2007;49(20):2003–9. [DOI] [PubMed]
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21(11):1539–58. [DOI] [PubMed]
- 22.Angioi M, Abdelmouttaleb I, Rodriguez RM, Aimone-Gastin I, Adjalla C, Gueant JL, Danchin N. Increased C-reactive protein levels in patients with in-stent restenosis and its implications. Am J Cardiol 2001;87(10):1189–93; A4. [DOI] [PubMed]
- 23.Gomma AH, Hirschfield GM, Gallimore JR Jr, Lowe GD, Pepys MB, Fox KM. Preprocedural inflammatory markers do not predict restenosis after successful coronary stenting. Am Heart J 2004;147(6):1071–7. [DOI] [PubMed]
- 24.Turker S, Guneri S, Akdeniz B, Ozcan MA, Baris N, Badak O, et al. Usefulness of preprocedural soluble CD40 ligand for predicting restenosis after percutaneous coronary intervention in patients with stable coronary artery disease. Am J Cardiol 2006;97(2):198–202. [DOI] [PubMed]
- 25.Skowasch D, Jabs A, Andrie R, Luderitz B, Bauriedel G. Progression of native coronary plaques and in-stent restenosis are associated and predicted by increased pre-procedural C reactive protein. Heart 2005;91(4):535–6. [DOI] [PMC free article] [PubMed]
- 26.Segev A, Kassam S, Buller CE, Lau HK, Sparkes JD, Connelly PW, et al. Pre-procedural plasma levels of C-reactive protein and interleukin-6 do not predict late coronary angiographic restenosis after elective stenting. Eur Heart J 2004;25(12): 1029–35. [DOI] [PubMed]
- 27.Yip HK, Hung WC, Yang CH, Chen YH, Cheng CI, Chen SM, Yeh KH. Serum concentrations of high-sensitivity C-reactive protein predict progressively obstructive lesions rather than late restenosis in patients with unstable angina undergoing coronary artery stenting. Circ J 2005;69(10):1202–7. [DOI] [PubMed]
- 28.Karaca I, Aydin K, Yavuzkir M, Ilkay E, Akbulut M, Isik A, Arslan N. Predictive value of C-reactive protein in patients with unstable angina pectoris undergoing coronary artery stent implantation. J Int Med Res 2005;33(4):389–96. [DOI] [PubMed]
- 29.Naumov VG, Sumarokov AB, Ezhov MV, Aleksandrova EN, Novikov AA, Raimbekova IR, et al. Markers of chronic inflammation in patients with ischemic heart disease with in-stent restenosis [in Russian]. Kardiologiia 2005;45(1):14–7. [PubMed]
- 30.Qi X, Li S, Li J. The prognostic value of IL-8 for cardiac events and restenosis in patients with coronary heart diseases after percutaneous coronary intervention. Jpn Heart J 2003;44(5): 623–32. [DOI] [PubMed]
- 31.Bennett MR. In-stent stenosis: pathology and implications for the development of drug eluting stents. Heart 2003;89(2): 218–24. [DOI] [PMC free article] [PubMed]
- 32.Lowe HC, Oesterle SN, Khachigian LM. Coronary in-stent restenosis: current status and future strategies. J Am Coll Cardiol 2002;39(2):183–93. [DOI] [PubMed]
- 33.Karthikeyan G, Bhargava B. Prevention of restenosis after coronary angioplasty. Curr Opin Cardiol 2004;19(5):500–9. [DOI] [PubMed]
- 34.Li JJ. Inflammatory response, drug-eluting stent and restenosis. Chin Med J (Engl) 2008;121(6):566–72. [PubMed]
- 35.Aggarwal A, Blum A, Schneider DJ, Sobel BE, Dauerman HL. Soluble CD40 ligand is an early initiator of inflammation after coronary intervention. Coron Artery Dis 2004;15(8): 471–5. [DOI] [PubMed]
- 36.Gach O, Biemar C, Nys M, Deby-Dupont G, Chapelle JP, Deby C, et al. Early release of neutrophil markers of activation after direct stenting in patients with unstable angina. Coron Artery Dis 2005;16(1):59–65. [DOI] [PubMed]
- 37.Inoue T, Uchida T, Yaguchi I, Sakai Y, Takayanagi K, Morooka S. Stent-induced expression and activation of the leukocyte integrin Mac-1 is associated with neointimal thickening and restenosis. Circulation 2003;107(13):1757–63. [DOI] [PubMed]
- 38.Saleh N, Tornvall P. Serum C-reactive protein response to percutaneous coronary intervention in patients with unstable or stable angina pectoris is associated with the risk of clinical restenosis. Atherosclerosis 2007;195(2):374–8. [DOI] [PubMed]
- 39.Gottsauner-Wolf M, Zasmeta G, Hornykewycz S, Nikfardjam M, Stepan E, Wexberg P, et al. Plasma levels of C-reactive protein after coronary stent implantation. Eur Heart J 2000; 21(14):1152–8. [DOI] [PubMed]
- 40.Liuzzo G, Buffon A, Biasucci LM, Gallimore JR, Caligiuri G, Vitelli A, et al. Enhanced inflammatory response to coronary angioplasty in patients with severe unstable angina. Circulation 1998;98(22):2370–6. [DOI] [PubMed]
- 41.Yazdani S, Simon AD, Vidhun R, Gulotta C, Schwartz A, Rabbani LE. Inflammatory profile in unstable angina versus stable angina in patients undergoing percutaneous interventions. Am Heart J 1998;136(2):357–61. [DOI] [PubMed]
- 42.Saleh N, Svane B, Jensen J, Hansson LO, Nordin M, Tornvall P. Stent implantation, but not pathogen burden, is associated with plasma C-reactive protein and interleukin-6 levels after percutaneous coronary intervention in patients with stable angina pectoris. Am Heart J 2005;149(5):876–82. [DOI] [PubMed]
- 43.Buffon A, Liuzzo G, Biasucci LM, Pasqualetti P, Ramazzotti V, Rebuzzi AG, et al. Preprocedural serum levels of C-reactive protein predict early complications and late restenosis after coronary angioplasty. J Am Coll Cardiol 1999;34(5):1512–21. [DOI] [PubMed]
- 44.Petitti DB. Approaches to heterogeneity in meta-analysis. Stat Med 2001;20(23):3625–33. [DOI] [PubMed]
- 45.Rifai N, Tracy RP, Ridker PM. Clinical efficacy of an automated high-sensitivity C-reactive protein assay. Clin Chem 1999;45(12):2136–41. [PubMed]
- 46.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315(7109):629–34. [DOI] [PMC free article] [PubMed]