Abstract
Noonan syndrome is an autosomal dominant dysmorphic syndrome. Pulmonary stenosis is the most common cardiac anomaly in Noonan patients, with an incidence of 60%. A 9-year-old girl was referred to our institution with pericardial effusion. Transthoracic echocardiography indeed confirmed massive pericardial effusion and revealed, further, valvular and arterial pulmonary vegetations that accompanied a dysplastic tricuspid pulmonary valve. We decided to perform emergency pericardial tube drainage and to continue the anti-biotic regimen for 2 more weeks before undertaking open-heart surgery. After 2 weeks, the patient underwent an operation wherein the valvular vegetations were excised and a pulmonary valve commissurotomy was performed, yielding a competent pulmonary valve with 3 distinct but moderately dysplastic cusps. In addition to the pulmonary valve, the main, left, and right pulmonary arteries were filled with mobile vegetations, which were removed during the procedure.
In this patient, a dysplastic and stenotic pulmonary valve may have contributed to the progression of endocarditis and to the growth of vegetations that occupied the pulmonary arteries. In conclusion, we hypothesize that although pulmonary stenosis is not considered a common predisposing factor for infective endocarditis, it can contribute to the progression of infective endocarditis in Noonan patients.
Key words: Endocarditis; heart defects, congenital; pulmonary artery/surgery; pulmonary valve stenosis/complications; Noonan syndrome/therapy
The pulmonary valve is the least commonly involved valve in infective endocarditis, and isolated pulmonary valve endocarditis is extremely rare: predisposing factors are congenitally abnormal pulmonary valve, intravenous drug abuse, and the presence of pulmonary artery catheters. In children, most cases of pulmonary valve endocarditis are secondary to congenitally abnormal pulmonary valves, whereas in adults most cases are due to intravenous drug abuse.1 Noonan syndrome, the estimated incidence of which is 1 in 1,000 to 2,500 live births, is an autosomal dominant dysmorphic syndrome characterized by hypertelorism, down-slanting palpebral fissures, low-set posterior-rotated ears, and short stature—together with cardiac anomalies, of which pulmonary stenosis is the most frequent feature.2,3 We herein report a case of pulmonary valve endocarditis with massive pulmonary artery vegetations, in a girl with Noonan syndrome.
Case Report
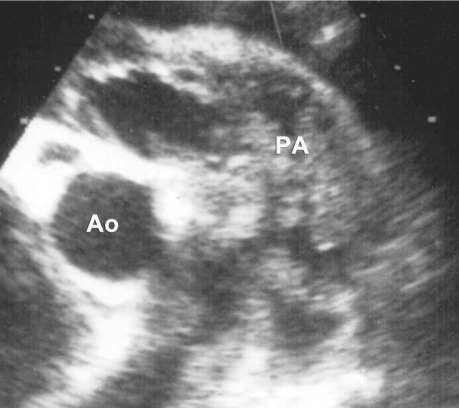
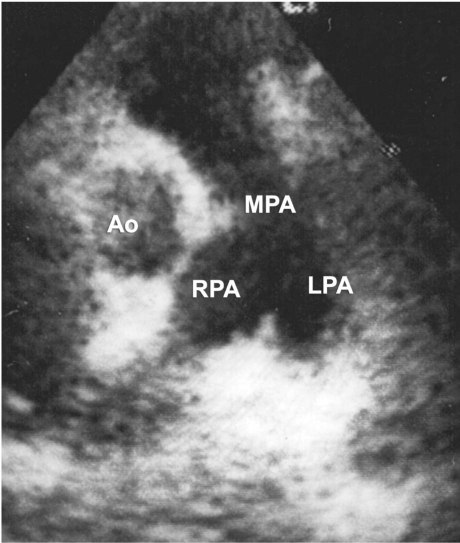
In March 2007, a 9-year-old girl was referred to our institution with a diagnosis of pericardial effusion. The patient had a dysmorphic facial appearance and a family history of mental retardation. Transthoracic echocardiography (TTE) revealed massive pericardial effusion and masses that occupied the pulmonary valve and arteries. The pulmonary valve leaflets appeared to be thickened and myxomatous, and there was mild isolated pulmonary artery stenosis (pulmonary artery peak systolic pressure gradient, 42 mmHg; pulmonary artery mean systolic pressure gradient, 22 mmHg). Trivial pulmonary regurgitation was also found (Fig. 1). Chest radiography revealed moderate cardiomegaly and bronchopneumonic infiltrations in the lung fields. The physical examination was significant for shortness of breath, ascites, tachycardia, and a 2/6 diastolic murmur. The electrocardiogram was normal. Hematologic examination showed leukocytosis (white cell count, 20,000/mm3), 85% of which leukocytes were polymorphonuclear lymphocytes that displayed a toxic granulation on the peripheral smear; and it showed further a normocytic, normochromic anemia with a hematocrit of 22% and hemoglobin level of 7.2 g/dL. The significant biochemical values were blood urea nitrogen, 49 mg/L; creatinine, 0.6 mg/dL; aspartate transaminase, 48 U/L; alanine transaminase, 53 U/L; C-reactive protein, 2.23 mg/L; and erythrocyte sedimentation rate, 69 mm/hr. Renal insufficiency was thought to be secondary to endocarditis, because the abdominal ultrasonography and urinalysis showed no pathologic findings. Although blood cultures had been negative, a broad-spectrum anti-biotic regimen (vancomycin and amikacin) was initiated. We decided to perform emergent pericardial tube drainage and to continue the antibiotic regimen for 2 more weeks before a scheduled open-heart procedure. Cultures of pericardial--drainage specimens were sterile. Four weeks after her admission, open-heart surgery was performed. At operation, the pulmonary valve and the main, left, and right pulmonary arteries were all filled with mobile vegetations. The pulmonary valve was tri-leaflet, but the leaflets were thickened and fused, leaving a small central orifice. Valvular vegetations were cleaned, and a commissurotomy was performed, yielding a competent pulmonary valve with 3 distinct but moderately dysplastic cusps. Similarly, mobile vegetations were removed from the main, left, and right pulmonary arteries. The pulmonary artery incision was closed primarily. Exploration of the mitral and submitral system revealed an anatomically normal, competent valve and subvalvular apparatus. The patient was weaned from cardiopulmonary bypass, and her postoperative recovery was uneventful. Culture of the vegetations was sterile, but those samples had been taken after 2 weeks of antibiotic treatment. Discharge from the hospital was on the 10th postoperative day. After discharge referral to the genetic department, the patient's dysmorphic features, mental retardation, and short stature resulted in a formal genetic diagnosis of Noonan syndrome. Transthoracic echocardiography, repeated at 1 and 6 postoperative months, revealed a fully competent pulmonary valve with a minimal residual gradient of 12 mmHg (Fig. 2); and the patient was in good health.
Fig. 1 Preoperative echocardiogram shows vegetations occupying the pulmonary arteries. Ao = aorta; PA = main pulmonary artery
Fig. 2 Postoperative echocardiogram shows clear and confluent pulmonary arteries. Ao = aorta; LPA = left pulmonary artery; MPA = main pulmonary artery; RPA = right pulmonary artery
Discussion
Various genetic diseases are associated with congenital heart defects, and these defects usually have decisive influence on patients' outcomes.4 Congenital heart defects are present in 85% of Noonan syndrome patients, in whom the most prevalent cardiac anomaly is pulmonary valve stenosis, with an incidence of 50% to 60%.5 Native pulmonary valve endocarditis is a very rare entity in childhood.
Right-heart endocarditis accounts for only 5% to 10% of all cases of infective endocarditis.6 Moreover, in patients with right-heart endocarditis, necropsy studies have shown vegetations to be 10 times more frequent in the tricuspid valve than in the pulmonary valve. Studies have reported isolated pulmonary valve endocarditis to be an extremely rare entity, accounting for approximately 1.5% to 2% of all admissions for endocarditis.7 The correlation between pulmonary valve stenosis and infective endocarditis is still controversial. Hypothetically, pulmonary valve stenosis can increase the risk of infective endocarditis by causing a jet flow, but this has never been proved; nowadays, Noonan patients with pulmonary valve stenosis are accepted as low risk, and antibiotic prophylaxis is no longer recommended.8 In a natural history study, only 1 pulmonary valve endocarditis case was observed among 592 patients with pulmonary valve stenosis.9 On the other hand, pulmonary artery vegetations are extremely rare. To our knowledge, the few other cases reported in the literature have been associated either with aortopulmonary window or patent ductus arteriosus.10–12
In regard to this specific case, we can speculate that stenosis secondary to a dysplastic, fibrotic pulmonary valve may have contributed to the progression of endocarditis and in this manner may have caused the vegetations that occupied the pulmonary arteries.
Although pulmonary stenosis is not considered a common predisposing factor for infective endocarditis, we conclude that a dysplastic valve can contribute to the progression and worsen the prognosis of infective endocarditis when vegetations remain undiagnosed and untreated.
Footnotes
Address for reprints: Ali Can Hatemi, MD, PhD, Abdi Ipekci Cad. Polat Saray Apt. 79/17 Macka, Sisli, 34365 Istanbul, Turkey
E-mail: hatemi@superonline.com
References
- 1.Akram M, Khan IA. Isolated pulmonic valve endocarditis caused by group B streprococcus [sic] (streptococcus agalactiae) a case report and literature review. Angiology 2001;52(3): 211–5. [DOI] [PubMed]
- 2.Allanson JE. Noonan syndrome. Am J Med Genet C Semin Med Genet 2007;145C(3):274–9. [DOI] [PubMed]
- 3.Jorge AAL, Malaquias AC, Arnhold IJ, Mendonca BB. Noo-nan syndrome and related disorders: a review of clinical features and mutations in genes of the RAS/MAPK pathway. Horm Res 2009;71:185–93. [DOI] [PubMed]
- 4.Hatemi AC, Gursoy M, Ceviker K, Tongut A, Cetin G, Celebi S, Kansiz E. Ventricular septal defect closure in a patient with VACTERL syndrome: anticipating sequelae in a rare genetic disorder. Tex Heart Inst J 2008;35(2):203–5. [PMC free article] [PubMed]
- 5.Sznajer Y, Keren B, Baumann C, Pereira S, Alberti C, Elion J, et al. The spectrum of cardiac anomalies in Noonan syndrome as a result of mutations in the PTPN11 gene. Pediatrics 2007;119(6):e1325-31. [DOI] [PubMed]
- 6.Farsak B, Yilmaz M, Oc M, Ozkutlu S, Demircin M. Non-valvular isolated pulmonary artery vegetations. Med Sci Monit 2002;8(4):CS39-41. [PubMed]
- 7.Cassling RS, Rogler WC, McManus BM. Isolated pulmonic valve infective endocarditis: a diagnostically elusive entity. Am Heart J 1985;109(3 Pt 1):558–67. [DOI] [PubMed]
- 8.Nishimura RA, Carabello BA, Faxon DP, Freed MD, Lytle BW, O'Gara PT, et al. ACC/AHA 2008 Guideline update on valvular heart disease: focused update on infective endocarditis: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;52(8):676–85. [DOI] [PubMed]
- 9.Gersony WM, Hayes CJ, Driscoll DJ, Keane JF, Kidd L, O'Fallon WM, et al. Bacterial endocarditis in patients with aortic stenosis, pulmonary stenosis, or ventricular septal defect. Circulation 1993;87(2 Suppl):I121-6. [PubMed]
- 10.Bain RC, Edwards JE, Scheifley CH, Geraci JE. Right-sided bacterial endocarditis and endarteritis; a clinical and pathologic study. Am J Med 1958;24(1):98–110. [DOI] [PubMed]
- 11.Unal M, Tuncer C, Serçe K, Bostan M, Gökçe M, Erem C. Non-valvular main pulmonary artery vegetation associated with aortopulmonary window. Acta Cardiol 1995;50(3):241–4. [PubMed]
- 12.Sadiq M, Latif F, Ur-Rehman A. Analysis of infective endarteritis in patent ductus arteriosus. Am J Cardiol 2004;93(4):513–5. [DOI] [PubMed]