Abstract
We recently introduced a new adjunct to myocardial preservation in patients with a patent left internal mammary artery graft who were undergoing reoperative cardiac surgery. The purpose of this study was to review our early experience with this technique.
The technique consists of preoperative insertion of a suitably sized angioplasty balloon catheter into the proximal part of the graft under fluoroscopic guidance. Intraoperative inflation of the balloon results in occlusion of the graft during aortic cross-clamping. We have used this technique in 9 patients. The case records of these patients were retrospectively reviewed.
There were 5 men and 4 women with a mean age of 71 ± 11 years and a mean Euro-SCORE of 10 ± 3. The median time interval from previous cardiac operation to reoperation was 6 years (interquartile range, 2–11 yr). After the balloon catheter was inserted successfully into the left internal mammary artery graft, the balloon was inflated intraoperatively for successful occlusion of the graft in all patients. There was no in-hospital death, and no significant complications were observed.
The early results of this technique seem favorable. A prospective randomized study is needed in order to evaluate the potential advantages of this method over other techniques of myocardial protection during cardiac reoperations.
Key words: Angioplasty balloon; catheterization, peripheral; coronary artery bypass; graft occlusion, vascular; internal mammary artery; reoperation/methods; vascular patency
Reoperative cardiac surgery is becoming increasingly common but is associated with a higher risk of perioperative morbidity and death than is primary surgery.1,2 This increased risk is partly due to the characteristics of the patient population, since patients undergoing reoperations tend to be older and have more comorbid conditions and worse cardiac function, but it is also due to the complexity and technical challenges of reoperations.1,2 One of the common technical challenges of cardiac reoperations is the dissection and external control of a patent left internal mammary artery (LIMA) graft in patients who have undergone previous coronary artery bypass surgery. Control of this artery becomes necessary when cardioplegic techniques are used for myocardial protection, because blood flowing through a patent graft would otherwise wash away the cardioplegic solution. Mobilization and external clamping of a patent LIMA graft can cause injury to the vessel, leading to adverse short- and long-term outcomes. Angioplasty balloon occlusion of the LIMA graft—an interesting alternative to external clamping—was first described in 2001 by Grinda and colleagues.3 The principle of the technique is preoperative placement of a suitably sized angioplasty balloon in the LIMA graft followed by intraoperative inflation of the balloon during aortic cross-clamping. The purpose of this retrospective study was to review our early experience with this technique.
Patients and Methods
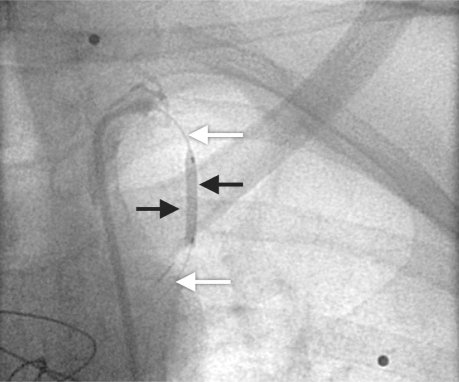
Over an 18-month period, from August 2004 through January 2006, we used the technique of angioplasty balloon occlusion of patent LIMA grafts in all suitable patients who underwent elective cardiac reoperations. All patients had previously undergone LIMA grafting of the left anterior descending coronary artery, and, in all cases, preoperative angiography had confirmed the patency of the graft. After signing informed-consent forms, the patients were taken to the cardiac catheterization laboratory immediately before reoperation. A common femoral approach was used, and systemic heparin (70 IU/kg) was administered. A 6F sheath was placed and an internal mammary artery (IMA) guide catheter (Medtronic Ltd.; Watford, UK) was inserted to cannulate the IMA and to obtain posteroanterior and left anterior oblique angiographic views of the IMA graft. The IMA size, estimated visually, varied from 2.5 to 3.5 mm in our patients. A Hi-Torque Balance 0.014-inch coronary guidewire (Abbott Laboratories; Abbott Park, Ill) was then positioned into the IMA, followed by an over-the-wire balloon that was 15 mm long with a nominal diameter of 2.5 to 3.5 mm. The balloon was briefly inflated at the nominal pressure (6 atm), and angiography was performed to ensure occlusion of the LIMA graft (Fig. 1). The balloon was then left, deflated, in a relatively straight segment of the proximal IMA, and the guidewire was removed under fluoroscopic guidance to prevent balloon displacement. Finally, the IMA guide catheter was partially withdrawn, proximal to the origin of the LIMA, to ensure that there would be no flow obstruction through the LIMA graft.
Fig. 1 A 15 × 2.5-mm balloon (black arrows) over the guidewire (white arrows) has been temporarily inflated in the proximal segment of the internal mammary artery graft.
The patients were then transferred to the anesthesia room for routine placement of monitoring lines and anesthetic induction, and subsequently were taken to the operating room. Resternotomy was begun, and the patients were placed on total cardiopulmonary bypass under moderate core hypothermia (32 °C). Aortic cross-clamping and intermittent antegrade cold-blood cardioplegic arrest were used in all patients for myocardial protection. Cardioplegic solution was administered either through a 12G aortic root cannula or directly into the coronary ostia after aortotomy for aortic valve surgery. Deep hypothermic circulatory arrest was not required in any patient. At the time of aortic cross-clamping, the LIMA balloon was inflated at nominal pressure (6 atm) to occlude the IMA. The balloon was deflated at the time of aortic cross-clamp release and then was removed in the intensive care unit.
The case records of the 9 patients were retrospectively examined. The data were presented as mean ± SD or as median and interquartile range when normal distribution could not be assumed.
Results
Nine patients, 5 men and 4 women, underwent cardiac reoperation. The mean age was 71 ± 11 years (range, 57–79 yr). The patients had a mean EuroSCORE of 10 ± 3. Left ventricular function was good in 5 patients, moderate in 3, and poor in 1. The median time from the 1st cardiac operation to reoperation was 6 years (interquartile range, 2–11 yr). Seven of the 9 reoperations involved valvular surgery on the aortic or mitral valve, or both. The nature of the primary and reoperative surgeries is presented in Table I.
TABLE I. Details of Primary Cardiac Operations and Reoperations
Endoluminal Occlusion. All IMA grafts were found to be patent, with no significant narrowing. The IMA balloon was inserted successfully in all cases, and no complications were encountered during preoperative catheterization. Satisfactory occlusion of LIMA flow with inflation of the endoluminal balloon was seen on preoperative angiography in all patients. Endoluminal occlusion of the LIMA graft was performed intraoperatively in all cases, and no patient required external occlusion of the graft. The cardiopulmonary bypass time was 142 ± 49 minutes, and the aortic cross-clamp time was 79 ± 44 minutes.
Postoperative Course. There were no in-hospital deaths and no incidents of myocardial infarction or cerebrovascular accident. No patient required an intra-aortic balloon pump; however, all patients required perioperative inotropic support. The patients were on ventilation for a mean duration of 18 ± 5 hours. The median stay in the intensive care unit was 2 days (range, 1–3 d). No false femoral artery aneurysm or any other vascular complications related to the preoperative catheterization were observed. One patient required placement of a permanent pacemaker after aortic valve replacement, mitral valve replacement, and coronary artery bypass grafting (CABG); 2 patients had transient renal failure that did not require renal replacement therapy; and 1 patient developed a leg-wound hematoma after aortic valve replacement and CABG. The median hospital stay was 12 days (interquartile range, 8–16 d).
Discussion
Myocardial protection during cardiac reoperation is crucial to short- and long-term outcomes. By far the most common technique used in such operations is aortic cross-clamping followed by the instillation of cardioplegic solution either through the aortic root or directly through the coronary ostia in aortic valve surgery. Although highly effective, this technique is potentially problematic in patients with patent mammary artery grafts, because cross-clamping does not prevent flow through the LIMA graft and hence allows washout of the cardioplegic solution. The most common approach to combat this effect is dissection and external clamping of the mammary artery graft. This technique of external occlusion is, however, associated with an incidence of arterial damage of up to 12.5%.2 For this reason, some surgeons have advocated the avoidance of cardioplegic arrest altogether, suggesting instead the use of hypothermic cross-clamp fibrillation or off-pump techniques for myocardial protection. Although these approaches are appropriate for patients who require repeat CABG, these techniques cannot always be applied in patients who require valvular surgery. For this subgroup, a number of alternatives to antegrade cardioplegia with mammary artery clamping have been described. For example, Byrne and associates4 proposed right thoracotomy and moderate hypothermia with fibrillatory cardiac arrest for reoperative mitral surgery, in order to avoid mobilization of the LIMA graft. Similarly, Kuralay and colleagues5 advocated deep systemic hypothermia without LIMA clamping to ensure myocardial protection during aortic valve surgery; others have suggested the use of continuous retrograde cardioplegia with moderate hypothermia.6
Although all these techniques have been shown to be technically feasible, there are few data concerning the long-term outcomes; hence, the optimal operative strategy is still in doubt. Given this uncertainty, we instituted the technique of endoluminal occlusion of the LIMA3 followed by aortic cross-clamping and antegrade cardioplegia via the coronary ostia for all appropriate reoperative patients. We selected this technique because of a number of perceived theoretical advantages over the other previously published approaches. For a start, our technique is equivalent to the standard method of external LIMA clamping—the only difference is that flow interruption is achieved via an intraluminal approach. This approach avoids unnecessary mobilization of the LIMA; moreover, external clamping of the IMA graft with the surrounding tissues appears cruder compared with endoluminal inflation of an appropriately sized balloon to a nominal pressure. In addition, we believe that that our technique has advantages over hypothermic techniques because it averts the potential adverse sequelae associated with systemic hypothermia. We also believe that this technique is superior to those involving continuous retrograde cardioplegia, because it avoids any retrograde flow of blood from the left main coronary ostia that might otherwise obscure the operative field.7 Our early experience with this method has been entirely satisfactory: there were no preoperative difficulties or complications, interruption of intraoperative LIMA flow was satisfactory, and the short-term outcome was excellent.
There are some potential problems associated with the technique that we describe. Heparin is administered to the patient preoperatively in order to place the guide catheter and balloon in the LIMA. This could increase perioperative blood loss, although this has not been our experience. Moreover, the administration of protamine while the guide catheter and the balloon catheter are still positioned in the LIMA could lead to thrombosis and damage to the graft. Removing the balloon and guide catheter in the operating theater before protamine administration might be a safer modification of the technique. Finally, there is a risk of movement or dislodgment of the balloon and guide catheter during movement of the patient from the catheterization laboratory to the operating theater or during surgery, especially if long-saphenous-vein harvesting from the ipsilateral leg is required.
Limitations. The number of patients involved in the study was small; however, these cases are uncommon, and it should be noted that this cohort represents the total patient population of a large supra-regional center over an 18-month period. In addition, we used solely clinical outcome measures to evaluate the efficacy of our technique. Although it would have been preferable to have obtained pre- and postoperative angiograms in order to prove that intraoperative endoluminal occlusion had no adverse effects on the LIMA, there are obvious ethical difficulties in performing unnecessary angiography. Finally, although we found excellent short-term outcomes in these patients, the long-term outcomes remain to be seen. The optimal strategy for myocardial protection in reoperative cardiac surgery patients with patent LIMA grafts requires study in a multicenter randomized controlled trial. However, in the absence of such a study at present, we advocate the use of endoluminal occlusion on the basis of our experience.
Footnotes
Address for reprints: Theodore Velissaris, MD, FRCS, Department of Cardiothoracic Surgery, Southampton General Hospital, Tremona Road, Southampton SO16 6YD, UK
E-mail: theovelissaris@googlemail.com
References
- 1.Yau TM, Borger MA, Weisel RD, Ivanov J. The changing pattern of reoperative coronary surgery: trends in 1230 consecutive reoperations. J Thorac Cardiovasc Surg 2000;120(1): 156–63. [DOI] [PubMed]
- 2.Borger MA, Rao V, Weisel RD, Floh AA, Cohen G, Feindel CM, et al. Reoperative coronary bypass surgery: effect of pa-tent grafts and retrograde cardioplegia. J Thorac Cardiovasc Surg 2001;121(1):83–90. [DOI] [PubMed]
- 3.Grinda JM, Latremouille CP, D'Attellis N, Berrebi A, Fabiani JN. Angioplasty balloon occlusion of internal thoracic artery in redo surgery in patients with coronary artery bypass operations. J Thorac Cardiovasc Surg 2001;122(1):182–3. [DOI] [PubMed]
- 4.Byrne JG, Aranki SF, Adams DH, Rizzo RJ, Couper GS, Cohn LH. Mitral valve surgery after previous CABG with functioning IMA grafts. Ann Thorac Surg 1999;68(6):2243–7. [DOI] [PubMed]
- 5.Kuralay E, Cingoz F, Gunay C, Oz BS, Kucukarslan N, Yil-dirim V, et al. Supraclavicular control of patent internal thoracic artery graft flow during aortic valve replacement. Ann Thorac Surg 2003;75(5):1422–8. [DOI] [PubMed]
- 6.Bar-El Y, Kophit A, Cohen O, Kertzman V. Continuous retrograde cardioplegia simplifies aortic valve replacement in the presence of a patent internal mammary artery. Ann Thorac Surg 2003;76(4):1337–8. [DOI] [PubMed]
- 7.Karavas AN, Byrne JG. Continuous retrograde cardioplegia simplifies aortic valve replacement in the presence of a patent internal mammary artery: reply. Ann Thorac Surg 2003;76 (4):1338. [DOI] [PubMed]