Abstract
We sought to critically assess the role of lipoprotein-associated phospholipase A2 (Lp-PLA2) in the prediction of cardiovascular events in primary and secondary prevention settings. The inclusion criteria for our study included population-based epidemiologic studies and the presence of clinical outcomes of interest, including atherosclerotic disease, coronary events, stroke, and cardiovascular death. Studies that lacked clinical outcomes or that involved animals were excluded. We included primary and secondary prevention studies of subjects in all ethnic groups and of either sex, with no age limitation. We searched MEDLINE, Google Scholar, and the Cochrane Library for studies with publication dates from January 1970 through July 2009, and we searched major cardiology meeting abstracts from 2000 through 2009. From each study, we used predictive ability—including relative risk, hazard ratio, odds ratio, and prevalence of high Lp-PLA2 levels, with adjustment—along with baseline population characteristics.
Of 33 studies that met our inclusion criteria, 30 showed a significant association between Lp-PLA2 and cardiovascular events. Most of the studies had been adjusted for major Framingham risk factors and other variables that might influence the effect under question. After multivariate adjustments in cohort and nested case-control studies, increased levels of Lp-PLA2 remained a significant predictor of cardiovascular events. The available body of evidence suggests that Lp-PLA2 is a reliable marker of risk for cardiovascular events.
Key words: Atherosclerosis/prevention & control, biological markers/blood, case-control studies, cohort studies, C-reactive protein, epidemiology, inflammation, lipoprotein-associated phospholipase A2, phospholipases A2, treatment outcome
Traditional risk factors can predict the risk of cardiovascular (CV) disease in many, but not all, patients. About 10% to 20% of persons with coronary heart disease (CHD) have no identifiable risk factor,1 and 35% of CHD deaths occur in patients who have total serum cholesterol levels of less than 200 mg/dL.2
Current risk-prediction methods (such as the Framingham risk table) are not able to accurately pinpoint which patient will develop a CV event, or at what time. Exhaustive research is under way to improve our ability to determine individuals' CV risks. Inflammation plays an important causal role in the development of atherosclerosis and its clinical complications3 (such as acute coronary syndrome [ACS], sudden cardiac death, acute myocardial infarction, unstable angina, and stroke). New diagnostic tests are being developed to identify individuals who are at high risk of inflammation.4 Several inflammatory markers, notably high-sensitivity C-reactive protein (hs-CRP)5 and white blood cells,6 have been studied as predictors of future CV events.
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is a novel inflammatory marker that has been the recent focus of multiple epidemiologic studies involving primary and secondary prevention populations.7 A growing body of evidence suggests that Lp-PLA2 is a CV risk marker independent of traditional risk factors like high-density-lipoprotein cholesterol (HDL-c), low-density-lipoprotein cholesterol (LDL-c), and hs-CRP. Lipoprotein-associated phospholipase A2, a 45-kDa protein with 441 amino acids, was cloned in 1995.8 The gene for Lp-PLA2 is located on chromosome 6p 21.2 to 12. Also known as platelet-activating factor acetylhydrolase (PAF-AH), Lp-PLA2 hydrolyzes the short acyl group at the Sn-2 position of the phospholipids in oxidized LDL, which leads to production of the pro-inflammatory compounds lyso-phosphatidylcholine and oxidized nonesterified fatty acids.9 Lipoprotein-associated phospholipase A2 is produced by several inflammatory cells (for example, monocytes, macrophages, T cells, hepatic Kupffer cells, and mast cells). In human plasma, it is associated mainly (70%–80%) with LDL particles, due to its affinity for the C-terminus of apolipoprotein B; the remaining 20%–30% is associated with HDL and other lipoproteins.10 Within LDL fractions, it shows more predilection for the denser LDL-4 and LDL-5, which are believed to be highly proatherogenic.11 Studies have also shown that oxidized phospholipids are chemotactic and promote monocyte–endothelial-cell interaction.12,13 A high level of circulating oxidized LDL has been shown to increase plaque vulnerability and mediate endothelial dysfunction, which can partly explain its higher levels in ACS patients.14,15
Recent reports suggest that human beings and rabbits with elevated Lp-PLA2 have higher plaque burden in the coronary arteries.16 The reduction of atherosclerotic lesions in hyperlipidemic rabbits, by inhibiting Lp-PLA2, supports the notion that Lp-PLA2 enhances atherosclerosis.16 Lipoprotein-associated phospholipase A2 is also expressed within the necrotic core and apoptotic macrophages of vulnerable and ruptured plaque in human coronary atheroma, which suggests its active role in acute coronary syndrome.17 Moreover, high levels of Lp-PLA2 mRNA have been noted in carotid plaque.
During myocardial ischemia, membrane phospholipids may undergo hydrolysis under stress, leading to accumulation of arachidonic acid and lysophophatidylcholine.18,19 Lipoprotein-associated phospholipase A2 levels are shown to be up-regulated by endotoxins.20,21 The proatherogenic contribution of Lp-PLA2 has been much studied; however, its anti-inflammatory and antiatherogenic potential should not go unnoticed.7
Platelet-activating factor is prothrombotic and a mediator of inflammation; platelet-activating factor acetylhydrolase hydrolyzes PAF, thereby negating its proinflammatory and prothrombotic effects.22 The opposing actions of Lp-PLA2 (PAF-AH) have led to a debate concerning which action is more potent and therefore warrants more attention. Initially, PAF-AH was thought to be atheroprotective by reducing platelet-activating factor.23,24 However, subsequent studies have shown its role in inflammation: lysophosphatidyl choline stimulates macrophage proliferation, up--regulates cytokines and CD40 ligands, and increases the expression of vascular adhesion molecules.25,26 This has led most researchers to believe that the proatherogenic effect of Lp-PLA2 has a greater importance in CV disease prevention and management.
Because of its low biologic fluctuation, high vascular specificity, and indication (at elevated levels) of plaque inflammation and endothelial dysfunction, Lp-PLA2 is useful in identifying patients at high risk of CHD. A recent report27 suggests that an Lp-PLA2 level >235 ng/mL in healthy populations and >225 ng/mL in clinical populations is a high-risk marker for CV events, and that Lp-PLA2 should be measured in patients who are at moderate to very high risk of CV events. An elevated Lp-PLA2 level also predicts increased risk of myocardial infarction and stroke in population studies that are fully adjusted for other CV risk factors. More epidemiologic and clinical studies in the future can help clarify the effect of Lp-PLA2 on atherosclerosis-related CV risk.
Secretory PLA2
Secretory PLA2 (sPLA2)—another enzyme from the phospholipase A2 family—has been the focus of many studies for its role in predicting coronary artery disease (CAD).28 Secretory PLA2 is an acute-phase reactant, the activity of which is up-regulated by other inflammatory markers.26 Unlike Lp-PLA2, its levels correlate with hs-CRP. Secretory PLA2 is not widely used in clinical practice; one explanation for this could be its nonspecific predictive ability. However, ongoing and future studies will help shed more light on the predictive potential of sPLA2 and its potential in comparison with Lp-PLA2.
The purpose of this particular review is to critically assess the role of Lp-PLA2 in the prediction of CV events (with a focus on coronary heart disease) in primary and secondary prevention settings.
Methods
The protocol for this review of the literature was designed prospectively to define study objectives, search strategy, patient populations of interest, study-selection criteria and methods for determining study eligibility, outcomes of interest, data elements to be abstracted and methods for abstraction, and methods for the assessment of study quality. Our review included studies on subjects of either sex, with no age limitation. Studies in all ethnic groups are included. Primary and secondary prevention studies are included.
Inclusion Criteria. In order to be included, all studies had to be population based and epidemiologic; further, they had to manifest clinical outcomes of interest (that is, atherosclerotic disease, coronary events, stroke, and CV death). We excluded studies that did not meet these inclusion criteria, as well as animal studies.
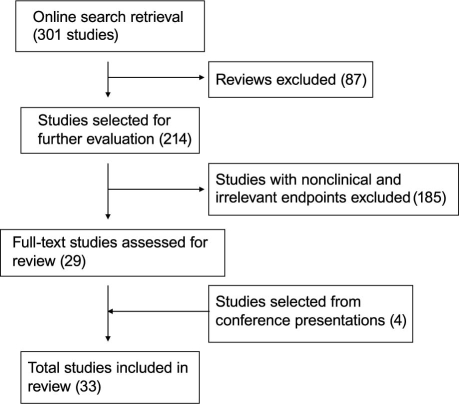
MEDLINE®, Google™ Scholar, the Cochrane Library, and the abstract books of major cardiology meetings were searched. Additional studies were sought and identified by reviewing the references of included articles. Figure 1 depicts the flow chart of the study's manuscript identification and selection process.
Fig. 1 Flow chart of the study's manuscript identification and selection process.
The MEDLINE database was searched using PubMed (National Library of Medicine). The search included all articles published from January 1970 through July 2009 that included at least 1 of the following key words in their titles, abstracts, MeSH headings and subheadings (including single words and phrases), or keyword lists:
lipoprotein-associated phospholipase A2 or platelet-activating factor acetylhydrolase (alternatively: Lp-PLA2 or PAF-AH)
AND
cardiovascular diseases or coronary disease or myocardial infarction or stroke or sudden cardiac death
The PubMed search was limited to “humans” and to publication dates from 1970/01/01 to 2009/07/29.
Abstracts presented at the 2000–2009 meetings of the American Heart Association, American College of Cardiology, and European Society of Cardiology were also searched using the keywords lipoprotein-associated phospholipase A2 and platelet-activating factor acetylhydrolase; then the searched results were screened, if they included CV events.
Each study was initially evaluated for its potential to cover any of the topics of interest and was studied in regard to all potentially relevant study-selection criteria. We included prospective and retrospective cohort studies, case-control studies, and nested case-control and case-cohort studies.
Description of Studies
From each study, we abstracted the predictive ability (for example, relative risk [RR], hazard ratio [HR], and odds ratio [OR]) and the prevalence of high Lp-PLA2 levels, along with baseline population characteristics and the effects of adjustment for established coronary risk factors.
Critical Appraisal of Selected Studies
The quality and validity of the studies were evaluated using the Critical Appraisal Tools guidelines developed by the Public Health Resource Unit of the United Kingdom National Health Services.29 The same critical appraisal tools were used to assess the cohort studies, the nested case-control studies, and the case-cohort studies.
Results
Total retrieval from our PubMed search was 301 references at the time of the study. After searching all described databases regarding the relationship between Lp-PLA2 and CV disease, we were left with 33 articles (18 cohort, 6 nested case-control or case-cohort, and 9 case-control studies) that met the study-selection criteria for inclusion in this systematic review.
A total of 52,995 subjects participated in 33 studies. Most of the participants were men, with an approximate age range of 21 to 84 years. The participants represented multiethnic backgrounds, and the studies were conducted in multinational centers. The follow-up period ranged from as short as 42 days to as long as 16 years. Sixteen studies assessed Lp-PLA2 mass assay, 11 studies assessed Lp-PLA2 activity assay, and 6 studies assessed both.
For the cohort and nested case-control studies, we abstracted data on the number of enrolled subjects, number of cases and controls, demographics of study subjects, definition of cases, list of Framingham risk factors for which the analyses were adjusted, list of other variables included in multivariate analyses, duration of follow-up, and major results after full adjustment for the described variables. After adjustment in cohort and nested case-control studies, the risk ratio and ORs ranged roughly between 1.40 and 2.00, provided that the relationship was statistically significant.
For case-control studies, we abstracted data on the number of cases and controls, demographics of study subjects, definition of cases and controls, list of Framingham risk factors for which the analyses were adjusted, list of other variables included in multivariate analyses, and major statistical findings (after full adjustment, when applicable).
These studies are abstracted in Tables I, II, and III.30–62
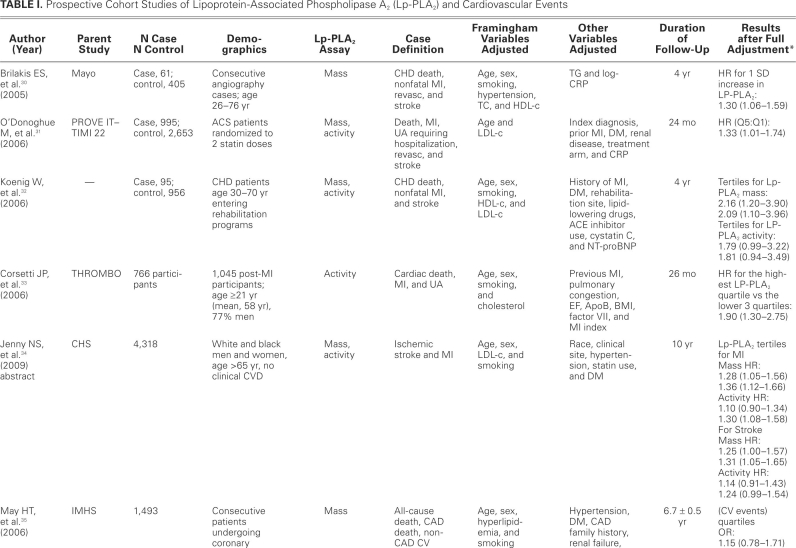
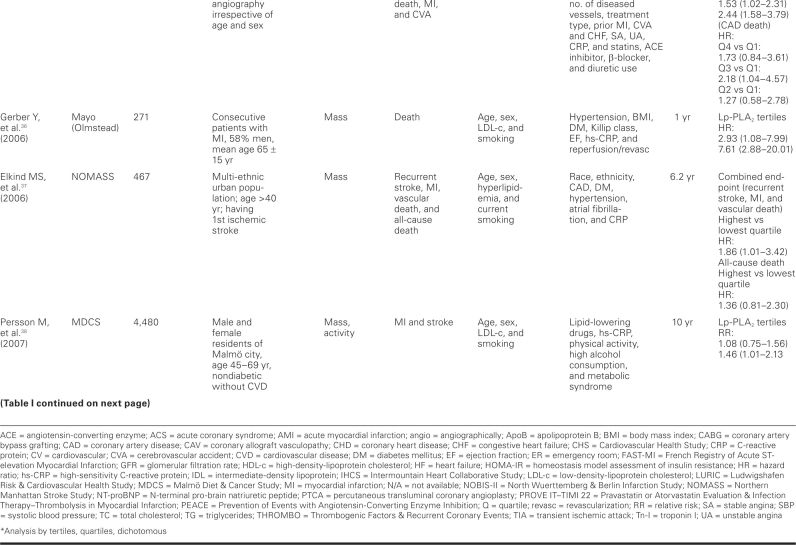
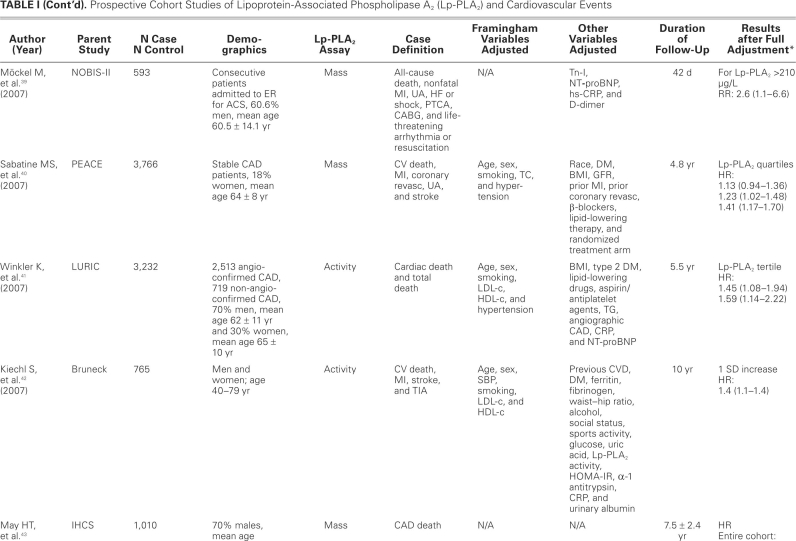
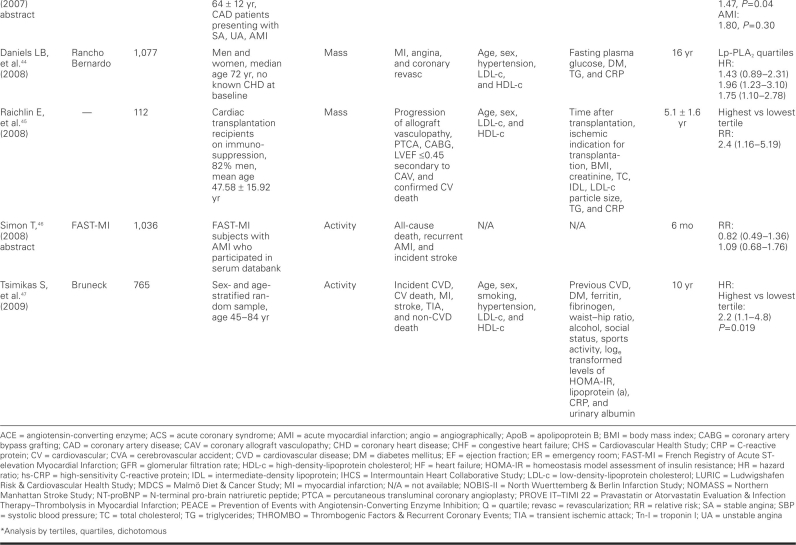
TABLE I. Prospective Cohort Studies of Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) and Cardiovascular Events
TABLE I. continued
TABLE I. continued
TABLE I. continued
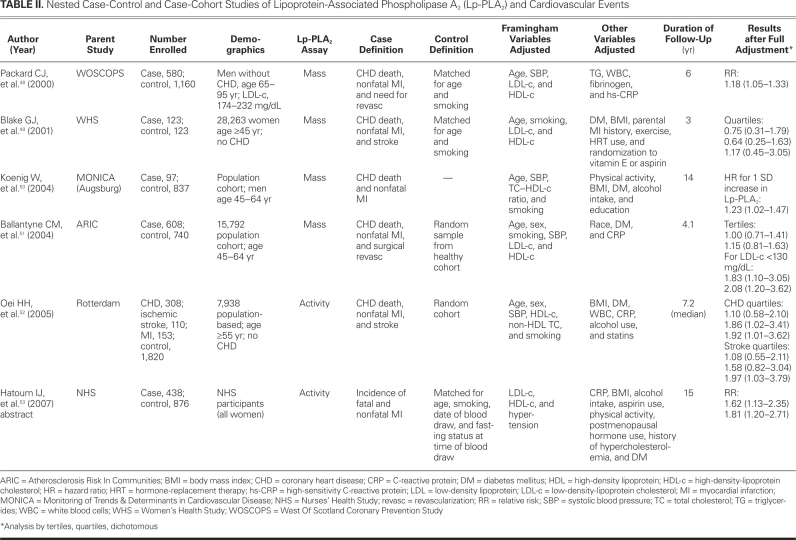
TABLE II. Nested Case-Control and Case-Cohort Studies of Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) and Cardiovascular Events
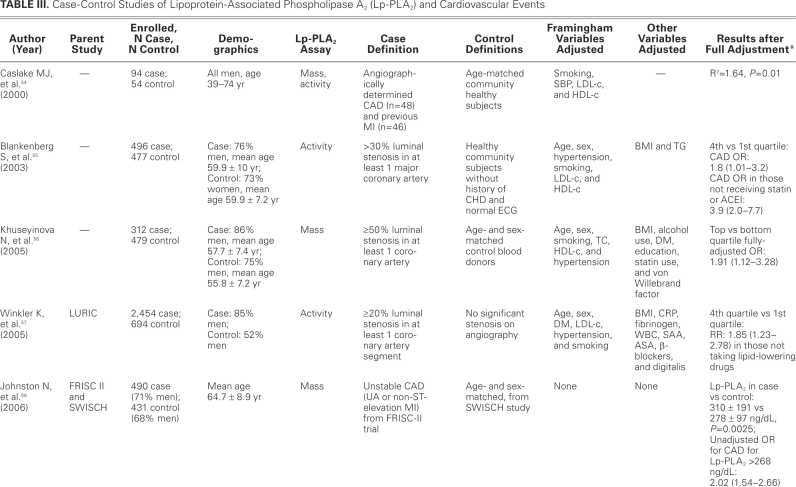
TABLE III. Case-Control Studies of Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) and Cardiovascular Events
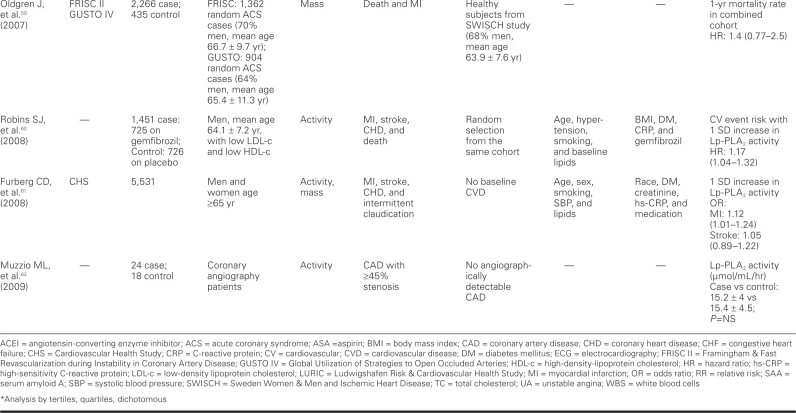
TABLE III. continued
Discussion
Although our case-control studies support a relationship between Lp-PLA2 and CV disease, the evidence from cohort and nested case-control studies is more reliable and provides more robust evidence in support of such a relationship. Because data from a good number of cohort and nested case-control studies are available, we focus our discussion on the data from these studies.
Most of the studies showed a positive association between Lp-PLA2 and CV events, but a few did not. One such study, by Blake and colleagues,49 failed to show a relationship between Lp-PLA2 levels and CV incidents; this may have been due to lack of power to detect such a relationship, because this study included the lowest number of subjects, and no power calculation was provided by the authors. The study included only middle-aged women, with the confounding effect of hormonal therapy, and the participants were few in number (123 cases, 123 controls). Lower-risk women were also included in this study. However, women with very high levels of Lp-PLA2 had increased risk of future CV events, as noted in the other trials. Oldgren and colleagues,59 in FRISC II and GUSTO IV, failed to find an association, in ACS patients, between Lp-PLA2 mass measurement and future CV events, including death, within 6 months to 1 year of follow-up. Similar results were found when measured early after an ACS event in the PROVE IT–TIMI 22 trial31; but at 30 days after the event, Lp-PLA2 activity in the highest quintile was associated with increased risk of additional CV events, when compared with the lowest quintile (P=0.002). Finally, a recent case-control study failed to show any difference in Lp-PLA2 activity between patients with CAD and without CAD.62
Limitations of the aforementioned negative studies include retrospective analysis of patients, reduction in Lp-PLA2 activity during the 1st few days after ACS,63 decrease in Lp-PLA2 activity by statin use, Lp-PLA2 measurement in frozen plasma, and effect of long-term storage on the level of Lp-PLA2. We should keep in mind, though, that a bias toward publishing positive findings and toward withholding negative findings might have prevented the publication of other studies with negative findings. Limitations in some of the positive-association studies include small sample size, retrospective nature, and the study of stored, frozen samples. However, recent studies have shown stability of Lp-PLA2 in properly stored samples.
All other published studies that we included in our analysis showed a significant association between Lp-PLA2 serum levels and CV events. These studies described the size of the effect by different measures of association, such as comparing HRs for the top tertiles or quartiles with those for the reference bottom tertiles or quartiles, or comparing HRs for 1 standard deviation64 increase in Lp-PLA2. Most of the studies did adjust for major Framingham risk factors and other variables that are believed to influence the effect in question, especially LDL-c and CRP. After investigators fully adjusted for potential confounders in cohort and nested case--control studies, the RRs and ORs ranged roughly between 1.40 and 2.00, provided that the relationship was statistically significant. This range of effect size is in accordance with the effect size observed in studies of newly emerging coronary risk markers. Lipoprotein-associated phospholipase A2 significantly retained its independence for prediction of CAD and also, in some cases, for prediction of ACS, after adjusting for LDL-c, CRP, and other variables; in some studies, however, the association weakened after adjustment. Lipoprotein-associated phospholipase A2 levels were in positive correlation with total cholesterol and LDL-c, while most of the studies showed a negative correlation with HDL. Lipoprotein--associated phospholipase A2 showed no correlation with CRP levels, except for 2 studies that showed a weakly positive correlation.40,44 Folsom and colleagues65 studied the influence of inflammatory markers, in addition to traditional risk factors, in the prediction of CAD. The traditional risk-factor model showed CAD prediction with a receiver operating characteristic area under curve of 0.8, but the addition of Lp-PLA2 showed a statistically significant increase in the area under curve. Notably, hs-CRP—an acute-phase reactant that increases both in obesity and in acute and chronic systemic inflammation—did not significantly increase the area under curve. Recently, it has been suggested that hs-CRP may be more than a simple marker of atherosclerosis. It can bind to oxidized LDL and activate the complement.66,67 When compared with hs-CRP, Lp-PLA2 in some studies is suggested to be a more promising marker of risk prediction. Winkler and associates41 showed that, in moderate-risk patients (Framingham risk, 10%–20%) with hs-CRP <3 mg/L, increased Lp-PLA2 levels doubled the HR for cardiac death. Similarly, the WOSCOPS study48 showed that in multivariate analysis, Lp-PLA2 was significantly associated with risk, compared with hs-CRP (P=0.005 vs P=0.09). However, further trials are needed to confirm an incremental prognostic value of Lp-PLA2 over hs-CRP. While Lp-PLA2 at this time could be speculated to have better specificity than CRP, evidence of better risk prediction is increasing when the two complement one another. The ARIC trial51 showed that low-to--moderate levels of both markers in combination significantly increase the risk, in comparison with a combination either of high Lp-PLA2 and low CRP or of high CRP and low Lp-PLA2. The MONICA study50 also showed better risk prediction when high Lp-PLA2 and high CRP were combined, when compared with each used alone (HR, 1.93; 95% confidence interval, 1.09–3.4). The IHCS trial43 revealed better prediction of CAD death with combinations of high CRP/low Lp-PLA2 and high CRP/high Lp-PLA2—but not with combinations of low CRP/low Lp-PLA2 and low CRP/high Lp-PLA2.
The association of Lp-PLA2 and CV events was consistent across a wide variety of subjects of both sexes, from different ethnic backgrounds and different countries. This relationship was observed in both primary and secondary prevention studies (that is, in subjects without and with clinically apparent disease at baseline). A dose–response relationship was observed in multiple studies, with higher risks associated with higher tertiles or quartiles of Lp-PLA2, after adjustment for the full range of known confounders. The absolute cutoff value of Lp-PLA2 is not clear at this time. It is different in women, smokers, and the elderly, compared with men, nonsmokers, and the young. Because these studies used different cutoff points, it is impossible (and outside the scope of this study) for us to determine the best cutoff value for Lp-PLA2 for the purpose of CV risk prediction. A consensus panel has recently proposed a set of cutoff points for interpreting Lp-PLA2 levels in clinical settings.68 According to the recommendations, a cutoff point of 235 ng/mL for Lp-PLA2 mass concentration should be lowered to 200 ng/mL, values above which would place patients in a high-risk category. Whether lowering the cutoff level of Lp-PLA2 will accord with the actual risk in these patients has not been proved in clinical trials.
Lipoprotein-associated phospholipase A2 is an enzyme that can be measured by either its mass or its activity.7 Sixteen studies included in this review calculated mass and 12 studies calculated activity, while 6 calculated both mass and activity. There is still controversy about the method of estimating Lp-PLA2 level. Only modest correlation has been found between Lp-PLA2 activity and Lp-PLA2 mass. Koenig and colleagues32 showed that Lp-PLA2 mass in multivariate analysis predicted increased risk of future CV events, when compared with Lp-PLA2 activity (4-yr follow-up, 1,051 patients). Persson and associates38 reported that Lp-PLA2 activity, but not mass, was associated with increased risk of CV disease. Jenny and co-authors,34 in their study, showed no difference in Lp-PLA2 activity and mass with respect to risk prediction. O'Donoghue and the PROVE IT–TIMI 22 trial investigators31 showed a moderate correlation between Lp-PLA2 activity and mass, both at baseline (r=0.35, P <0.001) and at 30-day follow-up (r=0.36, P <0.001). Recently, the United States Food and Drug Administration has approved the Immunoassay (PLAC test), which measures Lp-PLA2 mass, for screening patients who are at high risk for CV disease. A large-scale, randomized, multicenter trial is needed for a head-to-head comparison, to determine which assay method is the better predictor of future events.
On the basis of our evaluation of the studies included in this review, we can state that Lp-PLA2 fulfills most, but not all, of the criteria69 set forth by the American Heart Association for the evaluation of a novel risk marker. None of these studies, however, has evaluated the accuracy of Lp-PLA2, its ability to reclassify risk, the net effect that such reclassification would have, the fit of the model, or the cost-effectiveness. This needs to be done in future studies specifically designed to examine these issues.
The identification of Lp-PLA2 as a risk marker for CVD is consistent with our current understanding of atherosclerosis as an inflammatory disease. The available body of evidence suggests that Lp-PLA2 is a reliable marker of risk for CV events. A fundamental question remains: Is Lp-PLA2 a simple marker or does it have a causal impact on atherosclerosis? The relationship between Lp-PLA2 and CV events is consistent, strong, graded, and biologically plausible, thereby meeting a few (but not all) of several criteria proposed by Sir Bradford Hill to evaluate a cause–effect relationship.70 Obviously, it will be crucial to show that inhibition of Lp-PLA2 causes stabilization or regression of atherosclerosis and, preferably, that it prevents CV events. Recently, 2 randomized, placebo-controlled clinical trials studied the effects, in the vascular wall and systemic circulation, of the Lp-PLA2-specific inhibitor darapladib. Serruys and coworkers,71 in their trial, showed a 59% reduction in Lp-PLA2 levels in the darapladib-treated group (P <0.001 vs placebo) and a significant increase in necrotic core volume in the placebo group (P=0.009), when compared with the darapladib-treated group (P=0.71). Mohler and colleagues72 found a 66% decrease in Lp-PLA2 levels in the darapladib-treated group (P <0.001 vs placebo). Statins and fibrates are also shown to decrease Lp-PLA2 activity by 20% to 30% without an effect on de novo synthesis and secretion of Lp-PLA2 by macrophages.73 Whether inhibiting or decreasing its activity by the use of these drugs will lead to a decrease in CV events or death remains to be proved in clinical trials. Recently, a consensus panel has recommended decreasing LDL-c by 30 mg/dL in patients with high levels of Lp-PLA2, regardless of baseline LDL-c.68
If the reversibility criterion is met by future studies, a cause–effect relationship can be considered for Lp-PLA2 and CV disease, which is a multicausal disease with a complex pathogenesis.74
Footnotes
Address for reprints: Mohammad Madjid, MD, MSc, Texas Heart Institute, 6770 Bertner Ave., MC 2-255, Houston, TX 77030
E-mail: mmadjid@gmail.com
Dr. Madjid has received research support and honoraria from GlaxoSmithKline.
References
- 1.Khot UN, Khot MB, Bajzer CT, Sapp SK, Ohman EM, Brener SJ, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA 2003;290(7):898–904. [DOI] [PubMed]
- 2.Castelli WP. Lipids, risk factors and ischaemic heart disease. Atherosclerosis 1996;124 Suppl: S1–9. [DOI] [PubMed]
- 3.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med 1999;340(2):115–26. [DOI] [PubMed]
- 4.Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation 2004;109(21 Suppl 1):II2–10. [DOI] [PubMed]
- 5.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation 1998;98(8):731–3. [DOI] [PubMed]
- 6.Madjid M, Awan I, Willerson JT, Casscells SW. Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol 2004;44(10):1945–56. [DOI] [PubMed]
- 7.Ali M, Madjid M. Lipoprotein-associated phospholipase A2: a cardiovascular risk predictor and a potential therapeutic target. Future Cardiol 2009;5(2):159–73. [DOI] [PubMed]
- 8.Tjoelker LW, Wilder C, Eberhardt C, Stafforini DM, Dietsch G, Schimpf B, et al. Anti-inflammatory properties of a platelet-activating factor acetylhydrolase. Nature 1995;374(6522): 549–53. [DOI] [PubMed]
- 9.Zalewski A, Macphee C. Role of lipoprotein-associated phospholipase A2 in atherosclerosis: biology, epidemiology, and possible therapeutic target. Arterioscler Thromb Vasc Biol 2005;25(5):923–31. [DOI] [PubMed]
- 10.Karabina SA, Liapikos TA, Grekas G, Goudevenos J, Tselepis AD. Distribution of PAF-acetylhydrolase activity in human plasma low-density lipoprotein subfractions. Biochim Biophys Acta 1994;1213(1):34–8. [DOI] [PubMed]
- 11.Gazi I, Lourida ES, Filippatos T, Tsimihodimos V, Elisaf M, Tselepis AD. Lipoprotein-associated phospholipase A2 activity is a marker of small, dense LDL particles in human plasma. Clin Chem 2005;51(12):2264–73. [DOI] [PubMed]
- 12.Subbanagounder G, Leitinger N, Schwenke DC, Wong JW, Lee H, Rizza C, et al. Determinants of bioactivity of oxidized phospholipids. Specific oxidized fatty acyl groups at the sn-2 position. Arterioscler Thromb Vasc Biol 2000;20(10):2248–54. [DOI] [PubMed]
- 13.Subbanagounder G, Wong JW, Lee H, Faull KF, Miller E, Witztum JL, Berliner JA. Epoxyisoprostane and epoxycyclopentenone phospholipids regulate monocyte chemotactic protein-1 and interleukin-8 synthesis. Formation of these oxidized phospholipids in response to interleukin-1beta. J Biol Chem 2002;277(9):7271–81. [DOI] [PubMed]
- 14.Nishi K, Itabe H, Uno M, Kitazato KT, Horiguchi H, Shinno K, Nagahiro S. Oxidized LDL in carotid plaques and plasma associates with plaque instability. Arterioscler Thromb Vasc Biol 2002;22(10):1649–54. [DOI] [PubMed]
- 15.Ehara S, Ueda M, Naruko T, Haze K, Itoh A, Otsuka M, et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation 2001;103(15):1955–60. [DOI] [PubMed]
- 16.Hakkinen T, Luoma JS, Hiltunen MO, Macphee CH, Milliner KJ, Patel L, et al. Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase, is expressed by macrophages in human and rabbit atherosclerotic lesions. Arterioscler Thromb Vasc Biol 1999;19(12):2909–17. [DOI] [PubMed]
- 17.Kolodgie FD, Burke AP, Skorija KS, Ladich E, Kutys R, Makuria AT, Virmani R. Lipoprotein-associated phospholipase A2 protein expression in the natural progression of human coronary atherosclerosis. Arterioscler Thromb Vasc Biol 2006;26(11):2523–9. [DOI] [PubMed]
- 18.Sen A, Buja LM, Willerson JT, Chien KR. Membrane phospholipid metabolism during myocardial ischaemia: past, present and future. Basic Res Cardiol 1987;82 Suppl 1:121–5. [DOI] [PubMed]
- 19.Chien KR, Willerson JT, Buja LM. Phospholipid alterations and membrane injury during myocardial ischemia. Adv Myocardiol 1985;5:347–53. [DOI] [PubMed]
- 20.Watanabe J, Marathe GK, Neilsen PO, Weyrich AS, Harrison KA, Murphy RC, et al. Endotoxins stimulate neutrophil adhesion followed by synthesis and release of platelet-activating factor in microparticles. J Biol Chem 2003;278(35):33161–8. [DOI] [PubMed]
- 21.Memon RA, Fuller J, Moser AH, Feingold KR, Grunfeld C. In vivo regulation of plasma platelet-activating factor acetylhydrolase during the acute phase response. Am J Physiol 1999; 277(1 Pt 2):R94–103. [DOI] [PubMed]
- 22.Castro Faria Neto HC, Stafforini DM, Prescott SM, Zimmerman GA. Regulating inflammation through the anti-inflammatory enzyme platelet-activating factor-acetylhydrolase. Mem Inst Oswaldo Cruz 2005;100 Suppl 1:83–91. [DOI] [PubMed]
- 23.Morgan EN, Boyle EM Jr, Yun W, Kovacich JC, Canty TG Jr, Chi E, et al. Platelet-activating factor acetylhydrolase prevents myocardial ischemia-reperfusion injury. Circulation 1999;100(19 Suppl):II365–8. [DOI] [PubMed]
- 24.Theilmeier G, De Geest B, Van Veldhoven PP, Stengel D, Michiels C, Lox M, et al. HDL-associated PAF-AH reduces endothelial adhesiveness in apoE-/- mice. FASEB J 2000;14 (13):2032–9. [DOI] [PubMed]
- 25.Kume N, Gimbrone MA Jr. Lysophosphatidylcholine transcriptionally induces growth factor gene expression in cultured human endothelial cells. J Clin Invest 1994;93(2):907–11. [DOI] [PMC free article] [PubMed]
- 26.Tselepis AD, John Chapman M. Inflammation, bioactive lipids and atherosclerosis: potential roles of a lipoprotein-associated phospholipase A2, platelet activating factor-acetylhydrolase. Atheroscler Suppl 2002;3(4):57–68. [DOI] [PubMed]
- 27.Lanman RB, Wolfert RL, Fleming JK, Jaffe AS, Roberts WL, Warnick GR, McConnell JP. Lipoprotein-associated phospholipase A2: review and recommendation of a clinical cut point for adults. Prev Cardiol 2006;9(3):138–43. [DOI] [PubMed]
- 28.Boekholdt SM, Keller TT, Wareham NJ, Luben R, Bingham SA, Day NE, et al. Serum levels of type II secretory phospholipase A2 and the risk of future coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study. Arterioscler Thromb Vasc Biol 2005;25(4):839–46. [DOI] [PubMed]
- 29.Critical Appraisal Tools. Public Health Resource Unit. Available at: http://www.phru.nhs.uk/Pages/PHD/resources.htm
- 30.Brilakis ES, McConnell JP, Lennon RJ, Elesber AA, Meyer JG, Berger PB. Association of lipoprotein-associated phospholipase A2 levels with coronary artery disease risk factors, angiographic coronary artery disease, and major adverse events at follow-up. Eur Heart J 2005;26(2):137–44. [DOI] [PubMed]
- 31.O'Donoghue M, Morrow DA, Sabatine MS, Murphy SA, McCabe CH, Cannon CP, Braunwald E. Lipoprotein-associated phospholipase A2 and its association with cardiovascular outcomes in patients with acute coronary syndromes in the PROVE IT-TIMI 22 (PRavastatin Or atorVastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction) trial. Circulation 2006;113(14):1745–52. [DOI] [PubMed]
- 32.Koenig W, Twardella D, Brenner H, Rothenbacher D. Lipoprotein-associated phospholipase A2 predicts future cardiovascular events in patients with coronary heart disease independently of traditional risk factors, markers of inflammation, renal function, and hemodynamic stress. Arterioscler Thromb Vasc Biol 2006;26(7):1586–93. [DOI] [PubMed]
- 33.Corsetti JP, Rainwater DL, Moss AJ, Zareba W, Sparks CE. High lipoprotein-associated phospholipase A2 is a risk factor for recurrent coronary events in postinfarction patients. Clin Chem 2006;52(7):1331–8. [DOI] [PubMed]
- 34.Jenny NS, Solomon C, Cushman M, Tracy RP, Nelson JJ, Psaty BM, Furberg CD. Lipoprotein-associated phospholipase A2 (Lp-PLA2) and risk of cardiovascular disease in older adults: results from the Cardiovascular Health Study [abstract]. doi:10.1016/j.atherosclerosis/2009.09.021. [DOI] [PMC free article] [PubMed]
- 35.May HT, Horne BD, Anderson JL, Wolfert RL, Muhlestein JB, Renlund DG, et al. Lipoprotein-associated phospholipase A2 independently predicts the angiographic diagnosis of coronary artery disease and coronary death. Am Heart J 2006;152 (5):997–1003. [DOI] [PubMed]
- 36.Gerber Y, McConnell JP, Jaffe AS, Weston SA, Killian JM, Roger VL. Lipoprotein-associated phospholipase A2 and prognosis after myocardial infarction in the community. Arterioscler Thromb Vasc Biol 2006;26(11):2517–22. [DOI] [PubMed]
- 37.Elkind MS, Tai W, Coates K, Paik MC, Sacco RL. High-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2, and outcome after ischemic stroke. Arch Intern Med 2006;166(19):2073–80. [DOI] [PubMed]
- 38.Persson M, Hedblad B, Nelson JJ, Berglund G. Elevated Lp-PLA2 levels add prognostic information to the metabolic syndrome on incidence of cardiovascular events among middle-aged nondiabetic subjects. Arterioscler Thromb Vasc Biol 2007;27(6):1411–6. [DOI] [PubMed]
- 39.Mockel M, Muller R, Vollert JO, Muller C, Danne O, Gareis R, et al. Lipoprotein-associated phospholipase A2 for early risk stratification in patients with suspected acute coronary syndrome: a multi-marker approach: the North Wuerttemberg and Berlin Infarction Study-II (NOBIS-II). Clin Res Cardiol 2007;96(9):604–12. [DOI] [PubMed]
- 40.Sabatine MS, Morrow DA, O'Donoghue M, Jablonksi KA, Rice MM, Solomon S, et al. Prognostic utility of lipoprotein-associated phospholipase A2 for cardiovascular outcomes in patients with stable coronary artery disease. Arterioscler Thromb Vasc Biol 2007;27(11):2463–9. [DOI] [PubMed]
- 41.Winkler K, Hoffmann MM, Winkelmann BR, Friedrich I, Schafer G, Seelhorst U, et al. Lipoprotein-associated phospholipase A2 predicts 5-year cardiac mortality independently of established risk factors and adds prognostic information in patients with low and medium high-sensitivity C-reactive protein (the Ludwigshafen risk and cardiovascular health study). Clin Chem 2007;53(8):1440–7. [DOI] [PubMed]
- 42.Kiechl S, Willeit J, Mayr M, Viehweider B, Oberhollenzer M, Kronenberg F, et al. Oxidized phospholipids, lipoprotein(a), lipoprotein-associated phospholipase A2 activity, and 10-year cardiovascular outcomes: prospective results from the Bruneck study. Arterioscler Thromb Vasc Biol 2007;27(8):1788–95. [DOI] [PubMed]
- 43.May HT, Anderson JL, Horne BD, Pearson RR, Wolfert RL, Kolek MJ, et al. Lipoprotein-associated phospholipase A2 and high sensitivity C-reactive protein levels and the prediction of cardiovascular risk among coronary artery disease patients with differing clinical patients [abstract]. Circulation 2007;116:II 799–800.
- 44.Daniels LB, Laughlin GA, Sarno MJ, Bettencourt R, Wolfert RL, Barrett-Connor E. Lipoprotein-associated phospholipase A2 is an independent predictor of incident coronary heart disease in an apparently healthy older population: the Rancho Bernardo Study. J Am Coll Cardiol 2008;51(9):913–9. [DOI] [PMC free article] [PubMed]
- 45.Raichlin E, McConnell JP, Bae JH, Kremers WK, Lerman A, Frantz RP. Lipoprotein-associated phospholipase A2 predicts progression of cardiac allograft vasculopathy and increased risk of cardiovascular events in heart transplant patients. Transplantation 2008;85(7):963–8. [DOI] [PMC free article] [PubMed]
- 46.Simon T. Impact of circulating secretory phospholipase A2 (sPLA2) and lipoprotein-associated phospholipase A2 (Lp-PLA2) activities on 6 months-survival, recurrent AMI and incident stroke in patients with AMI [poster]. Presented at: ESC Congress 2008; Munich, Germany.
- 47.Tsimikas S, Willeit J, Knoflach M, Mayr M, Egger G, Notdurfter M, et al. Lipoprotein-associated phospholipase A2 activity, ferritin levels, metabolic syndrome, and 10-year cardiovascular and non-cardiovascular mortality: results from the Bruneck study. Eur Heart J 2009;30(1):107–15. [DOI] [PubMed]
- 48.Packard CJ, O'Reilly DS, Caslake MJ, McMahon AD, Ford I, Cooney J, et al. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland Coronary Prevention Study Group. N Engl J Med 2000;343(16):1148–55. [DOI] [PubMed]
- 49.Blake GJ, Dada N, Fox JC, Manson JE, Ridker PM. A prospective evaluation of lipoprotein-associated phospholipase A(2) levels and the risk of future cardiovascular events in women. J Am Coll Cardiol 2001;38(5):1302–6. [DOI] [PubMed]
- 50.Koenig W, Khuseyinova N, Lowel H, Trischler G, Meisinger C. Lipoprotein-associated phospholipase A2 adds to risk prediction of incident coronary events by C-reactive protein in apparently healthy middle-aged men from the general population: results from the 14-year follow-up of a large cohort from southern Germany. Circulation 2004;110(14):1903–8. [DOI] [PubMed]
- 51.Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Heiss G, Sharrett AR. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2004;109(7):837–42. [DOI] [PubMed]
- 52.Oei HH, van der Meer IM, Hofman A, Koudstaal PJ, Stijnen T, Breteler MM, Witteman JC. Lipoprotein-associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: the Rotterdam Study. Circulation 2005;111(5):570–5. [DOI] [PubMed]
- 53.Hatoum IJ, Nelson JJ, Rexrode K, Manson J, Rimm EB. Lipoprotein-associated phospholipase A2 and myocardial infarction in women [abstract]. Circulation 2007;116:II-818.
- 54.Caslake MJ, Packard CJ, Suckling KE, Holmes SD, Chamberlain P, Macphee CH. Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase: a potential new risk factor for coronary artery disease. Atherosclerosis 2000; 150(2):413–9. [DOI] [PubMed]
- 55.Blankenberg S, Stengel D, Rupprecht HJ, Bickel C, Meyer J, Cambien F, et al. Plasma PAF-acetylhydrolase in patients with coronary artery disease: results of a cross-sectional analysis. J Lipid Res 2003;44(7):1381–6. [DOI] [PubMed]
- 56.Khuseyinova N, Imhof A, Rothenbacher D, Trischler G, Kuelb S, Scharnagl H, et al. Association between Lp-PLA2 and coronary artery disease: focus on its relationship with lipoproteins and markers of inflammation and hemostasis. Atherosclerosis 2005;182(1):181–8. [DOI] [PubMed]
- 57.Winkler K, Winkelmann BR, Scharnagl H, Hoffmann MM, Grawitz AB, Nauck M, et al. Platelet-activating factor acetylhydrolase activity indicates angiographic coronary artery disease independently of systemic inflammation and other risk factors: the Ludwigshafen Risk and Cardiovascular Health Study [published erratum appears in Circulation 2005;111 (24):e444]. Circulation 2005;111(8):980–7. [DOI] [PubMed]
- 58.Johnston N, Jernberg T, Lagerqvist B, Siegbahn A, Wallentin L. Improved identification of patients with coronary artery disease by the use of new lipid and lipoprotein biomarkers. Am J Cardiol 2006;97(5):640–5. [DOI] [PubMed]
- 59.Oldgren J, James SK, Siegbahn A, Wallentin L. Lipoprotein-associated phospholipase A2 does not predict mortality or new ischaemic events in acute coronary syndrome patients. Eur Heart J 2007;28(6):699–704. [DOI] [PubMed]
- 60.Robins SJ, Collins D, Nelson JJ, Bloomfield HE, Asztalos BF. Cardiovascular events with increased lipoprotein-associated phospholipase A(2) and low high-density lipoprotein-cholesterol: the Veterans Affairs HDL Intervention Trial. Arterioscler Thromb Vasc Biol 2008;28(6):1172–8. [DOI] [PubMed]
- 61.Furberg CD, Nelson JJ, Solomon C, Cushman M, Jenny NS, Psaty BM. Distribution and correlates of lipoprotein-associated phospholipase A2 in an elderly cohort: the Cardiovascular Health Study. J Am Geriatr Soc 2008;56(5):792–9. [DOI] [PubMed]
- 62.Muzzio ML, Miksztowicz V, Brites F, Aguilar D, Repetto EM, Wikinski R, et al. Metalloproteases 2 and 9, Lp-PLA(2) and lipoprotein profile in coronary patients. Arch Med Res 2009;40(1):48–53. [DOI] [PubMed]
- 63.Stephens CJ, Graham RM, Sturm MJ, Richardson M, Taylor RR. Variation in plasma platelet-activating factor degradation and serum lipids after acute myocardial infarction. Coron Artery Dis 1993;4(2):187–93. [DOI] [PubMed]
- 64.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004;350(14):1387–97. [DOI] [PubMed]
- 65.Folsom AR, Chambless LE, Ballantyne CM, Coresh J, Heiss G, Wu KK, et al. An assessment of incremental coronary risk prediction using C-reactive protein and other novel risk markers: the atherosclerosis risk in communities study. Arch Intern Med 2006;166(13):1368–73. [DOI] [PubMed]
- 66.Bhakdi S, Torzewski M, Klouche M, Hemmes M. Complement and atherogenesis: binding of CRP to degraded, nonoxidized LDL enhances complement activation. Arterioscler Thromb Vasc Biol 1999;19(10):2348–54. [DOI] [PubMed]
- 67.Tracy RP. Inflammation markers and coronary heart disease. Curr Opin Lipidol 1999;10(5):435–41. [DOI] [PubMed]
- 68.Davidson MH, Corson MA, Alberts MJ, Anderson JL, Gorelick PB, Jones PH, et al. Consensus panel recommendation for incorporating lipoprotein-associated phospholipase A2 testing into cardiovascular disease risk assessment guidelines. Am J Cardiol 2008;101(12A):51F–57F. [DOI] [PubMed]
- 69.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation 2009;119(17):2408–16. [DOI] [PMC free article] [PubMed]
- 70.Madjid M, Aboshady I, Awan I, Litovsky S, Casscells SW. Influenza and cardiovascular disease: is there a causal relationship? Tex Heart Inst J 2004;31(1):4–13. [PMC free article] [PubMed]
- 71.Serruys PW, Garcia-Garcia HM, Buszman P, Erne P, Ver-heye S, Aschermann M, et al. Effects of the direct lipoprotein-associated phospholipase A(2) inhibitor darapladib on human coronary atherosclerotic plaque. Circulation 2008;118(11): 1172–82. [DOI] [PubMed]
- 72.Mohler ER 3rd, Ballantyne CM, Davidson MH, Hanefeld M, Ruilope LM, Johnson JL, Zalewski A. The effect of darapladib on plasma lipoprotein-associated phospholipase A2 activity and cardiovascular biomarkers in patients with stable coronary heart disease or coronary heart disease risk equivalent: the results of a multicenter, randomized, double-blind, placebo-controlled study. J Am Coll Cardiol 2008;51(17): 1632–41. [DOI] [PubMed]
- 73.Saougos VG, Tambaki AP, Kalogirou M, Kostapanos M, Gazi IF, Wolfert RL, et al. Differential effect of hypolipidemic drugs on lipoprotein-associated phospholipase A2. Arterioscler Thromb Vasc Biol 2007;27(10):2236–43. [DOI] [PubMed]
- 74.Labarthe DR. Epidemiology and prevention of cardiovascular diseases: a global challenge. Gaithersburg (MD): Aspen Publishers; 1998.