Abstract
Limited, controversial data exist regarding changes in high-sensitivity C-reactive protein (hs-CRP) levels over short times and the importance of detecting these changes in patients who have coronary artery disease (CAD). We investigated the variation of hs-CRP levels and their association with the severity of CAD in patients with stable CAD.
We measured morning, midday, evening, and midnight hs-CRP levels in 124 patients (94 with CAD, 30 with normal coronary arteries), who were evaluated via coronary angiography and Gensini scoring. Patients were divided into 3 groups (normal coronary arteries, mild CAD, or severe CAD) according to Gensini score.
Temporal hs-CRP levels varied significantly—the highest mean concentrations were found in the morning, and the lowest concentrations at midday (P <0.001). All temporal hs-CRP measurements and the absolute increase in hs-CRP levels were significantly higher in patients with severe CAD (both P <0.001). The most significant predictors of CAD severity were age (P=0.005), midday hs-CRP level (P <0.001), and brain natriuretic peptide level (P=0.045). Receiver operating characteristic curve analysis showed that cutoff values of hs-CRP taken at different times predicted severe CAD with similar sensitivity and specificity. Different cutoff values for temporal hs-CRP levels correlated with the severity of CAD. Serum levels of hs-CRP varied over 24 hours, whether patients had CAD or normal coronary arteries.
Key words: Analysis of variance, biological markers/ blood, C-reactive protein/analysis, coronary artery disease/blood, coronary stenosis, predictive value of tests, reference values, time factors
High-sensitivity C-reactive protein (hs-CRP) is secreted from the liver in response to inflammation. Consequently, patients who have coronary artery disease (CAD) have elevated hs-CRP levels.1,2 Moreover, high baseline hs-CRP levels are associated with the presence, severity, and prognosis of coronary arteriosclerosis in patients with stable CAD.3–8 In 2003, the American Heart Association and the Centers for Disease Control (AHA/CDC) recommended that CRP be regarded as an element of global coronary risk assessment in adults without known cardiovascular disease. In that recommendation, a CRP cutoff value of 3 mg/L was considered to indicate high risk for CAD.9
Previous studies have shown elevated hs-CRP levels in patients who have unstable CAD, and the severity of this elevation has been correlated with the extent of coronary atherosclerosis, myocardial damage, and relative prognosis.5,7,8,10–12 One study13 reported diurnal hs-CRP variation in a middle-aged population, whereas other investigators14 observed no variation among healthy subjects. Limited and controversial data exist regarding changes in hs-CRP levels during the day and the significance of these changes in patients who have stable CAD.
We investigated the variation of hs-CRP levels at 6-hour intervals over a 24-hour period and the association of any variation with the severity of CAD in patients with stable CAD.
Patients and Methods
We enrolled 124 patients (mean age, 56.7 ± 8.9 yr; 84 men) in this cross--sectional study. Each patient had stable angina and ischemia induced by an exercise or chemical stress test. All were in stable overall condition and were undergoing optimal, individualized medical therapy for their cardiovascular conditions. Persons were excluded from the study if they had renal disease (creatinine, >2 mg/dL), recent acute coronary syndrome, valvular heart disease, life-threatening arrhythmias, acute or chronic liver disease, infectious or noncoronary inflammatory disease, or symptomatic heart failure. Our local ethics committee approved the study, and written informed consent was obtained from all participants.
All patients were hospitalized for 36 hours and then underwent coronary angiography. We recorded their medical histories, including cardiovascular risk factors. Echocardiography was performed, and left ventricular ejection fractions were calculated by use of the modified Simpson method.15
Measurements of Biochemical Variables
On the day of the test, venous blood samples were taken from the antecubital vein every 6 hours: morning (6 AM), midday (12 PM), evening (6 PM), and midnight (12 AM). Each patient rested in a supine position for 20 minutes before blood was drawn. The samples were collected in tubes that contained EDTA. The hs-CRP levels were measured by means of immunonephelometric assay (BN ProSpec® System protein analyzer, Siemens Diagnostic Healthcare Inc.; Deerfield, Ill). Absolute change in hs-CRP (absolute Δhs-CRP) over time was calculated as the hs-CRP level in the morning minus the hs-CRP level at midday. Relative change in hs-CRP (relative Δhs-CRP) over time was calculated as absolute Δhs-CRP divided by the hs-CRP level at midday. Levels of serum cholesterol, glucose, homocysteine, brain natriuretic peptide (BNP), cystatin C, and creatinine were measured by routine laboratory methods.
Angiographic Analyses
Coronary angiography was performed via standard Judkins techniques. Two experienced investigators (blinded to the clinical data) analyzed the angiograms. Coronary arteries were considered to be normal (0, for no stenosis), obstructed (25%, 50%, 75%, 90%, or 99% stenosis), or 100% occluded, according to the maximum obstruction that was observed in any projection. The severity of coronary atherosclerosis was classified according to Gensini score, which grades narrowing of the lumen as 1 for 1%–25% stenosis, 2 for 26%–50%, 4 for 51%–75%, 8 for 76%–90%, 16 for 91%–99%, and 32 for total occlusion.16 This score was multiplied by a factor that accounted for the importance of a lesion's position in the coronary arterial tree: 5 for the left main coronary artery; 2.5 for the proximal left anterior descending coronary artery (LAD) or proximal circumflex artery; 1.5 for the mid-LAD; 1 for the proximal right coronary artery, distal LAD, obtuse marginal artery, or posterior lateral artery; and 0.5 for other stenoses. The severity of disease was expressed as the sum of the scores for the individual lesions. The study population was then divided into 3 groups according to CAD-severity score: normal coronary arteries (score, 0), mild CAD (score, 1–20), and severe CAD (score, >20).
Statistical Analyses
All analyses were performed with use of SPSS version 15.0 (SPSS, Inc.; Chicago, Ill). Categorical variables of the CAD groups were compared via the c 2 test. Continuous variables were expressed as mean ± SD. These variables were compared by 1-way analysis of variance, and, when indicated, by a post hoc Scheffé or Tamhane test. For the comparison of hs-CRP measurements that were taken at different times of the day, repeated--measures analysis of variance was applied. Correlations between continuous variables were evaluated by means of Pearson or Spearman rank correlation analysis. Multivariate logistic regression analyses were performed in order to determine significant predictors of CAD--severity scores above 20. Significant variables in univariate analysis at a P <0.1 level were entered in logistic regression analysis. Linear regression analysis was also applied for determining CAD-severity score. A receiver operating characteristic (ROC) curve analysis was performed in order to identify the optimal cutoff points of hs-CRP levels that were taken at different times, and the BNP level was added to this ROC curve analysis (to determine maximal sensitivity and specificity) for predicting CAD--severity scores above 20. The area under the curve was calculated to determine the accuracy of the test. A P value of less than 0.05 was considered statistically significant. All data conformed to each test that was used to analyze them.
Results
Clinical Characteristics and Laboratory Variables According to Severity Score
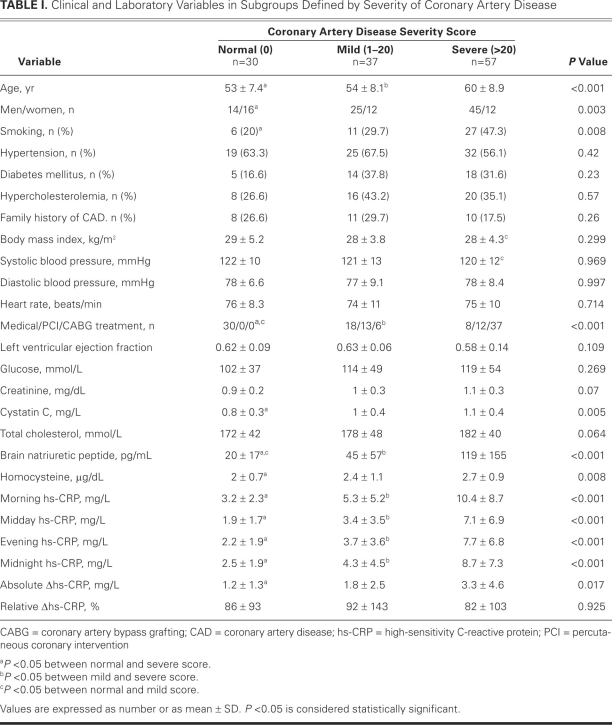
Table I shows the clinical characteristics and laboratory variables of the study population. Of the patients, 94 had CAD (75.8%) and 30 had normal coronary arteries (24.2%). The severe-CAD group comprised more men, a higher mean age, a greater incidence of smoking, and higher levels of serum BNP and cystatin C than did the other groups. Except for BNP level, there was no significant difference between the normal--artery and mild-CAD groups in their comparative clinical and laboratory values.
TABLE I. Clinical and Laboratory Variables in Subgroups Defined by Severity of Coronary Artery Disease
Temporal Variation of High-Sensitivity C-Reactive Protein Levels
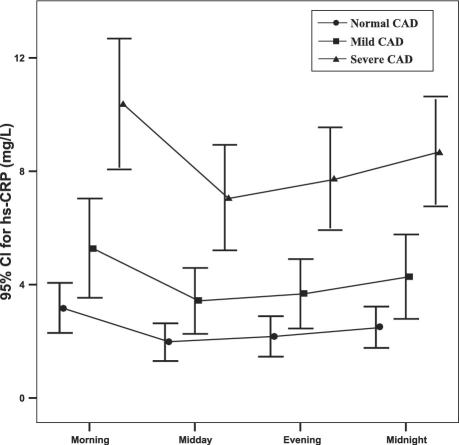
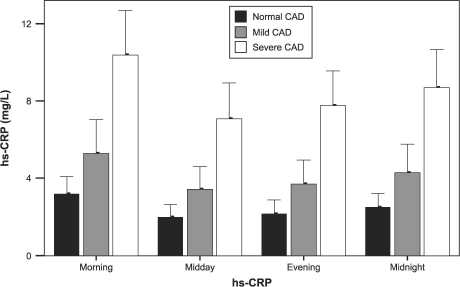
The hs-CRP measurements differed greatly throughout the day: the most significant difference was between the morning and midday measurements (P <0.001) (Fig. 1). In all 3 groups, maximum mean hs-CRP levels were observed in the morning, and minimum mean levels were observed at midday. Accordingly, in the determination of absolute and relative Δhs-CRP, the difference between the morning and midday hs-CRP measurements was used. Morning measurements were higher than other measurements in all of the study population, except for 3 patients in the mild-CAD group and 6 in the severe--CAD group. All 4 temporal hs-CRP levels and the absolute Δhs-CRP were significantly higher in the severe--CAD group, whereas no significant difference was observed for relative Δhs-CRP (Fig. 2 and Table I).
Fig. 1 Temporal variation of mean high-sensitivity C-reactive protein (hs-CRP) levels according to coronary artery disease (CAD) severity group. CI = confidence interval
Fig. 2 High-sensitivity C-reactive protein (hs-CRP) levels (mean values ± SD) taken at 6-hour intervals around the clock, according to coronary artery disease (CAD) severity group.
Correlation of High-Sensitivity C-Reactive Protein Levels with Clinical and Laboratory Variables
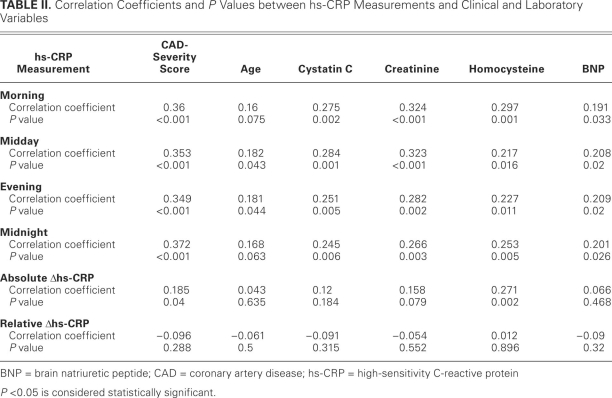
Table II shows the correlation of hs-CRP levels with the clinical and laboratory variables. All temporal hs--CRP measurements positively correlated with the CAD--severity score, patient age, and concentrations of serum creatinine, homocysteine, cystatin C, and BNP. Of the 4 temporal measurements, midnight hs-CRP most significantly correlated with CAD-severity score. Although absolute Δhs-CRP positively correlated with the CAD-severity score and with homocysteine concentration, no correlation was observed between relative Δhs-CRP and these 2 variables.
TABLE II. Correlation Coefficients and P Values between hs-CRP Measurements and Clinical and Laboratory Variables
Independent Predictors of Severity Score
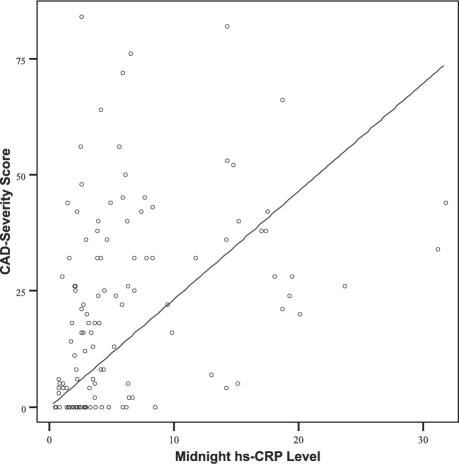
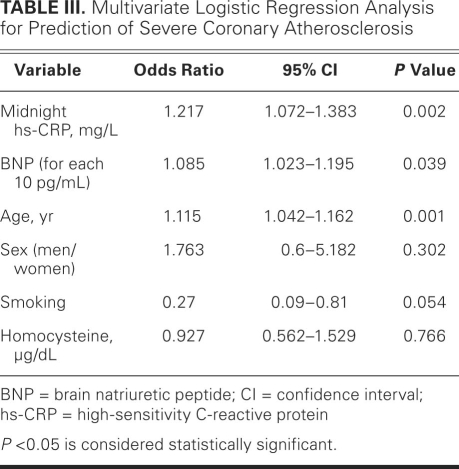
Multivariate linear regression analysis showed that age (β=0.234, P=0.005), midnight hs-CRP (β=0.3, P < 0.001), and BNP level (β=0.165, P=0.045) were the most important predictors of the CAD-severity score. The coefficient of determination (R2) for the CAD-severity score was 0.28. Figure 3 shows the relationship between the midnight hs-CRP level and the severity score. Upon multivariate logistic regression analysis, independent predictors of a severity score above 20 were age, midnight hs-CRP level, and BNP level; these were adjusted for sex, smoking, and homocysteine, and for morning, midday, and evening hs-CRP levels (Table III). Every 10-pg/mL increase in BNP, 1-mg/L increase in midnight hs-CRP, and 1-year increase in age caused corresponding increases of 10.5%, 20.1%, and 8.7% in the risk of having severe CAD.
Fig. 3 The relationship between midnight levels of high-sensitivity C-reactive protein (hs-CRP) and coronary artery disease (CAD) severity score.
TABLE III. Multivariate Logistic Regression Analysis for Prediction of Severe Coronary Atherosclerosis
Receiver Operating Characteristic Curve Analyses for Detecting Patients with Severe Coronary Artery Disease
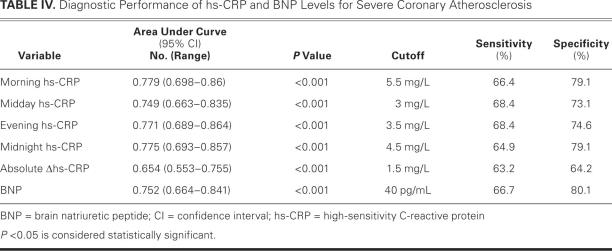
We performed ROC analyses in order to estimate the predictive value of hs-CRP levels in the diagnosis of severe CAD (Table IV). Area-under-ROC curves for the 4 temporal hs-CRP levels and for BNP provided good discriminatory power, particularly for morning hs-CRP. These analyses also disclosed that different cutoff values of hs-CRP levels at particular measurement times revealed similar sensitivities and specificities for predicting severe CAD (Table IV).
TABLE IV. Diagnostic Performance of hs-CRP and BNP Levels for Severe Coronary Atherosclerosis
Discussion
Our study shows significant temporal variation in hs-CRP levels over 24 hours, whether patients had CAD or normal coronary arteries. Morning hs-CRP was markedly higher than at other sampling times in all 3 groups. Moreover, different cutoff hs-CRP values for samples taken at different times had similar sensitivities and specificities for predicting severe CAD.
In our study, patients with stable CAD had significantly elevated hs-CRP levels regardless of the sampling time, which is in accordance with previous studies that reported high hs-CRP levels in stable and unstable patients alike.4,6,9,11,12 Our investigation of sampling time of hs-CRP revealed high hs-CRP at all measurement times in patients with mild and severe CAD.
To the best of our knowledge, temporal hs-CRP variation in patients with CAD had not previously been investigated over a time frame such as ours. The available data on diurnal variations of hs-CRP levels in healthy subjects are few and controversial. A recent study13 of hs-CRP, D-dimer, tissue plasminogen activator, and von Willebrand factor in a middle-aged population showed diurnal variation as high as 34% in hs-CRP, with maximum hs-CRP levels observed at 3 PM. However, that study was limited in that hs-CRP was measured between 9 AM and 10 PM. In our study, we measured hs-CRP 4 times over 24 hours at 6-hour intervals, and we observed the highest levels of hs-CRP in the morning, with a variation of 86%. These results also held true for our 30 patients who had normal coronary arteries. Previously, Meier-Ewert and colleagues14 found no diurnal variation in hs-CRP samples that were taken hourly from 13 healthy subjects. The variation in our study could be due to cardiovascular factors in all patients—even in those with normal coronary arteries—because such risk factors as obesity, hypertension, diabetes mellitus, and smoking have been shown to induce hs-CRP secretion.6
A high baseline level of hs-CRP has been associated with the severity and prognosis of coronary atherosclerosis in patients who have stable CAD.5–8 To indicate a high risk of CAD in adults without known cardiovascular disease, the AHA/CDC recommended a CRP cutoff value of 3 mg/L.9 Likewise, Sabatine and associates6 showed that an hs-CRP cutoff value of more than 3 mg/L predicted a 78% increased risk of adverse cardiovascular events in patients with stable CAD. Another study17 showed that hs-CRP is an independent predictor of CAD in patients with diabetes, and the cutoff value was determined to be 5.2 mg/L. In our study, our respective cutoff values of 5.5 mg/L, 3 mg/L, 3.5 mg/L, and 4.5 mg/L for morning, midday, evening, and midnight samplings strongly predicted severe CAD.
The number of angiographically detected critical coronary artery stenoses is a predictor of unfavorable events in patients with CAD.18 In previous studies,11,19 no significant association was reported between baseline serum CRP levels and CAD severity or the extent of disease in patients with unstable CAD. This may be related to lesion morphology and vulnerability. Conversely, in a large study of patients with stable CAD, Zebrack and colleagues5 reported a weak but significant correlation between hs-CRP and CAD severity. Our results similarly showed an independent relationship between midnight hs-CRP levels and severe CAD. Furthermore, all cutoff values of our temporal hs-CRP measurements predicted severe CAD with similar sensitivity and specificity.
Limitations of the Study
Our study is limited in several ways. The sample population was relatively small, considering the prevalence of CAD in any community. Whereas we measured hs-CRP 4 times during a 24-hour period, hourly measurements may more precisely disclose round-the-clock variations in hs-CRP levels. (Of note, hourly or more frequent sampling would be more costly, and arguably unethical because of frequent venipunctures.) In support of our quadripartite testing over 1 day, almost all of our patients had consistent hs-CRP measurements during a particular sampling time, so we conclude that our approach was sufficient and not of major concern. All of our patients were in stable overall condition, and all were on optimal medical regimens for their cardiovascular conditions. The individualized therapy involved different medications and dosages, which may have influenced hs-CRP levels,6 and we did not consider medications in our analyses. Furthermore, our CAD-severity groups differed greatly regarding age, sex, and treatment. Although these factors were adjusted in the analyses, it is a major concern that the severity groups were unmatched for these variables. Finally, because stable CAD and acute coronary syndromes have different pathogeneses, it remains to be determined whether our results would apply to patients with unstable angina pectoris and acute myocardial infarction.
Conclusions
This is the 1st study to show variation of hs-CRP levels in patients with stable CAD at 6-hour intervals over a 24-hour period. We have shown that the sampling time of hs-CRP is important, because only midnight hs-CRP measurements predicted severe CAD in our study population. This aside, when different cutoff values were applied to particular sampling times, severe CAD was predicted with similar sensitivity and specificity. Whether our results apply to other forms of CAD needs to be confirmed in future study populations.
Footnotes
Address for reprints: Mevlut Koc, MD, Department of Cardiology, Adana Numune Education & Research Hospital, 01330 Adana, Turkey
E-mail: mevlutkoc78@yahoo.com
References
- 1.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men [published erratum appears in N Engl J Med 1997;337(5):356]. N Engl J Med 1997;336(14):973–9. [DOI] [PubMed]
- 2.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000;342 (12):836–43. [DOI] [PubMed]
- 3.Arroyo-Espliguero R, Avanzas P, Quiles J, Kaski JC. Predictive value of coronary artery stenoses and C-reactive protein levels in patients with stable coronary artery disease. Atherosclerosis 2009;204(1):239–43. [DOI] [PubMed]
- 4.Tataru MC, Heinrich J, Junker R, Schulte H, von Eckardstein A, Assmann G, Koehler E. C-reactive protein and the severity of atherosclerosis in myocardial infarction patients with stable angina pectoris. Eur Heart J 2000;21(12):1000–8. [DOI] [PubMed]
- 5.Zebrack JS, Muhlestein JB, Horne BD, Anderson JL; Intermountain Heart Collaboration Study Group. C-reactive protein and angiographic coronary artery disease: independent and additive predictors of risk in subjects with angina. J Am Coll Cardiol 2002;39(4):632–7. [DOI] [PubMed]
- 6.Sabatine MS, Morrow DA, Jablonski KA, Rice MM, Warnica JW, Domanski MJ, et al. Prognostic significance of the Centers for Disease Control/American Heart Association high-sensitivity C-reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation 2007;115(12):1528–36. [DOI] [PubMed]
- 7.Kuller LH, Tracy RP, Shaten J, Meilahn EN. Relation of C-reactive protein and coronary heart disease in the MRFIT nested case-control study. Multiple Risk Factor Intervention Trial. Am J Epidemiol 1996;144(6):537–47. [DOI] [PubMed]
- 8.Iseki K, Tozawa M, Yoshi S, Fukiyama K. Serum C-reactive protein (CRP) and risk of death in chronic dialysis patients. Nephrol Dial Transplant 1999;14(8):1956–60. [DOI] [PubMed]
- 9.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107(3):499–511. [DOI] [PubMed]
- 10.Morrow DA, Rifai N, Antman EM, Weiner DL, McCabe CH, Cannon CP, Braunwald E. C-reactive protein is a potent predictor of mortality independently of and in combination with troponin T in acute coronary syndromes: a TIMI 11A substudy. Thrombolysis in Myocardial Infarction. J Am Coll Cardiol 1998;31(7):1460–5. [DOI] [PubMed]
- 11.Brunetti ND, Troccoli R, Correale M, Pellegrino PL, Di Biase M. C-reactive protein in patients with acute coronary syndrome: correlation with diagnosis, myocardial damage, ejection fraction and angiographic findings. Int J Cardiol 2006; 109(2):248–56. [DOI] [PubMed]
- 12.Avanzas P, Arroyo-Espliguero R, Cosin-Sales J, Quiles J, Zouridakis E, Kaski JC. Multiple complex stenoses, high neutrophil count and C-reactive protein levels in patients with chronic stable angina. Atherosclerosis 2004;175(1):151–7. [DOI] [PubMed]
- 13.Rudnicka AR, Rumley A, Lowe GD, Strachan DP. Diurnal, seasonal, and blood-processing patterns in levels of circulating fibrinogen, fibrin D-dimer, C-reactive protein, tissue plasminogen activator, and von Willebrand factor in a 45-year-old population. Circulation 2007;115(8):996–1003. [DOI] [PubMed]
- 14.Meier-Ewert HK, Ridker PM, Rifai N, Price N, Dinges DF, Mullington JM. Absence of diurnal variation of C-reactive protein concentrations in healthy human subjects. Clin Chem 2001;47(3):426–30. [PubMed]
- 15.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 1989;2(5):358–67. [DOI] [PubMed]
- 16.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 1983;51(3):606. [DOI] [PubMed]
- 17.Pu LJ, Lu L, Xu XW, Zhang RY, Zhang Q, Zhang JS, et al. Value of serum glycated albumin and high-sensitivity C-reactive protein levels in the prediction of presence of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol 2006;5:27–33. [DOI] [PMC free article] [PubMed]
- 18.Humphries JO, Kuller L, Ross RS, Friesinger GC, Page EE. Natural history of ischemic heart disease in relation to arteriographic findings: a twelve year study of 224 patients. Circulation 1974;49(3):489–97. [DOI] [PubMed]
- 19.Niccoli G, Biasucci LM, Biscione C, Fusco B, Porto I, Leone AM, et al. Independent prognostic value of C-reactive protein and coronary artery disease extent in patients affected by unstable angina. Atherosclerosis 2008;196(2):779–85. [DOI] [PubMed]