Abstract
The escape response of the cockroach is a well-studied example of sensorimotor behavior. Cockroaches respond to wind puffs, which may signal a predator attack, by making a swift turn followed by a forward acceleration. We have recently shown that their escape trajectories, measured relative to the position of the threatening stimulus, show preferred directions.1 Previous work has often distinguished between the most common type of escape turn, which begins as a rotation away from the stimulus, and the relatively rare turns initiated towards the stimulus. Here, we analyze these “away” and “towards” responses in light of our recent work on preferred escape trajectories (ETs). We find that the ETs of towards responses show a pattern of frequency distribution similar to that of away responses. The range of the bodyturn angles of towards responses, however, is much smaller than that of away responses, being <30° in most cases, which approximately corresponds to the angular distance between ET peaks. This suggests that cockroaches minimize their turn when making a towards response, which could represent an effective anti-predator behavior that allows cockroaches to reach one of the preferred ETs within a relatively short time.
Key words: Escape response, cockroaches, locomotion, anti-predator behavior, wind stimulation, sensory hairs, protean behavior
Escape responses consist of rapid movements in reaction to threats and can be observed in many animal species as a means of avoiding predation.2 In particular, the escape behavior of lower vertebrates and invertebrates has been used as a model in neurobiology for illustrating how specific sensory inputs can generate motor outputs. The escape response of the cockroach is one of the best known models in terms of escape neurophysiology, biomechanics and behavior.3–5 Perhaps unexpectedly, this system is providing one of the best models, using dsRNAi in a reverse-genetic approach, to understand how molecular-genetic information is used to construct circuits of neurons, and thereby mediates behavior.6,7
The escape directions taken by prey are highly variable in all species studied.8–11 This escape variability has been claimed to be a necessary component of anti-predator behavior, in which “protean” unpredictability of the prey can result in successful escapes.12,13 Our own recent work on cockroaches has demonstrated that variability in escape trajectories (ETs) can be generated by the animal choosing from a finite number of preferred ETs.1 These ETs are revealed by applying principles of circular statistics, using stimulus direction as the reference (i.e., zero) point.
Usually, the rotation accompanying an escape response is initiated in a direction away from the stimulus.8,9 However, in some rare instances animals respond by rotating their body towards the stimulus (Fig. 1). These “towards” responses have been sometime regarded as “incorrect” or “wrong” turns, since they tend to move the prey towards the threat.10,14 In light of our recent results on preferred ETs, here we analyze towards and away responses separately, to determine whether they show similar preferred ETs.
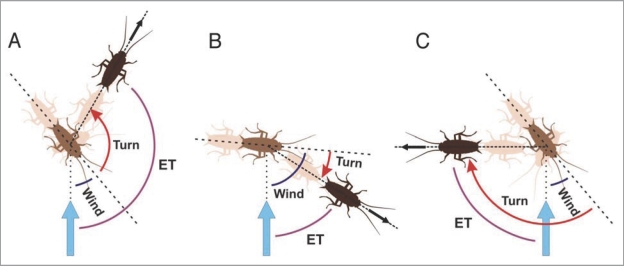
Figure 1.
Cockroaches respond to a wind stimulus (vertical blue arrow) with either away (A), towards (B) or overshooting towards responses (C). In away responses (A), cockroaches rotate their body in a direction opposite to the stimulus. The final direction of motion (black arrow) is along an ET (purple curve) which corresponds to the sum of wind angle (blue curve) and turn angle (red curved arrow). In towards responses (B), cockroaches rotate their body in the direction of the stimulus. The resulting ET corresponds to wind angle minus turn angle. In overshooting towards responses (C), after an initial rotation towards the stimulus, cockroaches continue the rotation in a direction away from the stimulus. As in other towards responses, the resulting ET is calculated as wind angle minus turn angle, i.e., in this case 45° − 135° = −90°, which in our previous circular statistical analysis1 would correspond to an ET of 270°.
Towards responses only occur rarely, and therefore they only permit a qualitative assessment of their distribution relative to away responses. We used four data sets [singleton;1 data-set 5i;1 data from Camhi and Tom (1978);9 data from Comer and Dowd (1987)10] of escape responses triggered by a wind stimulus (reviewed in ref. 1). The proportion of towards responses out of the total are: singletons 5.8% (N = 5 out of 86); data-set 5i 2.8% (N = 12 out of 431); Camhi and Tom (1978)9 data 13.7% (N = 22 out of 161); Comer and Dowd (1987)10 data 18.6% (N = 47 out of 253). A particular type of towards response, the “overshooting” towards response, is defined as a response that is initiated towards the stimulus (usually from a small wind angle), then reaches an orientation head-on towards the stimulus, and continues its rotation in a direction away from the stimulus (Fig. 1C). These overshooting responses were only present in the data from Camhi and Tom (1978)9 (N = 5 out of 22 towards responses) and in data from Comer and Dowd (1987)10 (N = 8 out of 47 towards responses) and resulted from a mean incident wind angle of 18.2 ± 6.0° (mean ± S.E.M.) (Camhi and Tom (1978)9 data, N = 5 ) and 21.7 ± 7.4° (Comer and Dowd (1987)10 data, N = 8). Another kind of “overshooting” responses may occur for escapes initiated away from the threat, i.e., responses that start as an “away” turn in the right semicircle (0–180°), and end in the left semicircle (180–360°). However, these were rare and most of the away responses ended their rotation with an ET <200°, i.e., within a few degrees from the peak at 180° [100% in singleton; 98.6% in dataset 5i; 99.3% in Camhi and Tom (1978)9 data; 96.6% in Comer and Dowd (1987)10 data] (Fig. 2).
Figure 2.
Though far less frequent, toward responses show similar ET peaks to away responses. Circular frequency distribution polygons of ETs in the four data sets analyzed in Domenici et al. (2008).1 (A) singletons, (B) data-set 5i, (C) data from Camhi and Tom (1978),9 (D) data from Comer and Dowd (1987).10 Frequency values (shown as concentric circles of 0.1 each) are relative proportions of away responses (blue lines), towards responses (red lines), and the multimodal curves fitted for all the data in Domenici et al. (2008)1 (grey-filled areas). Note that the away and towards ET peaks are in similar positions. Towards responses >180° are overshooting responses and are only seen in the data sets comprising (C and D). Red triangles in the 180−360° sector indicate mirror images of the peaks (grey triangles) found in the 0−180° sector for the distributions of away responses. Note that these overshooting ET peaks are approximately positioned at the mirror image of opposite away peaks.
ETs were calculated as the sum of the wind angle and the body turn of the cockroach (defined as the angle between the body’s midline before stimulation and its direction of motion at the end of the response), in which body turns away from the stimulus bear a positive sign and body turns toward the stimulus bear a negative sign (Fig. 1).1 ETs with stimulus head-on were designated as 0°, while ETs in a direction opposite to the stimulus corresponded to 180°. Escapes from left and right stimuli were pooled as if the stimuli were always from the right. ETs potentially spanned 360° and were treated as a circular variable. 15 Importantly, all data sets show that towards responses have similar ET peaks as the away responses (Fig. 2). One notable exception is the peak at the largest ET (i.e., approximately 180°), which is not present in towards responses. This is to be expected because, like all responses, towards responses start from a maximum wind angle of 180°, and their ET must be <180° since their rotation is towards the stimulus. Overshooting responses show up as escapes in the 180–360° sector, and are positioned at angles which mirror approximately some of the peaks found in the 0–180° sector (Fig. 2).
Our previous work showed that ETs in cockroaches are not generated by neural or mechanical constraints in the body turn, since neither body-turn angles nor wind-angles showed multimodal distributions. 1 ETs are however related to wind angle, since escapes triggered at small wind angles (i.e., almost head on) show all four main peaks, while escapes triggered by posterior wind show a smaller number of ET peaks.1 This is because most escape responses are a turn away from the stimulus; 9 therefore the angles of ETs are almost always larger than those of wind angle. However, when separating towards and away turns, it is apparent that the distribution of body-turn angles of these two response types are different in all data sets analyzed [Fig. 3; data-set 5i: χ2 = 11.6, df = 2, p < 0.005; Camhi and Tom (1978)9 data: χ2 = 10.7, df = 2, p < 0.005; Comer and Dowd (1987)10 data: χ2 = 19.6, df = 5, p < 0.005; singletons: N = 5, too low to allow distribution testing]. The body-turn angles of away responses are distributed over a larger range than those of towards responses and show a higher mean in all data sets (singletons: towards responses 18.8 ± 3.6°, N = 5, away responses 61.19 ± 4.0°, N = 81, Mann Whitney test, p < 0.01; data-set 5i: towards responses 21.1 ± 6.3°, N = 12, away responses 69.6 ± 2.1° N = 419, Mann Whitney test, p < 0.0001; Camhi and Tom (1978)9 data: towards responses 22.6 ± 7.5° N = 22, away responses 38.2 ± 3.3°, N = 139, Mann- Whitney test, p < 0.005; Comer and Dowd (1987)10 data: towards responses 32.4 ± 5.2°, N = 47, away responses 59.0 ± 3.1°, N = 206, Mann-Whitney test, p < 0.0001. Specifically, the body-turn angles of towards responses tend to be mostly <30° [100%, 83%, 82%, 68% of all towards responses in singleton, dataset 5i, Camhi and Tom (1978)9 data, and Comer and Dowd (1987)10 data, respectively]. Exceptions are the body turns of overshooting towards responses, which show body-turn angle ranges that are comparable (qualitatively, since the N is too low for statistical analysis) to those of away responses (Fig. 3C and D). An explanation for this pattern is that overshooting towards responses, once they pass through the zero point (i.e., facing the stimulus), become effectively away responses and thus behave as such.
Figure 3.
Body-turn angles have different distributions for away and towards responses. Frequency distribution of body-turn angles in the four data sets analyzed in Domenici et al. (2008).1 The centre of each 20° bin is indicated on the x-axis. (A) singletons, (B) data-set 5i, (C) data from Camhi and Tom (1978),9 (D) data from Comer and Dowd (1987).10 Frequency values are relative proportions of away responses (blue bars), non-overshooting towards responses (red bars) and overshooting towards responses (grey bars).
Our data show that the distribution of ETs of towards responses has a similar pattern as that of away responses, suggesting that the final trajectory undertaken by towards and away responses is controlled by similar neural mechanisms. Whether producing away or towards responses, neural processing ensures that trajectories are fixed relative to the external references (the wind stimulus), rather than producing an outcome where trajectories are generated relative to a body reference i.e., by fixed angles of body turn.
It is the case that the body-turn angles of towards responses appear to be minimized, unlike those of away responses which show a large range of angles. Interestingly, most of the body turn angles are <30° which is approximately the angular distance between the peaks of the ETs. This suggests that in making towards responses, cockroaches generally stop the rotation as soon as they reach one of the preferred ETs, unlike away responses which can stop the rotation at any one of the ETs. Given that cockroaches escape along preferred ETs which are separated by approximately 30° (e.g., 90 and 120°,1), it is conceivable that a cockroach stimulated, for example, with a wind orientation of 100° could reach a preferred ET of 90° (i.e., by making a towards responses with a 10° turn) in a shorter time than it would take to reach the preferred ET of 120° by making an away response with a 20° turn. Although away responses are the preferred option, presumably because they displace the prey further away from the threat, towards responses may nevertheless represent an effective anti-predator behavior allowing cockroaches to reach one of the preferred ETs within a relatively short time.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/9408
References
- 1.Domenici P, Booth D, Blagburn JM, Bacon JP. Cockroaches keep predators guessing by using preferred escape trajectories. Curr Biol. 2008;18:1792–1796. doi: 10.1016/j.cub.2008.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bullock TH. Comparative neuroethology of startle, rapid escape and giant fiber-mediated responses. In: Eaton RC, editor. Neural Mechanisms of Startle Behaviour. New York: Plenum Press; 1984. pp. 1–13. [Google Scholar]
- 3.Camhi JM. Neuroethology: nerve cells and the natural behavior of animals. Sunderland: Sinauer; 1983. [Google Scholar]
- 4.Rinberg D, Davidowitz H. Do cockroaches ‘know’ about fluid dynamics? Nature. 2000;405:756. doi: 10.1038/35015677. [DOI] [PubMed] [Google Scholar]
- 5.Stern M, Ediger VL, Gibbon CR, Blagburn JM, Bacon J-P. Regeneration of cercal filiform hair sensory neurons in the first-instar cockroach restores escape behavior. J Neurobiol. 1997;33:439–458. [PubMed] [Google Scholar]
- 6.Marie B, Bacon JP, Blagburn JM. Double-stranded RNA interference shows that Engrailed controls the synaptic specificity of identified sensory neurons. Curr Biol. 2000;10:289–292. doi: 10.1016/s0960-9822(00)00361-4. [DOI] [PubMed] [Google Scholar]
- 7.Booth D, Marie B, Domenici P, Blagburn JM, Bacon JP. Transcriptional control of behavior: Engrailed knock-out changes cockroach escape trajectories. J Neurosci. 2009;29:7181–7190. doi: 10.1523/JNEUROSCI.1374-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domenici P, Blake RW. Escape trajectories in angelfish (Pterophyllum eimekei) J Exp Biol. 1993;177:253–272. [Google Scholar]
- 9.Camhi JM, Tom W. The escape behaviour of the cockroach Periplaneta americana 1. Turning response to wind puffs. J Comp Physiol A. 1978;128:193–201. [Google Scholar]
- 10.Comer CM, Dowd JP. Escape turning behaviour of the cockroach. Changes in directionality induced by unilateral lesions of the abdominal nervous system. J Comp Physiol. 1987;160:571–583. [Google Scholar]
- 11.Eaton RC, Lavender WA, Wieland CM. Identification of Mauthner initiated response patterns in goldfish: evidence from simultaneous cinematography and electrophysiology. J Comp Physiol A. 1981;144:521–531. [Google Scholar]
- 12.Humphries DA, Driver PM. Protean defence by prey animals. Oecologia. 1970;5:285–302. doi: 10.1007/BF00815496. [DOI] [PubMed] [Google Scholar]
- 13.Godin J-GJ. Evading predators. In: Godin J-GJ, editor. Behavioural Ecology of Teleost Fishes. Oxford: Oxford University Press; 1997. pp. 191–236. [Google Scholar]
- 14.Camhi JM, Levy A. The Code for stimulus direction in a cell assembly in the cockroach. J Comp Physiol A. 1989;165:83–97. doi: 10.1007/BF00613802. [DOI] [PubMed] [Google Scholar]
- 15.Batschelet E. Circular Statistics in Biology. New York: Academic Press; 1981. [Google Scholar]