Abstract
Nociceptive sensitization is a conserved form of neuronal plasticity that serves an important survival function, as it fosters behavior that protects damaged tissue during healing. This sensitization may involve a lowering of the nociceptive threshold (allodynia) or an increased response to normally noxious stimuli (hyperalgesia). Although nociceptive sensitization has been intensively studied in vertebrate models, an open question in the field is the extent to which allodynia and hyperalgesia, which almost always occur in tandem, are truly separate events at the mechanistic level. We recently introduced a genetically tractable model for damage-induced nociceptive sensitization in Drosophila larvae, and identified a conserved cytokine signaling module that mediates development of allodynia following UV irradiation. This pathway includes the Drosophila homolog of Tumor Necrosis Factor-α (TNFα), Eiger, which is released from damaged epidermal cells and acts directly on its receptor, Wengen, located on nociceptive sensory neurons. Here we show that although Eiger and Wengen are both required for the development of thermal allodynia, they are dispensable for thermal hyperalgesia, suggesting, contrary to what is commonly assumed, that these two forms of hypersensitivity are initiated by separate genetic pathways.
Key words: Drosophila, nociception, allodynia, hyperalgesia, tissue repair, UV damage, cytokines, tumor necrosis factor, eiger, wengen
Nociceptive sensory neurons normally activate only in response to very intense stimuli,1 but they may become hypersensitive in response to inflammation and tissue damage. The two forms of nociceptive sensitization, allodynia and hyperalgesia, have traditionally been regarded as intertwined processes,2,3 with the key difference being the nature of the inducing stimulus: allodynia describes a response induced by a previously non-noxious stimulus, while hyperalgesia describes an increased response to a stimulus that is normally painful.4 A revision of the terminology of both allodynia and hyperalgesia has recently been proposed.5 By the new definition, hyperalgesia would become an umbrella term for any case where there is an increased nociceptive response, whereas allodynia would be restricted to nociception evoked by normally low-threshold fibers. One primary motivator for these new definitions, which are exclusively based on an analysis of the vertebrate and clinical literature, is that multiple methods of inducing sensitization always seemed to produce allodynia and hyperalgesia together, but never alone, obscuring the need for a distinction between the two. Our recent data in Drosophila, however, suggest that there are molecular distinctions between these processes that go beyond the nature of the inducing stimulus provided by the investigator. To clarify our terminology, here we use the term “thermal allodynia” to describe a response to a normally nonnoxious thermal stimulus, and “thermal hyperalgesia” to describe a heightened response to a normally supra-threshold thermal stimulus.
Studies of nociception in Drosophila have a brief history to date. Tracey, Wilson and Benzer reported an aversive withdrawal behavior (the typical experimental assay for nociception in whole animals) in Drosophila larvae when they were presented with thermal stimuli above 38°C.6 This behavioral assay was used to identify a conserved gene required to detect painful stimuli, suggesting that the molecular basis of nociception might be shared between vertebrates and invertebrates. The gene they identified, painless, encodes an ion channel belonging to the transient receptor potential (TRP) family6 which had been implicated in nociception in prior electrophysiological and vertebrate studies.7,8 Tracey’s group has subsequently pinpointed the particular sensory neurons that mediate this aversive withdrawal behavior in Drosophila larvae.9 Within each dorsal larval body segment there are two such nociceptive sensory neurons that elaborate impressive dendritic arbors that contact nearly every cell of the larval barrier epidermis.10 To assess whether the cellular and molecular mechanisms underlying nociceptive sensitization (as distinct from baseline nociception) are also conserved, we developed a model of UV-induced hypersensitization using Drosophila larvae.11
In our recent study, we demonstrated an important role in development of thermal allodynia following UV-induced epidermal damage for the Drosophila homolog of TNFα, Eiger, and its receptor, Wengen. Our data suggest that Eiger is released from UV-damaged epidermal cells and acts directly on its receptor Wengen whose function is required within nociceptive sensory neurons.11 This direct effect is distinct from the typical inflammatory cascade outlined in some mammalian models of nociceptive hypersensitivity.12 Our study also revealed some important phenomenological and molecular distinctions between UV-induced thermal allodynia and thermal hyperalgesia.
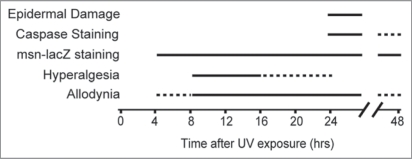
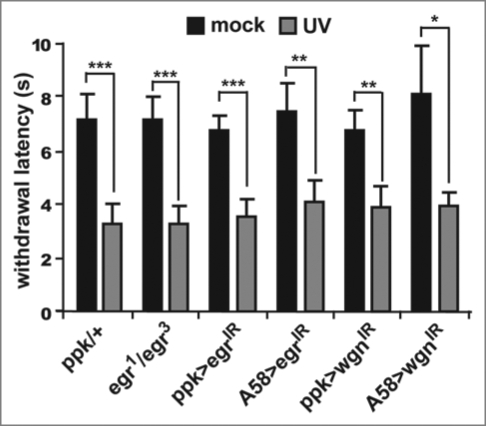
The first distinction was that the kinetics of hyperalgesia and allodynia were not the same in our assay (Fig. 1). Although both responses are observed approximately 8 hours after UV exposure, the hyperalgesic effects dissipated by 16 hours, while allodynia continued to increase until 24 hours after exposure. At the genetic level we also found that the apical caspase Dronc, which is required in epidermal cells for the development of thermal allodynia, plays no significant role in the development of thermal hyperalgesia. This suggests that the induction of thermal hyperalgesia is regulated by a distinct genetic pathway. One possibility is that the development of thermal hyperalgesia requires the release of a separate signaling molecule from damaged cells. To assess this, we have now tested whether Eiger/TNF and Wengen/TNFR play a significant role in the development of thermal hyperalgesia. Here we show that neither Eiger nor Wengen are required for the development of thermal hyperalgesia in our assay. In cases where epidermal expression of eiger or nociceptorspecific expression of wengen were knocked down via RNA interference transgenes, larvae still withdrew from a supra-threshold stimulus of 45°C significantly faster than their mock-treated counterparts (Fig. 2), suggesting that these genes are specifically involved in the induction of allodynia but not hyperalgesia. Thus, despite the fact that UV-induced epidermal damage results in the development of both thermal allodynia and thermal hyperalgesia, it is possible to genetically separate these nociceptive sensitization responses.
Figure 1.
Timing of UV-induced responses. Both allodynia and hyperalgesia develop before overt morphological evidence of epidermal damage and cleaved caspase immunoreactivity indicative of programmed cell death. Although mild allodynia arises earlier than hyperalgesia, the peak response and subsequent diminishment of hyperalgesia occur more rapidly. Staining using a stress response reporter (msn-lacZ, which reads out activation of the Jun N-Terminal Kinase signaling pathway) was evident from 4 to 48 hours after UV treatment. Solid lines, times response was seen most often. Dashed lines, times response was occasionally observed, or was mild or diminishing.
Figure 2.
Eiger and Wengen do not affect the development of thermal hyperalgesia. Whole-animal eiger null mutants (egr1/egr3) or tissue-specific expression of RNAi transgenes targeting eiger (UASeigerIR) or wengen (UAS-wengenIR) were used to test development of thermal hyperalgesia 8 hours following UV irradiation. Epidermal-specific (via A58-Gal4) knockdown of Eiger and nociceptor-specific (via ppk-Gal4) knockdown of Wengen do not prevent thermal hyperalgesia following UV damage. Withdrawal latency was measured (in seconds) in response to a thermal probe heated to 45°C. Larvae of all genotypes treated with UV (gray bars) respond nearly twice as fast as their mock-treated counterparts (black bars). Error bars = SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
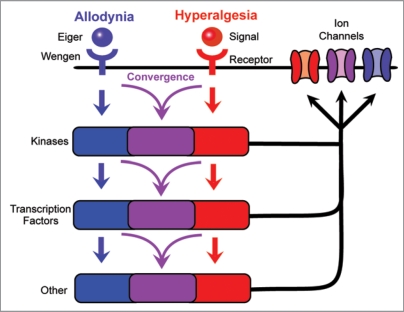
The gene(s) that mediate thermal hyperalgesia in our assay are still unknown, as are components of the thermal allodynia machinery downstream of Wengen/TNFR. A clear future priority is to identify the signaling factors and receptor(s) required for development of thermal hyperalgesia in our assay. It seems likely that sifting through the many conserved factors implicated in nociceptive sensitization in vertebrates will prove a fruitful place to start. A second key priority is to understand the downstream signaling cascades involved in development of allodynia and hyperalgesia and how they interact with one another.4 Of particular interest is whether or not these mediators activate parallel signaling cascades that do not intertwine or show crosstalk, or whether there is a downstream convergence of signals. Mediators could converge at some point in the signaling cascade, such as at the level of cytoplasmic kinases or nuclear transcription factors that regulate expression levels or activation properties of membrane-localized ion channels (Fig. 3). Such convergence is evident in mammalian models of thermal hyperalgesia, where several separate mechanisms come together to regulate Transient Receptor Potential Vanniloid-1 (TRPV1).13–18
Figure 3.
Possible relationships of signaling cascades mediating thermal allodynia and hyperalgesia. The development of thermal allodynia and thermal hyperalgesia is regulated by distinct receptors and may involve completely separate signaling pathways with minimal to no overlap. By contrast, these signals could converge downstream of receptor activation, allowing a single component to regulate multiple aspects of nociceptive sensitization. This convergence may take place at the level of cytoplasmic kinases, nuclear transcription factors, or other intracellular mediators, or the ion channels that are the likely endpoints of each signaling pathway.
Modeling nociceptive sensitization in Drosophila has demonstrated that specific aspects of thermal nociceptive sensitivity are mediated by distinct sets of genes. Given the resolving power of genetic analysis in Drosophila and the conservation of genes required for nociception and nociceptive sensitization we expect that further genetic dissection of UV-induced thermal allodynia and hyperalgesia will lead to more surprises in our understanding of these critical adaptive responses to injury.
Acknowledgements
This work was supported by an American Heart Association predoctoral fellowship (0815339F) to DTB and University of Texas MD Anderson institutional startup funds to MJG. The authors declare that they have no competing financial interests.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/9561
References
- 1.Woolf CJ, Ma Q. Nociceptors—noxious stimulus detectors. Neuron. 2007;55:353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Gottschalk A, Smith DS. New concepts in acute pain therapy: preemptive analgesia. Am Fam Physician. 2001;63:1979–1984. [PubMed] [Google Scholar]
- 3.Lewin GR, Lu Y, Park TJ. A plethora of painful molecules. Curr Opin Neurobiol. 2004;14:443–449. doi: 10.1016/j.conb.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55:365–376. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- 6.Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 7.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 8.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 9.Hwang RY, Zhong L, Xu Y, Johnson T, Zhang F, Deisseroth K, et al. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr Biol. 2007;17:2105–2116. doi: 10.1016/j.cub.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- 11.Babcock DT, Landry C, Galko MJ. Cytokine signaling mediates UV-induced nociceptive sensitization in Drosophila larvae. Curr Biol. 2009;19:799–806. doi: 10.1016/j.cub.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunha TM, Verri WA, Jr, Silva JS, Poole S, Cunha FQ, Ferreira SH. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci USA. 2005;102:1755–1760. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, et al. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proc Natl Acad Sci USA. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWt. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- 15.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5) P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 16.Jung J, Shin JS, Lee SY, Hwang SW, Koo J, Cho H, et al. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J Biol Chem. 2004;279:7048–7054. doi: 10.1074/jbc.M311448200. [DOI] [PubMed] [Google Scholar]
- 17.Planells-Cases R, Garcia-Sanz N, Morenilla-Palao C, Ferrer-Montiel A. Functional aspects and mechanisms of TRPV1 involvement in neurogenic inflammation that leads to thermal hyperalgesia. Pflugers Arch. 2005;451:151–159. doi: 10.1007/s00424-005-1423-5. [DOI] [PubMed] [Google Scholar]
- 18.Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]