Abstract
Objective
To quantify disuse atrophy using electrical impedance myography (EIM), a noninvasive technique that we have used successfully to study neurogenic and myopathic atrophy.
Design
We performed EIM of the tibialis anterior of 10 subjects with disuse atrophy secondary to cast immobilization and in their contralateral normal leg. Subjects were studied shortly after cast removal and again several weeks to months after the cast was removed and normal mobility was restored.
Setting
Outpatient neurology and orthopedic practices at a tertiary care medical center.
Participants
10 otherwise healthy subjects with unilateral leg fracture.
Main Outcome Measures
Resistance, reactance, and phase measured at 50 kHz.
Results
We found that the main EIM outcome parameter, phase at 50 kHz, was lower in the immobilized leg in 9 of 10 cases. Additionally, when normal mobility was restored, the phase of the casted leg increased relative to its initial measurement in all 10 cases, while it increased inconsistently in the contralateral leg.
Conclusions
EIM may be a powerful tool for the assessment of disuse atrophy.
Keywords: electrical impedance myography, disuse atrophy, muscle, fracture
Introduction
Changes in skeletal muscle morphology and physiology are largely governed by physical demands, with heavier workloads producing myocyte hypertrophy and inactivity resulting in “disuse atrophy”.1 Compared to atrophy secondary to neurogenic disorders such as amyotrophic lateral sclerosis or myopathic disorders such as the muscular dystrophies, disuse atrophy is relatively unappreciated by neurologists and neuromuscular specialists. It is particularly relevant to elderly persons with limited mobility2, patients who have sustained orthopedic injuries3, those who have sustained a stroke resulting in limb weakness4, ventilated patients 5, and in persons in microgravity environments. 6, 7 Although the precise epidemiology of disuse atrophy is not known, it is a common condition with substantial public health consequences.
One of the reasons that disuse atrophy has been largely unstudied is the difficulty in finding methods to reliably quantify it. Techniques such as limb circumference measurements, computed tomography, magnetic resonance imaging, dual-energy x-ray absorptiometry, electromyography, and muscle biopsy are hampered by insensitivity, invasiveness, or a lack of portability. Electrical impedance myography (EIM) is a technique that can potentially circumvent these obstacles. EIM involves measurement of muscle impedance by the application of alternating current via surface electrodes.8 Because low-intensity currents are used, muscle and nerve membranes are not depolarized, and the patient experiences no sensation during the study. The central concept of EIM is that skeletal muscle can be modeled as a network of resistors and capacitors. The intracellular and extracellular matrices of muscle tissue act as resistors, and any atrophy which reduces the cross-sectional area of muscle tissue would be expected to increase the resistance (R). The lipid bilayers which constitute muscle membranes act as capacitors, and as muscle atrophies, the cumulative capacitance of the muscle membranes increases. This capacitance is inversely proportional to reactance (X), and muscle atrophy, therefore, would be expected to decrease X. For most of our work, we have used the outcome parameter phase (θ), which is related to R and X by the equation θ = arctan (X/R). Obviously, the individual effects of muscle atrophy on X and R would be predicted to decrease θ, and indeed we have observed that neurogenic and myopathic atrophy do decrease θ. 8, 9 Most of our work uses 50 kHz current, the frequency used in traditional bioimpedance analysis and the frequency at which X is maximal relative to R.8 For the past 10 years, we have used EIM to successfully quantify the severity and progression of neuromuscular disease.8, 9 We undertook this study in an attempt to extend the technique to the evaluation of disuse atrophy.
While there are many causes of disuse atrophy, we sought an experimental model that would allow us to study a relatively homogeneous population free of as many confounding medical comorbidities as possible. Thus, we chose a group of otherwise healthy subjects who had sustained lower extremity fractures requiring below-knee casts. Casting is a well-established model of disuse atrophy1, 3, 10–12 used to study both the immobilization-related changes in skeletal muscle and their subsequent recovery once mobility is restored. The casting protocol we employed involved immobilization at the ankle joint, and thus the muscles which are responsible for its dorsiflexion (predominantly the tibialis anterior) and plantarflexion (predominantly the gastrocnemius-soleus complex). We hypothesized that muscles of immobilized limbs would show impedance changes which reflect disuse atrophy, and that when mobility was restored, impedance parameters would return towards their initial, normal values.
Methods
Subjects
Ten otherwise healthy subjects (six men and four women), ranging in age from 20 to 52, with distal leg and foot fractures requiring cast immobilization were recruited consecutively from the orthopedic surgery department at Beth Israel Deaconess Medical Center. All subjects were treated nonoperatively in below-knee casts which consisted of the serial application of a four-inch stockinette (BSN Medical, Hamburg, Germany), followed by four to six rolls of Webril undercast padding (Tyco Healthcare, Mansfield, MA), and then four rolls of 10 cm × 3.6m Delta Lite fiberglass cast rolls (BSN Medical, Hamburg, Germany). Casting was performed using standard technique with the ankle in the neutral position. At the first EIM testing visit, informed consent was obtained from each participant using forms approved by the Beth Israel Deaconess Medical Center Committee on Clinical Investigations.
Electrical Impedance Myography Measurements
Data were obtained using an RJL Model 101-A impedance instrument (RJL Electronics, Clinton Township, MI) operating at 50 kHz, a wide range lock-in amplifier (Signal Recovery Model 7280, Advanced Measurement Technology Inc., Oak Ridge, TN) with current supplied from the 1-volt reference channel output, or an Impedimed SFB7 bioimpedance spectroscopy device (Impedimed, San Diego, CA).13 Because the latter two devices collect data at multiple frequencies, 50 kHz data was extracted for analysis. All three devices were calibrated against the same resistor-capacitor network and tested in human subjects to verify consistency among the different instruments.
Electrode Montages
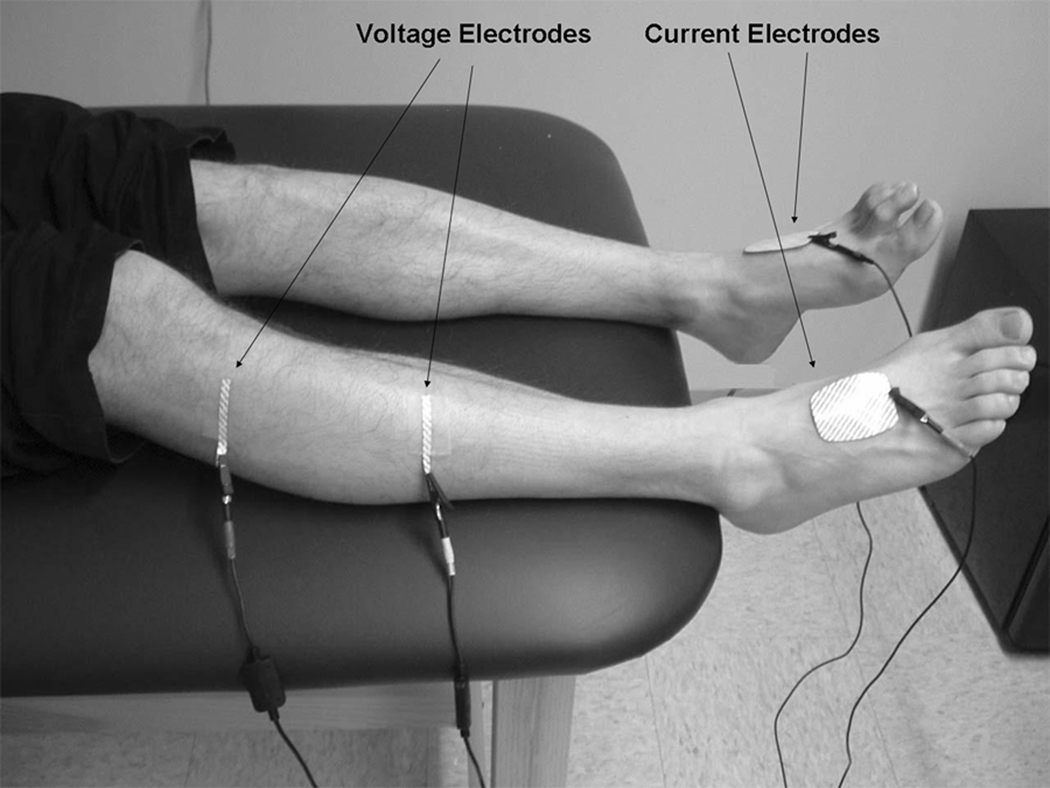
EIM data was obtained from tibialis anterior of both legs. For the purposes of this study, measurements were obtained using our standard electrode montage in which current-injecting electrodes (Disposable Ground Plate Electrodes, part number 019-400500, Viasys Healthcare/Nicolet Biomedical, Madison, WI) were attached over the dorsum of the feet and voltage measuring electrodes (Disposable Ring Electrodes, part number 019-766400, Viasys Healthcare/Nicolet Biomedical, Madison, WI, cut to half-length) were placed over tibialis anterior. The first voltage electrode was placed 15 cm distal to the fibular head, while the second voltage electrode was placed 2.5 cm distal to the fibular head. The electrode montage is shown in Figure 1.
Figure 1.
EIM recording of tibialis anterior. Current and voltage electrodes are displayed.
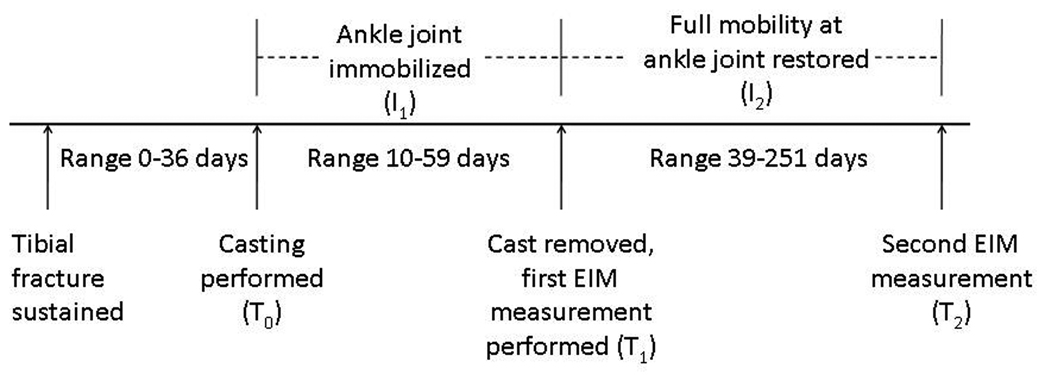
Study Timeline
The study timeline is shown in Figure 2. Casting was performed on the day of fracture (T0) in 9 of 10 cases. In Subject 9, the fracture was undiagnosed for several weeks and casting was not performed until 36 days after the initial injury. Subjects were immobilized in their casts for intervals ranging between 10 and 59 days (I1). The first set of EIM measurements was obtained on both legs within one day of cast removal (T1). EIM measurements were not obtained prior to cast removal due to the medical necessity of cast application. Subsequent measurements at T2 were made from both legs at intervals (I2) between 39 and 375 days after the initial measurement, during which time, full range of motion at the ankle joint and weight bearing were permitted. Formal physical therapy was not employed during this period of restored mobility. At both T1 and T2, all patients had full range of motion and strength in tibialis anterior. None of our patients had a complication (e.g. infection, bleeding, development of reflex sympathetic dystrophy) during their recovery processes.
Figure 2.
Study timeline.
Data Analysis
The main EIM outcome parameter, θ at 50 kHz was determined from the raw impedance values X and R using the equation θ = arctan (X/R). Side-to-side differences were computed at both T1 and T2, with Wilcoxon signed-rank tests being used to assess for a statistically significant difference between the two groups. Differences were also computed between θ measured between T1 and T2 for both legs. Again, Wilcoxon signed-rank tests were used to assess for a statistically significant difference between the two groups. Measurements obtained at T1 and T2 were graphically compared to previously established normal values.14 These normal values were derived from measurements of θ in 87 healthy subjects. Briefly, age-specific normal values were established by fitting the logarithmic transformation of θ versus age to a quadratic function and determining the lower 95% confidence interval of this curve for tibialis anterior.
Results
Table 1 lists patient characteristics including the durations of immobilization (I1) and time between serial EIM measurements (I2). All results discussed in sections 1–3 below are also detailed in Table 2.
Table 1.
Patient characteristics and timing of measurements
| Patient | Age, sex |
Side of Fracture |
Site of Fracture | I1,days | I2,days |
|---|---|---|---|---|---|
| 1 | 20, M | R | Base of 5th metatarsal | 46 | 251 |
| 2 | 22, M | L | Distal fibula | 49 | 70 |
| 3 | 26, F | L | Distal tibia | 10 | 181 |
| 4 | 29, M | L | Distal fibula | 45 | 104 |
| 5 | 42, M | R | Distal fibula | 41 | 178 |
| 6 | 46, F | L | Dorsal navicular bone | 29 | 140 |
| 7 | 48, M | R | Distal tibia | 20 | 192 |
| 8 | 51, F | L | Distal tibia | 35 | 28 |
| 9 | 49, F | R | Base of 5th metatarsal | 35 | 38 |
| 10 | 52, M | L | Calcaneus | 59 | 375 |
I1 = interval during which patient was casted and immobilized. I2 = interval between first and second measurements, during which time mobility was completely restored.
Table 2.
Measurement of θ at 50 kHz in casted and uncasted legs at time of cast removal and after restoration of mobility.
| Patient | θTA, Casted Leg, T1 |
θTA, Uncasted leg, T1 |
Side-to- side difference |
θTA, Casted Leg T2 |
θTA, Uncasted leg, T2 |
Side-to-side difference |
Difference between θTA, T1 and T2, Casted leg |
Difference between θTA, T1 and T2, Uncasted leg |
|---|---|---|---|---|---|---|---|---|
| 1 | 9.27 | 12.53 | 26.0% | 14.1 | 14.7 | 4.1% | 34.3% | 14.8% |
| 2 | 6.34 | 11.34 | 44.1% | 10.13 | 10.02 | −1.1% | 37.4% | −13.2% |
| 3 | 9.29 | 9.95 | 6.6% | 10.55 | 9.67 | −9.1% | 11.9% | −2.9% |
| 4 | 5.39 | 9.26 | 41.8% | 9.63 | 9.55 | −0.8% | 44.0% | 3.0% |
| 5 | 5.84 | 11.54 | 49.4% | 10.46 | 12.29 | 14.9% | 44.2% | 6.1% |
| 6 | 4.06 | 6.68 | 39.2% | 7.00 | 8.56 | 18.2% | 42.0% | 22.0% |
| 7 | 7.57 | 10.84 | 30.2% | 10.10 | 12.74 | 20.7% | 25.0% | 14.9% |
| 8 | 4.42 | 7.32 | 39.6% | 5.41 | 7.04 | 23.2% | 18.3% | −4.0% |
| 9 | 7.54 | 6.44 | −17.1% | 8.27 | 7.46 | −10.9% | 8.8% | 13.7% |
| 10 | 6.66 | 8.73 | 23.7% | 8.02 | 10.26 | 21.8% | 17.0% | 12.9% |
| Mean | 6.64 | 9.46 | 28.4% | 9.37 | 10.23 | 8.4% | 28.3% | 6.7% |
Legend - θTA = 50 kHz phase measured for tibialis anterior. All measurements of θ are in degrees. T1 = date of cast removal. T2 = date of second measurement after mobility had been restored.
1. Side-to-side comparison immediately after cast removal. Subjects were immobilized in their casts for between 10 and 59 days. Immediately after cast removal (T1), θ measured at 50 kHz in the tibialis anterior of the casted leg was lower than in the uncasted leg in 9 of 10 cases. The mean θ in the casted legs was 6.64 while the mean θ in the uncasted legs was 9.46, a mean side-to-side difference of 28.4% (p<0.01).
2. Comparison of θ in the casted leg before and after restoration of mobility. Tibialis anterior θ was measured again after complete weightbearing and mobility at the ankle joint was restored for intervals between 28 and 375 days. In all 10 cases, θ increased after restoration of mobility as compared to its measurement immediately after cast removal. As noted above, the mean θ immediately after cast removal was 6.64, while after mobility was restored, the mean θ was 9.37, an increase of 28.3% (p<0.01).
3. Comparison of θ in the uncasted leg before and after restoration of mobility. Tibialis anterior θ in the uncasted leg was also measured immediately after cast removal from the contralateral leg and then again after full restoration of mobility. θ changed inconsistently, with increases observed in 7 of 10 and decreases in 3 of 10. As a group, θ increased from a mean of 9.46 to a mean of 10.21, a non-significant increase of 6.7%.
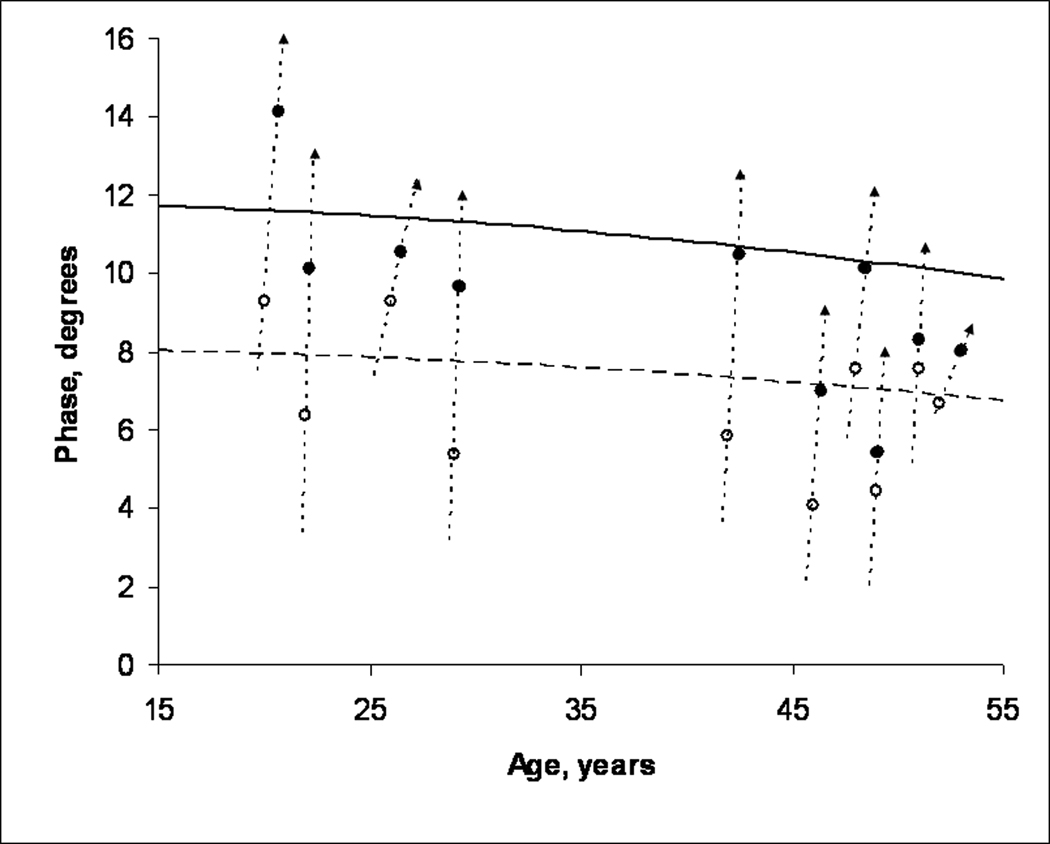
4. EIM measurements in subjects with disuse atrophy as compared to normal subjects. θ at 50 kHz in the casted tibialis anterior was compared to previously established age-dependent normal values for tibialis anterior14 as shown in Figure 3. In 6 of 10 cases, measurements obtained at the time of cast removal were smaller than the age-adjusted lower limit of normal. θ returned to a normal value in 4 of these 6 cases, and in 1 additional subject, the θ returned to a near normal value.
Figure 3.
The age-dependent mean value for 50 kHz θ for tibialis anterior is shown in the upper, solid curve, while the 95% lower confidence limit of normal is shown in the lower, dashed curve. For each patient, θ was measured in tibialis anterior at the time of cast removal (open circle) and then after mobility had been restored for several weeks to months (filled circle). In all 10 cases, an increase was noted between the two data points. In six cases, the initial measurement was smaller than the lower limit of normal. After mobility was restored, θ returned to a normal value in 4 of these cases and to near normal in a fifth.
Discussion
Disuse atrophy is an important phenomenon with health consequences that are potentially of great magnitude. A noninvasive method to quantify disuse atrophy could be helpful in monitoring rehabilitation efforts in patients with orthopedic injuries15, assessing sarcopenia of aging 16–18 and planning adaptive countermeasures for microgravity environments6, 7. Unfortunately, the methods used thus far are not ideal for the clinical study of disuse atrophy, as they are invasive, inconsistent, or lack portability. Because EIM can potentially circumvent these obstacles and has been successful in the study of muscle atrophy secondary to neurogenic and myopathic disease, we chose to extend the technique to patients with disuse atrophy.
We chose casting as our disuse atrophy model for several reasons. Casting assures a relatively homogeneous study population, as the muscles of interest and the techniques used to immobilize them can remain relatively consistent across patients. Healthy subjects could be recruited without concern for confounding medical illnesses which might be expected to change body composition, and therefore tissue impedance. Finally, the existence of established animal models for disuse atrophy secondary to casting would allow us to further explore the anatomic and physiologic basis of impedance changes in the condition.
Our data supports that EIM may be a potentially powerful tool to diagnose and quantify disuse atrophy. First, we found that θ measured immediately after cast removal was smaller in the casted leg as compared to the uncasted leg in 9 of 10 cases. This is consistent with our findings in other diseases such as ALS in which decreases in θ accompany progressive muscle atrophy.19 Perhaps of more direct relevance to this study is our finding of a decrease in θ in the affected limb of subjects with radiculopathy as compared to their normal contralateral limb.9 The precise explanation for why subject 9 had a larger phase in the affected leg at T1 is not immediately obvious, although the persistence of this difference at T2 suggests that pre-existing side-to-side differences between the two legs may have played a role. Another possible explanation is that subject 9 was the only patient not casted immediately after injury, and that possible overuse of the tibialis anterior during the uncasted period resulted in an increase in θ. Disuse atrophy results in several pathological changes including decreases in muscle cross-sectional area, reduction in contractile proteins, and increases in connective tissue.10, 20, 21 The exact methods by which these changes affect impedance parameters is currently undergoing study in animal models.
We also found that after mobility was restored, there was an increase in θ in the casted leg in all 10 cases. This change parallels that seen in animal and human models of disuse atrophy in which casting results in muscle atrophy followed by a resolution of that atrophy once the cast is removed and mobility is restored.3, 11 By comparison, there was no consistent change in the θ measured in the uncasted leg during the same period of mobility restoration. A related finding is that θ measured immediately after cast removal was smaller than the previously established lower limit of normal, and increased back into the normal range after normal mobility was restored. The two subjects in whom θ did not increase back into the normal range both had short durations of restored mobility (38 days for subject 8 and 39 days for subject 9). We hypothesize that further measurements of these subjects would likely show increases in θ back into the normal range.
There were several limitations to this study. We recognize that factors such as the age and sex of the patient, the degree of disuse compared with the normal level of muscle use, pre-existing muscle weakness, inflammation due to the fracture, noncompliance with non-weightbearing orders, and skin-electrode interface inconsistencies likely contributed to variability in our measurements.22 Compensatory hypertrophy of the non-casted leg during the casting period may have resulted in a supernormal θ on that side. Although casting should limit movements of tibialis anterior, we did not assess muscle activation during the casting period to verify that the muscle was truly in a state of disuse. We also acknowledge that there was considerable variation in the duration of I2. This was, however, entirely the result of difficulties with patient availability, and was not generated by any differences in patient health or progress of rehabilitation efforts. Finally, this is a small-scale study of 10 patients, and a confirmatory study on a larger cohort is obviously indicated.
We view this study as preliminary in the application of EIM as a tool to evaluate disuse atrophy. Larger scale studies to verify and extend these results would be valuable. In this study, we used single-frequency measurements of phase: multifrequency EIM is another variation of the technique in which measurements are made at multiple frequencies.13 This variation might be effective in helping to distinguish among disuse, neurogenic, and myopathic forms of atrophy and is worthy of further exploration. Studies in which interventions such as physical therapy programs or even medication trials are included are next logical steps. It may be useful to obtain EIM measurements prior to casting to verify that EIM measurements are similar before the casting takes place and after the leg has been allowed to heal. Finally, correlation of these results with our ongoing animal work will provide a better understanding of how changes in muscle pathology affect impedance measurements.
Conclusions
EIM may be a powerful tool for the assessment of disuse atrophy. Further study to verify these results in a larger population of patients with disuse atrophy from a variety of conditions is indicated.
Footnotes
Suppliers
BSN Medical, Hamburg, Germany
Tyco Healthcare, Mansfield, MA
RJL Electronics, Clinton Township, MI
Signal Recovery Model 7280, Advanced Measurement Technology Inc., Oak Ridge, TN
Impedimed, San Diego, CA
Viasys Healthcare/Nicolet Biomedical, Madison, WI
References
- 1.Appell H. Muscular atrophy following immobilisation: a review. Sports Medicine. 1990;10:42–58. doi: 10.2165/00007256-199010010-00005. [DOI] [PubMed] [Google Scholar]
- 2.Thomas DR. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr. 2007;26:389–399. doi: 10.1016/j.clnu.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Vandenborne K, Elliott MA, Walter GA, Abdus S, Okereke E, Shaffer M, Tahernia D, Esterhai JL. Longitudinal study of skeletal muscle adaptations during immobilization and rehabilitation. Muscle and Nerve. 1998;21:1006–1012. doi: 10.1002/(sici)1097-4598(199808)21:8<1006::aid-mus4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 4.Bourbonnais D, Vanden Noven S. Weakness in patients with hemiparesis. Am J Occup Ther. 1989;43:313–319. doi: 10.5014/ajot.43.5.313. [DOI] [PubMed] [Google Scholar]
- 5.Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrager JB. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358:1327–1335. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- 6.Fitts RH, Riley DR, Widrick JJ. Microgravity and skeletal muscle. J Appl Physiol. 2000;89:823–839. doi: 10.1152/jappl.2000.89.2.823. [DOI] [PubMed] [Google Scholar]
- 7.Vandenburgh H, Chromiak J, Shanksy J, del Tatto M, Lemaire J. Space travel directly induces skeletal muscle atrophy. FASEBJ. 1999;13:1031–1038. doi: 10.1096/fasebj.13.9.1031. [DOI] [PubMed] [Google Scholar]
- 8.Rutkove SB, Aaron R, Shiffman CA. Localized bioimpedance analysis in the evaluation of neuromuscular disease. Muscle Nerve. 2002 Mar;25(3):390–397. doi: 10.1002/mus.10048. [DOI] [PubMed] [Google Scholar]
- 9.Rutkove SB, Esper GJ, Lee KS, Aaron R, Shiffman CA. Electrical impedance myography in the detection of radiculopathy. Muscle Nerve. 2005;32:335–341. doi: 10.1002/mus.20377. [DOI] [PubMed] [Google Scholar]
- 10.Appell HJ. Morphology of immobilized skeletal muscle and the effects of a pre- and postimmobilization training program. Int J Sports Med. 1986;7:6–12. doi: 10.1055/s-2008-1025726. [DOI] [PubMed] [Google Scholar]
- 11.Booth FW, Seider MJ. Recovery of skeletal muscle after 3 mo of hindlimb immobilization in rats. J Appl Physiol: Respirat Environ Exercise Physiol. 1979;47:435–439. doi: 10.1152/jappl.1979.47.2.435. [DOI] [PubMed] [Google Scholar]
- 12.Chor H, Dolkart RE. A study of 'simple disuse atrophy' in the monkey. Am J Physiol. 1936;117:626–630. [Google Scholar]
- 13.Esper GJ, Shiffman CA, Aaron R, Lee KS, Rutkove SB. Assessing neuromuscular disease with multifrequency electrical impedance myography. Muscle Nerve. 2006;34:595–602. doi: 10.1002/mus.20626. [DOI] [PubMed] [Google Scholar]
- 14.Rutkove SB, Fogerson PM, Garmirian LP, Tarulli AW. Reference values for 50-kHZ electrical impedance myography. Muscle Nerve. 2008 doi: 10.1002/mus.21075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bloomfield SA. Changes in musculoskeletal structure and function with prolonged bed rest. Med Sci Sports Exerc. 1997;29:197–206. doi: 10.1097/00005768-199702000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Aaron R, Esper GJ, Shiffman CA, Bradonjic K, Lee KS, Rutkove SB. Effects of age on muscle as measured by electrical impedance myography. Physiol Meas. 2006;27:953–959. doi: 10.1088/0967-3334/27/10/002. [DOI] [PubMed] [Google Scholar]
- 17.Suetta C, Magnusson SP, Beyer N, Kjaer M. Effect of strength training on muscle function in elderly hospitalized patients. Scand J Med Sci Sports. 2007;17:464–472. doi: 10.1111/j.1600-0838.2007.00712.x. [DOI] [PubMed] [Google Scholar]
- 18.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 19.Rutkove SB, Zhang H, Schoenfeld DA, Raynor EM, Shefner JM, Cudkowicz ME, Chin AB, Aaron R, Shiffman CA. Electrical impedance myography to assess outcome in amyotrophic lateral sclerosis clinical trials. Clin Neurophysiol. 2007;118:2413–2418. doi: 10.1016/j.clinph.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oki S, Desaki J, Matsuda Y, Okumura H, Shibata T. Capillaries with fenestrae in the rat soleus muscle after experimental limb immobilization. J Electron Microsc. 1995;44:307–310. [PubMed] [Google Scholar]
- 21.Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2005;288:R337–R344. doi: 10.1152/ajpregu.00469.2004. [DOI] [PubMed] [Google Scholar]
- 22.Wills CA, Caiozzo VJ, Yasukawa DI, Prietto CA, McMaster WC. Effects of immobilization of human skeletal muscle. Orthop Rev. 1982;11:57–64. [Google Scholar]