Abstract
StAR family proteins, including StarD4, play a key role in steroidogenesis by transporting cholesterol (Ch) into mitochondria for conversion to pregnenolone. Using a model system consisting of peroxidized cholesterol (7α-OOH)-containing liposomes as donors, we showed that human recombinant StarD4 accelerates 7α-OOH transfer to isolated liver mitochondria, and to a greater extent than Ch transfer. StarD4 had no effect on transfer of non-oxidized or peroxidized phosphatidylcholine, consistent with sterol ring specificity. StarD4-accelerated 7α-OOH transfer to mitochondria resulted in greater susceptibility to free radical lipid peroxidation and loss of membrane potential than in a non-StarD4 control. The novel implication of these findings is that in oxidative stress states, inappropriate StAR-mediated trafficking of peroxidized Ch in steroidogenic tissues could result in damage and dysfunction selectively targeted to mitochondria.
Keywords: Oxidative Stress, Cholesterol Hydroperoxide, StAR proteins, Lipid Trafficking, Steroidogenesis
Introduction
The plasma membrane of most mature mammalian cells contains a substantial amount of non-esterified cholesterol (Ch), 45–50 mol % of the total membrane lipid. Like other unsaturated lipids, Ch is susceptible to oxidation under oxidative stress conditions, hydroperoxide species (ChOOHs) being prominent products/intermediates [1,2]. In membranes undergoing free radical-mediated (chain) peroxidation, the 7α- and 7β-hydroperoxides of Ch (7α- and 7β-OOH) are formed, along with end-products such as the 7α-OH and 7β-OH diols, 5,6-epoxide, and 7-ketone [1–4]. Like phospholipid counterparts (PLOOHs), ChOOHs are susceptible to reductive turnover, either undergoing iron-catalyzed one-electron reduction to free radicals, which expands peroxidative damage, or enzyme-catalyzed two-electron reduction to alcohols, which attenuates further damage [4]. One-electron turnover is not necessarily limited to a ChOOH’s membrane of origin, but can extend to other membranes via ChOOH translocation [5–7]. Spontaneous intermembrane ChOOH transfer was found to be nearly two orders of magnitude faster than that of Ch, and it sensitized acceptor membranes to iron-catalyzed chain peoxidation [5,6]. Subsequent studies revealed that recombinant sterol carrier protein-2 (SCP-2), a low specificity lipid trafficker [8], could accelerate ChOOH translocation in various model systems, including a liposomal donor-mitochondrial acceptor system [9]. This suggested that under oxidative stress, adventitious trafficking of lipid hydroperoxides (LOOHs) by lipid transfer proteins might be a general phenomenon.
Proteins of the steroidogenic acute regulatory (StAR) family contain a unique lipid binding/transfer (START) domain and play a key role in steroid hormone biosynthesis by transporting Ch to mitochondria, where it is converted to pregnenolone [10–12]. StAR proteins are highly selective for Ch, each binding one sterol molecule in a unique hydrophobic pocket [13]. StarD4 and StarD1 have been proposed to operate cooperatively in transporting Ch, the former moving it through the cytosol to mitochondria and the latter, in concert with other proteins, moving it from the outer to inner mitochondrial membrane, where the pregnenolone-generating side chain cleavage system (P450scc) is located [10–12]. We postulated that under conditions favoring oxidative stress, e.g. diminished antioxidant capacity with aging, ChOOHs will be recognized by StAR proteins of steroidogenic cells and inappropriately delivered to mitochondria, leading to free radical damage and loss of function. In this report, we provide the first evidence to support this hypothesis, based on experiments with 7α-OOH-containing liposomal donors, isolated mitochondrial acceptors, and recombinant StarD4.
Materials and methods
General materials
Sigma Chemical Co. (St. Louis, MO) supplied the antimycin A, CCCP, DFO, DTT, EDTA, Rh123, and rotenone. DMPC and POPC were obtained from Avanti Polar Lipids, (Birmingham, AL). Amersham Biosciences (Arlington Heights, IL) supplied the [4-14C]Ch (~50 mCi/ml) and 1-palmitoyl-2-[1-14C]oleoyl-sn-phosphocholine (~55 mCi/ml), referred to as [14C]Ch and [14C]POPC, respectively. [14C]7α-OOH was prepared by dye-sensitized photoperoxidation of [14C]Ch and isolated by reverse- and normal-phase HPLC, as described [14]. Radiolabeled POPC-OOH (also referred to as PCOOH) was also prepared photochemically [15].
Preparation of recombinant proteins
The preparation of an expression vector encoded for human StarD4 fused at its N-terminus to an enterokinase-cleavable glutathione S-transferase (GST) tag was described previously, along with details about expression and isolation of the fusion protein [16,17]. Isolated protein was treated with enterokinase (1% w/w for 1 h at 37 °C) to cleave off the GST tag, which was separated from StarD4 by incubating with glutathione-linked Sepharose 4B (10–12 h at 4 °C). On SDS-PAGE, isolated StarD4 appeared as a single band of Mr ~27 kDa, in agreement with previous findings [17]; the protein was stored in 300 mM NaCl/50 mM NaH2PO4/20 mM Tris-Cl (pH 7.4) at 4 °C.
Human recombinant SCP-2 was prepared as described previously [9], using a plasmid encoded for the mature, 13.2 kDa protein. The purified protein was stored at −20 ° C in 10 mM phosphate/0.5 mM EDTA/1 mM DTT/50% glycerol (pH 6.8). Immediately before being used in transfer experiments, SCP-2 was dialyzed against PBS/0.1 mM EDTA/0.1 mM DFO at 4 ° C to remove glycerol and DTT, the latter of which would otherwise reduce 7α-OOH during transfer incubation [9].
Preparation of liposomes
Small unilamellar liposomes (50 nm SUVs) were prepared by an extrusion procedure [5,6]. All steps were carried out under argon to minimize lipid autoxidation. A chloroform solution of chosen lipids was dried under argon and then in vacuum at room temperature. After hydration, followed by five cycles of freezing and thawing, the vesicles were passed through two 0.05 µm-pore polycarbonate filters in an Extruder apparatus (Lipex Biomembranes, Vancouver, BC). The resulting SUVs were stored at 4°C under argon for up to 48 h.
Preparation of mitochondria
Freshly isolated mouse livers in chilled MS buffer (0.2 M mannitol/50 mM sucrose/1 mM KH2PO4/2 mM MgCl2/1 mM EGTA/5 mM MOPS, pH 7.2) were homogenized and subjected to differential centrifugation [9]. The mitochondrial fraction was recovered, washed with MS buffer, and total protein concentration was determined by Bradford assay [18]. Molar concentration of total lipid was calculated using the known protein/lipid mass ratio and the overall lipid composition (wt %) [19].
[14C]Ch labeling of mitochondria
Mitochondria (15 mg protein/ml in MS buffer) were incubated with DMPC/[14C]Ch/DCP (100:50:1.5 by mol; 1.5 mM; 3 µCi/ml) SUVs and SCP-2 (2 µg/ml) for 1 h at 37° C with occasional mixing. This typically resulted in ~35 % [14C]Ch-labeling. Labeled mitochondria were washed twice with and resuspended in respiration buffer (210 mM mannitol/70 mM sucrose/10 mM HEPES, pH 7.4), and stored on ice under argon.
Transfer incubation conditions
Mitochondria (typically 2.8 mg protein/ml, ~0.6 mM total lipid) were incubated with POPC/7α-OOH, POPC/Ch, DMPC/POPC, or DMPC/POPC-OOH (0.8:0.2 mol.mol) SUVs at 25 °C or 37 °C with periodic gentle mixing. StarD4 or SCP-2 (1–2 µM) were included as indicated. At various time points, mitochondria were pelleted (12000 g, 5 min) and radioactivity of translocated [14C]lipid analytes (~0.1 µCi/ml) was assessed by scintillation counting.
Determination of free radical-mediated lipid peroxidation
[14C]Ch-labeled mitochondria were incubated with 7α-OOH-containing SUVs ± StarD4 as described above. [14C]ChOX-based free radical peroxidation was assessed immediately after transfer (0 min) and also after follow-up incubation in the presence of lipophilic ferric-8-hydroxyquinoline [Fe(HQ)3] and ascorbate (AH−), which promote pro-oxidant turnover of 7α-OOH [1,4]. After treatment, mitochondria were pelleted, washed, and lipid-extracted using chloroform-methanol (2:1, vol/vol) [20]. Recovered lipid fractions were analyzed for [14C]ChOX species by high performance thin layer chromatography with phosphorimaging detection (HPTLC-PI), as described previously [21].
Measurement of mitochondrial membrane potential
Following transfer incubation in the absence or presence of StarD4, mitochondria were isolated from SUVs and typically added to 5 mM succinate/1 µM rotenone/0.2 µM Rh123 in MS buffer at 37 °C, giving 0.2 mg protein/mL. After a Rh123 fluorescence baseline was attained, the uncoupler CCCP and respiratory inhibitor antimycin A were added (2 µM each), allowing ΔΨ measurement. Other details were as described [9].
Results
Kinetics of StarD4-mediated transfer of 7α-OOH vs. Ch to mitochondria
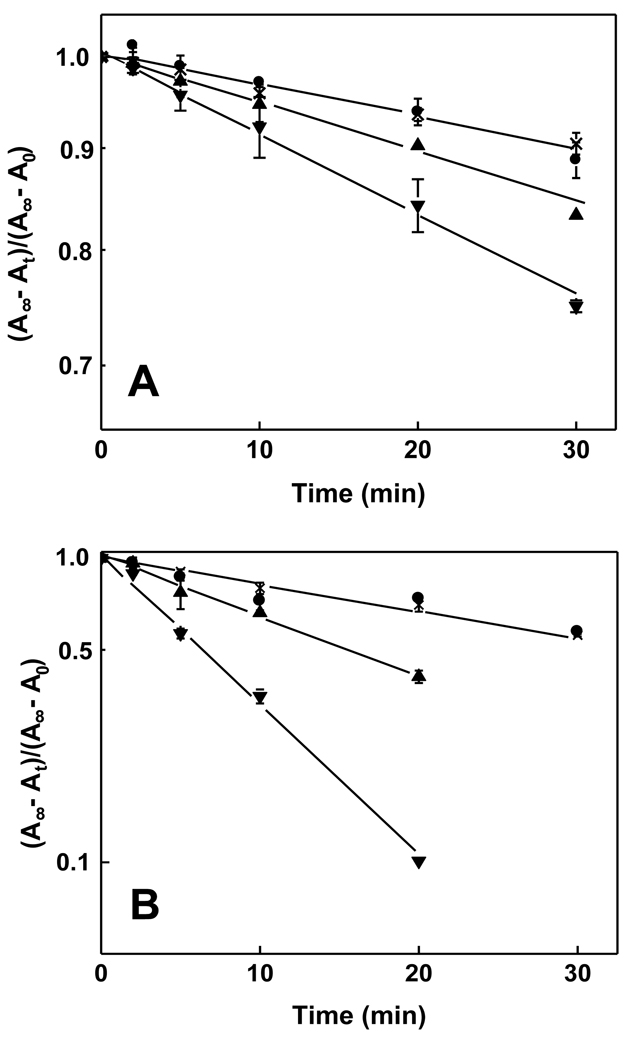
The ability of recombinant StarD4 to facilitate translocation of 7α-OOH (a free radical-generated ChOOH) was examined and compared with Star4-mediated translocation of Ch, using [14C]7α-OOH- or [14C]Ch-containing SUVs as donors and isolated liver mitochondria as acceptors. Mitochondria were in 10-fold lipid molar excess over SUVs, so most of the analyte at equilibrium was in the acceptor compartment. At various times during transfer incubation in MS buffer at 25 °C, samples were removed, centrifuged, and recovered mitochondrial pellets subjected to scintillation counting. As shown in Fig. 1A, in the absence of StarD4, [14C]Ch moved slowly and spontaneously from SUVs to mitochondria, the apparent first-order rate constant being ~3.8 × 10−3 min−1. StarD4 increased the transfer rate constant significantly and concentration-dependently (Fig. 1A), the value at 1 µM protein being ~1.6-times greater than background and at 2 µM protein ~2.6-times greater. Importantly, 2 µM BSA, used as a control, had no effect on transfer rate, whereas recombinant SCP-2 also accelerated Ch transfer (results not shown), confirming highly specific functional recognition of Ch by StarD4 and SCP-2 ( ). In the case of [14C]7α-OOH (Fig. 1B), spontaneous transfer was ~5-times faster than that of [14C]Ch, consistent with our previous findings on the two analytes [6,7]. As shown in Fig. 1B, StarD4 also enhanced the rate of [14C]7α-OOH transfer, 1 µM protein increasing it by ~2.8-fold above background and 2 µM protein increasing it by ~6-fold. Once again, no significant increase was observed when BSA was substituted for StarD4 (Fig. 1B), ruling out a non-specific general protein effect. StarD4-enhanced transfer rates (spontaneous-corrected) at each of the protein concentrations used were much more impressive for 7α-OOH than for Ch, viz. 14–15-times greater for the former (Fig. 1A, 1B), suggesting greater recognition by or accessibility to the protein.
Figure 1.
Spontaneous and StarD4-enhanced transfer of Ch and 7α-OOH from liposomes to mitochondria. Reaction mixtures in pH 7.5 MS buffer at 25 °C contained POPC/[14C]Ch or [14C]7α-OOH (8:2 mol/mol) SUVs (0.06 mM total lipid; 100 nCi/ml), liver mitochondria (2.8 mg protein/ml, 0.6 mM total lipid) and either StarD4 [1 µM (▲) or 2 µM (▼)] or BSA (2 µM (●)]. Spontaneous transfer for mixtures lacking StarD4 or BSA is also represented (x). At the indicated incubation times, samples were removed, centrifuged to separate SUVs from mitochondria, and radioactivity in the latter was determined by scintillation counting. (A) First-order kinetic plot for [14C]Ch acquisition by mitochondria; (B) First-order plot for [14C]7α-OOH acquisition by mitochondria. A∞, Ao, and At denote analyte level at infinite time, time zero and time t, respectively. Plotted values are means ± SD (n = 3).
Comparative ability of StarD4 and SCP-2 to transfer PCOOH vs. PC to mitochondria
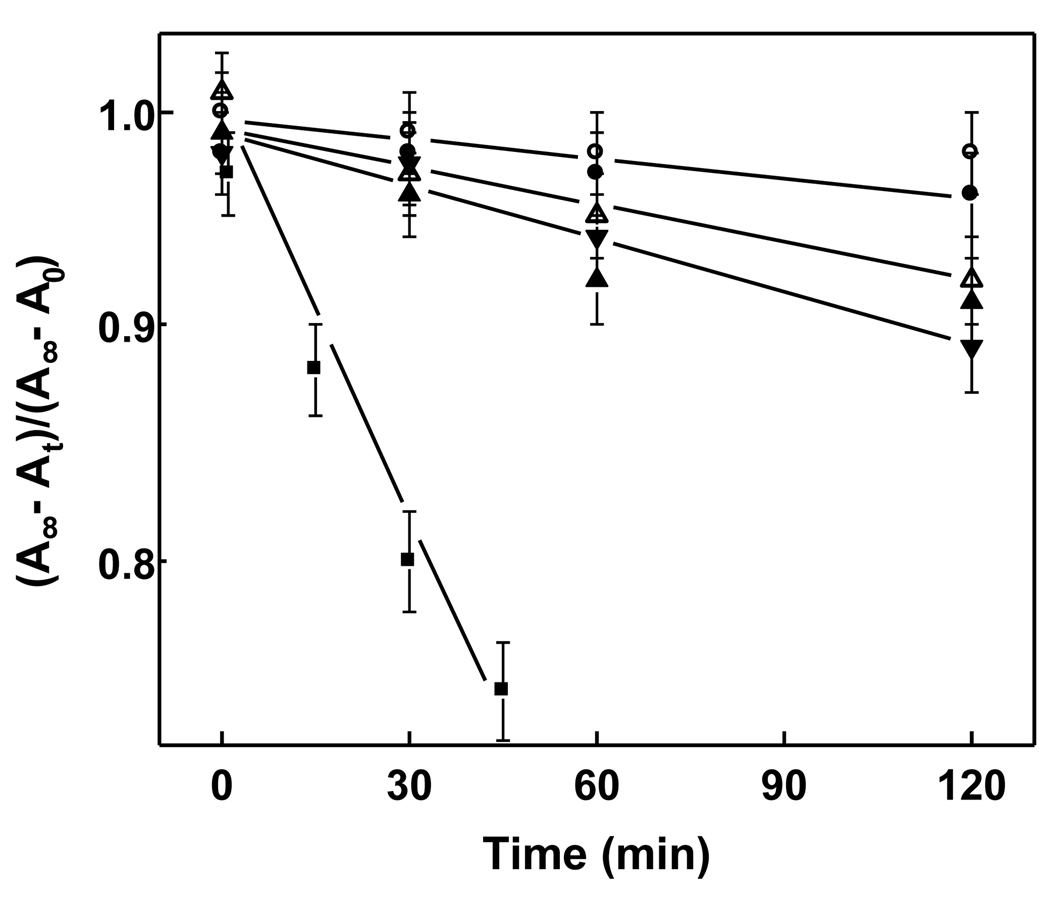
Previous studies have demonstrated that full-length recombinant StarD1 or N-62 truncated StarD1 can facilitate intermembrane transfer of Ch in donor/acceptor model systems [22]. By contrast, N-62 StarD1 exhibited no significant ability to facilitate phosphatidylcholine (PC) transfer, whereas SCP-2 was fully capable of this [22]. We asked whether StarD4 might also be selective in recognizing and transporting sterols like Ch and 7α-OOH, but not phospholipids or oxidized phospholipids. As shown in Fig. 2, the synthetic phospholipid [14C]POPC translocated slowly on its own from SUVs to mitochondria, the apparent first-order rate constant (3.2 × 10−4 min−1) being about one-tenth that determined for Ch (Fig. 1A); the difference would have been greater still had the Fig. 1 and Fig. 2 experiments been run at the same temperature. Whereas 1 µM SCP-2 increased the POPC transfer rate by ~3.3-fold, StarD4 at the same concentration produced no significant increase (Fig. 2). Similar to 7α-OOH vs. Ch, 14C-labeled peroxidized POPC (PCOOH ) translocated more rapidly than unoxidized POPC, in this case by a factor of ~2.5. Whereas PCOOH transfer, like that of PC, was not affected by StarD4, it was accelerated nearly 10-fold by SCP-2 at the same concentration (Fig. 2). In terms of LOOHs, therefore, StarD4 was found to be absolutely specific, recognizing and transporting at least one ChOOH species, but not a PCOOH species. It is reasonable to assume that StarD4’s specificity for ChOOHs vs. PLOOHs exists in general, but this remains to be established.
Figure 2.
Transfer of PC and PCOOH from liposomes to mitochondria in the absence vs. presence of StarD4 or SCP-2. Reaction mixtures containing liver mitochondria (0.6 mM total lipid) and DMPC/POPC (1:1 mol/mol) SUVs (0.06 mM total lipid) spiked with a trace of either [14C]POPC or [14C]POPC-OOH in pH 7.5 MS buffer at 37 °C were incubated in the absence or presence of 1 µM StarD4 or SCP-2. At the indicated times, samples were removed, centrifuged, and radioactivity in the mitochondrial fraction was measured. First-order kinetic plots for analyte acquisition by mitochondria are shown, the data points representing the following: POPC (○), POPC/StarD4 (●), POPC-OOH (△), POPC-OOH/StarD4 (▲), POPC/SCP-2 (▼), POPC-OOH/SCP-2 (■). Plotted values are means ± SD (n = 3).
Chain lipid peroxidation induced by StarD4-delivered 7α-OOH in mitochondria
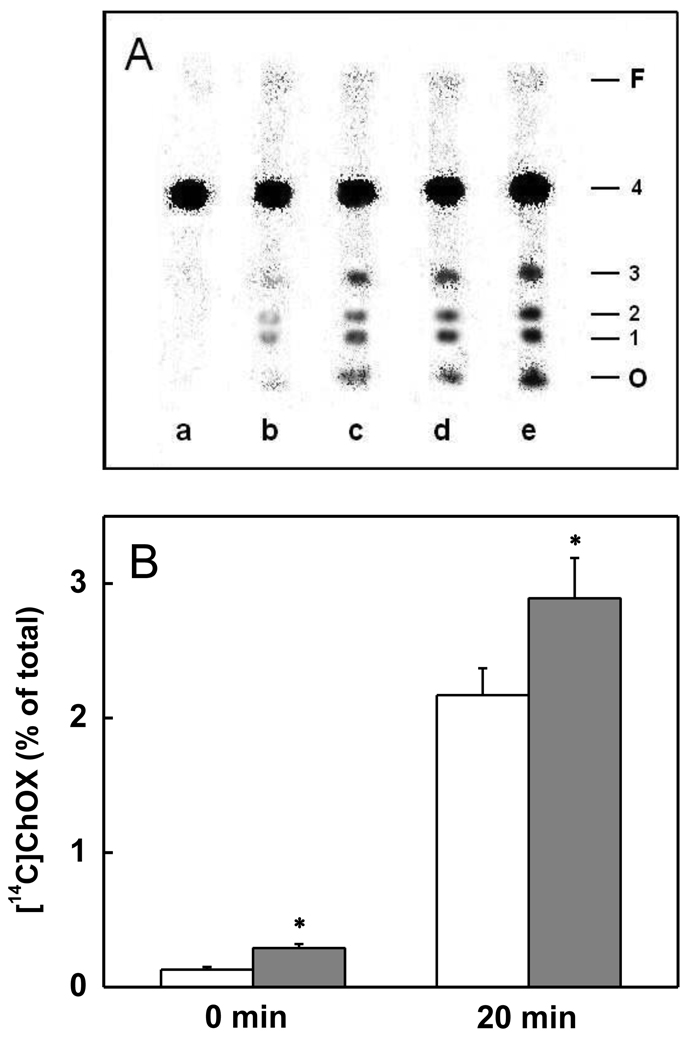
We examined the ability of transfer-acquired 7α-OOH to induce membrane-damaging lipid peroxidation in mitochondria, using [14C]Ch as an in situ probe and [14C]ChOX species as qualitative and quantitative indicators of chain peroxidation. As shown in Fig. 3A (0 min), [14C]ChOX (identified as 5,6-epoxide, 7α-OH, and 7β-OH) could be detected in mitochondria that had been incubated with 7α-OOH-containing SUVs for 30 min in the absence vs. presence of StarD4, the levels clearly being higher in the latter case. Post-incubation treatment with Fe(HQ)3 and AH− for 20 min further increased the [14C]ChOX levels, those in the StarD4-containing system remaining higher (Fig. 3A). Total integrated ChOX was approximately doubled when StarD4 was present during transfer incubation (Fig. 3B, 0 min) and ~40 % greater in the large increase that occurred after subsequent exposure to Fe(HQ)3/AH−. Thus, StarD4-enhanced 7α-OOH transfer greatly sensitized acceptor mitochondria to free radical-mediated peroxidative damage.
Figure 3.
Sensitization of mitochondria to free radical lipid peroxidation by StarD4-enhanced transfer uptake of 7α-OOH. [14C]Ch-labeled mitochondria (2.8 mg protein/ml, 0.6 mM lipid) were incubated with POPC/7α-OOH (8:2, mol/mol; 0/06 mM lipid) SUVs for 30 min in the absence or presence of StarD4 (2 µM) in MS buffer at 37 °C. Lipids were either extracted at this point or after additional 37 °C incubation in the presence of 5 µM Fe(HQ)3 and 10 mM AH−, and extracts containing the same amount of total lipid were subjected to HPTLC-PI analysis. (A) Analyte profiles showing origin (O), 7α-OH (1), 7β-OH (2), 5,6-epoxide (3), Ch (4), and solvent front (F). Sample lanes represent the following: (a) non-transfer-incubated control; (b) and (c) immediately after transfer in absence and presence of StarD4, respectively; (d) and (e) 20 min with Fe(HQ)3/AH− after transfer in absence and presence of StarD4, respectively. (B) Total ChOX before and after Fe(HQ)3/AH− treatment (percent of total lane radioactivity normalized for Ch and corrected for control); open bars, −StarD4; solid bars, +StarD4. Values are means ± SD (n = 3). *P<0.01 compared with corresponding sample lacking StarD4.
Effect of StarD4-delivered 7α-OOH on mitochondrial membrane potential
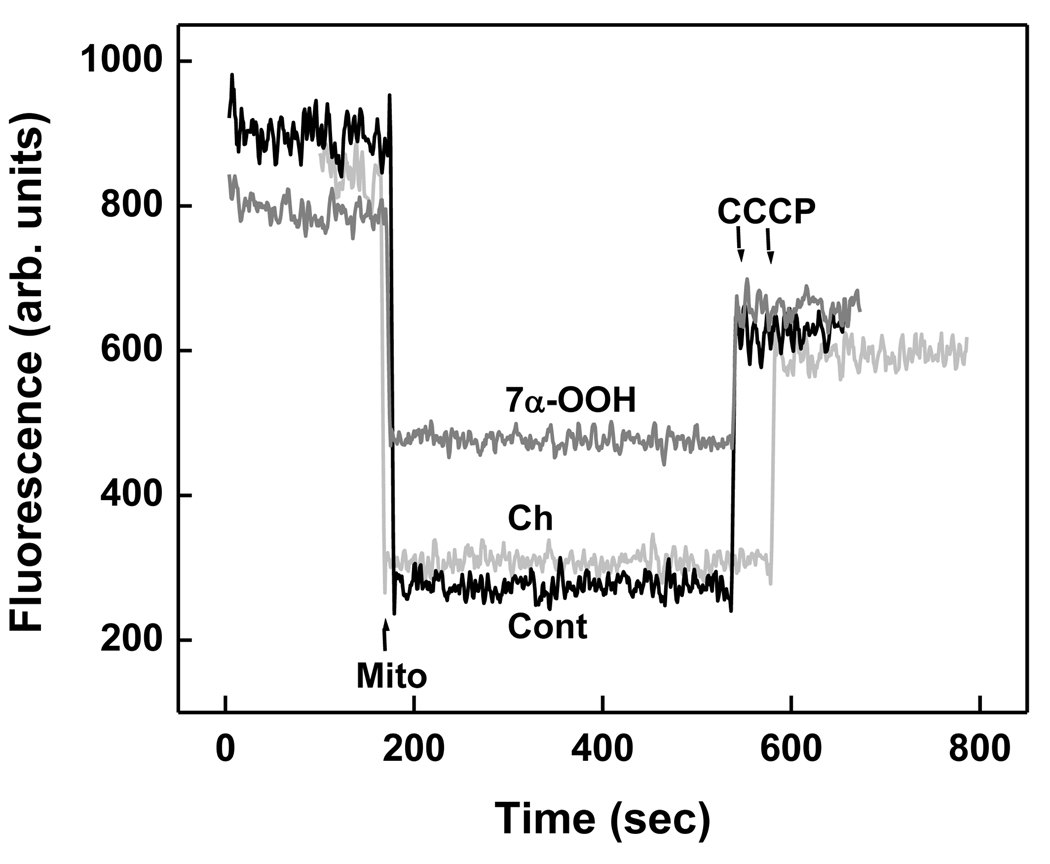
The functional significance of StarD4-enhanced 7α-OOH delivery to mitochondria with accompanying induction of free radical lipid peroxidation was evaluated by determining mitochondrial ability to generate and sustain a transmembrane potential (ΔΨ). Using mouse mitochondria that had been preincubated with 7α-OOH-SUVs for 30 min in the presence of 1 µM StarD4, we found that ΔΨ decreased tp ~40% of the value produced by a control not exposed to 7α-OOH (Fig. 4). When the same transfer incubation conditions were used except for switching from 7α-OOH to Ch in the donor membranes, there was no significant decrease in ΔΨ relative to the control value (Fig. 4). Thus, uptake of the redox-active sterol hydroperoxide was functionally detrimental, presumably by triggering chain peroxidation reactions (Fig. 3), whereas uptake of the unoxidized sterol was not. However, some caution is appropriate in deducing this for the Fig. 4 results, since considerably more 7α-OOH than Ch was delivered to mitochondria by StarD4 in the transfer time used (30 min).
Figure 4.
Effect of StarD4-facilitated 7α-OOH transfer on mitochondrial membrane potential (ΔΨ). Mitochondria (0.6 mM lipid) were incubated with POPC/7α-OOH or POPC/Ch (8:2, mol/mol; 0.06 mM lipid) SUVs for 30 min in the absence or presence of StarD4 (1 µM) in MS buffer at 37 °C. Immediately thereafter, the mitochondria were added to 5 mM succinate/1 µM rotenone/0.2 µM Rh123 in MS buffer at 37 °C, giving 0.2 mg protein/ml (arrow). After a baseline of Rh123 fluorescence was attained, CCCP and antimycin-A were added (2 µM each), allowing ΔΨ measurement. Notations: Cont (no SUVs/StarD4); 7α-OOH (7α-OOH-SUVs + StarD4); Ch (Ch-SUVs + StarD4).
Discussion
Free Ch required for steroid hormone synthesis by cells of the adrenal cortex, testis, and ovary can derive from external sources such as low density lipoprotein (via the LDL receptor) and high density lipoprotein (via the SR-BI receptor) [12]. Most of the Ch from these sources arises via hormone sensitive lipase-catalyzed hydrolysis of internalized cholesteryl esters. Ch can also be supplied internally, e.g. by hydrolysis of cholesteryl esters in lipid droplets or removal from plasma membrane, where most of the free Ch is located in cells. StAR family proteins play a key role in transporting intracellular Ch to mitochondria for pregnenolone synthesis [10–12]. Unlike SCP-2 and other lipid carriers, StAR proteins are highly selective for Ch or at least sterols with a ring structure similar to that of Ch [13]. The prototype, StarD1, acts at the mitochondrial level, whereas more recently identified homologues, StarD4, StarD5 and StarD6, lack organelle-targeting sequences and are believed to act exclusively in the cytosol [11,12]. StarD1 and StarD4 have been proposed to act cooperatively in steroidogenesis, the former transporting Ch through the cytosol to the mitochondrial outer membrane and the latter, in conjunction with translocator protein (TSPO, also known as peripheral benzodiazepine receptor), delivering it to the inner membrane for conversion to pregnenolone by the P450scc system [11].
There is a growing awareness that functionality of steroidogenic tissues may be compromised in physiological states associated with oxidative stress, e.g. diabetes, ischemia/reperfusion, chronic inflammation, and aging [23]. Among these, attenuation of natural antioxidant defenses with advancing age has received the most attention up to now. Because of their vigorous mitochondrial electron transport activity, adrenocortical cells in an animal model were reported to be highly susceptible to oxidative injury, which increased dramatically with aging [23]. Moreover, steroidogenic cells in vitro have been shown to become damaged and dysfunctional when exposed to various peroxides other than ChOOHs, e.g. H2O2 and fatty acid hydroperoxides [24,25]. The study involving hormone-activated mouse MA-10 Leydig cells [24] was highly relevant because in vivo such cells reside in the testicular interstitium with ROS-generating macrophages in close proximity. In the cases cited [ ], it is unlikely that the primary peroxides were targeted specifically to mitochondria by StAR proteins due to the selectivity of these proteins for sterol-type ligands, as shown in this and other studies [22].
Using a model construct mimicking a plasma membrane/mitochondrion donor/acceptor system in a steroidogenic cell, we show for the first time in this study that StarD4, can stimulate intermembrane transfer not only of Ch itself, but also a cholesterol B-ring hydroperoxide (7α-OOH). It is reasonable to assume that in promoting 7α-OOH translocation, StarD4 actually binds the hydroperoxide, but this has not been examined. Although 7α-OOH is more polar than Ch, it retains most of Ch’s amphiphilic properties, viz. the same hydrophobic “tail” portion and rigid “hydrophilic” head portion, where the B-ring hydroperoxyl group is relatively close to the A-ring hydroxyl group. Based on these considerations, it would not be surprising that Ch and 7α-OOH use the same binding pocket on StarD4 with similar orientation of their hydrophobic and hydrophilic groupings. Specificity for sterol-type ligands was demonstrated by showing that StarD4, unlike SCP-2, cannot accelerate PC or PCOOH translocation. In keeping with our expectations from previous studies on disseminated peroxidative stress [5,9], we found that StarD4-enhanced 7α-OOH transfer to mitochondria sensitized them to free radical lipid peroxidation with loss of functional integrity reflected by ΔΨ generation.
Conclusions
The present findings provide valuable new insights into how hormone production in steroidogenic tissues might be compromised under physiological conditions associated with oxidative stress. Under such conditions, redox-active ChOOHs from imported oxidized lipoproteins or Ch oxidation in existing intracellular pools (e.g. plasma membrane) could be transported to mitochondria by the StarD4/D1 system along with Ch, leading to site-specific free radical damage and dysfunction if resident antioxidant capacity is overwhelmed. Inadvertant ChOOH delivery in this fashion has not been recognized up to now. As more evidence for this accumulates, a key goal will be the rational application of antioxidant interventions to minimize mitochondrial damage.
Acknowledgements
This work was supported by NIH Grants CA72630 and HL85677 (AWG), Polish Ministry of Science Grant N301 08332/3232 (WK), and an American Heart Association Scientist Development Grant (DR).
Abbreviations
- AH−
ascorbate
- BSA
bovine serum albumin
- CCCP
carbonyl cyanide m-chlorophenylhydrazone
- Ch
cholesterol
- ChOOH(s)
cholesterol hydroperoxide(s)
- ChOX
cholesterol oxidation product(s)
- 7α/7β-OOH
3β-hydroxycholest-5-ene-7α/7β-hydroperoxide
- DCP
dicetyl phosphate
- DFO
desferrioxamine
- Fe(HQ)3
ferric-8-hydroxyquinoline
- HPTLC-PI
high performance thin layer chromatography with phosphorimaging detection
- LOOH(s)
lipid hydroperoxide(s)
- MOPS
3-(N-morpholino)propanesulfonic acid
- SCP-2
sterol carrier protein-2
- StarD1
type-1 steroidogenic acute regulatory domain protein
- StarD4
type-4 steroidogenic acute regulatory domain protein
- DMPC
1,2-dimyristoyl-sn-phosphocholine
- POPC
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- PLOOH(s)
phospholipid hydroperoxide(s)
- Rh123
rhodamine 123
- SUV(s)
small unilamellar vesicle(s)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Girotti AW. Cholesterol-derived hydroperoxides: generation and reactivity in biological systems. In: Fleischer S, editor. Sterols and Oxysterols: Chemistry, Biology and Pathobiology. Kerala: Research Signpost; 2002. pp. 121–139. [Google Scholar]
- 2.Murphy RC, Johnson KM. Cholesterol, reactive oxygen species, and the formation of biologically active mediators. J. Biol. Chem. 2008;283:15521–15525. doi: 10.1074/jbc.R700049200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith LL, Teng JI, Kulig MJ, Hill FL. Sterol metabolism XXIII. Cholesterol oxidation by radical-induced processes. J. Org. Chem. 1973;38:1763–1765. doi: 10.1021/jo00949a041. [DOI] [PubMed] [Google Scholar]
- 4.Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J. Lipid Res. 1998;39:1529–1542. [PubMed] [Google Scholar]
- 5.Vila A, Korytowski W, Girotti AW. Dissemination of peroxidative stress via intermembrane transfer of lipid hydroperoxides: model studies with cholesterol hydroperoxides. Arch. Biochem. Biophys. 2000;380:208–218. doi: 10.1006/abbi.2000.1928. [DOI] [PubMed] [Google Scholar]
- 6.Vila A, Korytowski W, Girotti AW. Spontaneous intermembrane transfer of various cholesterol-derived hydroperoxide species: kinetic studies with model membranes and cells. Biochemistry. 2001;40:14715–14726. doi: 10.1021/bi011408r. [DOI] [PubMed] [Google Scholar]
- 7.Girotti AW. Translocation as a means of disseminating lipid hydroperoxide-induced oxidative damage and effector action. Free Radic. Biol. Med. 2008;44:956–968. doi: 10.1016/j.freeradbiomed.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallegos AM, Atshaves BP, Storey SM, Starodub O, Petrescu AD, Huang H, McIntosh AL, Martin GG, Chao H, Kier AB, Schroeder F. Gene structure, intracellular localization, and functional roles of sterol carrier protein-2. Prog. Lipid Res. 2001;40:498–563. doi: 10.1016/s0163-7827(01)00015-7. [DOI] [PubMed] [Google Scholar]
- 9.Vila A, Levchenko VV, Korytowski W, Girotti AW. Sterol carrier protein-2-facilitated intermembrane transfer of cholesterol- and phospholipid-derived hydroperoxides. Biochemistry. 2004;43:12592–12605. doi: 10.1021/bi0491200. [DOI] [PubMed] [Google Scholar]
- 10.Soccio RE, Breslow JL. StAR-related lipid transfer (START) proteins: mediators of intracellular lipid metabolism. J. Biol. Chem. 2003;278:22183–22186. doi: 10.1074/jbc.R300003200. [DOI] [PubMed] [Google Scholar]
- 11.Miller WL. StAR search: what we know about how the steroidogenic acute regulatory protein mediates mitochondrial cholesterol import. Mol. Endocrinol. 2007;21:589–501. doi: 10.1210/me.2006-0303. [DOI] [PubMed] [Google Scholar]
- 12.Rone MB, Fan J, Papadopoulos V. Cholesterol transport in steroid biosynthesis: role of protein-protein interactions and implications in disease states. Biochim. Biophys. Acta. 2009;1791:646–658. doi: 10.1016/j.bbalip.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsujishita Y, Hurley JH. Structure and lipid transport mechanism of a StAR-related domain. Nat. Struct. Biol. 2000;7:408–414. doi: 10.1038/75192. [DOI] [PubMed] [Google Scholar]
- 14.Korytowski W, Geiger PG, Girotti AW. Lipid hydroperoxide analysis by high-performance liquid chromatography with mercury cathode electrochemical detection. Methods Enzymol. 1999;300:23–33. doi: 10.1016/s0076-6879(99)00109-3. [DOI] [PubMed] [Google Scholar]
- 15.Kriska T, Marathe GK, Schmidt JC, McIntyre TM, Girotti AW. Phospholipase action of platelet-activating factor acetylhydrolase, but not paraoxonase-1, on long fatty acyl chain phospholipid hydroperoxides. J. Biol. Chem. 2007;282:100–108. doi: 10.1074/jbc.M608135200. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Agudo D, Ren S, Hylemon PB, Redford K, Natarajan R, DelCastillo A, Gil G, Pandak WM. Human StarD5, a cytosolic StAR-related lipid binding protein. J. Lipid Res. 2005;46:1615–1623. doi: 10.1194/jlr.M400501-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Agudo D, Ren S, Wong E, Marques D, Redford K, Gil G, Hylemon PB, Pandak WM. Intracellular cholesterol transporter StarD4 binds free cholesterol and increases cholesteryl ester formation. J. Lipid Res. 2008;49:1409–1419. doi: 10.1194/jlr.M700537-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1986;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 19.Ardail D, Privat JP, Egret-Charlier M, Levrat C, Lerme F, Louisot P. Mitochondrial contact sites: lipid composition and dynamics. J. Biol. Chem. 1991;265:18797–18802. [PubMed] [Google Scholar]
- 20.Girotti AW, Korytowski W. Cholesterol as a singlet oxygen detector in biological systems. Methods Enzymol. 2000;319:85–100. doi: 10.1016/s0076-6879(00)19011-1. [DOI] [PubMed] [Google Scholar]
- 21.Korytowski W, Wrona M, Girotti AW. Radiolabeled cholesterol as a reporter for assessing one-electron turnover of lipid hydroperoxides. Anal. Biochem. 1999;270:123–132. doi: 10.1006/abio.1999.4070. [DOI] [PubMed] [Google Scholar]
- 22.Kallen CB, Billheimer JT, Summers SA, Stayrook SE, Lewis M, Strauss JF., III Steroidogenic acute regulatory protein (StAR) is a sterol transfer protein. J. Biol. Chem. 1998;273:26285–26288. doi: 10.1074/jbc.273.41.26285. [DOI] [PubMed] [Google Scholar]
- 23.Azhar S, Cao L, Reaven E. Alteration of the adrenal antioxidant defense system during aging in rats. J. Clin. Invest. 1995;96:1414–1424. doi: 10.1172/JCI118177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diemer T, Allen JA, Hales KH, Hales DB. Reactive oxygen disrupts mitochondria in MA-10 tumor Leydig cells and inhibits steroidogenic acute regulatory (StAR) protein and steroidogenesis. Endocrinology. 2003;144:2882–2891. doi: 10.1210/en.2002-0090. [DOI] [PubMed] [Google Scholar]
- 25.Kodaman PH, Atan RF, Behrman HR. Lipid hydroperoxides evoke antigonadotropic and antisteroidogenic activity in rat luteal cells. Endocrinology. 1994;135:2723–2730. doi: 10.1210/endo.135.6.7988463. [DOI] [PubMed] [Google Scholar]