Abstract
Activation of mu opioid receptors (MOR) makes animals hyperphagic and selectively increases their preference for a high fat diet independent of their dietary preference. The orexigenic peptide Agouti Related Peptide (AgRP) also produces hyperphagia and increased the preference for a high fat diet. In this paper, we tested the hypothesis that the effect of MOR on feeding behavior will be attenuated in the absence of the orexigenic peptide AgRP. Immunohistochemical studies demonstrated that MOR are co-localized on AgRP neurons located in the arcuate nucleus. This finding is consistent with a role of MOR in mediating the release of AgRP. Our data also demonstrated that the wild-type (FVB) animals preferred a diet high in fat whereas the AgRP knockout (AgRP KO) mice did not. mRNA expression of MOR in the hypothalamus was not significantly different between AgRP KO mice and their wild-type control. In a dose response experiment, the low dose (0.025μg) of a MOR agonist, DAMGO, increased cumulative food intake in wild-type and AgRP KO mice. The low and middle (0.25μg) dose of DAMGO significantly increased the amount of high fat diet eaten by the wild-type animals, but did not significantly change the amount of high fat diet eaten by the AgRP KO mice. The highest dose of DAMGO (2.5μg) reduced food intake in the control and AgRP KO mice, probably due to somnolence. These data demonstrate that the increased preference for a high fat diet after stimulation of MOR is attenuated in the absence of AgRP, but the increase in food intake (i.e. hyperphagia) is not.
Keywords: mu opioid receptors, Agouti Related Peptide (AgRP), dietary preference
Introduction
The level of fat in the diet, together with portion size, are thought to play a major role in the current epidemic of obesity (Hill and Peters 1998), and emphasize the need to understand the factors that modulate food intake as well as the preference for a high fat diet. Of the many peptides, neurotransmitters and receptor populations that affect food intake, only a few have been demonstrated to make animals hyperphagic and simultaneously increase their preference for a diet high in fat. Included in this list are mu opioid receptors (MOR) and Agouti Related Peptide (AgRP).
Mu opioid receptors (MOR) belong to a family of seven-transmembrane domain receptors that are coupled to G-proteins (Chen et al., 1993). This receptor population is located in many areas of the central nervous system that are involved in feeding such as the hypothalamus, nucleus accumbens, amygdala, ventral tegmental area and the nucleus tractus solitarius (Zhang et al., 1998, Zheng et al., 2005). In the early 1980s investigators made the observation that after the injection of a non-selective opioid agonist (i.e. morphine) animals increased their fat intake while suppressing carbohydrate intake and exhibiting little modification in protein intake (Marks-Kaufman 1982, Marks-Kaufman and Kanarek 1980). Later studies by Zhang et al (1998) suggested that endogenous opioids within the ventral striatum participate in the mechanisms governing preferences for highly palatable foods, especially those rich in fat. From these studies and others (Evans and Vaccarino 1990; Gosnell and Krahn 1993), it has been concluded that activation of MOR make animals hyperphagic and selectively increase their preference for a diet high in fat.
The mechanism(s) by which activation of this receptor population increases food intake and the preference for a high fat diet is unknown. This paper tested the hypothesis that the effect of MOR on feeding behavior would be attenuated in the absence of the orexigenic peptide Agouti Related Peptide (AgRP).
AgRP is an orexigenic peptide that is co-localized with neuropeptide Y (NPY) in neurons located in the arcuate nucleus (Chen et al., 1999, Hahn et al., 1998). When released from its neurons in the arcuate nucleus, AgRP increases food intake by functioning as an antagonist to the melanocortin 3 and 4 receptors (Fong et al., 1997, Ollmann et al., 1997). The effect of AgRP on food intake lasts up to 72 hours (Hagan et al., 2000). Administering AgRP into the central nervous system selectively increases the preference for a diet high in fat (Hagan et al., 2001).
Ubiquitous overexpression of AgRP in transgenic mice leads to hyperphagia, severe obesity and reduced corticosterone levels (Graham et al., 1997). However, AgRP knockout mice which we utilized in this manuscript have been reported to exhibit normal feeding behavior without changes in body weight and cumulative food intake (Qian et al., 2002). AgRP knockout mice live 10% longer than their wild-type littermates while consuming a high fat diet but without exhibiting differences in food intake or body weight (Redmann et al., 2006).
In the present studies, we demonstrate that MOR are co-localized on AgRP neurons located in the arcuate nucleus. We also demonstrate that activating MOR in AgRP knockout mice does not increase their preference for a high fat diet. However, activation of MOR does make AgRP knockout mice hyperphagic. Taken together, these data suggest that the effect of MOR on dietary preference for a high fat diet is dependent on the presence of the orexigenic peptide AgRP, but that stimulation of food intake is not.
Results
Experiment 1: Immunohistochemical (IHC) identification of mu opioid receptors and AgRP neurons
Figure 1 provides a representation of the co-localization of MOR and AgRP that was observed in the arcuate nucleus. Panel A is a representation of the area of the brain (medial arcuate nucleus; Bregma -3.30mm) that was used to identify the mu opioid receptors. High power (40×) magnification in Panel B provides an image of a neuron (green) that is positively labeled with MOR. Panel C shows positively labeled AgRP neurons (red). The final panel, Panel D is an overlay of Panel B and C which demonstrates that MOR are located on AgRP neurons located in the arcuate nucleus.
Figure 1A, 1B, 1C & 1D.
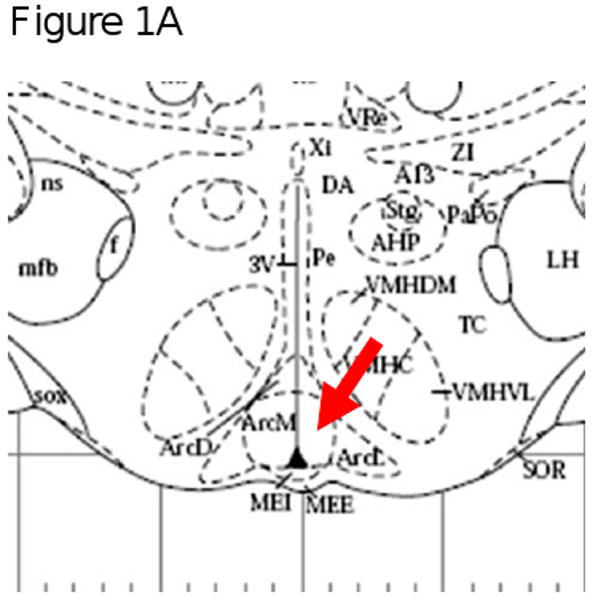
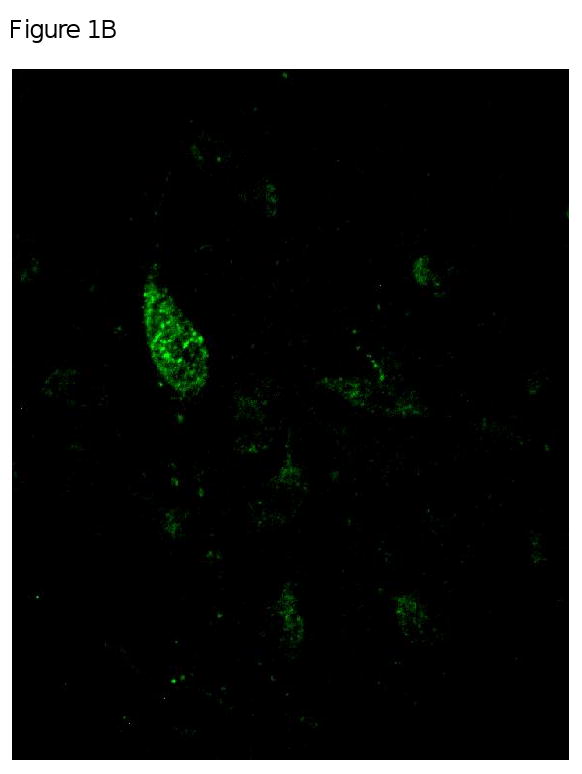
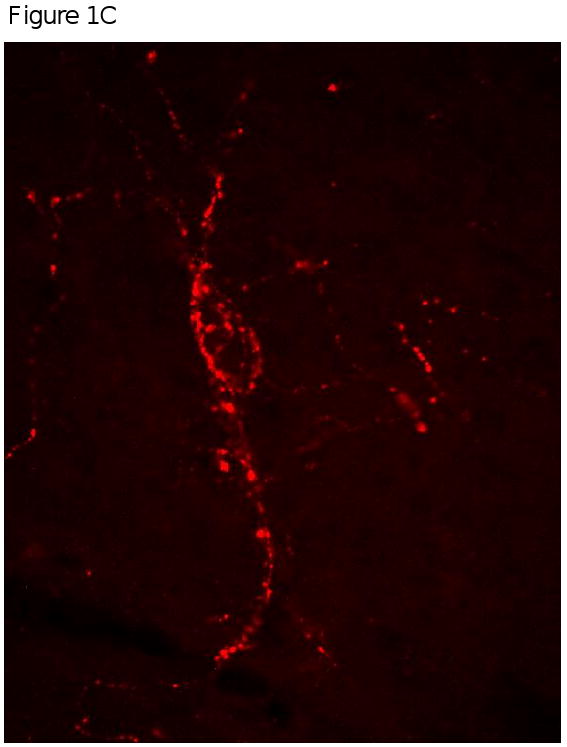
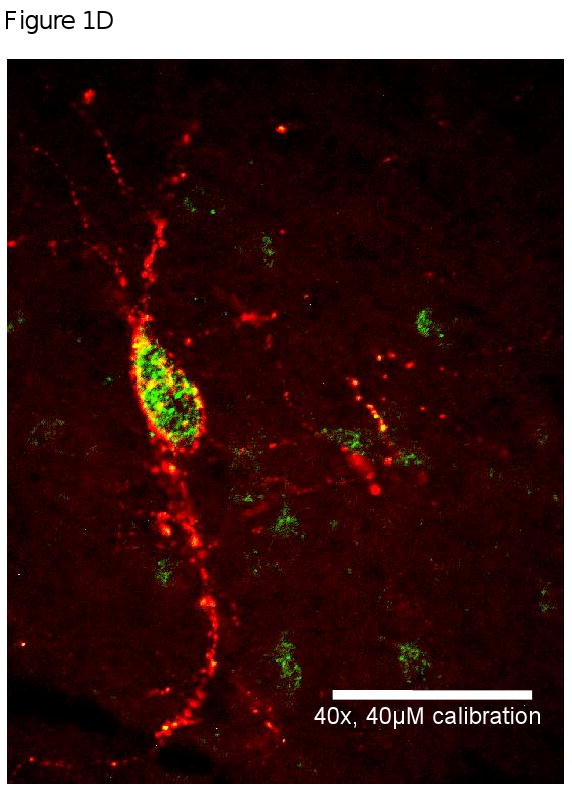
Figure 1A is a representation of the area of the brain (medial arcuate nucleus; Bregma -3.30mm) that was used to identify the mu opioid receptors from FVB mice. Figure 1B, 1C and 1D are high power magnification (40×) of the arcuate nucleus of FVB mice on a chow diet. Magnification for the three micrographs is indicated by the 40μm scale in Panel D. Panel B is a neuron positively immunostained for mu opioid receptors. Panel C is a representative of a neurons that is immunostained for Agouti gene Related Peptide (AgRP). Panel D is an overlay of Panel B and Panel C which demonstrate that mu opioid receptors are located on AgRP neurons within the arcuate nucleus.
Experiment 2: mRNA expression of MOR and AgRP in FVB and AgRP KO mice
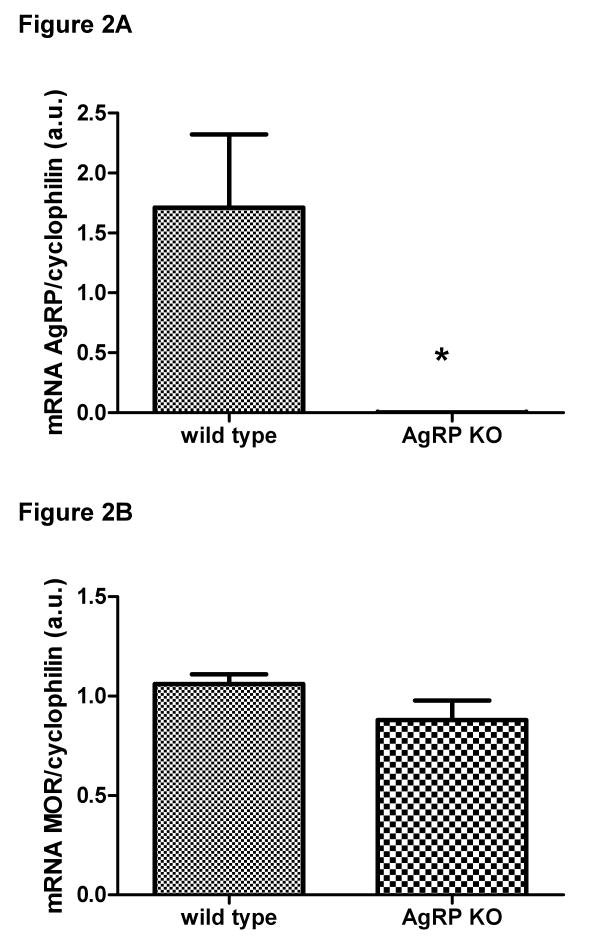
mRNA expression of AgRP was statistically different between the two groups, t(10) = 3.384, p<0.05 (Figure 2A). As expected, we were able to detect the mRNA expression of AgRP in the hypothalamus of the control animals, but it was not detectable in AgRP KO mice. In contrast, the expression of MOR was similar between the control and the AgRP KO mice, t(11) = 1.723, p>0.05 (Figure 2B).
Figure 2.
Hypothalamic mRNA expression of AgRP (Figure 2A) and MOR (Figure 2B). The mRNA expression of AgRP was not detectable in the knockout mice. The expression of MOR was similar between the wild-type control and AgRP knockout mice (p>0.05).
Experiment 3: Dietary preference of AgRP KO and FVB mice
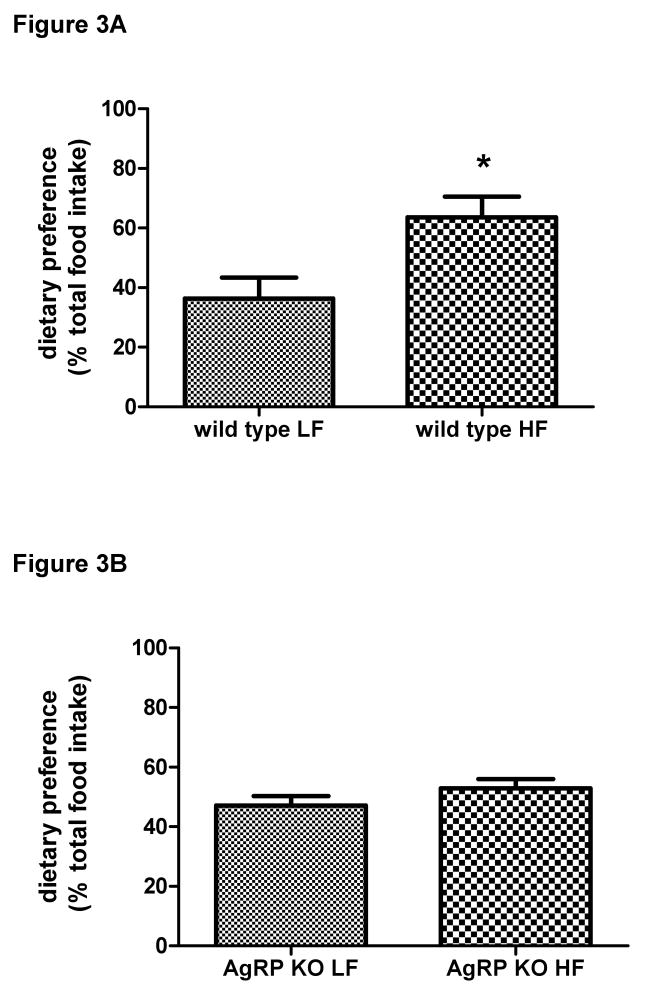
Figure 3 shows the intake of high fat and low fat diet as a percentage of total intake consumed when the FVB and AgRP knockout mice were given a choice between a low fat diet and a high fat diet. FVB mice ate significantly more of the high fat diet, t(6) = 2.760, p < 0.05 (Figure 3, Panel A). In contrast, the AgRP KO mice did not have a preference for either diet, t(6) = 1.287, p > 0.05 (Figure 3, Panel B).
Figure 3.
Dietary preference of wild-type control (3A) and AgRP knockout mice (3B). Preference is represented as a percentage of total food intake. Control animals preferred to eat significantly more of the high fat diet when given a choice between low fat and high fat diets simultaneously. In contrast, there was no preference for either diet with the AgRP knockout animals. Values are expressed as means ± SE; *p ≤ 0.05 vs. low fat.
Experiment 4: Effect of DAMGO (selective mu opioid receptor agonist) on high fat intake of AgRP KO and FVB mice
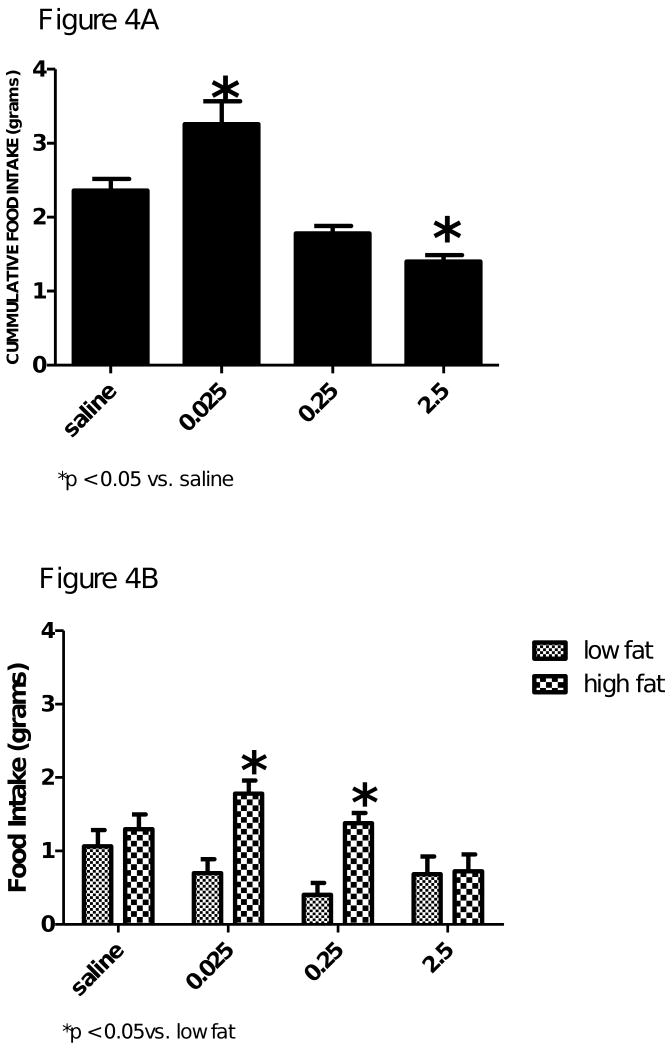
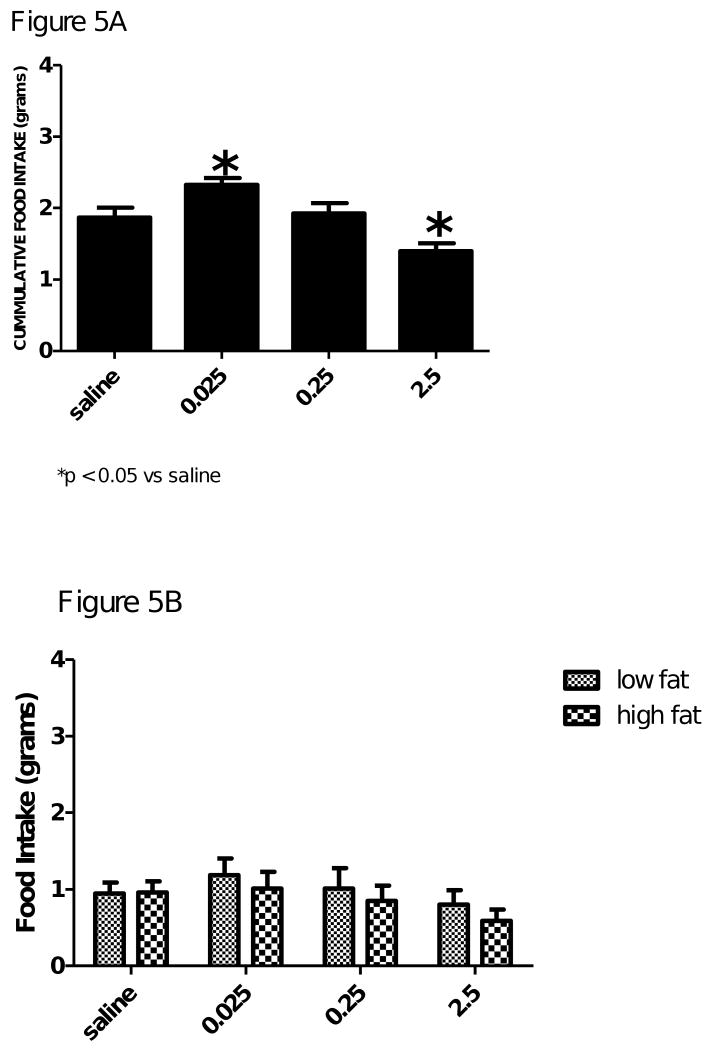
Figure 4 and 5 shows a dose response to a mu opioid receptor agonist DAMGO [(d-Ala2-N-Me-Phe4-Glycol5)-enkephalin] on cumulative food intake and dietary preference for either a low fat or a high fat diet, respectively in the control and AgRP KO mice. Cummulative food intake was increased in the wild-type mice and in the AgRP KO mice (Figure 4A and 5A, respectively). The low dose (0.025μg) caused a significant increase in cumulative food intake in the wild-type mice, F(3,4) = 20.61, p<0.05 and the AgRP KO mice, F(3,6) = 11.69, p<0.05. The middle (0.25 μg) and high (2.5μg) doses of DAMGO resulted in a decrease in cumulative food intake, probably due to somnolence.
Figure 4.
Figure 4A. Cumulative food intake of wild-type control mice after icv injection of saline, 0.025, 0.25 or 2.5μg of DAMGO. Food intake is represented in grams. DAMGO dose of 0.025μg significantly increased cumulative food intake. The highest dose of DAMGO 2.5μg significantly decreased food intake, probably due to somnolence. Values are expressed as means ± SE; *p ≤ 0.05 vs saline.
Figure 4B. The effect of saline, 0.025, 0.25, or 2.5μg of DAMGO on dietary selection (i.e. low fat or high fat diet) with FVB control mice. Food intake is presented in grams. The low (0.025μg) and high (2.5μg) dose of DAMGO significantly increased the grams of high fat diet consumed when compared to the low fat diet. The highest dose of DAMGO did not have any effect on dietary selection. Values are expressed as mean ± SEM. *p ≤ 0.05 vs low fat diet.
Figure 5.
Figure 5A. Cumulative food intake of AgRP knockout mice after icv injection of saline, 0.025, 0.25 or 2.5μg of DAMGO. Food intake is represented in grams. DAMGO dose of 0.025μg significantly increased cumulative food intake. The highest dose of DAMGO 2.5μg significantly decreased food intake, probably due to somnolence. Values are expressed as means ± SE; *p < 0.05 vs saline.
Figure 5B. The effect of saline, 0.025, 0.25, or 2.5μg of DAMGO on dietary selection (i.e. low fat or high fat diet) with AgRP knockout mice. Food intake is presented in grams. Neither treatment was able to significantly increase the preference for either diet. Values are expressed as mean ± SEM. *p > 0.05 vs low fat diet.
Main effects of treatment, F(3,24) = 3.53, p < 0.05, and diet, F(1,24) = 13.65, p<0.05, were found when the preference for either a low fat or high fat diet was analyzed in Figure 4. These data demonstrated that the low dose and the middle dose of DAMGO were able to significantly increase the wild-type animal's preference for a high fat diet. However, the same doses were not able to alter the dietary preference of the AgRP KO mice. There was not an effect of diet, F(3,56) = 0.13, p>0.05, or treatment, F(3,56) = 1.49, p>0.05.
Discussion
While the role of mu opioid receptors (MOR) in modulating the palatability and taste preference of animals has been established and is an active area of ongoing research, the precise mechanism(s) by which this occurs is unknown. In this manuscript, we used AgRP knockout mice to test the hypothesis that in the absence of AgRP, a mu opioid receptor agonist (i.e. DAMGO) would not stimulate hyperphagia nor increase the preference for a high fat diet.
Our histological data demonstrate that MOR are co-localized on AgRP neurons located in the arcuate nucleus. This finding is consistent with a role of MOR in modulating the release of AgRP. The possibility exists that when MOR are activated it initiates a signaling cascade within AgRP neurons which ultimately results in the release of this orexigenic peptide. Future testing of this hypothesis will provide an explanation as to 1) why MOR are located in a region of the central nervous system (i.e. arcuate nucleus) that has a well-established role in energy balance; and 2) test the hypothesis that another role for MOR may be to modulate the activity of orexigenic neurons (e.g. AgRP neurons) that is involved in the regulation of feeding behavior.
We found that the AgRP knockout mice did not increase their preference for a high fat diet after the DAMGO injection suggesting that the response depends on the release of AgRP. However, total food intake was increased when the AgRP knockout mice were administered DAMGO; suggesting that other orexigenic peptides besides AgRP are involved in the hyperphagic response to the MOR agonist. One possibility is the orexigenic peptide Neuropeptide Y (NPY).
NPY and AgRP are co-localized in neurons in the arcuate nucleus and both can increase food intake (Morton and Schwartz 2001). When animals are given a choice between a low fat and a high fat diet, administering NPY in the central nervous system results in the animals eating the diet they initially preferred (Smith et al., 1997). Giraudo et. al., (1999) demonstrated that NPY can increase energy intake without increasing the preference for a highly palatable meal. However, administering AgRP to animals result in the animals increasing their preference for a high fat diet independent of their baseline preference (Hagan et al., 2001).
It is plausible that activation of MOR on NPY/AgRP neurons increases the release of NPY to make animals hyperphagic and AgRP to increase their preference for a high fat diet. The data presented in Experiment 4 provide some support to our hypothesis that MOR can utilize different orexigenic peptides to make animals hyperphagic and increase their preference for a high fat diet. Experiment 4 demonstrated that the lowest (0.025μg) and intermediate dose of DAMGO (0.25μg) increased the preference for a high fat diet when control animals were given an ad libitum choice of a low fat and high fat diet. When the same doses were administered to the AgRP knockout mice there was no significant increase in the preference for the high fat diet when compared to the amount of low fat diet eaten after each dose of DAMGO was administered. Although the doses of DAMGO did not increase the preference for a high fat diet in the AgRP knockout mice it did make the animals hyperphagic. Cumulative food intake of the AgRP knockout mice after icv injection of 0.025μg of DAMGO was statistically different from the saline control.
The highest dose of DAMGO, 2.5μg, to the control and AgRP knockout mice resulted in a decrease in food intake. We are attributing the decrease in food intake to the sedative effect of the high dose of DAMGO.
The difference in the response to DAMGO injection between wild-type and AgRP knockout mice was not a result of a reduced number of MOR in the hypothalamus of the AgRP knockout mice. mRNA expression of this gene was not statistically different between the two groups.
The dietary selection between the animals was different. The control animals preferred a diet high in fat, whereas the AgRP knockout mice displayed no preference. Administering DAMGO potentiated the preference for a high fat diet in the control animals. Administering DAMGO to the AgRP knockout mice did not selectively increase their preference for a high fat diet. This finding was not anticipated because previous reports have demonstrated that activation of MOR can change the dietary selection of animals to prefer a high fat diet independent of their initial preference (Zhang et al., 1998). The lack of an effect of DAMGO on dietary preference in AgRP knockout mice provides further support to our hypothesis that MOR mediates dietary preference through AgRP.
Although the methodology is completely different, data from Brugman et. al., (2002) and Hagan et. al., (2000) provides support to our hypothesis. In their manuscripts, they gave an opioid antagonist 20 hours prior to giving AgRP and demonstrated that AgRP was still able to maintain its long term effect on feeding. This suggests that AgRP does not need MOR to mediate its feeding effect. We propose that when they blocked MOR with an opioid antagonist, they prevented the release of AgRP from its neurons. However, the injection of AgRP that occurred following the opioid antagonist increased endogenous AgRP levels which ultimately resulted in an increase in food intake and the preference for a palatable diet. This manuscript took a different approach and was able to demonstrate that after activation of MOR, if AgRP is not present, animals will not increase their preference for a high fat diet.
In summary, these studies have shown that the hypothalamic expression of MOR are the same in the AgRP knockout mice compared to the wild-type control animals and that MOR are located on AgRP neurons in the arcuate nucleus. Wild-type mice preferred a high fat diet, but AgRP knockout mice did not. DAMGO, a MOR agonist, stimulates food intake in both the wild-type and AgRP knockout mice, but only stimulated the preference for a high fat diet in the wild-type mice suggesting that the effect of MOR on dietary preference is dependent on AgRP.
Experimental Procedure
Animals
All procedures were performed in accordance with NIH guidelines for the care and use of animals and were approved by the Animal Care and Use Committee of Pennington Biomedical Research Center. Six to eight week old male and female AgRP KO mice were used in the experiments. The AgRP KO mice were originally the kind gift of Dr. Qian (2002) to Dr. Argyropoulos and were re-derived in his laboratory onto FVB genetic background. Control wild type mice were also of the FVB strain. There was no difference in body weight between the wild-type and AgRP KO mice. Animals were individually housed with a 12:12 light/dark cycle with ad libitum access to water and their respective diet (i.e. standard laboratory chow diet, low fat diet and/or a high fat diet). Low and high fat diets were purchased from Research Diets (New Brunswick, NJ), and had the composition listed in Table 1.
Table 1.
Composition of the high fat (55%) and low fat (10%) diet that was used
| Diets | ||||
|---|---|---|---|---|
| Low fat | High Fat | |||
| Ingredients | gm% | kcal% | gm% | kcal% |
| Casein | 21.99 | 87.96 | 28.69 | 114.8 |
| DL-Methionine | 0.11 | 0.44 | 0.14 | 0.56 |
| Corn Starch | 42.32 | 169.3 | 23.91 | 95.64 |
| Sucrose | 18.14 | 72.56 | 0 | 0 |
| Cellulose | 9.16 | 0 | 11.96 | 0 |
| Corn Oil | 2.46 | 22.14 | 3.2 | 28.8 |
| Crisco | 1.61 | 14.49 | 26.6 | 239.4 |
| AIN-76 Salt Mix | 3.22 | 0 | 4.21 | 0 |
| AIN-76A Vitamin Mix | 0.81 | 3.24 | 1.05 | 4.2 |
| Choline Bitartrate | 0.18 | 0 | 0.24 | 0 |
| Energy Density (kcal/g) | 3.7 | 4.8 | ||
Experiment 1: Immunohistochemical (IHC) identification of mu opioid receptors and AgRP neurons
Tissue Preparation and immunohistochemistry
FVB mice (n=4) with ad libitum access to a standard laboratory chow were injected with 30mg/ml of colchine (Sigma-Aldrich, St. Louis, MO). Twenty-four (24) hours after the injection, animals were anesthetized with nembutal (100mg/kg) before being perfused transcardially with 4% paraformaldehyde. The brains were removed and stored in 30% sucrose for 24 hours at 4°C. The brains were cut in 30 micron thick sections using a cryostat (HM550, Microm, Richard Allan Scientific, Kalamazo MI). A profile of various sections throughout the brain was processed at the same time to ensure uniformity of immunostaining. Sections were immersed in a Biocare (Concord, CA) Rodent Decloaking solution and placed in the Biocare Decloaker for 30 minutes at 80°C. After cooling, brain sections were washed with 0.1M PBS (pH 7.5), and then treated with 1% sodium borohydride for 20 minutes. Sections were washed with 0.1M PBS before incubating in Rodent Block M (Biocare Medical, Concord CA) for thirty minutes. Sections were placed in an avidin block (Vector Laboratories, Burlingame, Ca) for fifteen minutes, washed with 0.1M PBS before being placed in a biotin block (Vector Laboratories, Burlingame, Ca) for fifteen minutes. Sections were incubated in guinea pig anti mu opioid receptor antibody serum (1:250, Catalog Number AG375, Millipore, Billerica MA) and rabbit anti-Agouti gene Related Peptide serum (1:250, Catalog Number H-003-57, Phoenix Pharmaceuticdal, Inc, Belmont, CA) overnight at room temperature. The following morning the sections were washed with 0.1M PBS and treated with a secondary antibody consisting of Alexa 488 goat anti guinea pig and Alexa 594 goat anti rabbit (1:200, Catalog Numbers A-11073 & A-11012, Jackson Laboratories, West Grove, PA) for one hour at room temperature in the dark. Sections were washed with 0.1M PBS before being mounted and cover-slipped with Prolong Gold fluorescent mounting media (Invitrogen, Carlsbad, CA).
Immunohistochemistry Negative Control
Following the protocol described above, sections of the brain were exposed to preabsorbed solutions of the control peptide for the mu opioid receptor (Millipore, Billerica MA) and a control peptide for the rabbit anti-Agouti gene Related Peptide (Phoenix Pharmaceuticdal, Inc, Belmont, CA) at the same concentration of the AgRP and mu opioid receptor antibody.
Microscopy
Tissue sections were examined with a Nikon E800 microscope equipped with a Perkin Elmer CSU21 spinning disk laser confocal system. Images were collected with a Hamamatsu ORCA CCD camera using Ultraview system integration software. Final figure plates were assembled in Adobe Photoshop (version 7.0), and the brightness and contrast of the images were adjusted to uniform levels when necessary.
Experiment 2: mRNA expression of AgRP and mu opioid receptors in FVB and AgRP knockout mice
AgRP KO (n=8) and FVB (n=8) mice were given ad libitum access to a standard laboratory chow diet. The hypothalamus of each animal was collected and processed for isolation of RNA.
Real Time Polymerase Chain Reaction (Real Time-PCR)
Frozen tissue was homogenized in QIAzol (trizol) using a motorized tissue homogenizer (Tekmar, Cincinnati, OH). Homogenate was transferred to a phase lock gel tube and chloroform was added. Samples were centrifuged and the upper aqueous phase was placed in a new collection tube containing 70% ethanol. RNA from the punches was isolated using RNeasy Kit (Qiagen, Valencia, CA). Reverse transcriptase (RT) was conducted using iScript procedures (BioRad, Hercules, CA). For RT, 1μg of RNA from each sample was added to 5× iScript Reaction Mix, iScript Reverse Transcriptase, and Nuclease-free water. Samples were placed in a thermal cycler (PTC-100, MJ Research Inc., Watertown, MA) for 5 minutes at 25°C, 30 minutes at 42°C, and 5 minutes at 85°C. Tubes were removed and placed in minus 20°C for storage. Real-Time PCR was conducted with primers designed for mice MOR and AgRP. For Real-Time PCR, SYBR Green 2× Master Mix (Applied Biosystems, Foster City, CA), forward and reverse primers (10μM), and RT product (10ng) were added to 384 well plates. The cycling parameters consisted of initial 2 minutes incubation at 50°C, followed by 10 minutes at 95°C, and a 1 minute annealing step at 60°C (40 cycles). A dissociation step (15 seconds at 95°C) was added following 40 cycles to determine specificity of primers. Quantity of MOR and AgRP was based on a standard curve and normalized to cyclophilin RNA (ABI prism 7900 Sequence Detection System, Applied Biosystems).
Experiment 3: Dietary preference of AgRP KO and FVB mice
AgRP KO (n=10) and FVB (n=10) mice were given ad libitum access to a choice between a low fat and a high fat diet for one week. One week after the animals had access to each diet, the testing phase began. Animals were given pre-weighed low fat and high fat diet simultaneously. Food intake was measured for seven days. Utilizing statistical analysis (p < 0.05), dietary preference was determined by the type of diet (i.e. low fat or high fat) that was observed to be statistically greater than the other.
Experiment 4: Effect of DAMGO (selective mu opioid receptor agonist) on low fat intake, high fat intake and cumulative intake of AgRP KO and FVB mice
FVB (n=10) and AgRP KO (n=10) mice housed individually were given ad libitum access to water, a high fat non-pelleted diet and a low fat non-pelleted diet for one week. On test day, animals were fasted overnight. On the following day, animals were anesthetized with isoflourane before receiving an injection (3μl) into the dorsal third ventricle (a detailed description of this procedure has been previously reported, Laursen and Belknap 1986) of one of four treatments: 0.9% saline, 0.025, 0.250, or 2.5μg of DAMGO [d-Ala2-N-Me-Phe4-Glycol5]-enkephalin (Sigma Aldrich, St. Louis, MO) over one minute. Food intake was measured for the first, third and sixth hour after injections was completed. Utilizing statistical analysis (p < 0.05), dietary preference was determined by the type of diet (i.e. low fat or high fat) that was demonstrated to be statistically greater than the other.
Injection Verification
At the conclusion of Experiment 4 animals were anesthetized with isoflourane before receiving an icv injection (3μl) of a blue dye. Only animals observed to have blue die in the third ventricle was used in the data analysis.
Primers
Primers were designed using Primer Express (Applied Biosystems, Foster City CA). The following primers were used for mouse mu opioid receptor (MOR-Forward) -TAGTGGGCCTCTTCGGAAAC and (MOR-Reverse)-GTTGGTGGCAGTCTTCATTTTG; the primers used for mouse AgRP were: (AgRP-Forward) – CCTCCATGAACTCGCTGGAT and (AgRP-Reverse) – CTTCTTTTCTGCTGATCTTCTTGGAT; the primers used for mouse cyclophilin were: (cyclophilin – Forward) – GCTGGATGGCAAGCATGTG and (cyclophilin – Reverse) – TGTCTTGGTGCTCTCCACCTT
Statistical Analysis
Data are presented as mean ± SE. Statistical analyses were performed using GraphPad Prism Version 5.01 (LaJolla, CA). Results were analyzed by using two-way ANOVA for repeated measures or by using t-test where appropriate. Bonferroni's post-hoc analysis was used to identify significant differences between groups. Differences were considered statistically significant at p < 0.05.
Acknowledgments
The project described was supported by Award Numbers DK078588-01A2 to MJB, DK R34-32089 to GAB and DK62156 to GA from the National Institute of Diabetes And Digestive And Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes And Digestive And Kidney Diseases or the National Institutes of Health.
We would like to thank Ms. Rachel Dauterive for excellent care of the mouse colonies and Dr. Richard Rogers for his help in taking pictures of the histological images.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature References
- Brugman S, Clegg DJ, Woods SC, Seeley RJ. Combined blockade of both micro – and kappa-opioid receptors prevent the acute orexigenic action of Agouti-related Protein. Endocrinology. 2002;143(11):4265–4270. doi: 10.1210/en.2002-220230. [DOI] [PubMed] [Google Scholar]
- Chen P, Li C, Haskell-Luevano C, Cone RD, Smith MS. Altered expression of agouti-related protein and its colocalization with neuropeptide Y in the arcuate nucleus of the hypothalamus during lactation. Endocrinology. 1999;140:2645–2650. doi: 10.1210/endo.140.6.6829. [DOI] [PubMed] [Google Scholar]
- Chen Y, Mestek A, Liu J, Hurley JA, Yu L. Molecular cloning and functional expression of a mu-opioid receptor from rat brain. Mol Pharmacol. 1993;44:8–12. [PubMed] [Google Scholar]
- Evans KR, Vaccarino FJ. Amphetamine- and morphine- induced feeding: evidence for involvement of reward mechanism. Neurosci Biobehav Rev. 1990;14:9–22. doi: 10.1016/s0149-7634(05)80156-3. [DOI] [PubMed] [Google Scholar]
- Fong TM, Mao C, MacNeil T, Kalyani R, Smith T, Weinberg D, Tota MR, Van der Ploeg LH. ART (protein product of agouti-related transcript) as an antagonist of MC-3 and MC-4 receptors. Biochem Biophys Res Commun. 1997;237:629–631. doi: 10.1006/bbrc.1997.7200. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Giraudo SQ, Grace MK, Billington CJ, Levine AS. Differential effects of neuropeptide Y and the mu-agonist DAMGO on ‘palatability’ vs. ‘energy’. Brain Res. 1999;834(12):160–163. doi: 10.1016/s0006-8993(99)01512-7. [DOI] [PubMed] [Google Scholar]
- Graham M, Shutter JR, Sarmiento U, Sarosi I, Stark KL. overexpression of Agrt leads to obesity in transgenic mice. Nat Genet. 1997;17:273–274. doi: 10.1038/ng1197-273. [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Krahn DD. The effects of continuous morphine infusion on diet selection and body weight. Physiol Behav. 1993;54(5):853–859. doi: 10.1016/0031-9384(93)90292-n. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Rushing PA, Benoit SC, Woods SC, Seeley RJ. Opioid receptor involvement in the effect of AgRP- (83-132) on food intake and food selection. Am J Physiol Regul Integr Comp Physiol. 2001;280:R814–821. doi: 10.1152/ajpregu.2001.280.3.R814. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Rushing PA, Pritchard LM, Schwartz MW, Strack AM, Van Der Ploeg LH, Woods SC, Seeley RJ. Long-term orexigenic effects of AgRP-(83---132) involve mechanisms other than melanocortin receptor blockade. Am J Physiol Regul Integr Comp Physiol. 2000;279:R47–52. doi: 10.1152/ajpregu.2000.279.1.R47. [DOI] [PubMed] [Google Scholar]
- Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1:271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- Hill JO, Peters JC. Environmental Contributions to the Obesity Epidemic. Science. 1998;280(5368):1371–1374. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]
- Laursen SE, Belknap JK. Intracerebroventricular injections in mice. Some methodological refinements. J Pharmacol Methods. 1986;16:355–357. doi: 10.1016/0160-5402(86)90038-0. [DOI] [PubMed] [Google Scholar]
- Marks-Kaufman R. Increased fat consumption induced by morphine administration in rats. Pharmacol Biochem Behav. 1982;16:949–955. doi: 10.1016/0091-3057(82)90051-x. [DOI] [PubMed] [Google Scholar]
- Marks-Kaufman R, Kanarek RB. Morphine selectively influences macronutrient intake in the rat. Pharmacol Biochem Behav. 1980;12:427–430. doi: 10.1016/0091-3057(80)90048-9. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Schwartz MW. The NPY/AgRP neuron and energy homeostasis. Int J Obes Relat Metab Disord. 2001;25 5:S56–62. doi: 10.1038/sj.ijo.0801915. [DOI] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- Redmann SM, Jr, Argyropoulos G. AgRP-deficiency could lead to increased lifespan. Biochem Biophys Res Commun. 2006;351:860–864. doi: 10.1016/j.bbrc.2006.10.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BK, Berthoud HR, York DA, Bray GA. Differential effects of baseline macronutrient preferences on macronutrient selection after galanin, NPY, and an overnight fast. Peptides. 1997;18:207–211. doi: 10.1016/s0196-9781(96)00318-x. [DOI] [PubMed] [Google Scholar]
- Qian S, Chen H, Weingarth D, Trumbauer ME, Novi DE, Guan X, Yu H, Shen Z, Feng Y, Frazier E, Chen A, Camacho RE, Shearman LP, Gopal-Truter S, MacNeil DJ, Van der Ploeg LH, Marsh DJ. Neither Agouti-Related Protein nor Neuropeptide Y Is Critically Required for the Regulation of Energy Homeostasis in Mice. Mol Cell Biol. 2002;22:5027–35. doi: 10.1128/MCB.22.14.5027-5035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Gosnell BA, Kelley AE. Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther. 1998;285:908–914. [PubMed] [Google Scholar]
- Zhang M, Gosnell BA, Kelley AE. Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther. 1998;285:908–914. [PubMed] [Google Scholar]
- Zheng SX, Bosch MA, Ronnekleiv OK. mu-opioid receptor mRNA expression in identified hypothalamic neurons. J Comp Neurol. 2005;487:332–344. doi: 10.1002/cne.20557. [DOI] [PubMed] [Google Scholar]