Abstract
(−)-Epigallocatechin-3-gallate (EGCG), the most abundant and most biologically active polyphenolic compound in tea, has been proposed to have many health beneficial effects. The metabolic fate of EGCG, however, is not well understood. In the present study, we identified a novel EGCG metabolite, 7-O-β-D-glucopyranosyl-EGCG-4″-O-β-D-glucupyranoside, in a mouse urine sample using liquid chromatography/electrospray ionization tandem mass spectrometry. The structure of this metabolite was confirmed by analyzing the MSn (n = 1–4) spectra as well as comparing the MS/MS spectra of its product ions with those from EGCG and EGCG-4″-O-β-D-glucupyranoside standards. To our knowledge, this is the first report of the identification of a glucoside metabolite of EGCG in mammals. Our results indicate that glucosidation represents a novel pathway in the metabolism of EGCG in mice.
Tea (Camellia sinensis, Theaceae) has been proposed to have many health beneficial effects, including the prevention of cancer, heart disease, and diabetes.1–3 These effects have been attributed mostly to the polyphenol compounds in tea, in which the most abundant and biologically active polyphenol is (−)-epigallocatechin-3-gallate (EGCG), representing ~16.5 wt % of the water-extractable fraction of tea.4 Many mechanisms of action have been proposed based on in vitro assays; however, their relevance to the physiological functions of EGCG and tea in vivo remains to be determined. The bioavailability and biotransformation of EGCG play a key role in determining the importance of various mechanisms in vivo. However, the metabolic fate of EGCG in animals or humans is not fully understood.
We and others have reported that EGCG is subject to phase II biotransformation and undergoes methylation, glucuronidation, and sulfation.5,6 4″-O-Methyl-(−)-EGCG and 4′,4″-O-dimethyl-(−)-EGCG are the major methylated metabolites of EGCG formed by human, mouse, and rat liver cytosol.7 At low concentrations of EGCG, the dimethylated EGCG is the major product, whereas, at high EGCG concentrations, mono-methylated EGCG metabolites predominate.7 EGCG-4″-O-glucuronide is the major metabolite formed in human, mouse, and rat microsomes.8 Mouse small intestinal microsomes have the greatest catalytic efficiency (Vmax/Km) for glucuronidation followed by mouse liver, human liver, rat liver, and rat small intestine. EGCG is also time- and concentration-dependently sulfated by human, mouse, and rat liver cytosol.9 In addition to conjugation reactions, EGCG is metabolized by intestinal flora to form ring fission products 5-(3′,4′,5′-trihydroxyphenyl)-γ-valerolactone (M4), 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone (M6), and 5-(3′,5′-dihydroxyphenyl)-γ-valerolactone (M6′).10–12 More recently, we have identified two new cysteine conjugated metabolites of EGCG, EGCG-2″-cysteine and EGCG-2′-cysteine, in vivo.13 These thiol conjugates can be detected in the urine following administration of toxic doses of EGCG, 200–400 mg/kg, i.p., or 1500 mg/kg, i.g., to mice.
Glucosidation is a common metabolic pathway in plants, but rarely in mammals. Glucosidation of exogenous and endogenous compounds has been described previously.14–17 Mammalian UDP-glucosyltransferases utilizing phenols and UDP-β-D-glucose as substrates have been demonstrated.18–20 It has been reported that EGCG can react with sucrose to form three EGCG glycosides catalyzed by glucansucrase produced by Leuconostoc mesenteroides B-1299CB.21 The active sites of EGCG for glucosidation are positions 7 of the A-ring and 4′ of the B-ring. However, it is unknown whether glucosidation of EGCG occurs in mammals.
In the present study, we identified a new EGCG metabolite, 7-O-β-D-glucopyranosyl-EGCG-4″-O-β-D-glucu-pyranoside, in a mouse urine sample using liquid chromatography/electrospray ionization tandem mass spectrometry (LC/ESI-MS/MS). The structure of this metabolite was confirmed by analyzing the MSn (n = 1–4) spectra as well as comparing the MS/MS spectra of its product ions with those from EGCG and EGCG-4″-O-β-D-glucupyranoside standards.
EXPERIMENTAL
Materials
EGCG (>97% pure) was provided by Mitsui Norin Co. Ltd. (Tokyo, Japan). EGCG-4″-O-β-D-glucupyranoside was prepared previously in our laboratory.8 HPLC-grade solvents and other reagents were obtained from VWR Scientific (South Plainfield, NJ, USA). HPLC-grade water was prepared using a Milli-Q purification system (Millipore, Bedford, MA, USA).
Treatment of mice and urine collection
Experiments with mice were carried out according to a protocol approved by the Institutional Review Board for the Animal Care and Facilities Committee (IRB-ACFC no. 91–024) at Rutgers University. Male CF-1 mice (25–30 g) were purchased from Charles River Laboratories and allowed to acclimate for at least 1 week prior to the start of the experiment. The mice were housed 10 per cage and maintained in air-conditioned quarters with a room temperature of 20 ± 2°C, relative humidity of 50 ± 10%, and an alternating 12-h light/dark cycle. Mice were fed Purina Rodent Chow #5001 (Research Diets) and water, and were allowed to eat and drink ad libitum.
EGCG was administered to mice, i.p. (50 mg/kg) or i.g. (200 mg/kg), and urine samples were collected in metabolism cages for 24 h after administration of vehicle or EGCG. These samples were stabilized with 0.1 volume of an ascorbate/EDTA solution (1.14 mol/L ascorbic acid, 1.3 mmol/L EDTA) and stored at −80°C before analysis.
Urine sample preparation
Urine samples (200 μL) were added to 1 mL acetonitrile to precipitate proteins. After centrifugation, the supernatant was dried under vacuum, and the solid was resuspended in 200 μL 50% aqueous acetonitrile containing 0.2% ascorbic acid. These samples were analyzed by LC/MS.
LC/ESI-MS method
LC/MS analysis was carried out with a Finnigan Spectra System which consisted of a Surveyor MS pump, a Surveyor refrigerated autosampler, and a LTQ linear ion trap mass detector (Thermo Electron, San Jose, CA, USA) incorporated with an electrospray ionization (ESI) interface. A 50 × 2.0 mm i.d., 3 μm Gemini C18 column (Phenomenex) was used for separation at a flow rate of 0.2 mL/min. The column was eluted with 100% solvent A (5% aqueous methanol with 0.2% acetic acid) for 10 min, followed by linear increases in B (95% aqueous methanol with 0.2% acetic acid) from 0 to 40% from 10 to 45 min, to 80% from 45 to 55 min, and to 100% from 55 to 60 min. The mobile phase was then re-equilibrated with 100% A for 10 min. The LC elute was introduced into the ESI interface. The negative ion polarity mode was set for the ESI source with the voltage on the ESI interface maintained at approximately 5 kV. Nitrogen gas was used as the sheath gas at a flow rate of 30 units and the auxiliary gas at 5 units. Optimized parameters include ESI capillary temperature, capillary voltage, ion spray voltage, sheath gas flow rate, tube lens offset voltage, and ion optics settings. These parameters were tuned by EGCG-4″-O-β-D-glucupyranoside standard. The collision-induced dissociation (CID) was conducted with an isolation width of 2 Da and normalized collision energy of 35 for MS2, MS3, and MS4. Default automated gain control target ion values were used for MS, MS2, MS3, and MS4 analyses. Data acquisition was performed with Xcalibur 2.0 version (Thermo Electron, San Jose, CA, USA).
RESULTS AND DISCUSSION
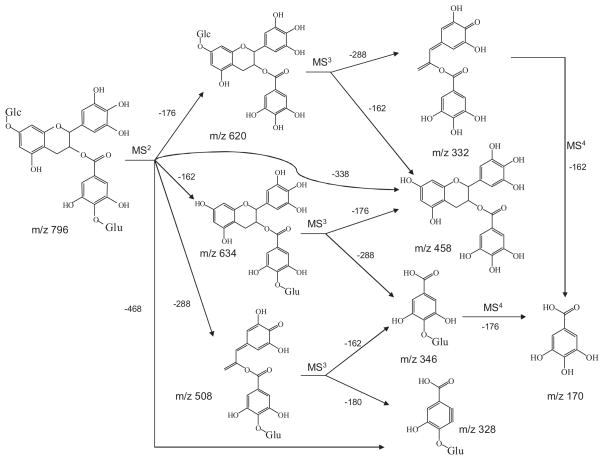
In our studies on the urinary metabolites of EGCG, the observation of the glucoside metabolite was a surprise. For further characterization, we predicted that the ionization and polarity features of EGCG glucoside were quite similar to those of EGCG glucuronide. Therefore, we used an EGCG glucuronide standard, EGCG-4″-O-β-D-glucupyranoside, to tune the instrument and optimize the LC/MS conditions. We used selected-ion monitoring (SIM) mode to search for all the possible EGCG glucoside metabolites from mouse urine samples collected after administration of EGCG, 50 mg/kg i.p. or 200 mg/kg i.g. Those metabolites included EGCG mono-glucoside (m/z 620), mono- and dimethylated EGCG mono-glucoside (m/z 634 and 648, respectively), EGCG mono-glucoside and mono-glucuronide (m/z 796), EGCG mono-glucoside and mono-sulfate (m/z 700), EGCG digluco-side (m/z 782), and its related methylated (m/z 796 and m/z 810), glucuronide (m/z 958), and sulfate (m/z 862) metabolites.
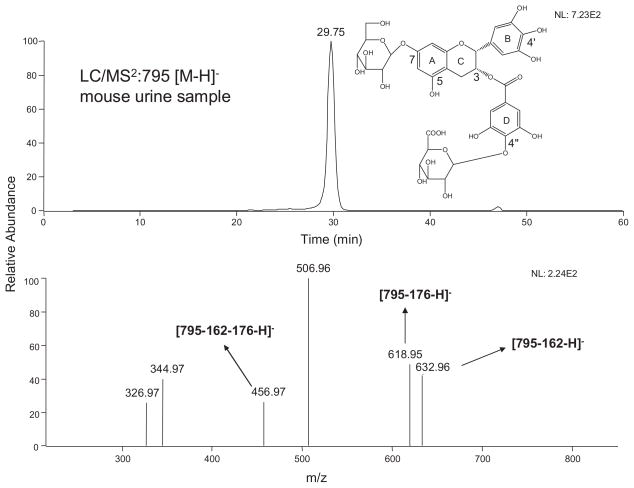
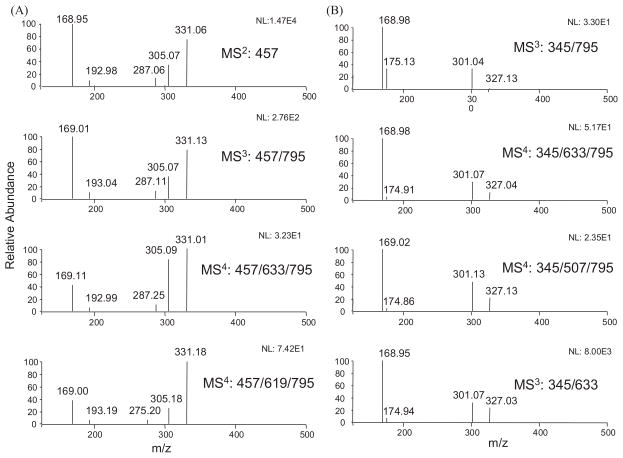
Among all the possible metabolites that we searched for, only one peak (m/z 795 [M–H]−) corresponding to the EGCG mono-glucoside and mono-glucuronide was exhibited in mouse urine samples (Fig. 1). This peak had fragment ions m/z 633 [M–162–H]−, m/z 619 [M–176–H]−, and m/z 457 [M–162–176–H]−, which lost one glucoside, one glucuronide, and one glucoside and one glucuronide units, respectively (Fig. 1). All of these features indicated that this compound was the possible mono-glucoside and mono-glucuronide metabolite of EGCG. To further confirm that this compound was the metabolite of EGCG, we compared the MS/MS spectra of the product ion m/z 457 [M–H]− of the precursor ion m/z 795 [M–H]− (MS3: 457/795) and its two major product ions m/z 633 [M–H]− (MS4: 457/633/795) and m/z 619 [M–H]− (MS4: 457/619/795) with the MS2 spectrum of the EGCG standard (Fig. 2(A)). Figure 2(A) clearly shows that the MS/MS spectrum of this product ion was identical to the MS/MS spectrum of the EGCG standard, indicating this compound was a metabolite of EGCG.
Figure 1.
LC/ESI-MS2 (negative ion) spectra of 7-O-β-D-glucopyranosyl-EGCG-4″-O-β-D-glucupyranoside in mouse urine sample after receiving 50 mg/kg EGCG i.p.
Figure 2.
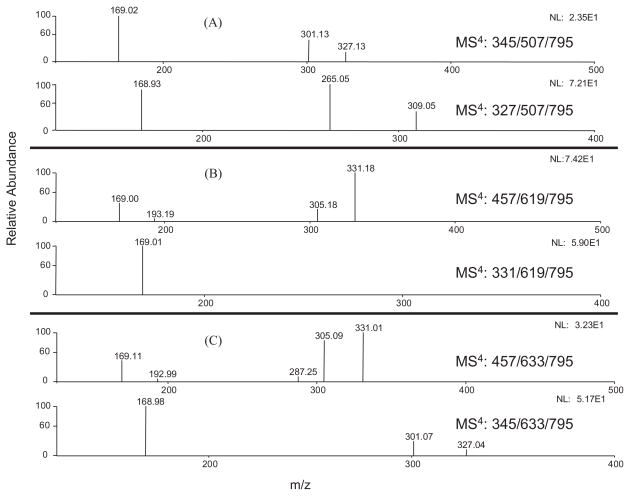
(A) ESI-MS/MS spectra of EGCG standard and the fragment ion m/z 457 [M–H]− from 7-O-β-D-glucopyranosyl-EGCG-4″-O-β-D-glucupyranoside (m/z 795 [M–H]−) and its product ions m/z 633 [M–H]− and 619 [M–H]−. (B) ESI-MS/MS spectra of fragment ion m/z 345 [M–H]− of 7-O-β-D-glucopyranosyl-EGCG-4″-O-β-D-glucupyranoside, its product ions m/z 633 [M–H]− and 507 [M–H]−, and EGCG-4″-O-β-D-glucupyranoside standard.
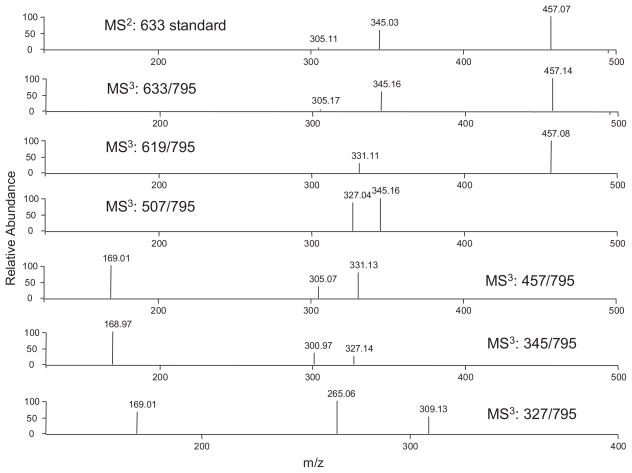
In order to elucidate the structure of this metabolite, we determined the position of the glucuronidation and the glucosidation, respectively. Our previous study has identified EGCG-4″-O-β-D-glucupyranoside as the major glucuronide metabolite of EGCG in mice, rats, and humans.8 The MS3 spectrum of the product ion m/z 633 [M–162]− (MS3: 633/795) of this metabolite was almost identical to the MS2 spectrum of the EGCG-4″-O-β-D-glucupyranoside standard (Fig. 3). It also had the product ion m/z 457, which lost one glucuronide unit. As shown in Fig. 2(A), the MS/MS spectrum of this product ion (MS4: 457/633/795) was identical to that of the EGCG standard (MS2: 457). In addition, the MS/MS spectrum of its product ion m/z 345 (MS4: 345/633/795) was identical to that of the same product ion from the EGCG-4″-O-β-D-glucupyranoside standard (MS3: 345/633), and both of them had the loss of one glucuronide unit to generate gallic acid ion (m/z 169) as the major product ion (Fig. 2(B)). All of these features suggested that the glucuronidation occurred at the 4″ position of the gallate ring.
Figure 3.
ESI-MS/MS spectra of EGCG-4″-O-β-D-glucupyranoside standard (m/z 633 [M-H]−) and the major ESI-MS3 (negative ion) spectra of 7-O-β-D-glucopyranosyl-EGCG-4″-O-β-D-glucupyranoside.
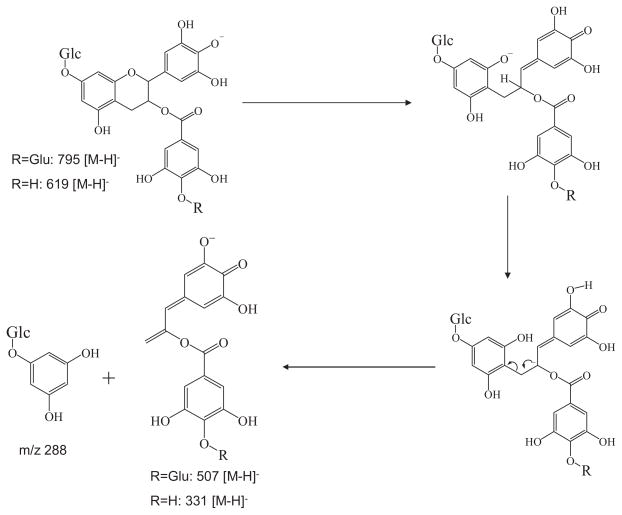
A previous in vitro study showed that the active sites for glucosidation of EGCG were position 4′ on the B-ring and position 7 on the A-ring.21 In our study, the MS2 spectrum of the EGCG mono-glucoside and mono-glucuronide metabolite had the base product ion m/z 507 [M–126–162–H]−, which was the fragment ion that lost the A-ring structure (Fig. 4), indicating there was no conjugation at position 4′ on the B-ring.22 The MS/MS spectrum of this product ion (MS3: 507/795) had the base product ion m/z 345, which was the glucuronide gallic acid (Fig. 2(B)). All of these features suggested that the glucosidation occurred at the A-ring of EGCG. This was further confirmed by the observation of the product ion m/z 331 from the MS/MS spectrum of the fragment ion m/z 619 (MS3 619/795) (Fig. 3). The ion at m/z 331 [M–126–162–H]− was formed following the same formation mechanism as the ion at m/z 507 (Fig. 4). The MS/MS spectrum of this product ion (MS4: 331/619/795) had the base product ion m/z 169 (Fig. 5(B)), further indicating that glucosidation had not occurred at the B-ring and the D-ring. Previous studies suggested that position 7 not position 5 was the active site of the EGCG A-ring for glucuronidation and glucosidation.8,21 Therefore, the glucosidation of this metabolite occurred at position 7 of the A-ring. In addition, this metabolite was tentatively identified as 7-O-β-D-glucopyranosyl-EGCG-4″-O-β-D-glucupyranoside (Fig. 6). This structure needs to be further confirmed by NMR analysis.
Figure 4.
Formation mechanism of the major fragment ions m/z 507 [M–H]− and 331 [M–H]−.
Figure 5.
The major ESI-MS4 (negative ion) spectra of 7-O-β-D-glucopyranosyl-EGCG-4″-O-β-D-glucupyranoside: (A) the major MS4 spectra of the product ion m/z 507 [M–H]−; (B) the major MS4 spectra of the product ion m/z 619 [M–H]−; and (C) the major MS4 spectra of the product ion m/z 633 [M–H]−.
Figure 6.
Proposed fragmentation pathway of 7-O-β-D-glucopyranosyl-EGCG-4″-O-β-D-glucupyranoside.
CONCLUSIONS
In this study, we identified novel mono-glucoside and mono-glucuronide metabolites of EGCG from mouse urine samples collected after administration of EGCG, 50 mg/kg, i.p. or 200 mg/kg i.g., using LC/MS analysis with multi-stage tandem mass spectrometry. To our knowledge, this is the first study on the identification of the glucoside metabolite of EGCG in mammals, and this is also the first report of glucosidation as a novel pathway in the metabolism of EGCG. It is worthwhile to further study whether glucosidation of EGCG occurs in humans. It has been reported that rabbit liver microsomes could efficiently catalyze the glucosidation of genistein to form genistein 7-glucoside.17 Many other dietary flavonoids, such as quercetin, epicate-chin, luteolin, and cyanidin, have the same A-ring structure as that of EGCG and genistein. Further studies are needed to determine whether glucosidation is similar to glucuronidation as a general biotransformation pathway to most of the dietary flavonoids.
A complete understanding of the biotransformation and bioavailability of EGCG will aid in designing future intervention studies and in interpreting the results of laboratory and epidemiological studies of green tea. Although biotransformation pathways are typically considered as the means of inactivating biologically active compounds and facilitating excretion, examples of biologically active metabolites of drugs and dietary compounds have been reported.23,24 The most prominent example is that morphine-6-glucuronide has a more potent analgesic action than morphine.24 Relatively few studies have been conducted on the biological activities of the metabolites of EGCG. We found that the major EGCG glucuronide metabolite, EGCG-4″-O-β-D-glucupyranoside, retained the activity of EGCG in inhibiting the release of arachidonic acid from HT-29 human colon cancer cells.8 It has been reported that EGCG-7-O-α-D-glucopyranoside had similar radical-scavenging effects to EGCG.21 Whether the newly identified EGCG mono-glucoside and mono-glucuronide metabolites are bioactive or not remains to be studied. The results would help us assess relative contribution of EGCG metabolites to the disease preventive effects of EGCG and green tea.
Acknowledgments
Contract/grant sponsor: NIH; contract/grant numbers: CA121390 and CA88961.
Contract/grant sponsor: Jewel of Charity to the Cancer Institute of New Jersey.
This work was supported by NIH grants CA121390 to S. Sang and CA88961 to C.S. Yang, as well as by a grant from the Jewel of Charity to the Cancer Institute of New Jersey.
References
- 1.Yang CS, Maliakal P, Meng X. Annu Rev Pharmacol Toxicol. 2002;42:25. doi: 10.1146/annurev.pharmtox.42.082101.154309. [DOI] [PubMed] [Google Scholar]
- 2.Hou Z, Lambert JD, Chin KV, Yang CS. Mutat Res. 2004;555:3. doi: 10.1016/j.mrfmmm.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 3.Lambert JD, Hong J, Yang GY, Liao J, Yang CS. Am J Clin Nutr. 2005;81:284S. doi: 10.1093/ajcn/81.1.284S. [DOI] [PubMed] [Google Scholar]
- 4.Balentine DA, Wiseman SA, Bouwens LC. Crit Rev Food Sci Nutr. 1997;37:693. doi: 10.1080/10408399709527797. [DOI] [PubMed] [Google Scholar]
- 5.Sang S, Hou Z, Lambert JD, Yang CS. Antioxid Redox Signal. 2005;7:1704. doi: 10.1089/ars.2005.7.1704. [DOI] [PubMed] [Google Scholar]
- 6.Feng WY. Curr Drug Metab. 2006;7:755. doi: 10.2174/138920006778520552. [DOI] [PubMed] [Google Scholar]
- 7.Lu H, Meng X, Yang CS. Drug Metab Dispos. 2003;31:572. doi: 10.1124/dmd.31.5.572. [DOI] [PubMed] [Google Scholar]
- 8.Lu H, Meng X, Li C, Sang S, Patten C, Sheng S, Hong J, Bai N, Winnik B, Ho CT, Yang CS. Drug Metab Dispos. 2003;31:452. doi: 10.1124/dmd.31.4.452. [DOI] [PubMed] [Google Scholar]
- 9.Lu H. PhD thesis. Rutgers, The State University of New Jersey; New Brunswick: 2002. [Google Scholar]
- 10.Li C, Lee MJ, Sheng S, Meng X, Prabhu S, Winnik B, Huang B, Chung JY, Yan S, Ho CT, Yang CS. Chem Res Toxicol. 2000;13:177. doi: 10.1021/tx9901837. [DOI] [PubMed] [Google Scholar]
- 11.Li C, Meng X, Winnik B, Lee MJ, Lu H, Sheng S, Buckley B, Yang CS. Chem Res Toxicol. 2001;14:702. doi: 10.1021/tx0002536. [DOI] [PubMed] [Google Scholar]
- 12.Meng X, Sang S, Zhu N, Lu H, Sheng S, Lee MJ, Ho CT, Yang CS. Chem Res Toxicol. 2002;15:1042. doi: 10.1021/tx010184a. [DOI] [PubMed] [Google Scholar]
- 13.Sang S, Lambert JD, Hong J, Tian S, Lee MJ, Stark RE, Ho CT, Yang CS. Chem Res Toxicol. 2005;18:1762. doi: 10.1021/tx050151l. [DOI] [PubMed] [Google Scholar]
- 14.Matern H, Matern S, Gerok W. Proc Natl Acad Sci USA. 1984;81:7036. doi: 10.1073/pnas.81.22.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boberg M, Angerbauer R, Kanhai WK, Karl W, Kern A, Radtke M, Steinke W. Drug Metab Dispos. 1998;26:640. [PubMed] [Google Scholar]
- 16.Tang C, Hochman JH, Ma B, Subramanian R, Vyas KP. Drug Metab Dispos. 2003;31:37. doi: 10.1124/dmd.31.1.37. [DOI] [PubMed] [Google Scholar]
- 17.Labow RS, Layne DS. Biochem J. 1972;128:491. doi: 10.1042/bj1280491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radominska A, Little J, Pyrek JS, Drake RR, Igari Y, Fournel-Gigleux S, Magdalou J, Burchell B, Elbein AD, Siest G, et al. J Biol Chem. 1993;268:15127. [PubMed] [Google Scholar]
- 19.Gessner T, Jacknowitz A, Vollmer CA. Biochem J. 1973;132:249. doi: 10.1042/bj1320249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magdalou J, Antoine B, Ratanasavanh D, Siest G. Enzyme. 1982;28:41. doi: 10.1159/000459083. [DOI] [PubMed] [Google Scholar]
- 21.Moon YH, Lee JH, Ahn JS, Nam SH, Oh DK, Park DH, Chung HJ, Kang S, Day DF, Kim D. J Agric Food Chem. 2006;54:1230. doi: 10.1021/jf052359i. [DOI] [PubMed] [Google Scholar]
- 22.Miketova P, Schram KH, Whitney J, Li M, Huang R, Kerns E, Valcic S, Timmermann BN, Rourick R, Klohr S. J Mass Spectrom. 2000;35:860. doi: 10.1002/1096-9888(200007)35:7<860::AID-JMS10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 23.Bai F, Lau SS, Monks J. Chem Res Toxicol. 1999;12:1150. doi: 10.1021/tx990084t. [DOI] [PubMed] [Google Scholar]
- 24.Kroemer HK, Klotz U. Clin Pharmacokinet. 1992;23:292. doi: 10.2165/00003088-199223040-00005. [DOI] [PubMed] [Google Scholar]