Abstract
Three conditioned lick suppression experiments with rats examined the role of the context in the selection and integration of independently acquired interval relationships. In Experiment 1, rats were exposed to separate CS1-CS2 pairings with two different interval relationships, each in its own distinctive context, X or Y. The resultant integration was determined by the training context (X or Y) in which US-CS2 backward pairings occurred, as assessed in a third neutral context (Z). In Experiment 2, rats experienced CS1-CS2 pairings with two different interval relationships as in Experiment 1, and then received US-CS2 pairings in both contexts X and Y. The testing context (i.e., X or Y) determined the resultant integration. In Experiment 3, rats were exposed to CS1-CS2 pairings in two different interval relationships each in different phases (i.e., Phases 1 and 2), and then in Phase 3 received US-CS2 pairings. The temporal context of testing (i.e., short or long retention interval) determined the resultant integration. Thus, context can be used to disambiguate conflicting temporal information.
Keywords: temporal coding, temporal integration, context, decision processes
In Pavlovian conditioning, an association is formed between an initially neutral stimulus and the unconditioned stimulus (US) such that presentation of the now conditioned stimulus (CS) activates an anticipatory representation of the US, which in turn causes the animal to emit a conditioned response (CR). Within such a perspective, a fundamental theoretical question is the informational content of these representations and how associations between CSs, USs, and conditioned responses should be characterized (see Rescorla, 1988; Wasserman & Miller, 1997). Considerable evidence suggests that the representation of the CS activates the what (e.g., Holland, 1988), when (e.g. Arcediano, Escobar, & Miller, 2005), and where (e.g., Bouton & Swartzentruber, 1986) regarding the representation of the US. In other words, the representation of the CS actives not only a memory of what the US was (i.e., content) but also when it occurred (e.g., time relative to the CS) and where it was presented (i.e., place).
Within the timing literature, Miller and colleagues have proposed the temporal coding hypothesis (TCH; Matzel, Held, & Miller, 1988; Savastano & Miller, 1998). The tenets of TCH can be summarized as follows: (1) Close temporal contiguity between events is sufficient for the formation of an association. (2) The temporal relationship between the associated events is automatically encoded as part of the association (i.e., subjects create temporal maps that link events in memory; also see Honig, 1981). (3) This temporal information plays a critical role in the nature, magnitude, and timing of the conditioned response elicited when one of the associates is subsequently presented. Lastly, (4) subjects can superimpose temporal maps when the maps share a common element, even when the maps were independently acquired, thereby allowing for the expression of temporal relationships between cues that were never actually paired.
The strongest support for the TCH comes from studies of backward conditioning in rats and humans (e.g., Arcediano, Escobar, & Miller, 2003, 2005). For example, Arcediano et al. (2003) used a modified sensory preconditioning preparation with rats in which they administered CS1→CS2 pairings in a forward relationship with either a 5-s gap or no gap between termination of CS1 and onset of CS2 in Phase 1, followed by CS2-footshock US pairings presented in a backward relationship (i.e., US→CS2) with a 4-s gap between termination of the US and onset of CS2 in Phase 2. When tested on CS1, rats trained with a 5-s gap in Phase 1 showed a large amount of conditioned suppression, whereas rats trained with no gap in Phase 1 showed less conditioned suppression. Arcediano et al. hypothesized that the rats had encoded the temporal relationships between CS1 and CS2 and between the US and CS2, thereby forming two independent temporal maps (with order and interval) between the paired events. These temporal maps presumably were integrated by superimposing the representation of the common element from the two phases of training (i.e., CS2), thereby allowing CS1 to predict an impending US when rats learned a temporal map with a 5-s gap in Phase 1 but not when rats learned a temporal map with no gap in Phase 1. Under the latter circumstances, superposition of maps would have caused the US to be expected simultaneously with the onset of CS1, a relationship which is not conducive to appreciable behavioral control. This and other studies with different Pavlovian paradigms suggest that animals can encode and integrate interval relations. Among these paradigms are conditioned inhibition (e.g., Denniston, Cole, & Miller, 1998), cue competition (e.g., Blaisdell, Denniston, & Miller, 1998), and occasion setting (e.g., Holland, Hamlin, & Parsons, 1997).
Surely, in real world situations animals store multiple interval relations of co-occurring events and subsequently retrieve such interval information. Moreover, when the temporal information of different memories is contradictory, the subject is challenged to decide which information to use to determine behavior. It is well established that when there are simple conflicts of information concerning whether or not an outcome will follow a cue (as after simple Pavlovian acquisition followed by extinction), context can play a strong role in determining which information will be expressed (e.g., Bouton, 1997). Perhaps the context also plays a role in resolving discrepancies between two conflicting interval representations concerning a pair of events. However, we currently know little about the contextual determinants of such interval encoding and expression. This is potentially important because real world situations do not happen in a vacuum, but rather in physical settings, settings that often differ from one another. Even if the physical attributes of a setting are held constant, time itself provides a context that is inevitably always changing. This thinking was the motivation for the present research. Recently, Miller and Escobar (2002) proposed that the context is critical whenever there are two conflicting associations that share a common element. That is, Bouton's (1993, 1997) principle of contextual modulation of memory selection, which was originally applied to situations in which one cue signals two different outcomes (i.e., extinction, counterconditioning, and latent inhibition), was extended to situations in which one outcome is predicted by two different cues (i.e., proactive and retroactive cue interference). In Miller and Escobar's view, when there is ambiguity provided by two conflicting associations that share a common element, then subjects use the context to disambiguate the situation. That is, the context determines which of the two conflicting associations is best primed for retrieval (e.g., Escobar, Matute, & Miller, 2001). By extending this priming principle to situations in which two conflicting interval relations between two stimuli have been trained, one may ask: Is selective retrieval of information concerning the interstimulus interval also subject to priming by the context?
One strategy that might be used to assess whether context can modulate the selection of one bit of interval information as opposed to another conflicting bit of interval information would be to train two forward intervals in different contexts and then explore the role of priming by the spatiotemporal context in integration of independent interval maps. Using the basic design of Arcediano et al. (2003), we conducted three experiments in which CS1 was paired with CS2 with two different interstimlus intervals each in a distinct context. In one experiment we varied the physical context of first-order conditioning, and other two experiments we varied either the physical context of testing or the temporal context of testing. A demonstration of spatiotemporal contextual control of interval integration of independently acquired intervals would add to the growing evidence that animals are capable of learning and storing inconsistent relationships and that the spatiotemporal context influences which associations will be retrieved and expressed at test.
Experiment 1
Experiment 1 tested whether animals would encode diverse interstimulus interval information and integrate it across separate phases of training as a function of the physical training context. We asked, when rats experience CS1-CS2 pairings (i.e., phase 1 of sensory preconditioning) with two different interval relationships, each unique to one of two distinctive Contexts X and Y, does the context in which CS2-US pairings (i.e., phase 2 of a sensory preconditioning procedure) take place determine which phase 1 interval relationship participates in the interval integration that is expressed at test?
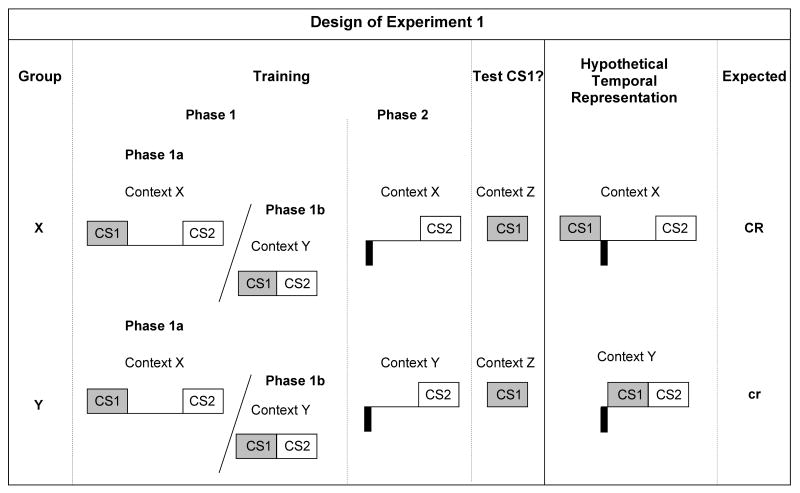
In Experiment 1, there were two treatment groups (see Figure 1). During Phase 1, both Groups X and Y received Click (CS1) and Tone (CS2) pairings with a 5-s gap between the termination of CS1 and the onset of CS2 in Context X, intermixed with CS1-CS2 pairings with no gap in Context Y. During Phase 2, Group X received US-CS2 pairings with a 4.5-s gap between the termination of the US (footshock) and the onset of CS2 in Context X, whereas Group Y received the same training in Context Y. Thus, the designations Group X and Group Y refer to the context in which the Phase 2 backward pairings of US and CS2 took place. Subsequently, all subjects were tested for lick suppression to CS1 in a third neutral context (Z) different from both of those used for training. Of central interest was the pattern of responding to CS1 as a function of the context used during Phase 2 conditioning. According to TCH (Savastano & Miller, 1998), the rats should have encoded the two different interval relationships between CS1 and CS2 as well as the interval relationship between the US and CS2, forming interval maps between the paired events. Additionally, interval maps are integrated by superposing the representation of the common element from the two phases of training (i.e., CS2). According to Miller and Escobar's (2002) retrieval model, the CS1-CS2 interval map acquired in the same context as the US-CS2 interval map should have been more strongly primed than the CS1-CS2 interval map learned in a context different from that of the US-CS2 interval map. Accordingly, presentation of CS1 at test should allow subjects to anticipate an impending US based on a CS1-CS2 interval map with a 5-s gap in Context X and a US-CS2 interval map with a 4.5-s gap in Context X. However a predictive relationship between the CS1 and the US did not exist when rats learned a CS1-CS2 interval map with no gap in Context Y and a US-CS2 interval map with a 4.5-s gap in Context Y. Figure 1 summarizes the design of Experiment 1 with the hypothetical interval maps for each group.
Figure 1.
Design and hypothetical interval representation of Experiment 1. The light gray and the white squares represent cues CS1 (a 3-s click train) and CS2 (a 3-s tone), respectively. Footshock unconditioned stimuli (1.0-mA for 0.5-s) are represented by black rectangles under the time line. The ISI between the termination of CS1 and the onset of CS2 was either 5 s (denoted by large spaces between the cue CS1 and the cue CS2 representations) or 0 s (denoted by the lack of space between the cue CS1 and the cue CS2 representations). The ISI between the termination of the US and the onset of CS2 was 4.5 s. “/” means “unpaired with”; “CR” and “cr” indicate the expectation of robust and weak conditioned response, respectively, based on the TCH and Miller and Escobar's (2002) retrieval model.
Method
Subjects
The subjects were 12 male (275-367 g) and 12 female (197-236 g), experimentally naïve, Sprague-Dawley-descended rats from our breeding colony. Subjects were individually housed and maintained on a 16-hr light / 8-hr dark cycle with experimental sessions occurring approximately midway through the light portion. Each animal was assigned to one of two groups (ns=12), counterbalanced for sex. Subjects had free access to food in the home cage. A progressive water deprivation was imposed over the week prior to the beginning of the experiment until water availability was limited to 30-min per day. All rats were handled for 30 s three times per week from weaning until the initiation of the study.
Apparatus
Three physical contexts were used in this study, two for training (Contexts X and Y) and one for testing (Context Z). The physical identities of Contexts X and Y were counterbalanced within each group. Six instances of each of three different types of experimental chambers were used. Type R and V chambers were rectangular and V-shaped, respectively. They were used for training. There were no lick tubes in these chambers. Chamber R was a clear Plexiglas chamber rectilinear in shape, measuring 22.75 × 8.25 × 13.0 cm (l × w × h). The floor was constructed of stainless steel rods 0.48 cm in diameter, spaced 1.5 cm apart center to center. The rods were connected by NE-2 neon bulbs that allowed a 0.5-s, 1.0-mA constant-current footshock to be delivered by means of a high voltage AC circuit in series with a 1.0-MΩ resistor. Each R chamber was housed in a separate light- and sound-attenuating environmental isolation chest, which was dimly illuminated by a 2-W (nominal at 120 V AC, driven at 80 V AC) incandescent house light mounted on the ceiling of the environmental chest, approximately 26 cm from the center of the experimental chamber.
Chamber V was a 25.5 cm long box shaped like a vertical truncated V, 28 cm high, 21 cm wide at the top, and 5.25 cm wide at the bottom. The floor and sides were constructed of stainless steel sheets, and the ceiling was constructed of clear Plexiglas. The floor of each chamber consisted of two parallel metal plates, each 2.0 cm wide, with a 1.25-cm gap between them. The floor plates were wired to permit the delivery of a 0.5-s, 1.0-mA constant-current footshock. Each V chamber was housed in its own environmental isolation chest, which was dimly illuminated by a 7-W (nominal at 120 VAC, driven at 80 VAC) incandescent house light mounted on an inside wall of the environmental chest, approximately 30 cm from the center of the experimental chamber. The light entering the animal chamber was primarily that reflected from the roof of the environmental chest. The light intensities in the two chambers were approximately equal because of the differences in opaqueness of the walls in Chambers R and V.
The Context Z chambers were modified R chambers, but with lick tubes present, the houselight turned off, an odor cue present (two drops of 98% methyl salicylate onto a small block of wood located inside the isolation chest), and a clear Plexiglas floor plate that covered the grid floor. Additionally, for each subject Context Z was a different instance than the regular R chamber used in training. In all chambers, background noise, primarily from ventilation fans, was 74 dB (C-scale). Two 45-Ω speakers mounted on the interior walls of each environmental chest could deliver an auditory stimulus, either a click train CS1; (6 Hz) or a complex tone CS2; (1000 and 800 Hz presented simultaneously), each at 8 dB (C-scale) above the background. All CSs were 3 s in duration.
Procedure
Acclimation
All rats were acclimated to Contexts X, Y, and Z on Day 1. Session durations were 30 min for Contexts X and Y, and 60 min for Context Z. The rats had access to water-filled lick tubes in Context Z only. The subjects were first acclimated to Contexts X and Y (the order of context acclimation being counterbalanced within groups) and then to Context Z. The intersession interval, which was spent in the home cage, was 40-210 min.
Sensory preconditioning (Phase 1a)
On Days 2, 4, 6, and 8, all subjects were exposed to four CS1-CS2 pairings during each daily 60-min session in Context X. CS1 and CS2 were each 3 s in duration, with the onset of CS2 occurring 5 s after CS1 terminated. On Days 2 and 6, the trials began 5, 16, 26, and 42 min into the session. On Days 4 and 8, trials began 10, 20, 37, 50 min into the session.
Sensory preconditioning (Phase 1b)
On Days 3, 5, 7 and 9, all subjects were exposed to four CS1-CS2 pairings during each daily 60-min session in Context Y. CS2 was presented immediately upon termination of CS1. On Days 3 and 7, the trials began 5, 16, 26, and 42 min into the session. On Days 5 and 9, the trials began 10, 20, 37, and 50 min into the session.
First-order conditioning (Phase 2)
On Day 10, rats in Group X were exposed to four US-CS2 pairings during a 60-min session in Context X, while rats in Group Y were exposed to four US-CS2 pairings during a 60-min session in Context Y. There was a 4.5-s gap between the termination of the US and the onset of CS2. The trials began 5, 21, 37, and 49 min into the session.
Reacclimation
On Days 11 and 12, all rats were exposed to the Context Z for 60 min to re-establish a steady rate of drinking prior to testing in this associatively neutral context.
Testing
On Day 13, all subjects were tested for conditioned lick suppression to the test stimulus (CS1). After five cumulative seconds of licking in the absence of any nominal stimulus, CS1 was presented for 15 min. Thus, all subjects were drinking at the time of test stimulus onset. Time to complete an additional five cumulative seconds of licking after the onset of the test stimulus was recorded. The latencies recorded during the presentation of the test stimulus reflected conditioned suppression (responding) to the test stimulus. The test session was 16 min in duration, with a ceiling score of 15 min imposed on the time to complete five cumulative seconds of drinking in the presence of CS1. Any rat that took longer than 60 s to complete its first five cumulative seconds of licking (i.e., prior to CS onset) was excluded from the experiment to preclude subjects with unusually high fear of the test context. No animal reached this criterion but the data from one animal in Group X had to be eliminated due to equipment problems. All test scores were transformed to log time (base 10) to better approximate the within-group normal distributions assumed by parametric statistics. An alpha level of .05 (two tailed) was adopted for tests of statistical significance. When appropriate, we report effect sizes calculated using the algorithm provided by Myers and Well (2003, p. 210).
Results and Discussion
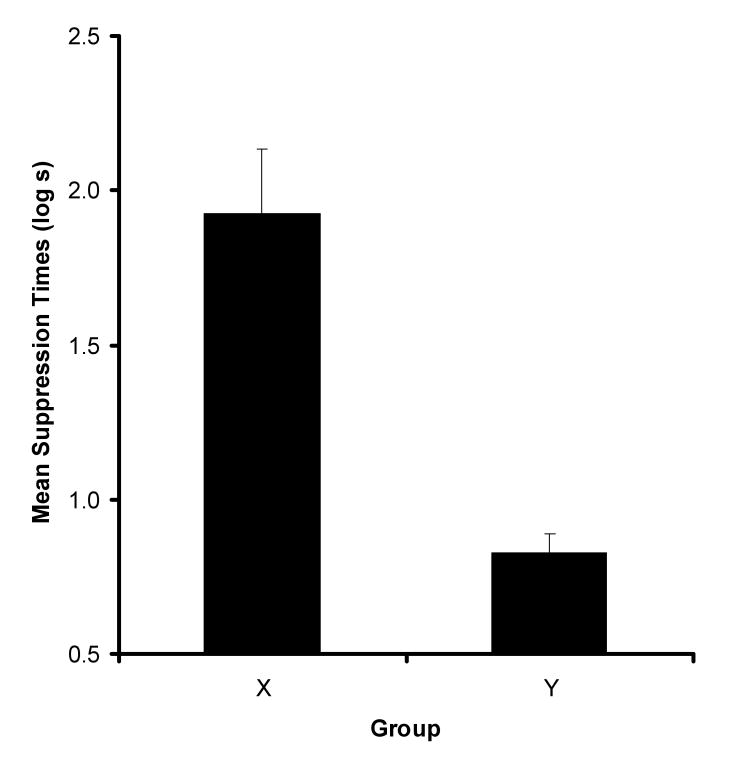
Figure 2 depicts the mean suppression to CS1 at test as a function of the context (X or Y) used during Phase 2 conditioning. As can be seen, the subjects in Group X suppressed drinking after the presentation of CS1 more than did the subjects in Group Y. A t-test performed on the data recorded prior to the test presentation of CS1 (i.e., baseline scores) revealed no difference between Groups X and Y (means in log s ± MSE, X = 1.05 ± .06 and Y = 1.08 ± .02), p > .65, which suggests similar levels of baseline drinking across groups. The analysis performed on the scores recorded during the presentation of CS1 revealed that suppression to CS1 in Group X was indeed greater than in Group Y, t(21) = 5.29, p < .01, MSE = .24, Cohen's f = 1.09.
Figure 2.
Results of Experiment 1. Mean suppression to CS1 for both Groups X and Y, respectively. Error bars represent the standard error of the mean.
On the basis of Miller and Escobar's (2002) retrieval model, in Group X, acquiring a US-CS2 interval map with a 4.5-s gap in Context X should have retrieved the CS1-CS2 interval map with a 5-s gap which was also trained in Context X, and should have interfered with retrieval of the CS1-CS2 interval map with no gap which was trained in a different context (i.e., Y). According to the temporal coding hypothesis, for rats in Group X the presentation of CS1 at test should have retrieved a representation of CS2 with an 8-s interval gap between the onset of CS1 and the onset of CS2. Moreover, this activated representation of CS2 should have activated a representation of the onset of the US 5-s back in time. Thus, those rats should have expected the US to be delivered immediately after termination of CS1. Thus, CS1 should have elicited strong conditioned responding in Group X. Following the same logic to analyze Group Y, acquiring a US-CS2 interval map with a 4.5-s gap in Context Y should have primed retrieval of the CS1-CS2 interval map with no gap which was also trained in Context Y, and should have interfered with retrieval of the CS1-CS2 interval map with a 5-s gap which was trained in Context X. By superimposing activated interval maps, rats in Group Y should have retrieved a representation of CS2 occurring immediately after the termination of CS1. Additionally, the representation of CS2 onset would activate a representation of the onset of the US 5-s back in time. Accordingly, those rats should have expected the US to be delivered 2-s before the onset of CS1 (i.e., backward conditioning). Thus, CS1 should elicit little or no anticipatory conditioned responding in Group Y.
In summary, the specific context (X or Y) used for US-CS2 pairings (with 4.5-s gap) appears to have influenced (i.e., primed) which CS1-CS2 interval map (with either a 0 or 5-s gap) was retrieved and superimposed with the US-CS2 interval map. Neither the TCH or the retrieval model by Miller & Escobar alone are able to fully account for the above observations. However, a hybrid of the TCH and the Miller & Escobar's retrieval model complement each other in providing a complete explanation of the present results.
Experiment 2
The results of Experiment 1 indicate that the selection between two conflicting interstimulus intervals for integration with a third interval depends on the similarity between the physical training context of the third interval and those in which the two conflicting interval relations were acquired. To go one step further, we now sought to determine if the test context could prove critical when the training contexts fail to resolve the ambiguity in interstimulus interval. Consequently, Experiment 2 looked at training-test similarity in physical context. The purpose was to ask, if rats experienced CS1-CS2 pairings (i.e., phase 1 of sensory preconditioning) with two different interval relationships, each unique to one of two distinctive Contexts X and Y, and then received pairings of US and CS2 in both Contexts X and Y, would the context of testing (X or Y) determine which of the potential CS1-CS2 interval maps would be integrated with the US-CS2 interval map?
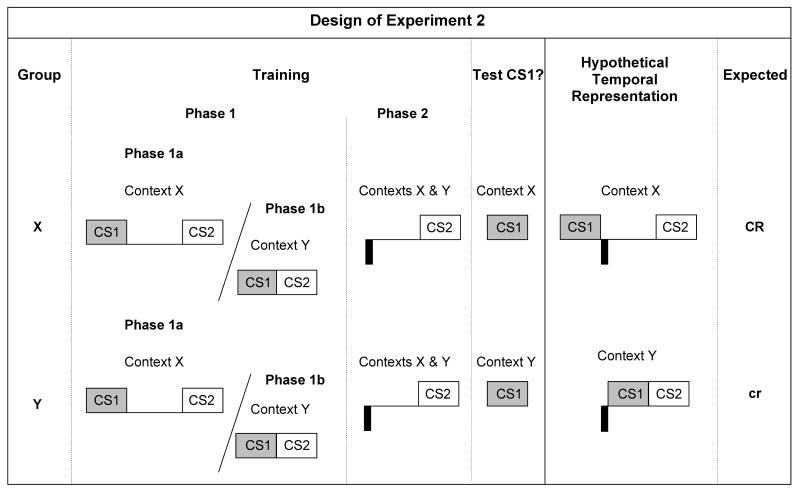
In Experiment 2, there were two treatment groups (see Figure 3). Phase 1 of the experiment was identical to the Phase 1 of Experiment 1. During Phase 2, both groups received US-CS2 pairings with a 4.5-s gap between the termination of the US and the onset of CS2 in both Contexts X and Y. Subsequently, Group X was tested for lick suppression to CS1 in Context X, whereas Group Y was tested in Context Y. The designations Group “X” and Group “Y” now refer to the test context. Central interest was in the pattern of responding to CS1 as a function of the test context. According to the TCH (Savastano & Miller, 1998) combined with the Miller & Escobar's (2002) retrieval model, the CS1-CS2 interval map acquired in the same context as testing should be more strongly primed than the CS1-CS2 interval map learned in a context different from testing. The presentation of CS1 at test in Context X presumably allowed subjects to anticipate the US based on a CS1-CS2 interval map with a 5-s gap and a US-CS2 interval map with a 4.5-s gap both acquired in the same context. However, the anticipation of the US is less likely when CS1 is tested in Context Y when rats learned a CS1-CS2 interval map with no gap and a US-CS2 interval map with a 4.5-s gap in that context. Figure 3 summarizes the design of Experiment 2 with the hypothetical interval maps for each group.
Figure 3.
Design and hypothetical interval representation of Experiment 2. The light gray and the white squares represent cues CS1 (a 3-s click train) and CS2 (a 3-s tone), respectively. Footshock unconditioned stimuli (1.0 mA for 0.5 s) are represented by black rectangles under the time line. The ISI between the termination of CS1 and the onset of CS2 was either 5 s (denoted by large spaces between the cue CS1 and the cue CS2 representations) or 0 s (denoted by the lack of space between the cue CS1 and the cue CS2 representations). The ISI between the termination of the US and the onset of CS2 was 4.5 s. “/” means “unpaired with”; “CR” and “cr” indicate the expectation of robust and weak conditioned response, respectively, based on the TCH and Miller and Escobar's (2002) retrieval model.
Method
Subjects and Apparatus
The subjects were 12 male (243-305 g) and 12 female (200-230 g), experimentally naïve, Sprague-Dawley-descended rats from our breeding colony. Twelve rats were randomly assigned to each group, counterbalanced for sex. The animals were housed and maintained as in Experiment 1. Six each of two different types of experimental chambers (R and V) were used as Contexts X and Y, counterbalanced within groups. The six copies of Chambers R and V were identical to those described in Experiment 1.
Procedure
Acclimation
All rats were acclimated to Contexts X and Y on Day 1. Session durations were 30 min for Contexts X and Y, with order of context exposure counterbalanced within groups. All rats had access to water-filled lick tubes.
Sensory preconditioning (Phases 1a and 1b)
On Days 2-9, all subjects received CS1-CS2 pairings as in Experiment 1. There was a 5-s gap between termination of CS1 and onset of CS2 in Context X and no gap between CS1 and CS2 in Context Y. The lick tubes were not available during Phases 1a and 1b.
First-order conditioning (Phase 2)
On Day 10, all subjects received four trace backward conditioning trials, with a 4.5-s gap between US termination and CS2, during each of two 60-min sessions, one in each X and Y contexts. The order of context exposure was counterbalanced within groups. The trials began 5, 21, 37, and 49 min into the first session, and 9, 24, 33, and 54 min into the second session. The water-filled lick tubes were not available during Phase 2.
Reacclimation
On Days 11 and 12, all rats were exposed to both Contexts X and Y for 60 min to re-establish a steady rate of drinking prior to testing. The daily order of exposure to Contexts X and Y was counterbalanced within groups.
Testing
On Day 13, all subjects were tested for conditioned lick suppression to CS1. Subjects in Group X were tested in Context X, whereas subjects in Group Y were tested in Context Y. Otherwise, testing was identical to Experiment 1.
Results and Discussion
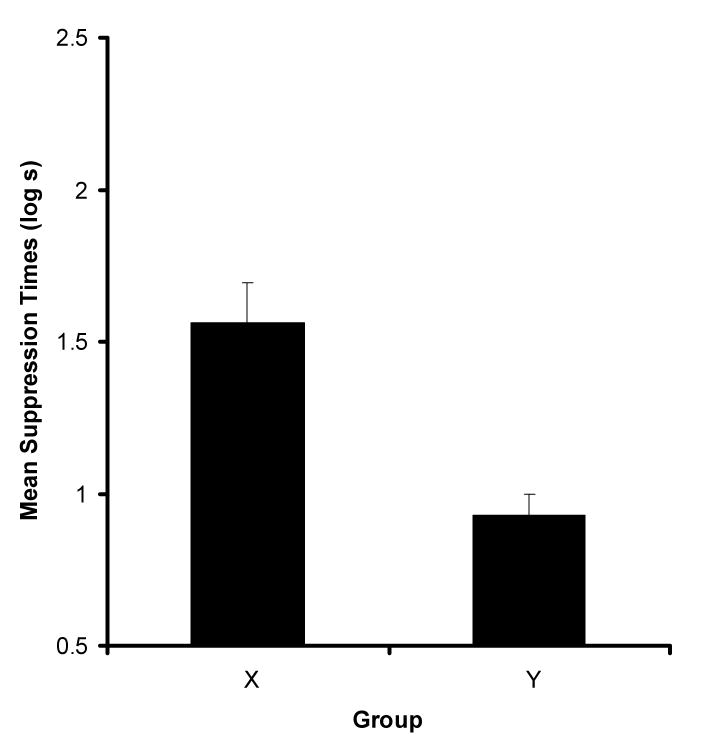
Figure 4 depicts the mean suppression to CS1 at test as a function of the context (X or Y) used during testing. As can be seen, the subjects in Group X suppressed drinking after the presentation of CS1 more than did the subjects in Group Y. A t-test performed on the data recorded prior to the test presentation of CS1 (i.e., baseline scores) revealed no difference between Groups X and Y (means in log s ± MSE, X = 1.08 ± .04 and Y = .94 ± .06), p > .10, which suggests similar levels of baseline drinking across groups. An analysis performed on the scores recorded during presentation of CS1 revealed that suppression to CS1 in Group X was indeed greater to that of CS1 in Group Y, t(22) = 4.07, p < .001, MSE = .14, Cohen's f = 1.19.
Figure 4.
Results of Experiment 2. Mean suppression to CS1 for both Groups X and Y, respectively. Error bars represent the standard error of the mean.
On the basis of Miller and Escobar's (2002) retrieval model, testing in Context X should have retrieved the CS1-CS2 interval map with a 4.5-s gap which was trained in Context X, and should have interfered with retrieval of the CS1-CS2 interval map with no gap which was trained in a different context (i.e., Y). According to the TCH, when CS1 was presented at test, subjects in Group X should have retrieved a representation of CS2 with an 8-s temporal gap between the onset of CS1 and the onset of CS2 and there should have been interference with retrieval of the no-gap temporal relationship between CS1 and CS2 that was trained in Context Y. Similarly, the representation of CS2 onset should have activated a representation of the onset of the US 5 s back in time. Thus, subjects should have expected the US to be delivered immediately after termination of CS1, and consequently suppressed strongly to CS1. Following the same logic, Group Y, which was tested in Context Y, should have retrieved the CS1-CS2 interval map with no gap which was trained in Context Y, and this should also have interfered with retrieval of the CS1-CS2 interval map with a 4.5-s gap which was trained in Context X. According to the TCH, subjects should have retrieved a representation of CS2 occurring immediately after termination of CS1. Similarly, the representation of CS2 onset should have activated a representation of the onset of the US 5 s back in time. Hence, subjects should have expected the US to be delivered 2 s before the onset of CS1, which would not be conducive to conditioned suppression.
In summary, the specific physical context of testing (X or Y) appears to have influenced (i.e., primed) which CS1-CS2 interval map (with either a 0 or 5-s gap) was preferentially superimposed on the US-CS2 interval map. This account, based on a hybrid of the TCH and the Miller & Escobar's retrieval model, seems able to provide a parsimonious account for the present results.
Experiment 3
In Experiment 3 we again used Miller and Escobar's (2002) retrieval model that builds on Bouton's (1993, 1997) proposal that time can serve as a context. Here temporal context is viewed as potentially able to prime retrieval of different interval relationships between associated events that were acquired at different times. Specifically, Experiment 3 was designed to examine the context dependence of integration due to a change in the temporal context between the last of two separate phases of CS1-CS2 pairings and testing. This would add generality to the findings of Experiment 2 by extending the finding of interval integration being regulated by physical attributes of the context to interval integration being regulated by temporal attributes of the context. We asked, if animals are sequentially trained with pairings of CS1 and CS2 with two different interval relationships in two successive phases (e.g., CS1-CS2 pairings with no gap in phase 1 followed by CS1-CS2 pairings with a 5-s gap in phase 2) and then trained with pairings of US and CS2, would the temporal context of testing (i.e., a short or long retention interval) determine which of the potential CS1-CS2 interval maps would be integrated with the US-CS2 interval map (i.e., recency of primacy)? It is well known that, when subjects acquire conflicting information in a phasic manner (e.g., A-B in phase 1 and A-C in phase 2), an immediate test is apt to yield behavior consistent with the later-learned information (A-C, i.e., recency). But when a long retention interval follows the second phase, recency effects wane. The observed behavior then either reflects a melding of the earlier and later phases of training (A-B/C) or sometimes even results in a clear primacy effect (A-B; Wright, Santiago, Sands, Kendrick, Cook, 1985). Such a shift from recency to primacy with increasing retention interval was obtained in our laboratory by Wheeler and Miller (2007) and Wheeler, Stout, and Miller (2004). We capitalized on this prior work and borrowed procedures and parameters from those studies for the present experiment.
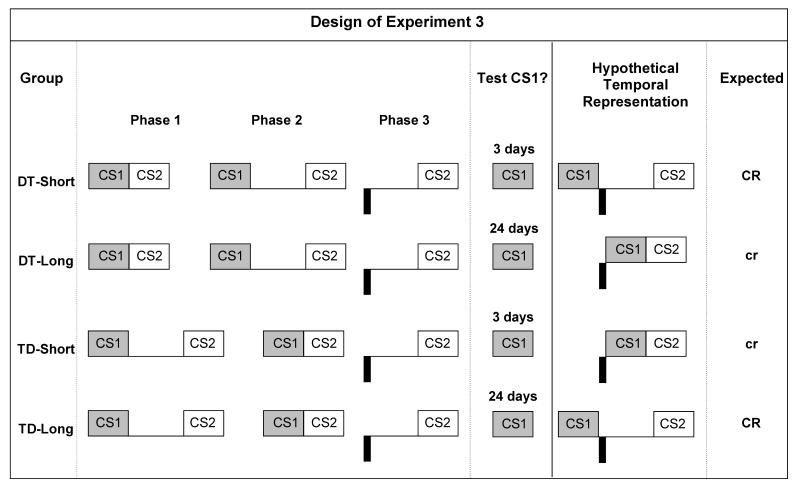
There were four groups constituting a 2 × 2 factorial design (see the Figure 5). Two groups were trained with CS1-CS2 delay conditioning in Phase 1 and CS1-CS2 trace conditioning in Phase 2 (condition Delay Trace [DT]), whereas the order of these two types of pairing was reversed in the two other groups (condition Trace Delay [TD]). Rats in Condition DT received CS1 and CS2 pairings with no gap between the termination of CS1 and the onset of CS2 in Phase 1 (i.e., delay conditioning), followed by CS1-CS2 pairings with a 5-s gap in Phase 2 (i.e., trace conditioning). Rats in Condition Trace Delay (TD) received similar training, with the order of treatments reversed. Phase 3 consisted of US-CS2 pairings with a 4.5-s gap between the termination of the US and the onset of CS2 for all groups. Orthogonal to the TD vs. DT conditions, half of the subjects were tested 3 days after the US-CS2 pairings of Phase 3 (Short retention interval condition), whereas the remaining subjects were tested 24 days after the US-CS2 pairings of Phase 3 (Long retention interval condition). We used interval parameters identical to those used in Experiments 1 and 2, but we conducted all phases of training and testing in the same physical context. Of central interest was the pattern of responding to CS1 as a function of the retention interval (short or long). According to the TCH, the rats had encoded the two different interval relationships between CS1 and CS2 and the interval relationship between the US and CS2, forming three interval maps between the paired events. Additionally, according to Miller & Escobar's (2002) retrieval model, each interval map was coded with its temporal context. On the basis of this retrieval model, with a short retention interval, the recent temporal context of Phase 2 should have primed retrieval of the more recently acquired CS1-CS2 interval map. When a long retention interval was interposed, the subjects should have been in a new temporal context and the more recently acquired CS1-CS2 interval map should no longer be strongly primed. The US-CS2 interval map is presumably unaffected by this change in retention interval. This should result in a relative increase in the likelihood of integration with the first-learned CS1-CS2 interval map. Figure 5 summarizes the design of Experiment 3 and the hypothetical interval maps for each group.
Figure 5.
Design and hypothetical interval representation of Experiment 3. CS1 = a 3-s click train) and CS2 = a 3-s tone, respectively. US = footshock unconditioned stimuli (1.0 mA for 0.5 s) is indicated by black rectangles. The ISI between the termination of CS1 and the onset of CS2 was either 5 s (denoted by large space between the cue CS1 and the cue CS2 representations) or 0 s (denoted by the lack of spaces between the cue CS1 and the cue CS2 representations). The ISI between the termination of the US and the onset of CS2 was 4.5 s. “CR” and “cr” indicate the expectation of robust and weak conditioned response, respectively. Based on the TCH and Miller and Escobar's (2002) retrieval model. The designations “TD” and “DT” refer to the interval arrangement between CS1 and CS2 in Phases 1 and 2 (T = trace and D = delay). “Short” = short retention interval (3 days). “Long” = long retention interval (24 days).
Method
Subjects and Apparatus
The subjects were 24 male (175-269 g) and 24 female (170-234 g) experimentally naïve Sprague-Dawley-descended rats. Animals were assigned to one of four treatment groups (ns=12) counterbalanced for sex. Animal care, maintenance, and the apparatus were the same as in Experiment 2. Six each of two different types of experimental chambers (R and V) were used. For each rat, all training and testing occurred in the same context. Chamber type was counterbalanced within groups. The use of two different chambers was due to the available apparatus and was not relevant to the issues addressed in this study, as for each subject all training and testing occurred in the same physical context.
Procedure
Acclimation
On Day 1, all subjects had free access to the water-filled lick tubes in the experimental context during a 60-min session. No nominal stimuli were presented.
Sensory preconditioning (Phase 1)
On Days 2-5, all subjects received four daily CS1-CS2 pairings. For Groups TD-Short and TD-Long, there was a 5-s gap between the termination of CS1 and the onset of CS2. For Groups DT-Short and DT-Long, there was no gap; that is, CS1 termination was followed immediately by CS2 onset. On Days 2 and 4, the trials began 5, 16, 26, and 42 min into the session. On Days 3 and 5, trials began 10, 20, 37, 50 min into the session. The water-filled lick tubes were absent.
Sensory preconditioning (Phase 2)
On Days 6-9, all subjects were presented daily with four CS1-CS2 pairings in the experimental context. For Groups TD-Short and TD-Long, there was no gap. For Groups DT-Short and DT-Long, there was a 5-s gap between the termination of CS1 and the onset of CS2. On Days 6 and 8, the trials began 5, 16, 26, and 42 min into the session. On Days 7 and 9, the trials began 10, 20, 37, and 50 min into the session. The water-filled lick tubes were absent.
First-order conditioning (Phase 3)
On Day 10, all subjects received backward trace conditioning trials, with a 4-s gap between US termination and CS onset. Subjects received eight US-CS2 pairings. We increased the number of US-CS2 pairings to ensure that fear would be retained throughout the retention interval in the Long condition. We also doubled the session length. The US onsets occurred at 9, 24, 33, 54, 65, 84, 97, and 114 min into the 120-min session. The water-filled lick tubes were absent.
Short-retention interval reacclimation and testing
On Days 11 and 12, rats in Groups TD-Short and DT-Short were exposed to the experimental context for 60-min to re-establish a steady rate of drinking prior to testing. On Day 13, subjects in Groups TD-Short and DT-Short were tested for conditioned lick suppression to CS1. The procedure was identical to Experiment 1.
Long-retention interval reacclimation and testing
Over the retention interval, the 30-min/day water deprivation schedule was maintained and the animals were handled trice weekly for 30 s. On Days 32 and 33, rats in Groups TD-Long and DT-Long were given daily 60-min reacclimation sessions. On Day 34, all rats were tested for suppression to CS1. The testing procedure was identical to that used after the short-retention interval.
Results and Discussion
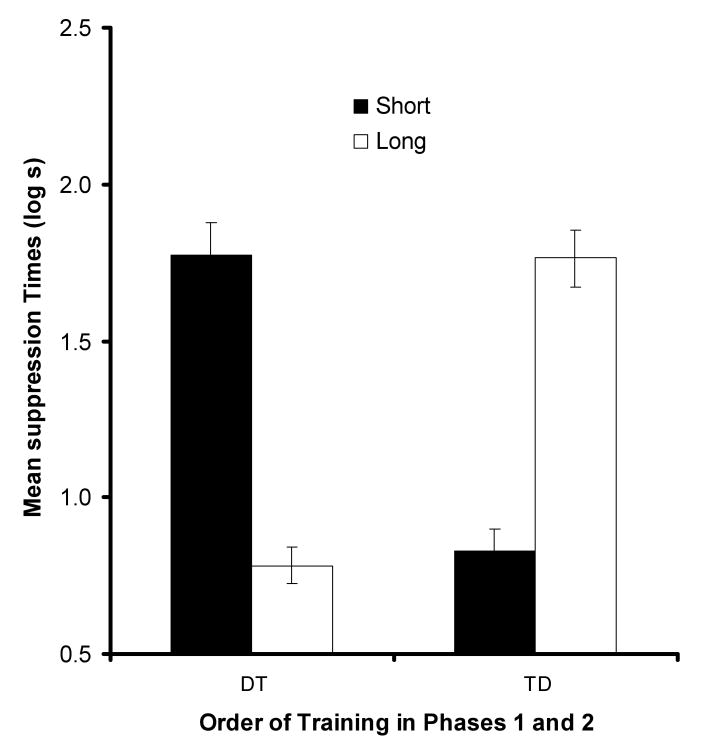
Figure 6 depicts the mean suppression at test for each group. As can be seen, after the short retention interval, rats in Condition DT (i.e., CS1-CS2 pairings with no gap followed by CS1-CS2 pairings with a 5-s gap) suppressed drinking at test more than did the rats in Condition TD (i.e., CS1-CS2 pairings with a 5-s gap followed by CS1-CS2 pairings with no gap). Importantly, these relations were reversed after a retention interval. That is, after the long retention interval rats in Condition DT showed less suppression than did rats in Condition TD. The following statistical analyses supported these observations.
Figure 6.
Results of Experiment 3. Mean suppression to CS1 for Groups DT and TD tested after a short or a long retention interval. Error bars represent the standard error of the mean.
Before analysing the suppression data, a 2 (order of training; DT vs. TD) × 2 (retention interval; Short vs. Long) ANOVA test was performed on the data recorded prior to the test presentation of Cue CS1 (i.e., baseline scores). This revealed no significant effects (means in log s ± MSE, DT-Short = 1.03 ± .03, DT-Long = 1.01 ± .05, TD-Short = 1.04 ± .05, TD-Long = 1.08 ± .05), all Fs < 1, which suggests similar levels of baseline drinking across groups. A similar ANOVA was performed on the scores recorded during the presentation of CS1. This analysis revealed a crossover interaction, F(1, 44) = 134.24, p < .01, MSE = .08, Cohen's f = 1.66 suggesting that suppression to CS1 between the DT and TD conditions differed as a function of the retention interval (Short vs. Long). Neither main effect was significant, Fs < 1. Planned comparisons using the overall error term from the ANOVA found that with the short retention interval (3 days) suppression to CS1 in Condition DT was stronger to that of in Condition TD, F(1, 44) = 64.80, p < .01. In contrast, with the long retention interval (24 days) suppression to CS1 in Condition TD was stronger to that of in Condition DT, F(1, 44) = 69.47, p < .01.
In the Short retention interval conditions (3 Days), the pattern of responding to CS1 appeared to reflect integration of the first-order conditioning interval map with the more recent sensory preconditioning interval relation (Phase 2). Upon presentation of CS1 at test, subjects in Group DT-Short behaved as if they retrieved a representation of CS2 with an 8-s interval gap between the onset of CS1 and the onset of CS2. Presumably, the representation of the onset of CS2 activated a representation of the onset of the US 5-s back in time. Accordingly, subjects should have expected the US to be delivered immediately after termination of CS1. Thus, CS1 should have elicited strong conditioned suppression based on the expectation of the impending US. In contrast, subjects in Group TD should have retrieved a representation of CS2 occurring immediately after termination of CS1. This representation of the onset of CS2 should have activated a representation of the onset of the US 5 s back in time. Accordingly, subjects should have expected the US to be delivered 2 s before the onset of CS1. Thus, CS1 should have elicited little or no conditioned suppression.
In the Long retention interval conditions (24 days), the pattern of responding to CS1 appeared to reflect integration of the first-order conditioning interval map with the initial sensory preconditioning interval relation (Phase 1). Upon presentation of CS1, subjects in Group DT-Long behaved as if they retrieved a representation of CS2 occurring immediately after termination of CS1. Presumably, the representation of CS2 onset activated a representation of the onset of the US 5 s back in time. Accordingly, subjects should have expected the US to be delivered 2 s before the onset of CS1. Thus, CS1 should have elicited little or no conditioned suppression, as there should have been no expectation of an impending US. In contrast, subjects in Group TD-Long should have retrieved a representation of CS2 with an 8-s interval gap between the onset of CS1 and the onset of CS2. This representation of CS2 onset should have activated a representation of the onset of the US 5 s back in time. Accordingly, subjects should have expected the US to be delivered immediately after termination of CS1. Thus, CS1 should have elicited strong conditioned suppression.
In summary, when all the phases of training were conducted in the same physical context, the specific temporal context of testing (Short or Long retention interval) appears to have influenced (i.e., primed) which CS1-CS2 interval map (with either a 0 or 5-s gap) was preferentially superimposed on the US-CS2 interval map. Congruent with Experiment 2, the rats behaved as if they had encoded as part of the association the context of acquisition along with each interval map of the relationship between paired events, only this time the context was a temporal context rather than a physical context. Consequently, in the short retention interval condition, the similarity of the test temporal context to the Phase 2 temporal context produced a recency effect. However, in the long retention interval condition, this similarity of temporal contexts waned with a consequent loss of the recency effect, and in fact allowed the emergence of a primacy effect.
General Discussion
The present series of experiments was aimed at understanding whether ambiguous interval relationships between stimuli can be selectively expressed based on the contextual attributes surrounding encoding and retrieval. In each experiment, a sensory preconditioning design was used (i.e., CS1-CS2) with different interstimulus intervals trained in each of two contexts. This was followed by backward first-order trace conditioning. In Experiment 1, we observed that which interval was used to control behavior was influenced by the context in which first-order conditioning occurred (US-CS2). We then extended the findings of Experiment 1 by obtaining evidence suggesting that such integration can also be influenced by the context of testing, the physical test context in Experiment 2 and the temporal test context in Experiment 3.
Here we sought to determine if the choice of which of two conflicting interval relationships between a pair of associated events (i.e., CS1-CS2) would be integrated with a complementary interval relationship (US-CS2) was context dependent. In other words, can the context of the complementary interval relationship (US-CS2) or the context of test favor the expression of one of two conflicting CS1-CS2 interval relationships? In one part of a sensory preconditioning procedure, we paired CS1 with CS2 with no gap and a 5-s gap. This was done in three experiments, Experiments 1 and 2 in which we manipulated the physical context, that is, each of the two interval relationships between CS1 and CS2 was trained in its own distinctive context (X and Y); and the Experiment 3 in which we manipulated the temporal context, that is, the two different interval relationships between CS1-CS2 were sequentially learned in different phases (two different temporal contexts). In the framework of the TCH, we expected that animals would encode both interval relationships between CS1 and CS2 (e.g., Arcediano et al., 2005). Moreover, on the basis of the Miller and Escobar's (2002) retrieval model, we anticipated that animals would encode the context of each interval relationship. In other words, we expected that the contextual information would be stored along with the interval information. In the second part of our sensory preconditioning procedure, animals were exposed to US-CS2 pairings with 4.5-s gap. In Experiment 1 the physical context of Phase 2 determined which interval relationship from Phase 1 was integrated with the Phase 2 interval information. In Experiment 2 the physical context of testing determined which interval relationship from Phase 1 was integrated with the interval information acquired in Phase 2. Finally, in Experiment 3 the temporal context of testing determined whether the interval relation learned last (i.e., recency) or the interval relation learned first (i.e., primacy) was integrated with the interval information from first-order conditioning. In each experiment, the encoding of two conflicting interval relationships between CS1 and CS2 created ambiguity with respect to the relative temporal location of those CSs with respect to each other. Presumably this ambiguity was resolved by the context. Taken together, our results support the view that animals are capable of learning, storing, retrieving, and using ambiguous interval information under contextual control in the same manner that they do concerning ambiguous cues (e.g., Bouton, 1997) and ambiguous outcomes (Escobar et al., 2001). Our evidence lends support to the conjecture that a rich representation is encoded between the associated events (e.g., Bonardi & Jennings, 2007; Chamizo, Rodrigo, & Mackintosh, 2006; Rosas & Alonso, 1997; Sawa, Leising, & Blaisdell, 2005) and that different aspects of this rich representation (in this case, the interval relation) can gain behavioral control.
The present experiments focused on contextual control of temporal integration when ambiguity exists. Putting these observations into a larger context, we mention recent evidence of integration of spatiotemporal stimulus representations acquired at different times in human associative learning (Molet, Jozefowiez, & Miller, 2009). These authors used a sensory preconditioning preparation to assess integration of interval and spatial cognitive maps in humans. Participants were exposed to a horizontal spatial relationship between A and B with a 3-s ISI, followed by a vertical spatial relationship between B and the outcome with a 5-s ISI. Results showed that A-B spatiotemporal training followed by B-outcome spatiotemporal training resulted in spatiotemporal integration that created both spatial and interval relationships between A and the outcome. This result encourages the view that a stimulus representation encodes the spatiotemporal location of the stimulus with respect to its associate. The larger claim is that both human and nonhuman subjects encode and retrieve the when and where of the associated events, and can integrate events with a common element.
In summary, in three experiments we observed that rats are able to integrate interval information from independently acquired associations provided the associations share a common element, and that they can use physical and temporal contextual information to select which specific interval information to integrate. This is important because much prior research has been devoted to how contextual information can be used to resolve ambiguity concerning the presence and absence of events, but to our knowledge this is the first clear demonstration of contextual information being used to resolve ambiguity concerning interval information in Pavlovian situations.
Acknowledgments
This research was supported by National Institute of Mental Health Grant 33881. Mikaël Molet was supported by a postdoctoral fellowship from the Fyssen Foundation. We thank Jérémie Jozéfowiez, Mario Laborda, Bridget McConnell, Cody Polack, and James Witnauer for their comments on earlier versions of this manuscript. Thanks are also due to James Esposito for his technical assistance. Correspondence concerning to this article should be addressed to Ralph R. Miller, Department of Psychology, SUNY at Binghamton, Binghamton, NY 13902-6000 USA. E-mail: rmiller@binghamton.edu.
References
- Arcediano F, Escobar M, Miller RR. Temporal integration and temporal backward associations in humans and nonhuman subjects. Learning & Behavior. 2003;31:242–256. doi: 10.3758/bf03195986. [DOI] [PubMed] [Google Scholar]
- Arcediano F, Escobar M, Miller RR. Bidirectional associations in humans and rats. Journal of Experimental Psychology: Animal Behavior Processes. 2005;31:301–318. doi: 10.1037/0097-7403.31.3.301. [DOI] [PubMed] [Google Scholar]
- Blaisdell AP, Denniston JC, Miller RR. Temporal encoding as a determinant of overshadowing. Journal of Experimental Psychology: Animal Behavior Processes. 1998;24:72–83. doi: 10.1037//0097-7403.24.1.72. [DOI] [PubMed] [Google Scholar]
- Bonardi C, Jennings D. Occasion setting of timing behavior. Journal of Experimental Psychology: Animal Behavior Processes. 2007;33:339–348. doi: 10.1037/0097-7403.33.3.339. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Signals for whether versus when an event will occur. In: Bouton ME, Fanselow MS, editors. Learning, motivation, and cognition: The functional behaviorism of Robert Bolles. Washington, DC: American Psychological Association; 1997. pp. 385–409. [Google Scholar]
- Bouton ME, Swartzentruber D. Analysis of the associative and occasion-setting properties of contexts participating in a Pavlovian discrimination. Journal of Experimental Psychology: Animal Behavior Processes. 1986;12:333–350. [Google Scholar]
- Chamizo VD, Rodrigo T, Mackintosh NJ. Spatial integration with rats. Learning & Behavior. 2006;34:348–354. doi: 10.3758/bf03193198. [DOI] [PubMed] [Google Scholar]
- Denniston JC, Cole RP, Miller RR. The role of temporal relationships in the transfer of condditioned inhibition. Journal of Experimental Psychology: Animal Behavior Processes. 1998;24:200–214. doi: 10.1037//0097-7403.24.2.200. [DOI] [PubMed] [Google Scholar]
- Escobar M, Matute H, Miller RR. Cues trained apart compete for behavioral control in rats: Convergence with the associative interference literature. Journal of Experimental Psychology: General. 2001;130:97–115. doi: 10.1037/0096-3445.130.1.97. [DOI] [PubMed] [Google Scholar]
- Holland PC. Extinction and inhibition in unblocking. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:261–279. [PubMed] [Google Scholar]
- Holland PC, Hamlin PA, Parsons JP. Temporal specificity in serial feature positive discrimination learning. Journal of Experimental Psychology: Animal Behavior Processes. 1997;23:95–109. doi: 10.1037//0097-7403.23.1.95. [DOI] [PubMed] [Google Scholar]
- Honig WK. Working memory and the temporal map. In: Spear NE, Miller RR, editors. Information processing in animals: Memory mechanisms. Hillsdale, NJ: Erlbaum; 1981. pp. 167–197. [Google Scholar]
- Matzel LD, Held FP, Miller RR. Information and the expression of simultaneous and backward associations: Implications for contiguity theory. Learning & Motivation. 1988;19:357–344. [Google Scholar]
- Miller RR, Escobar M. Associative interference between cues and between outcomes presented together and presented apart: An integration. Behavioural Processes. 2002;57:163–185. doi: 10.1016/s0376-6357(02)00012-8. [DOI] [PubMed] [Google Scholar]
- Molet M, Jozefowiez J, Miller RR. Integration of spatiotemporal maps in humans. 2009 Manuscript submitted for publication. [Google Scholar]
- Myers JL, Well AD. Research design and statistical analysis. 2nd. Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers; 2003. [Google Scholar]
- Rescorla RA. Pavlovian conditioning: It's not what you think it is. American Psychologist. 1988;43:151–160. doi: 10.1037//0003-066x.43.3.151. [DOI] [PubMed] [Google Scholar]
- Rosas JM, Alonso G. The effect of context change upon long-term memory of CS duration. Behavioural Processes. 1997;39:69–76. doi: 10.1016/s0376-6357(96)00045-9. [DOI] [PubMed] [Google Scholar]
- Sawa K, Leising KJ, Blaisdell AP. Sensory preconditioning in spatial learning using a touch screen task in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 2005;31:368–375. doi: 10.1037/0097-7403.31.3.368. [DOI] [PubMed] [Google Scholar]
- Savastano HI, Miller RR. Time as content in Pavlovian conditioning. Behavioral Processes. 1998;44:147–162. doi: 10.1016/s0376-6357(98)00046-1. [DOI] [PubMed] [Google Scholar]
- Wasserman EA, Miller RR. What's elementary about associative learning? Annual Review of Psychology. 1997;48:573–607. doi: 10.1146/annurev.psych.48.1.573. [DOI] [PubMed] [Google Scholar]
- Wheeler DS, Miller RR. Primacy effects induced by temporal or physical context shifts are attenuated by a preshift test trial. Quarterly Journal of Experimental Psychology. 2007;60:191–210. doi: 10.1080/17470210600790240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DS, Stout SC, Miller RR. Interaction of retention interval with CS-preexposure and extinction effects: Symmetry with respect to primacy. Learning & Behavior. 2004;32:335–347. doi: 10.3758/bf03196032. [DOI] [PubMed] [Google Scholar]
- Wright AA, Santiago HC, Sands SF, Kendrick DF, Cook RG. Memory processing of serial lists by pigeons, monkeys, and people. Science. 1985;229:287–289. doi: 10.1126/science.9304205. [DOI] [PubMed] [Google Scholar]