Abstract
Pattern separation, the process of transforming similar representations or memories into highly dissimilar, non-overlapping representations is a key component of many functions ascribed to the hippocampus. Computational models have stressed the role of the hippocampus and in particular the dentate gyrus and its projections into the CA3 subregion in pattern separation. We used high-resolution (1.5 mm3) functional magnetic resonance imaging to measure brain activity during incidental memory encoding. While activity consistent with a bias toward pattern completion was observed in CA1, the subiculum, and the entorhinal and parahippocampal cortices, activity consistent with a strong bias towards pattern separation was observed in, and limited to, the CA3/dentate gyrus. These results provide compelling evidence of a key role of the human CA3/dentate gyrus in pattern separation.
Introduction
The medial temporal lobe (MTL) is critically involved in the ability to store and retrieve facts and events (declarative memory) (1). Theoretical models suggest that declarative memory relies on both the ability to orthogonalize overlapping or similar patterns of activation (pattern separation) and the ability to complete partial patterns or “clean up” similar patterns of activity into a single common representation (pattern completion) (2–6). Such models of the MTL have stressed the role of the hippocampus and in particular the dentate gyrus and its projections into the CA3 subregion in the process of pattern separation. Specifically one computational model (4) proposed that the dentate gyrus creates a sparse, orthogonalized representation of its input from the entorhinal cortex and projects this into the CA3 via the mossy fibers. The model also suggests that the recurrent interconnected pyramidal cells of the CA3 subregion operate as an auto-association network capable of reestablishing previously stored patterns of activation based on noisy or degraded cues (pattern completion) (see also 2,3,6,7,18).
Only recently has empirical evidence been provided supporting the role of the hippocampus, and the dentate gyrus and CA3 in particular, in pattern separation. Rodent studies have shown striking differences in the stability of place cells in the CA1 and CA3 subfields in response to changes in the environment by means of early gene expression (8) and single cell recordings (9–13). Depending on the degree of changes in the environment, distorted cues lead to either very similar neuronal representations (completion) or very dissimilar neuronal representations (separation) with the bias towards separation or completion varying across hippocampal subfields. In one example (10), rodents were exposed to enclosures of varying similarity during multi-unit recording. In CA3, small changes in the enclosure resulted in the activation of a different, non-overlapping set of neurons (separation). In contrast, CA1 neurons were relatively insensitive to change with many (although not all) showing a strong overlap in activation between the different enclosures (with the degree of overlap correlated with enclosure similarity). Pattern separation and pattern completion can thus be observed in the rodent with a clear functional distinction between neuronal ensembles in the CA1 and CA3 subregions of the hippocampus.
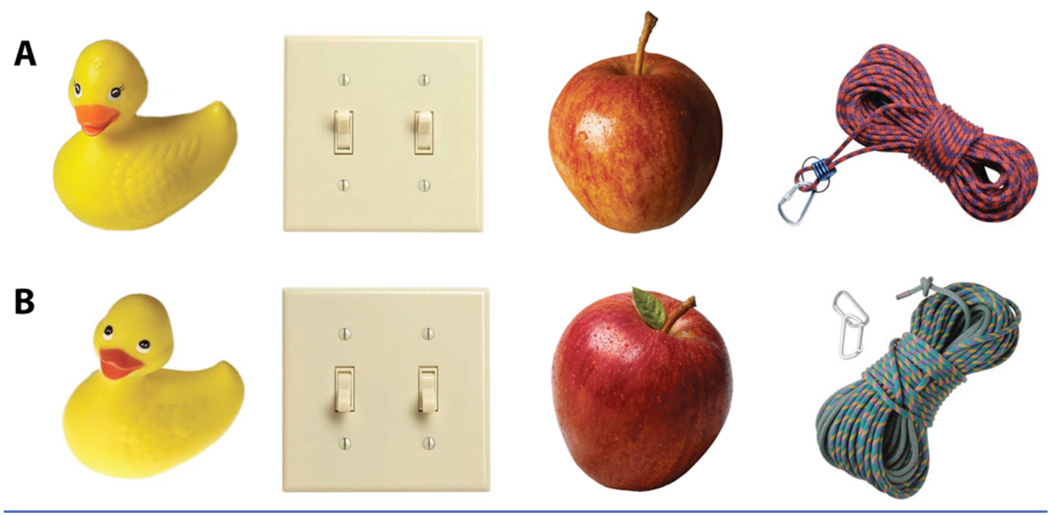
Here, we used high-resolution fMRI (1.5 mm3) to measure brain activity while subjects performed an incidental encoding task designed to parallel many of the rodent studies including Leutgeb et al. (10) described above. Eighteen subjects viewed a series of pictures of everyday objects (making the task not overtly spatial). The series included 144 sets of slightly different pictures of the same object as well as unrelated single pictures of objects used as foils (Fig. 1). Thus, on each trial, a presented object could be either 1) new, 2) a repetition of a previously shown object or, 3) a slightly different version of a previously shown object (lure). All trials were presented in pseudorandom order with the limitation that a repeat or a lure trial would be presented within approximately 30 trials of each other. During each trial subjects were not asked to determine whether the stimulus was a target, a repetition, or a lure but rather were asked to make an unrelated determination about the object (whether it was typically an indoor or outdoor object). As such, the encoding was incidental and not overtly mnemonic, paralleling the studies in the rodent described above (14).
Fig. 1.
Sample stimuli sets showing version A and B of the same object
While the resolution of 1.5 mm3 represents the likely limit for fMRI (15), it is still far too coarse to resolve the pattern of activity across individual neurons and assess pattern separation directly. However, if regions exhibit a change in activity with repetition, we can assess pattern separation versus pattern completion indirectly. We hypothesized that, if a given subregion was engaged in processes of pattern separation, the lure would more likely be treated like a new stimulus than a repetition and show activity similar to that for a first presentation of a stimulus. In contrast, if a given subregion was engaged in processes of pattern completion, the lure would be more likely treated as a repetition of the original stimulus and show activity consistent with a repetition.
Results
To examine the effects of the incidental encoding task, functional data from all trials was subjected to a 2-way ANOVA with participants as a random factor and trial type (1st presentation, first repeat and first lure) as a fixed factor. Functional regions of interest (ROIs) were based on the resulting F-maps and defined by setting a voxelwise threshold of p = 0.05 and a spatial extent threshold of a minimum ROI volume of 100 mm3. This threshold is deliberately somewhat liberal as the clusters form the basis for a functional ROI analysis in which all voxels within each ROI are collapsed and the final alpha is set at p < 0.05.
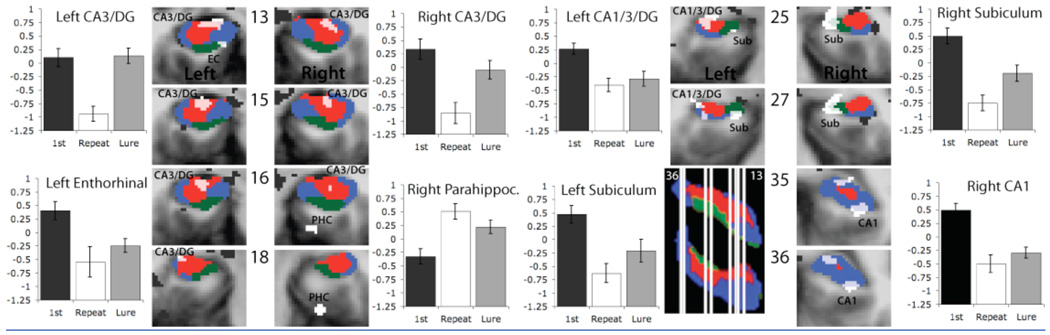
This cluster analysis resulted in eight regions in which activity varied in some way with some form of repetition in the MTL (Fig. 2). Using ROI-LDDMM (Region of Interest, Large Deformation Diffeomorphic Metric Mapping), segmentations of each subject’s individual anatomy are used to align his or her brain to a high-resolution model consisting of bilateral segmentations of the CA1 field, the CA3/dentate gyrus (including CA2 and CA4), the subiculum, and the entorhinal, perirhinal, and parahippocampal cortices (16, 17, and supporting online material). The model is further used to localize each region identified in the group analysis (Fig. 2). Note that we cannot confidently isolate some regions from each other and some activations cannot be confidently assigned to one region. In particular, we cannot confidently isolate CA3 from the dentate gyrus (DG) and therefore will refer to a single, combined region (CA3/DG) in discussing the results. In addition, if activity spans several regions (e.g., an ROI that covers both the CA1 and CA3/DG regions as observed below), we prefer to acknowledge this ambiguity in localization rather than strictly adopting one location.
Fig. 2.
Anatomical location and mean activity in the three task conditions for each of the eight MTL regions of interest. A model segmentation of hippocampal subfields is overlaid on each brain slice to indicate the location of the subiculum (green), CA1 (blue) and CA3/DG (red). Regions of activity within the MTL are shown in white and labeled within each slice (PHC=parahippocampal cortex, EC=entorhinal cortex. Regions of activity outside the MTL (not part of the analysis) are shown in black. 3D rendering (lateral, superior view) shows the location of each slice (white lines). The distance of each slice from the anterior commissure (y=0 in Talairach coordinates) is indicated for each slice as well (y=13–36 mm). The thicker white lines represent two adjoining slices. Bar graphs show mean activity (summed beta-coefficients) in each ROI for each trial condition.
Using these techniques, the activations were localized to the right CA1 subregion of the hippocampus (CA1), bilateral CA3/dentate gyrus subregions of the hippocampus (CA3/DG), a region spanning both the left CA1 and CA3/dentate gyrus subregion of the hippocampus (CA1/3/DG), the left entorhinal cortex (EC), the right parahippocampal cortex (PHC) and finally bilateral subiculum (Sub). Figure 2 shows these activities on representative coronal slices through the MTL (Note, only the tip of the EC activity is shown. In more anterior slices, the activity extends well into the gyrus). Mean activity within each of the ROIs was then calculated for the 1st presentation trials, the repeat trials and the lure trials by collapsing all voxels within an ROI (Fig. 2). Reliable differences were observed between the three trial types in each ROI. In all but one of the ROIs the repeat trials showed a lower mean activity compared to the 1st presentation and the lure trials. Only in the right parahippocampal cortex did the repeat trials show a greater mean activity compared to the 1st condition and the lure trials. In all, activity associated with lure trials was either in between the activity of the repetition trials and the 1st presentation trials, or was indistinguishable from the activity of the repetition trials or the 1st presentation trials. In response to the presentation of a lure, activity in the bilateral CA3/DG was significantly different from activity in response to a repeat presentation (right CA3/DG, t17 = −5.147, p < 0.001 and left CA3/DG t17 = −6.463, p < 0.001). Activity in response to the presentation of a lure stimulus in the bilateral CA3/DG was not significantly different from activity in response to a first presentation (right CA3/DG t17 = 1.89, p = 0.075 and left, CA3/DG t17 = −0.165, p = 0.871). This in contrast to other areas of activation observed in the medial temporal lobe where activity in response to the presentation of a lure was significantly different from activity in response to a first presentation (CA1/3/DG, t17 = 3.151, p < 0.001; left enthorhinal cortex, t17 = 3.697, p < 0.01 and right CA1, t17 = 6.224, p < 0.001) and not significantly different from the repeat presentation of a stimulus (CA1/3/DG, t17 = −0.822, p = 0.422; left enthorhinal cortex, t17 = −1.117, p = 0.279 and right CA1, t17 = −1.25, p = 0.228).
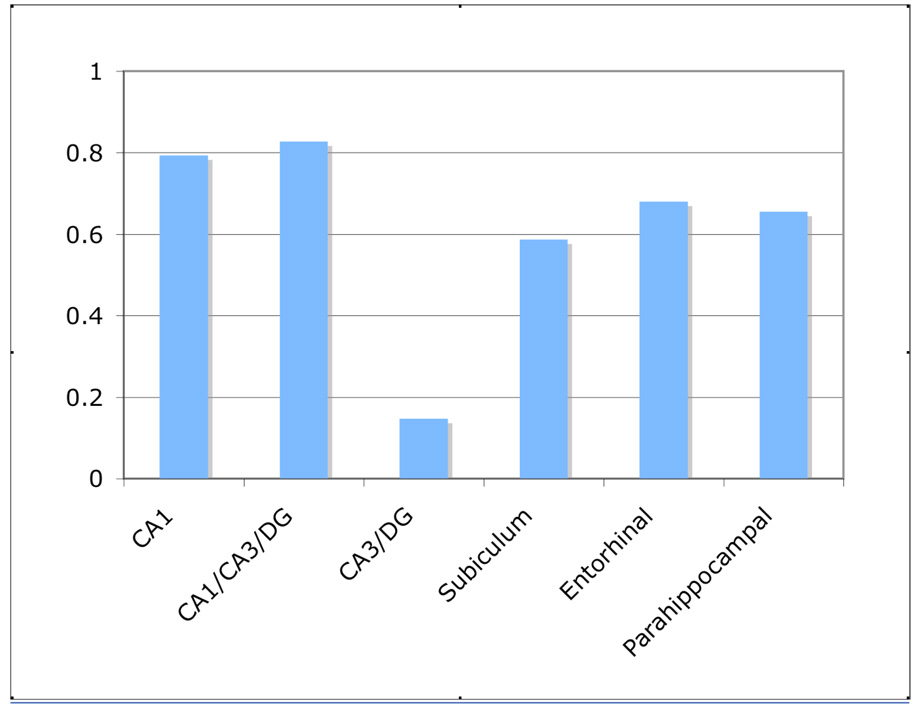
A simple bias score indexing how similar lure activity is to repetition [(1st – Lure) / (1st – Repetition)] can be used to assess the tendency towards completion (bias approaching 1) or separation (bias approaching 0) across regions. This bias score is shown in Fig 3. Note, a single bias score for each region is shown, collapsing across ROIs in the same region (e.g., bilateral CA3/DG). Bias scores for CA1, the region that spanned across the CA1/3/DG, the subiculum and the entorhinal and parahippocampal cortices ranged from 0.59 to 0.83. Thus, while somewhat mixed and not purely connoting completion, lure stimuli were treated more like true repetitions than first presentations of stimuli. In stark contrast, the bias score of 0.15 in the CA3/DG showed that lure stimuli were treated almost exactly the same as the presentation of a novel stimulus, consistent with a strong bias towards pattern separation.
Fig. 3.
Bias scores in the MTL. A single bias score for each of the six different areas in the MTL was calculated by collapsing the bias scores for multiple ROI’s in the same area. Bias scores closer to zero connote separation while scores closer to one connote completion.
Discussion
We used high-resolution fMRI to study processes of pattern separation in the human MTL by scanning subjects while they performed an incidental encoding task using pictures of common objects. Embedded in the task were direct repetitions of pictures and lure stimuli that were only similar to previously presented objects. A bias towards pattern separation was observed in the CA3/DG and biases towards completion were observed in several MTL regions including CA1.
Our design used an incidental encoding task with no overt behavioral assessment of whether or not pattern separation occurred on a given trial. This was done both to more closely parallel the rodent literature and to remove any explicit memory components from the task that might confound our findings. In an explicit memory task, correctly rejecting the lure as only similar to, but not the same as the target could provide behavioral evidence that pattern separation occurred. However, as previously suggested (17), this approach does not allow us to cleanly isolate processes of pattern separation. If subjects are aware of the pattern separation demands of the task, the subject might employ a “recall to reject” strategy (18). This strategy implies that when presented with a lure, subjects first retrieve the original presentation from memory (thereby performing pattern completion) and subsequently compare the representations to determine if the stimulus is a lure or a target. The subject can then make a determination based on the comparison between the stimulus and the representation of the initial presentation retrieved from memory. As such this task does not merely rely on pattern separation but also on pattern completion and even processes of encoding and retrieval. Our indirect measure that simply examines activity for lure items relative to first presentations and repetitions is not only closer to the free exploration of an environment used in the rodent studies, but also suffers far less from this difficulty.
Our observation of activity consistent with pattern separation specifically in the CA3/DG subregion of the hippocampus is consistent with computational models that have stressed the role of particularly the dentate gyrus in creating sparse orthogonalized representations (2, 4, 6, 18). We must note that although high-resolution fMRI allows us to study these processes within the hippocampus, the resolution remains insufficient to distinguish the neuronal activity of the dentate gyrus from the activity of the CA3 subregion and these two areas were combined and treated as one. As such, task-related activity of the dentate gyrus cannot be distinguished from activity of the CA3 subregion and processes of pattern separation cannot be ascribed to specifically the dentate gyrus. However, because the sparse orthogonalized representations created by the dentate gyrus are projected predominantly to the CA3 subregion, one would expect to observe activity related to processes of pattern separation in both subregions of the hippocampus. Animal studies have shown pattern separation signals in both the dentate gyrus as well as the CA3 subregion (19, 20, 21). Thus our findings remain consistent with both the computational models as well as the animal studies described earlier.
There are limitations of our method. First, if pattern separation was present in a region, but overall activity remained stable across the various repetition conditions, we would fail to detect pattern separation in this region. Indeed, all findings in this study are dependent on a change in BOLD activity across the conditions in our task (e.g., dropping activity with repetition of the stimulus), as fMRI is based on contrasts and is only sensitive to differences across conditions.
Second, pattern separation and pattern completion are processes that are not limited to the MTL (see 22, 23). Indeed, one could argue that pattern separation and pattern completion occurs in all sensory modalities in response to different or similar stimuli. It is thus possible that activity in the CA3/DG reflects the mere processing of patterns already separated elsewhere in the brain rather than active pattern separation. Although computational models and animal studies suggest the CA3/DG serves an active role in pattern separation the current data do not allow us to differentiate between these possibilities.
Third, as novelty, recency, and familiarity signals have played important roles in understanding the MTL (24), we must consider the present results in light of these codes. Each of the regions showed activity consistent with novelty (and recency) detection in that simple repetition was associated with less activity than the first presentation (with the exception that the parahippocampal cortex showed an inverted signal, increasing activity with repetition). In the regions outside the CA3/DG, the same change in activity was observed for lures, while the CA3/DG responded with elevated activity. These results would be consistent with the hypothesis that the same pattern of activity was present (i.e., the same neurons were firing and no pattern separation occurred), but at rates that coded for novelty. Importantly, the novelty coding in the CA3/DG must be less tolerant of small changes than other regions. While a plausible account of our data, this view is inconsistent with several other sources of data. First, it is inconsistent with the pattern of novelty signals typically observed in the hippocampus (24). We observed multiple patterns in the hippocampus and it is not clear that the CA3/DG would best be interpreted as coding for item-place (or similar) associations. Further, the simpler pattern of activity that is consistent with novelty signals was not observed in the perirhinal cortex. Finally, this account would ignore the data showing that neuronal activity in the dentate gyrus and the CA3 sub region of the hippocampus do in fact exhibit changes in the representation as the environment changes (10–13). Our results are in no way inconsistent with novelty signals in the hippocampus that differ from novelty signals elsewhere in the MTL (24). However, we suggest that the present results are more indicative of pattern separation and completion and that they are not synonymous with various novelty codes.
With the ability to perform fMRI with sufficiently high resolution pattern separation can be observed in the human MTL and even specifically be ascribed to the bilateral CA3/DG subregions of the hippocampus. These finding provide compelling evidence of a key role of the human CA3/DG in pattern separation and further evidence supporting the computational models' account of hippocampal function. The process of pattern separation is central to and heavily taxed by many of the functions ascribed to the hippocampus in various cognitive theories (e.g. recollection, episodic memory, conjunctive memory, etc.). We suggest that an understanding of the division of labor within the MTL and between the MTL and other structures will require a greater understanding of how pattern separation and completion function throughout the MTL and elsewhere in cortex.
Supplementary Material
References and Notes
- 1.Squire LR, Stark CEL, Clark RE. Annu. Rev. Neurosci. 2004;27:279. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 2.Marr D. Philos. Trans. R. Soc. London Ser. B. 1971;262:23. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 3.O’Reilly RC, McClelland JL. Hippocampus. 1994;4:661. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- 4.Treves A, Rolls ET. Hippocampus. 1994;4:374. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- 5.McClelland JL, Goddard NH. Hippocampus. 1996;6:654. doi: 10.1002/(SICI)1098-1063(1996)6:6<654::AID-HIPO8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 6.O’Reilly RC, Rudy JW. Psych Rev. 2001;108:311. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- 7.Amaral DG, Witter MP. Neuroscience. 1989;31:571. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- 8.Vazdarjanova A, Guzowski JF. J. Neurosci. 2004;24:6489. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee I, Yoganarasimha D, Rao G, Knierim JJ. Nature. 2004;430:456. doi: 10.1038/nature02739. [DOI] [PubMed] [Google Scholar]
- 10.Leutgeb S, Leutgeb JK, Treves A, Moser M, Moser EI. Science. 2004;305:1295. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- 11.Leutgeb S, Leutgeb JK, Moser MB, Moser EI. Cur. Opin. Neurobiol. 2005;15:738. doi: 10.1016/j.conb.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Wills TJ, Lever C, Cacucci F, Burgess N, O’Keefe J. Science. 2005;308:873. doi: 10.1126/science.1108905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Science. 2007;315:947. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 14.Materials and methods are available as supporting material on Science Online.
- 15.Hyde JS, Biswal BB, Jesmanowicz A. Magn. Reson. Med. 2001;46:114. doi: 10.1002/mrm.1166. [DOI] [PubMed] [Google Scholar]
- 16.Kirwan CB, Jones CK, Miller MI, Stark CEL. Hum Brain Mapp. 2007;28:959. doi: 10.1002/hbm.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirwan CB, Stark CE. Learn Mem. 2007;14:625. doi: 10.1101/lm.663507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norman KA, O'Reilly RC. Psychol. Rev. 2003;110:611. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert PE, Kesner RP, Lee I. Hippocampus. 2001;11:626. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- 20.Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Science. 2007;315:947. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 21.McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Science. 2007;317:94. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- 22.Buckner RL, Goodman J, Burock M, Rotte M, Koutstaal W, Schacter D, Rosen B, Dale AM. Neuron. 1998;20:285. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- 23.Rotshtein P, Henson RNA, Treves A, Driver J, Dolan RJ. Nature Neurosci. 2005;8:107. doi: 10.1038/nn1370. [DOI] [PubMed] [Google Scholar]
- 24.Brown MW, Aggleton JP. Nature Rev. Neurosci. 2001;2:51. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- 25.This work was supported by NSF grant BCS 0544959 to CS and NIH grant P41 RR15241-01A1 and NIMH grant R01 EB00975-01 to MM.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.