Abstract
Maternal aggression (maternal defense) is a fierce aggression produced by lactating females towards intruders that plays an important role in protection of vulnerable offspring. Enhancement of GABAA receptor signaling by benzodiazepines increases maternal aggression and we recently found indirect evidence that lateral septum (LS) could be a key site where benzodiazepines elevate aggression. In this study, we directly tested the hypothesis that activation of GABAA receptors in LS would promote maternal aggression while inhibition of this receptor would decrease aggression. Site-directed injections to LS were made using the GABAA receptor antagonist, bicuculline (3-30 ng), or the GABAA receptor agonists, chlordiazepoxide, a benzodiazepine (2.5-5 μg), and muscimol (0.05–5 ng). Maternal aggression and other behavioral measures were then evaluated in lactating mice. Neither GABAA receptor agonist elevated aggression, which could reflect a ceiling effect. However, 7 ng of the GABAA receptor antagonist, bicuculline, in LS significantly decreased maternal aggression without altering other maternal behaviors or light-dark box performance, suggesting some GABAA receptor signaling in LS is required for full maternal aggression expression. Together these results confirm a role for GABAA receptor signaling in LS in the regulation of maternal aggression.
Keywords: Maternal aggression, Lateral septum, GABA, Maternal behaviors
Introduction
During the postpartum period, various neuromodulators orchestrate the expression of maternal aggression, a fierce protective behavior whereby lactating females attack an intruder to protect offspring (Lonstein & Gammie, 2002). Gamma-aminobutyric acid (GABA), a prominent inhibitory neurotransmitter in the mammalian brain, has previously been linked to maternal aggression (maternal defense) regulation. Prior studies showed that benzodiazepine (BDZ) agonists (which increase the binding affinity of GABA onto GABAA receptors) alter maternal aggression in rats (Ferreira, Picazo, Uriarte, Pereira, & Fernandez-Guasti, 2000; Mos & Olivier, 1986, 1989) and mice (Lee & Gammie, 2007; Palanza, Rodgers, Ferrari, & Parmigiani, 1996; Yoshimura & Ogawa, 1989, 1991) with low doses increasing and higher doses inhibiting aggression. For example, the BDZ, chlordiazepoxide (CDP), triggers lactating females to be significantly more aggressive towards heavier male intruders (Mos, Olivier, & van Oorschot, 1987). In addition, a recent microarray study suggested lactating mice that were selected for high maternal aggression had increase expression of GABRA1, a gene that encodes a GABAA receptor alpha subunit, in the preoptic and hypothalamic brain regions (Gammie et al., 2007). Together, these studies suggest that activation of GABAA receptor can promote offspring protection.
The lateral septum (LS) has been linked to the regulation of maternal aggression. Previously, large septal lesions were found to disrupt maternal defense (Flannelly, Kemble, Blanchard, & Blanchard, 1986) and we recently found that activation of corticotropin releasing factor (CRF) receptor 2 in LS is a mechanism for inhibiting maternal aggression (D'Anna & Gammie, 2009). In other studies, we found that manipulations that decrease maternal aggression also trigger increases in Fos (an indirect marker for neuronal activity) in LS (D'Anna, Stevenson, & Gammie, 2005; Gammie, D'Anna, Gerstein, & Stevenson, 2008; Gammie, Negron, Newman, & Rhodes, 2004), suggesting LS neuronal activity contributes to aggression output. Importantly, we recently demonstrated that when GABAA agonist, CDP, is peripherally injected and elevates maternal aggression, that it also decreases Fos in LS (Lee & Gammie, 2007). GABAA receptors are expressed at high levels in LS, including those that are BDZ sensitive (Speth et al., 1980; Wisden, Laurie, Monyer, & Seeburg, 1992), and our findings suggest that the LS could be a critical site for CDP's pro-aggressive actions. To date, no study has directly examined the effects of GABAA receptor signaling in LS on maternal aggression in mice.
Together then, a number of studies suggest GABAA receptor signaling in LS could regulate maternal aggression, but this relationship has never been directly tested. In this study, we investigate a role for GABAA receptor in LS in the regulation of offspring protection. We hypothesize that GABAA agonists, CDP and muscimol, will enhance maternal aggression when injected into the LS, whereas GABAA antagonist, bicuculline, will impair maternal aggression. Because LS (Sheehan, Chambers, & Russell, 2004) and GABAA receptor signaling (Bourin & Hascoet, 2003; Dean, 1965; Frye, Edinger, & Sumida, 2007; Griebel, Sanger, & Perrault, 1996; Liu et al., 2007; Raud et al., 2005) have been linked to anxiety control, in this study we also wanted to evaluate whether treatments affected anxiety-like behavior using the light-dark box. This study is designed to provide insight into the role of GABAA receptor signaling in LS on maternal aggression.
Materials and Methods
Animal Conditions
Female mice selected for high maternal aggression (Gammie, Garland, & Stevenson, 2006) and male outbred hsd: ICR (Harlan, Madison, WI) mice were used in this study. Mice were housed in polypropylene cages with access to tap water and breeder chow (for females) and regular chow (for males) ad libitum. Females were paired with a breeder male and after 10 days, the male was removed from the cage. The day after birth (postpartum day 1), litters were culled to eleven pups. Outbred, sexually naïve male mice were grouped housed and were used as intruders. Intruder males were never used more than once per day and were used for ∼ 3 tests each. Cages were changed weekly prior to parturition after which they were unchanged for the remainder of the experiment. All animals were housed on a 14:10 light/dark cycle with lights on at 06:00 CST. All procedures followed the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the University of Wisconsin.
Cannulae Surgeries
On postpartum day (PPD) 3, under isoflurane anesthesia and using a stereotaxic apparatus (David Kopf Instruments, Tunjunga, CA), a midline incision across the top of the skull was made. Prior to the cut, the hair above the skull was shaved and the skin cleaned and treated with alcohol and Betadine (Purdue Frederick, Stamford, CT). Sedation from anesthesia was determined by a lack of movement in response to a foot and tail pinch. Based on chemoarchitecture and connections, LS has been divided into rostral, and caudal regions (Risold & Swanson, 1997a, 1997b). The Paxinos mouse brain atlas divides LS into dorsal, ventral, and intermediate (LSi) regions (Paxinos & Franklin, 2001), which is more strictly based on gross anatomy. In the present study we focused our cannulae placements on LSi in the rostral portion as determined by these two classifications. We targeted this portion of LS based on our previous work with CDP (Lee & Gammie, 2007). For LS unilateral injections, a small hole was drilled +0.45 mm posterior and ± 0.14 mm lateral to Bregma. In the hole, a 26 gauge stainless-steel indwelling cannula (Plastics One, Roanoke, VA) was implanted to -2.0 mm below the skull surface. In some cases, the cannula hit medial septum (MS) or lateral ventricle, and was analyzed separately. Two small screws were drilled into the skull lateral and anterior to the guide cannula. Each cannula was secured to the skull using dental cement (Plastics One). A dummy cannula was inserted to maintain patency until injections were made. Injections were made using a 33 gauge stainless-steel injector attached to PE-50 tubing (Becton Dickenson, Sparks, MD) fitted to a 10 μL Hamilton syringe (Hamilton, Reno, NV). On PPD 3 and 4, a cannula was briefly put into the guide to prepare the tissue for later injections on test days. This approach was used to ensure more consistent conditions of tissue across test days. Before and after surgery, all animals received s.c. injections of ketoprofen (1 mg/kg) to minimize any discomfort.
Pharmacological Treatment and Site-Directed Injections
Separate groups were used for evaluating bicuculline methiodide (Sigma Chemical, St. Louis, MO), CDP hydrochloride (Sigma), and muscimol (Sigma). For any given group, vehicle and two doses of a drug were used. The following groups were used: vehicle, 3 ng and 7 ng bicuculline; vehicle, 15 ng and 30 ng bicuculline; vehicle, 2.5 μg and 5 μg CDP; vehicle, 0.05 and 0.5 ng muscimol; and vehicle, 1 ng and 5 ng muscimol. Within each group, a within-subjects repeated measures design was used whereby each animal received vehicle and the two drug treatments. Order of injections was always counterbalanced. This approach was used because if there were any effect of multiple injections or multiple behavioral testing, then those effects would be equally distributed across treatments. Final N's for the separate treatments and intra-LS injections are provided below. The bicuculline and muscimol doses were chosen based on previous literature (Arrati, Carmona, Dominguez, Beyer, & Rosenblatt, 2006; Hansen & Ferreira, 1986b; Numan et al., 2005; Salzberg, Lonstein, & Stern, 2002) and CDP doses were based on observations in the lab that intracerebroventricular (ICV) injections of 2.5 μg revealed a non-significant positive effect on maternal aggression. Beginning on PPD 6, single injections were delivered each day for up to 3 consecutive days to animals under light isoflurane anesthesia. All unilateral injections were made using 0.4 μL volume. Based on previous studies and histological analysis of our brain sections following injection of Chicago sky blue dye (see below), it was expected that with each site-specific injection, that the fluid would diffuse ∼ 200 μm from the injector tip and that there would be trend for some fluid to flow up the cannula (Henry, Vale, & Markou, 2006; Lohman, Liu, Morris, & O'Brien, 2005; Nicholson, 1985). Infusions were verified by following movement of an air bubble in the tubing and the cannula remained in place for 60 seconds following each injection. 20 minutes after injection, a female was exposed to a 5 minute aggression test, a 5 minute light-dark box test, and then observed for maternal behaviors for 30 minutes.
Behavioral Testing
All behavioral testing occurred between 0800 and 1200 hours on PPD 6, 7, and 8. In previous studies, we have found that maternal aggression and other maternal behaviors are expressed consistently across this time period (D'Anna & Gammie, 2006, 2009; D'Anna et al., 2005). Each test session was recorded on videotape and subsequently analyzed off-line to quantify maternal behaviors and stress behaviors by individuals blind to testing conditions. Total behavioral testing lasted for 40 min. To maintain consistency among scorers, all scorers had to pass a calibrator aggression test just prior to scoring. Further, each mouse was always evaluated by the same scorer to ensure that any lingering rater bias was equally spread across each treatment.
Maternal Aggression Testing
Twenty minutes following injection, females were moved into the testing room, and the pups were separated from the dam. A male intruder was placed into the female's cage for 5 minutes. Intruder mice from the same cage were used equally to test different treatments. Removal of pups from the home cage of a dam before an aggression test does not diminish the expression of maternal aggression in mice (Svare, Betteridge, Katz, & Samuels, 1981). For quantification of maternal aggression, the following features were measured: latency to first attack, number of attacks, and total duration of attacks (Gammie, Huang, & Nelson, 2000; Gammie & Nelson, 1999).
Light-Dark Box Testing
After the intruder male was removed, the female was exposed to a light-dark box test for 5 minutes. The apparatus consisted of a large box (30 × 24.5 × 25.5 cm) with a black plastic box in one half with an opening to connect the dark compartment (∼9.3 lux) to the light compartment (∼250 lux). At the beginning of the test, the female was placed directly in front of the opening to the dark box. For quantification of light–dark box test the following features were measured: latency to enter the dark compartment, number of transitions between light and dark compartments, and total duration spent in the light and dark compartments (Bourin & Hascoet, 2003).
Maternal Behavior Observations
Immediately after the light-dark box test, the female was placed back into her home cage, pups were scattered evenly away from the nest allowing the female to retrieve pups, and the female was allowed to perform maternal behaviors for 30 minutes. Pup retrieval was quantified by measuring the time elapsed to retrieve the first and fourth pup. Other maternal behaviors were surveyed every 30 seconds and quantified. Maternal behaviors include nursing (all forms, such as high and low arched - back nursing), licking/grooming of pups by the female, nest building activity, self-grooming, and time on and off nest. Latency to nursing, nest building, and grooming of pups, and rates of each maternal behavior were quantified in each observation.
Nissl Staining for Cannulae Placement
On PPD 9 and prior to brain collection, a 0.4 μL volume of 0.01% Chicago sky blue in saline was injected into the brain to verify cannula placement. Mice were anesthetized, decapitated and their brains were removed from the skull. Brains were postfixed overnight in 6% acrolein in phosphate buffered saline (PBS) and stored in 30% sucrose in PBS for 2 days. Brains were frozen on a platform and cut into 40 μm thick section using a sliding microtome (Leica, Microsystems, Heidelberg, Germany) and stored in cryoprotectant solution at -20 degrees C until processing for Nissl staining with Thionin. Brain sections were mounted, stained with Thionin, cover slipped, and images of the section were projected from an Axioskop Zeiss light microscope through an Axiocam Zeiss high-resolution digital camera attach to the microscope and interfaced with a computer.
Analysis of Maternal and Anxiety-like Behaviors
All behavioral testing variables were analyzed using a one-way repeated measures (RM) analysis of variance (ANOVA). In the cases where data were not normally distributed, a non-parametric Friedman RM ANOVA on Ranks test was performed. For maternal behavior proportions, if data were not normally distributed, data was transformed to 2arcsinsqrt(x) (Lehner, 1996), and then a one–way RM ANOVA was performed. If an overall significant effect of treatment was found, the subsequent Holm-Sidak (parametric) or Dunn's method (nonparametric) post hoc tests were performed. In the case of latency to first attack, if an animal was not aggressive, a time of 300 seconds (the maximum time of the test) was assigned. The following indicate sites of cannula placement and sample size for behavioral analysis (See Figure 1): low dose of bicuculline (3 ng and 7 ng) relative to vehicle: LS, n = 7; high dose of bicuculline (15 ng and 30 ng) relative to vehicle: LS, n = 7 (See note below); CDP (2.5 μg and 5 μg) relative to vehicle: LS, n = 8; low dose of muscimol (0.05 ng and 0.5 ng) relative to vehicle: LS, n = 5; high dose of muscimol (1 ng and 5 ng) relative to vehicle: LS, n = 5. For the high dose of bicuculline, four hits were within the defined rostral portion of LS and three additional hits to LS occurred in a slightly more caudal portion of LS. The aggression results were similar when analyzed separately, so these numbers were combined. In a few cases the hits to LS were near to MS and we cannot exclude the possibility of diffusion to MS. Additional injection sites outside LS are presented in the results section.
Figure 1.
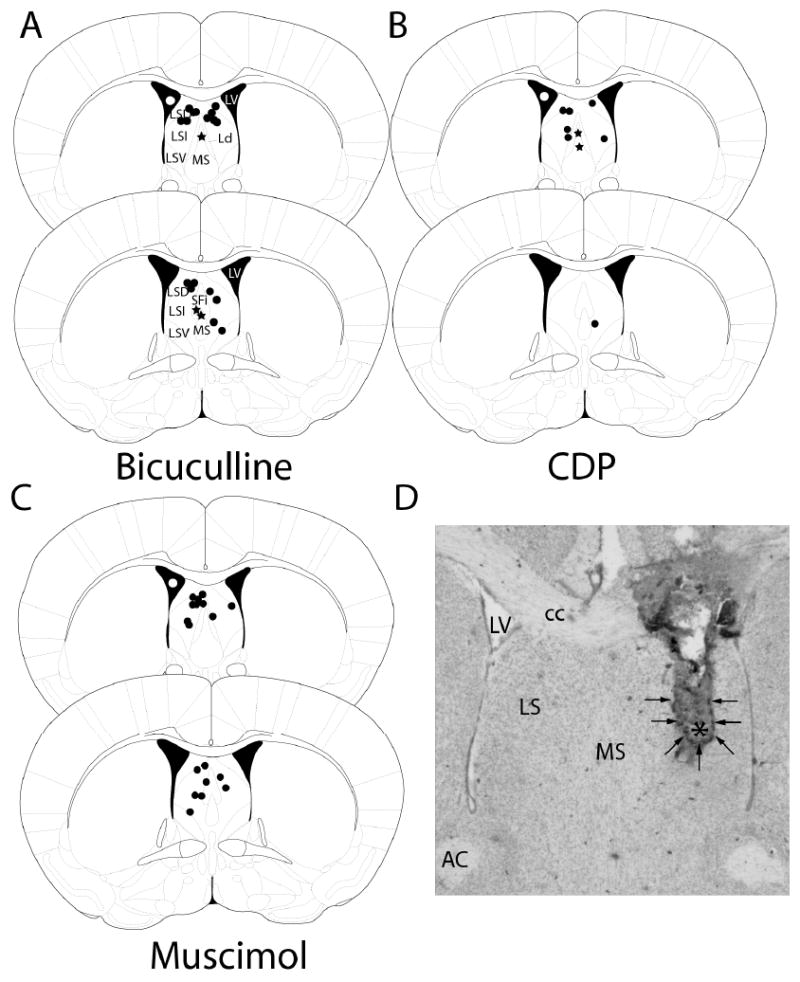
Cannulae placement for all treatment groups. Black dots represent the location of the tips of cannula injections in LS and stars represent the tips of cannula injections into MS for each treatment group. See Methods section for information on likely area of diffusion for each injection. White dots represents ventricular injections A: Cannula placement for bicuculline treatments. Ventricle injections = 20. B: Cannula placement for CDP treatments. Ventricle injections = 3. C: Cannula placement for muscimol treatments. Ventricle injections = 14. The two coronal brain sections represent Bregma 0.38 mm and 0.26 mm. Each injection was within 0.12 mm of the shown section. D: Representative photomicrograph of LS tissue following cannula implantation and injections. Darker staining in LS and arrows surrounding dark staining indicates spread of dye injection just prior to fixation (see Methods for details). Asterisk indicates approximate location of the tip of the cannula. Diagrams are adapted from The Mouse Brain in Stereotaxic Coordinates (2nd. Ed), G. Paxinos and K.B.J. Franklin, 2001. LV = lateral ventricle, LSD = lateral septum, dorsal, LSI = lateral septum, intermediate, LSV = lateral septum ventral, Ld = lambdoid septal zone, MS = medial septum, SFi = septofimbrial nucleus, CC = corpus callosum, AC = anterior commissure.
Results
Effects of Bicuculline Injections on Maternal Aggression
In LS injections, there was an overall significant effect of bicuculline on number of attacks in the low dose group (3 ng and 7 ng), F(2, 12) = 7.135, p = 0.009, RM ANOVA (See Figure 2A), and the high dose group (15 ng and 30 ng), F(2, 12) = 16.839, p < 0.001, RM ANOVA (See Figure 2A). Holm–Sidak post hoc tests revealed 7 ng, t(6) = 3.597, p = 0.004, 15 ng, t(6) = 4.161, p = 0.001, and 30 ng, t(6) = 5.584, p < 0.001, of bicuculline significantly decreased number of attacks relative to vehicle. Additionally, Holm–Sidak post hoc tests revealed 7 ng of bicuculline significantly decreased number of attacks relative to 3 ng of bicuculline, t(6)= 2.798, p = 0.016. Furthermore, one–way RM ANOVA revealed an overall significant effect of treatment in the high dose of bicuculline group on total time of aggression, χ2(2, 7) = 8.857, p = 0.008), RM ANOVA on ranks (See Figure 2B). Dunn's method post hoc tests indicated that 30 ng bicuculline, Q = 2.940, p < 0.05, significantly inhibited total time of aggression relative to vehicle. Total time of aggression was decreased by the lower doses of bicuculline, but was not significant, F(2, 12) = 3.054, p = 0.085, RM ANOVA (See Figure 2B). No differences on latency to first attack were found between vehicle and the low doses, F(2, 12) = 1.488, p = 0.265, RM ANOVA and the high doses showed a non-significant overall trend, F(2, 12) = 3.460, p = 0.065, RM ANOVA, of bicuculline (See Figure 2C).
Figure 2.
Effects of LS-bicuculline injection on maternal aggression. A: 7 ng, 15 ng, and 30 ng of bicuculline significantly decreased the mean of number attacks relative to vehicle and 7 ng of bicuculline significantly decreased the mean of numbers of attacks relative to 3 ng. B: Only 30 ng of bicuculline significantly lowered the mean total time spent being aggressive towards an intruder relative to vehicle. C: No treatments of bicuculline affected the mean latency to first bite. Bars represent means + SEM between treatments. * = p <0.05; ** = p ≤0.01; *** = p ≤0.001.
With ICV injections, the lower doses of bicuculline (3 ng and 7 ng) (n = 7) had no effect on either number of attacks, F(2, 12) = 0.601, p = 0.564, RM ANOVA, total time of aggression, F(2,12) = 0.726, p = 0.504, RM ANOVA, or latency to first attack, χ2(2, 7) = 8.222, p =0.079, RM ANOVA on ranks (Not shown). There was an overall significant effect of treatment with high doses (n = 13) on aggression expression in two behavioral measures: number of attacks, χ2 (2, 13) = 8.588, p = 0.014, RM ANOVA on ranks, and total time of aggression F(2, 24) = 12.989, p < 0.001, RM ANOVA (Not shown). Dunn's method post hoc tests revealed 30 ng significantly decreased the number of attacks relative to vehicle, Q = 2.648, p <0.05. The means and standard error for number of attacks are: vehicle: 18.2 ± 2.4, 15 ng: 8.8 ± 2.0, 30 ng: 6.8 ± 3,1. Holm-Sidak post hoc tests revealed a significant reduction of total time of aggression for 15 ng, t(12) = 4.470, p < 0.001, and 30 ng, t(12) = 4.356, p < 0.001, relative to vehicle. The means and standard error for total time of aggression are: vehicle: 37.6 ± 6.1, 15 ng: 10.6 ± 2.3, 30 ng: 11.3 ± 5.6. Latency to first attack was not affected by any of the high dose bicuculline treatments relative to vehicle, χ2(2, 13) = 3.640, p = 0.162, RM ANOVA on ranks.
In three mice, injections targeted to LS instead hit MS. Here, low doses of bicuculline had an overall significant affect on total time of aggression, F(2, 4) = 11.238, p = 0.023, RM ANOVA (Not shown). Holm-Sidak post hoc tests revealed an increase of total time of aggression in 7 ng of bicuculline relative to 3 ng of bicuculline, t(2) = 4.576, p = 0.010, and relative to vehicle, t(2) = 3.362, p = 0.028. Neither of the doses had significant effects on either number of attacks, F(2, 4) = 1.772, p = 0.281, RM ANOVA, or latency to first attack, F(2, 4) = 0.956, p = 0.458, RM ANOVA.
Effects of CDP Injections on Maternal Aggression
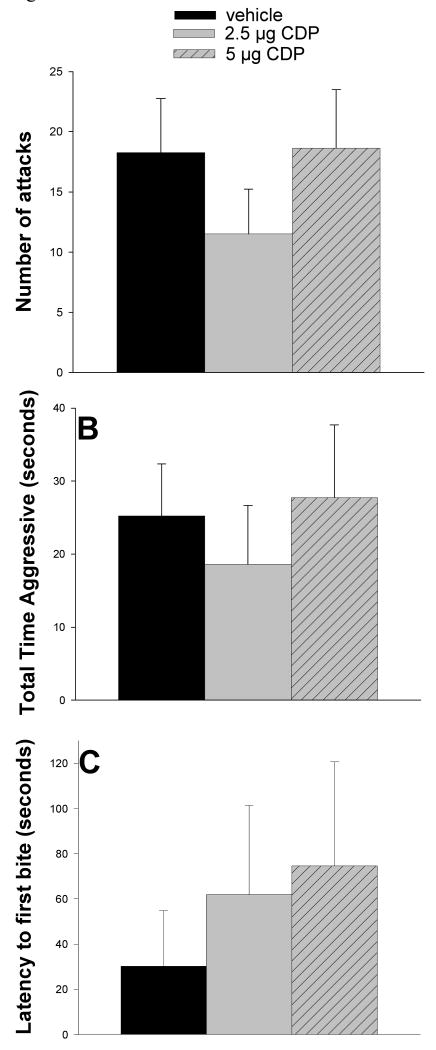
Intra-LS injections of CDP had no impact on any measure of aggression: number of attacks, F(2, 14) = 2.115, p = 0.158, RM ANOVA (See Figure 3A), total time of aggression, F(2, 14) = 1.705, p = 0.217, RM ANOVA (See Figure 3B), and latency to first attack, χ2(2, 8) = 0.519, p = 0.794, RM ANOVA on ranks (See Figure 3C).
Figure 3.
Effects of LS- CDP injection on maternal aggression. A: CDP had no significant effects on mean number of attacks. B: CDP had no influence on mean total time of aggression. C: CDP did not alter latency to first bite relative to vehicle. Bars represent means + SEM.
In addition, ICV injections of CDP (n = 3) did not affect number of attacks, F(2, 4) = 0.817, p = 0.504, RM ANOVA, or total time of aggression, F(2, 4) = 0.622, p = 0.582, RM ANOVA. However, there was a trend towards a longer latency to first attack with CDP, F(2, 4) = 6.649, p = 0.053, RM ANOVA.
Effects of Muscimol Injections on Maternal Aggression
Muscimol infused into LS had no influence on any of the three aggressive behavior measures. The mean levels of aggression for treatment and injection site are shown in Table 1.
Table 1.
Effects of LS-muscimol injection on maternal defense. No significant effects were found for any treatments on any measures.
| vehicle | 0.05 ng | 0.5 ng | vehicle | 1 ng | 5 ng | |
|---|---|---|---|---|---|---|
| ATT | 27.2±7.8 | 21.0+3.2 | 23.8±5.4 | 21.4±6.5 | 19.6±5.8 | 13.2±3.8 |
| AGG | 37.8±8.9 | 36.8±9.1 | 31.8±7.2 | 31.0±7.8 | 28.8±7.8 | 15.8±5.5 |
| FB Latency | 9.4±3.2 | 11.2±6.5 | 9.0±6.5 | 4.8±1.4 | 3.2±1.0 | 29.0±26.8 |
Mean (±SEM) of raw data in seconds.
ATT = number of attacks, AGG = total time aggression, FB latency = latency to first bite.
For ICV injections, there were no effects of muscimol in the low dose group (n = 10) in terms of latency to first bite, χ2(2, 10) = 0.743, p = 0.690, RM ANOVA on ranks, number of attacks, χ2(2, 10) = 4.421, p = 0.110, RM ANOVA on ranks, and total time of aggression, F(2,18) = 0.579, p = 0.571, RM ANOVA. Moreover, high doses (15 ng and 30 ng) of muscimol (n = 4) injected into the ventricle had no effect on latency to first attack, χ2(2, 4) = 2.000, p = 0.431, RM ANOVA on ranks, number of attacks, F(2, 6) = 1.086, p = 0.396, RM ANOVA, and total time of aggression, F(2, 6) = 1.287, p = 0.343, RM ANOVA.
Effects of Bicuculline, CDP, and Muscimol Injections on the Light-Dark Box Test
For intra-LS injections of bicuculline, CDP, and muscimol, no effect on light-dark box performance was found (See Supplemental Table 1), except for high doses of bicuculline. There was an overall significant difference on the number of transitions from light to dark compartments, F(2, 12) = 5.266, p = 0.023, RM ANOVA and total time spent in the light, F(2, 12) = 4.713, p = 0.031, RM ANOVA. Holm-Sidak post hoc tests revealed that 30 ng of bicuculline significantly decreased the number of transitions relative to vehicle, t(6) = 3.215, p = 0.007. Additionally, Holm-Sidak post hoc tests revealed that 30 ng of bicuculline significantly decreased the total time spent in the light compartment relative to 15 ng, t(6) = 3.029, p = 0.010.
For ICV injections, the only overall significant effect was for low dose bicuculline treatment on total time spent in the light compartment, F(2, 12) = 4.105, p = 0.044, RM ANOVA (Not shown). Holm-Sidak post hoc tests showed that subjects injected with 7 ng spend less time in the light compartment relative to vehicle, t(6) = 2.694, p = 0.020, and 3 ng of bicuculline, t(6) = 2.192, p = 0.049. For all other ICV injections, high doses of bicuculline did not affect total time in light (p = 0.337) or number of transitions (p = 0.065). High doses of ICV injections of muscimol did not affect either time in the light (p = 0.069), number of transitions (p = 0.069), or latency to enter the dark (p = 1.000). Low doses of ICV injections of muscimol did not affect total time in the light (p = 0.221), number of transitions (p = 0.516), or latency to enter the dark (p = 1.000). ICV injections of CDP did not affect total time spent in the light (p = 0.332), number of transitions (p = 0.174), or latency to enter the dark (p = 1.000).
Effects of Bicuculline, CDP, and Muscimol Injections on Maternal Behaviors
For intra-LS injections, neither bicuculline, CDP, nor muscimol had any effect on any of the maternal behaviors monitored (See Supplemental Table 2). However, there was a non–significant trend for CDP to decrease the latency to nurse, F(2, 14) = 3.158, p = 0.074, RM ANOVA, and the frequency of on-nest behavior, F(2, 14) = 3.164, p = 0.073, RM ANOVA. In addition, high doses of muscimol revealed a non–significant longer latency to lick/groom the pups, F(2, 6) = 4.101, p = 0.075, RM ANOVA.
ICV injections of low doses of bicuculline had an overall significant effect on latency to nurse, χ2(2, 7) = 8.222, p = 0.016, RM ANOVA on ranks (Not Shown). Dunn's method post hoc tests revealed that 7 ng decreased the latency to nurse relative to vehicle, Q = 2.806, p < 0.05. The means and standard error for the latency to nurse are: vehicle: 964.2 ± 158.1, 3 ng: 724.2 ± 185.7, 7 ng: 492.8 ± 55.4. Additionally, low doses of bicuculline infused into the ventricle had an overall significant effect on the latency to lick/groom the pups, F(2, 12) = 5.057, p = 0.026, RM ANOVA. Holm-Sidak post hoc tests revealed that 7 ng, t(6) = 3.042, p = 0.010, and 3 ng, t(6) = 2.323, p = 0.039, decreased the latency to lick/groom their pups relative to vehicle. The means and standard error for the latency to lick/groom the pups are: vehicle: 1658.5 ± 141.4, 3 ng: 1050.0 ± 257.9, 7 ng: 861.4 ± 269.8. Furthermore, ICV injection of low doses of bicuculline had an overall significant effect on lick/groom rates, F(2, 12) = 4.606, p = 0.033, RM ANOVA. Holm–Sidak post hoc tests revealed that 7 ng increased the frequency of licking/grooming the pups relative to vehicle, t(6) = 2.983, p = 0.011. The means and standard error of frequency for licking/grooming pups are: vehicle: 0.01 ± 0.01, 3 ng: 0.02 ± 0.007, 7 ng: 0.05 ± 0.02.
ICV injections of CDP, muscimol, and high doses of bicuculline did not alter maternal behaviors. Furthermore, there were no effects of intra-MS injections of CDP or low doses of bicuculline on maternal behaviors (Data not shown).
Discussion
In a recent study we found that CDP, a benzodiazepine, significantly elevated maternal aggression in mice while also decreasing Fos activity in LS and caudal periaqueductal gray (cPAG) (Lee & Gammie, 2007), suggesting that LS and/or cPAG were key sites for GABAA receptor modulation of maternal aggression. In addition, previous studies demonstrated a link between LS and maternal aggression (D'Anna & Gammie, 2009; Flannelly et al., 1986) and that GABAA receptor activity regulates maternal aggression (Arrati et al., 2006; Hansen & Ferreira, 1986b; Mos & Olivier, 1989; Olivier, Mos, & Oorschot, 1985; Palanza et al., 1996; Yoshimura & Ogawa, 1989), possibly via LS (Lee & Gammie, 2007). This is the first study to date to investigate directly a role for GABAA receptor activity in LS on maternal defense. The finding that antagonism of GABAA receptor by bicuculline (7 ng) significantly impaired number of attacks strongly supports a role for the GABAA receptor in LS in regulating maternal aggression. Importantly, 7 ng bicuculline inhibited aggression only when injected into LS, but not into neighboring regions or the ventricle, which highlights the role for LS in this response. At higher doses bicuculline impaired aggression when injected into either LS or the ventricle, but whether injections into ventricle were acting via LS alone is not known.
Although we used a wide range of doses, intra-LS injections of GABAA receptor agonists, CDP and muscimol did not elevate or alter aggression. One possible reason for a lack of agonist effect is a ceiling effect, whereby a baseline of GABAA receptor signaling exists in LS that promotes aggression and further increases in signaling do not alter the behavior. We used unilateral injections in this study in order to minimize tissue damage in LS and because in previous work we were able to modulate maternal aggression with unilateral LS injections (D'Anna & Gammie, 2009). However, it is possible that if we had used bilateral injections that an effect would have been found. It is also possible that activation of GABAA receptor in LS alone is not sufficient to elevate the behavior, but that increases in GABAA receptor activity in other regions, such as cPAG, are needed to obtain this response. The finding that inhibition of GABAA receptor activity in LS impairs aggression indicates that some GABAA receptor activity is needed for full maternal aggression expression.
GABA-positive neurons that release GABA into LS can be found both within LS and outside of LS (DeFrance, Yoshihara, McCrea, & Kitai, 1975; Fonnum, Walaas, & Iversen, 1977; McLennan & Miller, 1974; Panula, Revuelta, Cheney, Wu, & Costa, 1984). Although a high number of GABA-positive neurons are found within LS, many project outside of LS (Risold & Swanson, 1997a, 1997b), so it is unclear if these play a primary role in regulating LS activity via the GABAA receptors.
GABAA receptors, including those that are BDZ sensitive, are expressed at high levels in LS (Speth et al., 1980; Wisden et al., 1992). To understand the mechanism by which inhibition of GABAA receptors in LS reduces maternal aggression, it will be important to know the neuronal targets in LS that contain these receptors. However, to date this information is lacking although there are some interesting candidate neurons. LS projects to a number of brain regions, including PAG, amygdala, paraventricular nucleus, medial preoptic area, and MS (Deller, Leranth, & Frotscher, 1994; Risold & Swanson, 1996, 1997a, 1997b; Risold, Thompson, & Swanson, 1997; Sheehan et al., 2004). Interestingly, each of these regions has previously been linked to maternal aggression regulation (Arrati et al., 2006; Bosch, Sartori, Singewald, & Neumann, 2007; Consiglio, Borsoi, Pereira, & Lucion, 2005; Consiglio & Lucion, 1996; de Almeida, Giovenardi, da Silva, de Oliveira, & Stein, 2005; de Almeida & Lucion, 1997; Giovenardi, Padoin, Cadore, & Lucion, 1997; Hansen & Ferreira, 1986a; Lonstein & Stern, 1997, 1998; Lubin, Elliott, Black, & Johns, 2003; McGregor & Herbert, 1992; Olazabal & Ferreira, 1997). By inhibiting GABAA receptor activity in LS, bicuculline is expected to have a net excitatory effect on LS neuronal activity. One possibility is that the main targets of GABAA receptor inhibition in LS are GABA-positive projection neurons. In this scenario, there would be a depolarization of these GABA projection neurons that would lead to release of inhibitory neurotransmitter into these downstream brain regions linked to maternal aggression regulation. In future studies, it would be valuable to determine the extent to which GABA-positive projection neurons in LS also contain GABAA receptors.
We recently demonstrated that CRF and related peptides, urocortin 1 and 3, inhibit maternal aggression when infused into LS and that this action is dependent upon the CRF receptor 2 (D'Anna & Gammie, 2009). The neurons containing CRF receptor 2 in LS are not known, but it would be interesting if some of the target neurons in LS that respond to CRF-related peptides are GABA-positive projection neurons. In this scenario, CRF and bicuculline could both be inhibiting aggression by triggering an excitation of GABA-positive projection neurons, but other targets are possible.
Unexpectedly, the 3 mice that received intra–MS injections of 7 ng bicuculline showed significant increases in total duration of attacks relative to vehicle. Another study found that serotonin 1A agonist infused in MS significantly enhances maternal aggression (de Almeida & Lucion, 1997). Due to the small sample size in the current study, conclusions cannot be made. Although LS projects to MS (Risold & Swanson, 1997b) and MS project to LS (Sheehan et al., 2004), it is unclear whether maternal aggression is modulated via changes in signaling between these two regions. Our preliminary finding suggests it may be worthwhile to investigate further whether or how MS regulates maternal defense.
LS has been linked to regulating behavioral responses to stressors and here we wanted to evaluate whether any of our treatments altered anxiety-like behaviors as measured through the light–dark box test. Intra-LS 7 ng of bicuculline that decreased aggression did not alter any light-dark box measures. Therefore, the impairment of maternal aggression at this dose occurred without an alteration in anxiety, at least as evaluated by this one measure. In general, despite a wide range of treatments, only modest effects on light-dark box performance were found.
In terms of maternal behaviors, LS injections of bicuculline, CDP, and muscimol had no significant effects, suggesting GABA release in LS acting on the GABAA receptor may not play a role in regulation of maternal behaviors. This finding supports the idea that maternal aggression and other maternal behaviors use different neural pathways (Gammie, 2005). One interesting observation in this study is that ICV injections of low doses of bicuculline increased the frequency of licking/grooming of the pups. This result suggests that inhibition of GABAA receptor in the CNS can regulate the level of licking/grooming behavior in mothers. However, where in the CNS this action occurred is not clear. We recently found that ICV injections of the neuropeptide, hypocretin, increased levels of licking/grooming of pups in mice (D'Anna & Gammie, 2006), but whether ICV bicuculline modulates hypocretin signaling is not known. Our current study provides evidence that altered GABAA receptor activity within the CNS can promote the frequency of licking/grooming of the pups.
One possible issue is whether the results from mice selected for high maternal aggression are applicable to other mouse strains. We feel the results are applicable for the following reasons. One, these mice were derived from outbred hsd:ICR and we have been using within family selection to minimize inbreeding over time (Gammie et al., 2006). Thus, these mice still have high similarity to outbred mice. Two, we have regularly evaluated aggression in these females when they are non-lactating and as expected, they show little or no aggression. Therefore, the high aggression is specific to maternal aggression. Third, we have examined intermale aggression in these mice and found no changes relative to a control line (S. C. Gammie, unpublished observations), indicating again that the elevated aggression is specifically associated with maternal aggression and not general aggression levels. However, as for results from any given mouse strain, it is useful to be cautious in assuming our results will generalize from one strain to another.
From this study it is suggested that some level of GABAA receptor activity in LS is necessary for full expression of maternal aggression. In addition, the finding that manipulations of LS can alter defense without modifying other maternal behaviors further supports that GABAA receptor modulation in LS can specifically regulate maternal defense. To date this is the first study to link the GABAA receptor activity in LS with maternal aggression.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant R01 MH066086 to S.C.G. The authors wish to thank Terri Driessen, Katie Engh, Erin Griffith, Jessica Hilmelman, Jen Homer, Patrick Klevens, Claire Kostechka, Breann Kroll, Martin Lea, Kate Lentz, Caleigh Mandel – Brehm, Sarang Patel, Ashley Peterson, Derek Powell, Alexandra Ostromecki, Amy Toberman, and Kimberly D'Anna, for technical assistance and Kate Skogen and Jeff Alexander for animal care.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Arrati PG, Carmona C, Dominguez G, Beyer C, Rosenblatt JS. GABA receptor agonists in the medial preoptic area and maternal behavior in lactating rats. Physiology & Behavior. 2006;87:51–65. doi: 10.1016/j.physbeh.2005.08.048. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Sartori SB, Singewald N, Neumann ID. Extracellular amino acid levels in the paraventricular nucleus and the central amygdala in high- and low-anxiety dams rats during maternal aggression: regulation by oxytocin. Stress. 2007;10:261–270. doi: 10.1080/10253890701223197. [DOI] [PubMed] [Google Scholar]
- Bourin M, Hascoet M. The mouse light/dark box test. European Journal Pharmacology. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- Consiglio AR, Borsoi A, Pereira GA, Lucion AB. Effects of oxytocin microinjected into the central amygdaloid nucleus and bed nucleus of stria terminalis on maternal aggressive behavior in rats. Physiology & Behavior. 2005;85:354–362. doi: 10.1016/j.physbeh.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Consiglio AR, Lucion AB. Lesion of hypothalamic paraventricular nucleus and maternal aggressive behavior in female rats. Physiology & Behavior. 1996;59:591–596. doi: 10.1016/0031-9384(95)02117-5. [DOI] [PubMed] [Google Scholar]
- D'Anna KL, Gammie SC. Hypocretin-1 dose-dependently modulates maternal behaviour in mice. Journal of Endocrinology. 2006;18:1–14. doi: 10.1111/j.1365-2826.2006.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Anna KL, Gammie SC. Activation of corticotropin-releasing factor receptor 2 in lateral septum negatively regulates maternal defense. Behavioral Neuroscience. 2009;123:356–368. doi: 10.1037/a0014987. [DOI] [PubMed] [Google Scholar]
- D'Anna KL, Stevenson SA, Gammie SC. Urocortin 1 and 3 impair maternal defense behavior in mice. Behavioral Neuroscience. 2005;119:161–171. doi: 10.1037/0735-7044.119.4.1061. [DOI] [PubMed] [Google Scholar]
- de Almeida RM, Giovenardi M, da Silva SP, de Oliveira VP, Stein DJ. Maternal aggression in Wistar rats: effect of 5-HT2A/2C receptor agonist and antagonist microinjected into the dorsal periaqueductal gray matter and medial septum. Brazilian Journal of Medical & Biological Research. 2005;38:597–602. doi: 10.1590/s0100-879x2005000400014. [DOI] [PubMed] [Google Scholar]
- de Almeida RM, Lucion AB. 8-OH-DPAT in the median raphe, dorsal periaqueductal gray and corticomedial amygdala nucleus decreases, but in the medial septal area it can increase maternal aggressive behavior in rats. Psychopharmacology. 1997;134:392–400. doi: 10.1007/s002130050476. [DOI] [PubMed] [Google Scholar]
- Dean SR. Diazepam as an Adjuvant in Clinical Psychotherapy. Diseases of the Nervous System. 1965;26:181–183. [PubMed] [Google Scholar]
- DeFrance JF, Yoshihara H, McCrea RA, Kitai ST. Pharmacology of the inhibiton in the lateral septal region. Experimental Neurology. 1975;48:502–523. doi: 10.1016/0014-4886(75)90009-6. [DOI] [PubMed] [Google Scholar]
- Deller T, Leranth C, Frotscher M. Reciprocal connections of lateral septal neurons and neurons in the lateral hypothalamus in the rat: a combined phaseolus vulgaris-leucoagglutinin and Fluoro-Gold immunocytochemical study. Neuroscience Letters. 1994;168:119–122. doi: 10.1016/0304-3940(94)90430-8. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Picazo O, Uriarte N, Pereira M, Fernandez-Guasti A. Inhibitory effect of buspirone and diazepam, but not of 8-OH-DPAT, on maternal behavior and aggression. Pharmacology, Biochemistry, & Behavior. 2000;66:389–396. doi: 10.1016/s0091-3057(00)00211-2. [DOI] [PubMed] [Google Scholar]
- Flannelly KJ, Kemble ED, Blanchard DC, Blanchard RJ. Effects of septal-forebrain lesions on maternal aggression and maternal care. Behavioral & Neural Biology. 1986;45:17–30. doi: 10.1016/s0163-1047(86)80002-4. [DOI] [PubMed] [Google Scholar]
- Fonnum F, Walaas I, Iversen E. Localization of GABAergic, cholinergic and aminergic structures in the mesolimbic system. Journal Neurochemistry. 1977;29:221–230. doi: 10.1111/j.1471-4159.1977.tb09612.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Edinger K, Sumida K. Androgen Administration to Aged Male Mice Increases Anti-Anxiety Behavior and Enhances Cognitive Performance. Neuropsychopharmacology. 2008;33:1049–61. doi: 10.1038/sj.npp.1301498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC. Current models and future directions for understanding the neural circuitries of maternal behaviors in rodents. Behavioral and Cognitive Neuroscience Reviews. 2005;4:1–17. doi: 10.1177/1534582305281086. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Auger AP, Jessen HM, Vanzo RJ, Awad TA, Stevenson SA. Altered gene expression in mice selected for high maternal aggression. Genes, Brain, & Behavior. 2007;6:432–443. doi: 10.1111/j.1601-183X.2006.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC, D'Anna KL, Gerstein H, Stevenson SA. Neurotensin inversely modulates maternal aggression. Neuroscience. 2008;158:1215–1223. doi: 10.1016/j.neuroscience.2008.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC, D'Anna KL, Lee G, Stevenson SA. Role of corticotrophin releasing factor-related peptides in the neural regulation of maternal defense. In: Bridges RS, editor. Neurobiology of the Parental Brain. Oxford: Elsevier; 2008. pp. 103–114. [Google Scholar]
- Gammie SC, Garland T, Jr, Stevenson SA. Artificial selection for increased maternal defense behavior in mice. Behavior Genetics. 2006;36:713–722. doi: 10.1007/s10519-006-9071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC, Huang PL, Nelson RJ. Maternal aggression in endothelial nitric oxide synthase-deficient mice. Hormones and Behavior. 2000;38:13–20. doi: 10.1006/hbeh.2000.1595. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Negron A, Newman SM, Rhodes JS. Corticotropin-releasing factor inhibits maternal aggression in mice. Behavioral Neuroscience. 2004;118:805–814. doi: 10.1037/0735-7044.118.4.805. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Nelson RJ. Maternal aggression is reduced in neuronal nitric oxide synthase-deficient mice. Journal of Neuroscience. 1999;19:8027–8035. doi: 10.1523/JNEUROSCI.19-18-08027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovenardi M, Padoin MJ, Cadore LP, Lucion AB. Hypothalamic paraventricular nucleus, oxytocin, and maternal aggression in rats. Annals of the New York Academy of Sciences. 1997;807:606–609. doi: 10.1111/j.1749-6632.1997.tb51981.x. [DOI] [PubMed] [Google Scholar]
- Griebel G, Sanger DJ, Perrault G. Further evidence for differences between non-selective and BZ-1 (omega 1) selective, benzodiazepine receptor ligands in murine models of “state” and “trait” anxiety. Neuropharmacology. 1996;35:1081–1091. doi: 10.1016/s0028-3908(96)00080-9. [DOI] [PubMed] [Google Scholar]
- Hansen S, Ferreira A. Effects of bicuculline infusions in the ventromedial hypothalamus and amygdaloid complex on food intake and affective behavior in mother rats. Behavioral Neuroscience. 1986;100:410–415. doi: 10.1037//0735-7044.100.3.410. [DOI] [PubMed] [Google Scholar]
- Henry B, Vale W, Markou A. The effect of lateral septum corticotropin-releasing factor receptor 2 activation on anxiety is modulated by stress. Journal of Neuroscience. 2006;26:9142–9152. doi: 10.1523/JNEUROSCI.1494-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Gammie SC. GABA enhancement of maternal defense in mice: possible neural correlates. Pharmacology, Biochemistry, & Behavior. 2007;86:176–187. doi: 10.1016/j.pbb.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner PN. Handbook of ethological methods. 2nd. Cambridge; New York: Cambridge University Press; 1996. [Google Scholar]
- Liu GX, Cai GQ, Cai YQ, Sheng ZJ, Jiang J, Mei Z, Wang ZG, Guo L, Fei J. Reduced anxiety and depression-like behaviors in mice lacking GABA transporter subtype 1. Neuropsychopharmacology. 2007;32:1531–1539. doi: 10.1038/sj.npp.1301281. [DOI] [PubMed] [Google Scholar]
- Lohman RJ, Liu L, Morris M, O'Brien TJ. Validation of a method for localised microinjection of drugs into thalamic subregions in rats for epilepsy pharmacological studies. Journal of Neuroscience Methods. 2005;146:191–197. doi: 10.1016/j.jneumeth.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Gammie SC. Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neuroscience & Biobehavioral Reviews. 2002;26:869–888. doi: 10.1016/s0149-7634(02)00087-8. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Stern JM. Role of the midbrain periaqueductal gray in maternal nurturance and aggression: c-fos and electrolytic lesion studies in lactating rats. Journal of Neuroscience. 1997;17:3364–3378. doi: 10.1523/JNEUROSCI.17-09-03364.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS, Stern JM. Site and behavioral specificity of periaqueductal gray lesions on postpartum sexual, maternal, and aggressive behaviors in rats. Brain Research. 1998;804:21–35. doi: 10.1016/s0006-8993(98)00642-8. [DOI] [PubMed] [Google Scholar]
- Lubin DA, Elliott JC, Black MC, Johns JM. An oxytocin antagonist infused into the central nucleus of the amygdala increases maternal aggressive behavior. Behavioral Neuroscience. 2003;117:195–201. doi: 10.1037/0735-7044.117.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor A, Herbert J. Differential effects of excitotoxic basolateral and corticomedial lesions of the amygdala on the behavioural and endocrine responses to either sexual or aggression-promoting stimuli in the male rat. Brain Research. 1992;574:9–20. doi: 10.1016/0006-8993(92)90793-9. [DOI] [PubMed] [Google Scholar]
- McLennan H, Miller JJ. Gamma-aminobutyric acid and inhibition in the septal nuclei of the rat. Journal of Physiology. 1974;237:625–633. doi: 10.1113/jphysiol.1974.sp010501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mos J, Olivier B. RO 15-1788 does not influence postpartum aggression in lactating female rats. Psychopharmacology. 1986;90:278–280. doi: 10.1007/BF00181259. [DOI] [PubMed] [Google Scholar]
- Mos J, Olivier B. Quantitative and comparative analyses of pro-aggressive actions of benzodiazepines in maternal aggression of rats. Psychopharmacology. 1989;97:152–153. doi: 10.1007/BF00442238. [DOI] [PubMed] [Google Scholar]
- Mos J, Olivier B, van Oorschot R. Maternal aggression towards different sized male opponents: effect of chlordiazepoxide treatment of the mothers and d-amphetamine treatment of the intruders. Pharmacology, Biochemistry & Behavior. 1987;26:577–584. doi: 10.1016/0091-3057(87)90169-9. [DOI] [PubMed] [Google Scholar]
- Nicholson C. Diffusion from an injected volume of a substance in brain tissue with arbitrary volume fraction and tortuosity. Brain Research. 1985;333:325–329. doi: 10.1016/0006-8993(85)91586-0. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Schwarz JM, Neuner CM, Flood TF, Smith CD. Medial preoptic area interactions with the nucleus accumbens-ventral pallidum circuit and maternal behavior in rats. Behavioural Brain Research. 2005;158:53–68. doi: 10.1016/j.bbr.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Ferreira A. Maternal behavior in rats with kainic acid-induced lesions of the hypothalamic paraventricular nucleus. Physiology & Behavior. 1997;61:779–784. doi: 10.1016/s0031-9384(96)00567-7. [DOI] [PubMed] [Google Scholar]
- Olivier B, Mos J, Oorschot R. Maternal aggression in rats: Effects of chlordiazepoxide and fluprazine. Psychopharmacology. 1985;86:68–76. doi: 10.1007/BF00431686. [DOI] [PubMed] [Google Scholar]
- Palanza P, Rodgers RJ, Ferrari PF, Parmigiani S. Effects of chlordiazepoxide on maternal aggression in mice depend on experience of resident and sex of intruder. Pharmacology, Biochemistry & Behavior. 1996;54:175–182. doi: 10.1016/0091-3057(95)02109-4. [DOI] [PubMed] [Google Scholar]
- Panula P, Revuelta AV, Cheney DL, Wu JY, Costa E. An immunohistochemical study on the location of GABAergic neurons in rat septum. Journal of Comparative Neurology. 1984;222:69–80. doi: 10.1002/cne.902220107. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 2001. [Google Scholar]
- Raud S, Innos J, Abramov U, Reimets A, Koks S, Soosaar A, Matsui T, Vasar E. Targeted invalidation of CCK2 receptor gene induces anxiolytic-like action in light-dark exploration, but not in fear conditioning test. Psychopharmacology. 2005;181:347–357. doi: 10.1007/s00213-005-2255-x. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Structural evidence for functional domains in the rat hippocampus. Science. 1996;272:1484–1486. doi: 10.1126/science.272.5267.1484. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Chemoarchitecture of the rat lateral septal nucleus. Brain Research. Brain Research Reviews. 1997a;24:91–113. doi: 10.1016/s0165-0173(97)00008-8. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Connections of the rat lateral septal complex. Brain Research. Brain Research Reviews. 1997b;24:115–195. doi: 10.1016/s0165-0173(97)00009-x. [DOI] [PubMed] [Google Scholar]
- Risold PY, Thompson RH, Swanson LW. The structural organization of connections between hypothalamus and cerebral cortex. Brain Research. Brain Research Reviews. 1997;24:197–254. doi: 10.1016/s0165-0173(97)00007-6. [DOI] [PubMed] [Google Scholar]
- Salzberg HC, Lonstein JS, Stern JM. GABA(A) receptor regulation of kyphotic nursing and female sexual behavior in the caudal ventrolateral periaqueductal gray of postpartum rats. Neuroscience. 2002;114:675–687. doi: 10.1016/s0306-4522(02)00358-5. [DOI] [PubMed] [Google Scholar]
- Sheehan TP, Chambers RA, Russell DS. Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Research. Brain Research Reviews. 2004;46:71–117. doi: 10.1016/j.brainresrev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Speth RC, Johnson RW, Regan J, Reisine T, Kobayashi RM, Bresolin N, Roeske WR, Yamamura HI. The benzodiazepine receptor of mammalian brain. Federation Proceedings. 1980;39(12):3032–3038. [PubMed] [Google Scholar]
- Svare B, Betteridge C, Katz D, Samuels O. Some situational and experiential determinants of maternal aggression in mice. Physiology & Behavior. 1981;26(2):253–258. doi: 10.1016/0031-9384(81)90020-2. [DOI] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. Journal of Neuroscience. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura H, Ogawa N. Acute and chronic effects of psychotropic drugs on maternal aggression in mice. Psychopharmacology. 1989;97:339–342. doi: 10.1007/BF00439447. [DOI] [PubMed] [Google Scholar]
- Yoshimura H, Ogawa N. Ethopharmacology of maternal aggression in mice: effects of diazepam and SM-3997. European Journal of Pharmacology. 1991;200:147–153. doi: 10.1016/0014-2999(91)90677-i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.