Abstract
The intention of this review is to provide information about the rapidly evolving field of molecular imaging and its potential impact on the clinical practice of nuclear medicine. Upon completing this article the reader should be able to 1) define molecular imaging, 2) describe the ways in which molecular imaging can be used, 3) identify some of the biological processes that can be targeted with molecular imaging agents, and 4) list the modalities that can be used for molecular imaging along with the strengths and weaknesses of each.
INTRODUCTION
Molecular imaging has become something of a buzzword in recent years and often is portrayed as a key component of personalized medicine, itself another popular buzzword. SNM and its Molecular Imaging Center of Excellence (MICoE) have adopted an official definition of molecular imaging (1): “Molecular imaging is the visualization, characterization, and measurement of biological processes at the molecular and cellular levels in humans and other living systems.” The objective of this article is to elucidate what molecular imaging is, how it is done, and how it is used both clinically and in research. Molecular imaging comprises a range of techniques, spanning not only several imaging modalities but also many diseases and organ sites. Many of the examples presented in what follows focus on cancer, primarily because that is the research area in which the authors are most heavily involved.
HOW CAN MOLECULAR IMAGING BE USED?
Traditionally, the primary role of medical imaging has been to aid medical diagnosis through visualization of the presence, location, and extent of pathologies. Because molecular imaging techniques are capable of providing functional information at the cellular level, they offer the potential to move beyond mere identification and localization of diseased tissues to the characterization of the molecular processes involved.
The ability to characterize disease at the molecular level via imaging provides a powerful tool for clinical treatment planning as well as in the characterization of novel therapeutic regimens in the preclinical setting (clinical trial modeling). Increasingly, therapeutic strategies for oncology utilize pharmaceutics that directly inhibit the activity of specific molecular pathways. For example, there are currently numerous drugs in the clinic that are designed to inhibit epidermal growth factor receptor (EGFR), human epidermal growth factor receptor- type 2 (HER-2), and vascular endothelial growth factor receptor (VEGFR) signaling. These agents are frequently used as single agents or in combination with other molecularly targeted therapies and/or standard chemotherapy. The current clinical emphasis on molecularly targeted therapeutics underscores a key objective of personalized medicine; pairing the most appropriate treatment with each patient on an individualized basis. However, the enormous complexity of employing multi-agent, molecularly targeted therapeutic regimens to treat cancer increases the already considerable pressure upon clinical trialists and the pharmaceutical industry to develop and validate efficient and robust biomarkers for assaying the clinical and biological activity of these interventions. Molecular imaging can play a key role in this area.
Again using cancer as the example, knowing something about the molecular characteristics of the tumor prior to starting treatment decreases the chances that an ineffective therapy will be used. Molecular imaging can be used as a means of stratifying patients into those likely to respond to a molecularly targeted therapy from likely non-responders, such as identifying HER-2 overexpressing breast cancers for treatment with trastuzumab (Herceptin) (2). Similarly, baseline tumor [18F]-FES uptake, a validated measure of estrogen receptor expression (3), and metabolic flare assessed by [18F]-FDG PET following estradiol challenge are both predictive of responsiveness to endocrine therapy in ER+ breast cancer (4). By individualizing treatment plans in this way, the hope is that patient outcomes will be improved while at the same time controlling health care costs through better utilization of resources. While in certain cases such molecular information can be obtained through biopsy, there are many tumor sites, such as liver and brain, where biopsies are sufficiently risky that there is a clear preference for a less invasive readout. Furthermore, sampling errors can occur in biopsies and there also can be discordance in expression profiles between primary and metastatic tumor sites.
Another area of patient care in which molecular imaging has great potential is in the evaluation of treatment response. The current standard method for using imaging to assess treatment response in clinical cancer trials is the application of the RECIST criteria (Response Evaluation Criteria in Solid Tumors). This approach is based on the assessment of the largest linear dimension of a tumor as assessed by X-ray CT or MRI. Research has shown, however, that tumor shrinkage exhibits a considerable lag time after therapy, being preceded by changes in metabolism and cellular proliferation, for example, which can be detected through molecular imaging. Molecular imaging techniques also can be used to test whether specific pathways targeted by therapies have indeed been altered, such as probing angiogenesis via expression of the vascular-endothelial growth factor (VEGF) receptor.
Looking beyond the clinic, molecular imaging has become a key component of the modern drug development process. A major driver of the enormous costs of bringing a new drug to market is failure in late stages of the clinical trial process, and many pharmaceutical firms now incorporate molecular imaging with the objective of lowering these costs through earlier identification not only of drugs that are likely to be successful but also, more importantly, of those likely to fail, as halting those trials earlier leads to significant cost savings. There are a number of ways in which molecular imaging can be incorporated into drug development. One way is through direct labeling of the novel drug to turn it into a molecular imaging agent. This approach provides a useful means of assessing the pharmacokinetics (PK) of the drug. The ability to obtain biodistribution information using tracer levels of the drug, sometimes referred to as microdosing, can provide insight into potential toxicity concerns, while at the same time helping to verify that the drug reaches its target.
Another approach for investigating the properties of new drugs with molecular imaging is to use a radiotracer that shares a pathway or target with the drug in question as a means of determining the appropriate dosage and dosing schedule. Through kinetic modeling it is possible to determine the occupancy of the drug at the target site based on the degree of binding of the molecular imaging agent. Such studies employ molecular imaging to probe the relationship between receptor occupancy levels of the drug under investigation and clinically observable pharmacological effects at a given dosage. This data can be used to inform whether a drug under investigation has a sufficient safety margin with respect to possible toxicity or side effects at an efficacious dosage.
Another important area of drug development in which molecular imaging can play an important role is as a biomarker in clinical trials. The basic concept of the biomarker is to use some measurable quantity as an indicator of a biological process and its response to treatment. An example of such a biomarker is the use of cholesterol levels as a measure of risk for coronary artery disease. The advantage of biomarkers in drug development is that they can significantly shorten the length of time required to complete a clinical trial, since in many cases the recognized endpoints, such as five-year survival rate in cancer treatments, make the process slow and expensive. The SNM Clinical Trials Network was established recently to facilitate the incorporation of imaging biomarkers into multi-center clinical trials. In particular, this network will help in coordinating the production of the molecular imaging agents and standardizion of the imaging protocols across multiple sites to streamline the inclusion of molecular imaging in the development of new therapeutics. A key task in such studies is to validate that the imaging readout serves as a biomarker. However, once a particular biomarker has been validated it can be used in clinical trials for any therapies that share the same outcome for which that biomarker serves as surrogate.
Moving beyond drug development, molecular imaging currently plays an important role in a great deal of biomedical research. The ability to study cellular and molecular processes using these minimally invasive techniques can provide insight into the mechanisms of disease onset and progression. The coupling of molecular imaging with reporter genes and transgenic mouse models of human diseases enables scientists to probe molecular pathways in ways that can reveal not only the fundamental processes that characterize these diseases but also identify possible targets for diagnosis and treatment. While it remains to be seen whether molecular imaging will reach its full potential in the clinical realm, it already is well established as a critical tool in basic and translational research.
MOLECULAR IMAGING MODALITIES
While molecular imaging as an identifiable field of research is a fairly recent phenomenon, it turns out that much of nuclear medicine as it is practiced can be considered molecular imaging. Those radiotracers that target specific receptors (Octreotide), transporters (FP-CIT), or molecular processes (FDG) are considered molecular imaging agents. On first glance, then, the nuclear medicine practitioner may be left wondering what the big deal is. The relationship between nuclear medicine and molecular imaging is not a one-to-one match; however, for there are nuclear medicine procedures that are not molecular imaging, while molecular imaging techniques can be applied using imaging modalities other than PET and SPECT. An example of the former would be the measurement of pulmonary perfusion with [99mTc]MAA, where the radiotracer does not have a specific molecular target, but rather is designed to stay within the vasculature. In the following several paragraphs, we present examples of the latter in the form of brief descriptions of non-nuclear molecular imaging approaches. Table 1 provides an overview and rough comparisons of the major molecular imaging modalities.
TABLE 1.
Comparison of molecular imaging modalities
| Modality | Signal | C | R | Sensitivity # | Quantification | Acq. Time (sec) |
|---|---|---|---|---|---|---|
| PET | 11C, 18F, 64Cu, 68Ga | Y | Y | 1 | Very Good | 10s–100s |
| SPECT | 99mTc, 123I, 111In, 177Lu | Y | Y | 10−1 - 10−2 | Good | 100s–1000s |
| Fluorescence | Fluorescent proteins, fluorochromes, quantum dots |
P | Y | 10−2 -1 * | Poor to Fair* | 1–10 |
| BLI | Luciferase | N | Y | 1-102 * | Poor to Fair* | 1–10 |
| MRI | Gd, SPIO, USPIO, 19F | P | Y | 10−5 | Fair | 100s–1000s |
| MRS | Endogenous compounds, hyperpolarized 13C |
Y | Y | <10−5 | Fair | 100s–1000s |
| US | microbubbles | P | Y | ** | Poor | <1 |
C=Clinical, R=Preclinical (rodent), P=Potential;
Relative to PET;
Depth dependent;
Not well characterized
While magnetic resonance imaging (MRI) is generally thought of as offering anatomical information, there are a number of methods by which physiological and functional information can be obtained. An example of a physiological readout is blood flow assessed through dynamic contrast-enhanced MRI (DCE-MRI). Molecular imaging with MRI typically involves contrast agents that incorporate gadolinium or iron oxide in the form of superparamagnetic iron oxide (SPIO) or ultrasmall superparamagnetic iron oxide (USPIO). These materials alter the relaxation times of nearby water protons (gadolinium) or perturb the local magnetic field (iron oxide) thereby influencing the MR signal. Although MRI techniques can offer very good spatial resolution (around 1 mm for clinical applications and down to 0.1 mm in animal studies), a major drawback of molecular imaging using MRI is that the sensitivity (minimum detectable concentration of agent) is typically several orders of magnitude lower than that of standard nuclear imaging methods, which means that any receptors targeted by the contrast agent must be available in large numbers. Another difficulty in using MRI for molecular imaging is that quantitative measurements cannot be easily made. In nuclear medicine the signal is generated solely from the radiotracer, whereas the signal in MRI comes from a vast number of hydrogen protons, with the contrast agent altering the signal properties of some of these protons. Absolute quantification requires knowledge of both how the contrast agent alters signal and what the signal would have been in the absence of the contrast agent.
Another molecular imaging method that is closely related to MRI is magnetic resonance spectroscopy (MRS). MRS can be used to measure the relative abundance of endogenous compounds, removing the need for contrast agents entirely. MRS relies on the fact that the resonant frequency of a specific nuclear species (i.e., hydrogen protons) depends on its molecular environment, an effect known as the chemical shift. By measuring the strength of the MR signal while scanning across a suitable range of frequencies, it is possible to measure the relative abundance of certain molecules. When such measurements are conducted with appropriate pulse sequences, these signals can be confined to a specific region of the body and repeated measurements used to create spatial maps of molecular abundances (this is sometimes referred to as MRSI), although typically at much coarser spatial resolution than standard MRI. MRS measurements of major metabolites in the brain such as N-acetyl aspartate and choline can be useful in characterizing brain tumors (5).
There are other nuclei besides that of 1H that possess magnetic moments suitable for use in MRI/MRS. Some of these, for example 13C and 19F, are of sufficiently low natural abundance in the body that, when used as a label on an exogenous contrast agent, the in vivo distribution of that agent can be measured with minimal background. While this approach is similar to nuclear imaging of radiotracers, it suffers from lower sensitivity because the net magnetization that creates the signal arises from the roughly 1 part in 100,000 asymmetry in the populations of the energy levels (parallel and anti-parallel spin states). The sensitivity can be increased through the use of hyperpolarization to create a less even distribution of energy levels, thereby enhancing the magnetization. This hyperpolarization must be created outside the body, and in the case of 13C it has been used to label metabolic substrates, such as pyruvate, prior to injection (6). Through MR spectroscopy it is then possible to measure the rate of metabolic turnover, for example the conversion of pyruvate to lactate, since the 13C signal depends on the chemical state. While such approaches offer the potential to measure biochemical pathways in exquisite detail, the short lifetime of the magnetization enhancement from the hyperpolarization (tens of seconds or less) makes this challenging.
An important recent development in ultrasound (US) imaging has been the use of microbubbles as a contrast agent. These microbubbles are gas-filled (often perfluorocarbon) lipid shells that are highly echogenic due to the large acoustic impedance mismatch between blood/tissue and gas. The major application of microbubbles has been as a vascular contrast agent to assess blood flow and perfusion (7). More recently, several investigators have turned microbubbles into molecular imaging agents by functionalizing the bubbles through the addition of ligands to the shells (8). Because the microbubbles are relatively large (microns), their use is limited to vascular targets, such as the epidermal growth factor receptor. To acquire these images the microbubbles are injected intravenously and the ultrasound transducer is positioned to image microbubbles as they appear in the body region of interest. The real-time nature of ultrasound acquisition enables visualization of the delivery, accumulation, and washout of the microbubbles. Periodic ultrasound pulses of higher power can be used to collapse the bubbles within the field of view, allowing the uptake and binding of the microbubbles in that region to be imaged multiple times and potentially quantified.
Probably the most widely used imaging modality for preclinical molecular imaging is optical imaging. The optical imaging paradigm is very attractive for preclinical studies for numerous reasons, including throughput, sensitivity, ease of use, and overall cost. Optical imaging in vivo covers a range of techniques, but can be broken down at the highest level into fluorescence and bioluminescence imaging. Bioluminescence and fluorescence imaging are theoretically capable of similar levels of sensitivity, yet in practice, bioluminescence imaging typically exhibits a considerable sensitivity advantage (several hundreds to a few thousand cells) over fluorescence imaging (tens of thousands of cells) due its fundamental lack of background emission. Signal detection in all varieties of optical imaging is most commonly accomplished using a lens-coupled CCD.
Bioluminescence imaging (BLI) is based upon a biochemical reaction in which optical photons are created--the exact same process, in fact, by which fireflies create their characteristic glow on summer nights. Using molecular biology techniques researchers have isolated the gene, luciferase, responsible for this reaction and have been able to genetically engineer mammalian cells, such as tumor cells, to express this gene. A standard BLI study involves injecting luciferin, the substrate required for the reaction, into a subject in which luciferase expressing cells are present. When luciferin enters into a cell in which the luciferase enzyme is present, a chemical reaction involving enzyme, substrate, ATP, and oxygen produces a detectable photon. BLI provides a sensitive means of detecting the presence and location of the luciferase-expressing cells, making it useful for studying such things as tumor growth and metastases. An extension of this technique involves cloning the luciferase gene with the promoter region of another gene of interest. The amount of luciferase activity is then related to the expression level of the gene under study, enabling researchers to study temporal and environmental factors influencing gene expression. One example of this approach is the creation of transgenic mice expressing luciferase under the control of the mouse insulin promoter, enabling investigators to monitor beta cell function in models of diabetes using BLI (9). Although BLI is unlikely ever to find a clinical application, it is a powerful preclinical tool that is having enormous impact on many areas of research.
Fluorescence imaging can be performed using dyes, quantum dots, and even proteins (The 2008 Nobel Prize in Chemistry was awarded to Chalfie, Shimomura, and Tsien for the development of green fluorescent protein (GFP) for biomedical research). In all cases the image acquisition first involves the excitation of the agent with an external light source at the appropriate wavelength, followed by detection of the resulting photon emissions from the decay of the excited states. Like nuclear medicine, optical imaging can have a low background, but it has the added advantages of not involving ionizing radiation and offering the possibility of repeatedly obtaining signal from each molecule. The fluorescent (emissive) state can be accessed multiple times (although not infinitely many due to a process known as photobleaching), making it possible for an individual group of molecules to contribute to multiple images over the course of a study, in contrast to radiotracers in which each probe can contribute (at most) only once to an image when its radionuclide decays. Signal generation is tied to the application of an external light source and the lifetime of the excited states is quite short, meaning that the temporal resolution is quite good. The imaging instruments themselves are simple to use, and images can be acquired rapidly.
The major drawback to optical imaging arises from the strong absorption and scattering of photons at that wavelength scale. These effects limit the depth of penetration, result in modest, depth-dependent spatial resolution, and complicate quantification. The attenuation problem is particularly complicated because it impacts both the excitation of the signaling molecules and their emissions. Many researchers are working on methods for creating tomographic images using optical probes, but the fact that nearly all of the detected photons undergo multiple scattering within the object makes the reconstruction process an ill-posed inverse problem for which unique solutions are difficult to obtain.
While molecular imaging using optical methods will not be broadly applicable to clinical imaging due to the strong attenuation of optical photon wavelengths in tissue, there are some possible areas in which it may be viable. It may be possible to use fluorescent agents for imaging superficial sites, perhaps in identifying melanoma. Another arena in which such agents could be put to use is in surgery, particularly in the resection of tumors. A tumor-specific ligand labeled with a fluorescent dye could be injected prior to the surgery. Fluorescent imaging could then be done during the resection to identify tumor cells to ensure that none were left behind. One other possible area of clinical application for optical imaging involves coupling fluorescent probes with endoscopy. An example here would be the detection of colorectal cancer, where the use of an endoscope eliminates the limitation of depth of penetration of the fluorescence photons (10).
The specificity of targeted molecular imaging agents can make interpretation of the images challenging. The benefit of anatomical information for image interpretation has led to the rapid adoption of so-called hybrid imaging devices, combining PET or SPECT with CT, for example. An added benefit of the CT in these cases is the ability to use the anatomical information in carrying out attenuation and scatter correction, resulting in improved quantification. There is great interest in the molecular imaging community in multi-modality imaging approaches that extend beyond the addition of anatomical information. Combining molecular imaging readouts, for instance assessing glucose metabolism via [18F]FDG and apoptosis via [99mTc]Annexin V, provides a fuller picture of the disease state or its response to treatment. The emergence of hybrid PET/MRI systems will usher in new opportunities not only for integrating anatomical and molecular information but also for adding functional and physiological information to the mix. This enhanced integration may allow, for instance, the physiological response to a drug challenge to be monitored in concert with the displacement of a radiotracer by that drug.
MOLECULAR IMAGING AGENTS
Attributes common to all molecular imaging agents
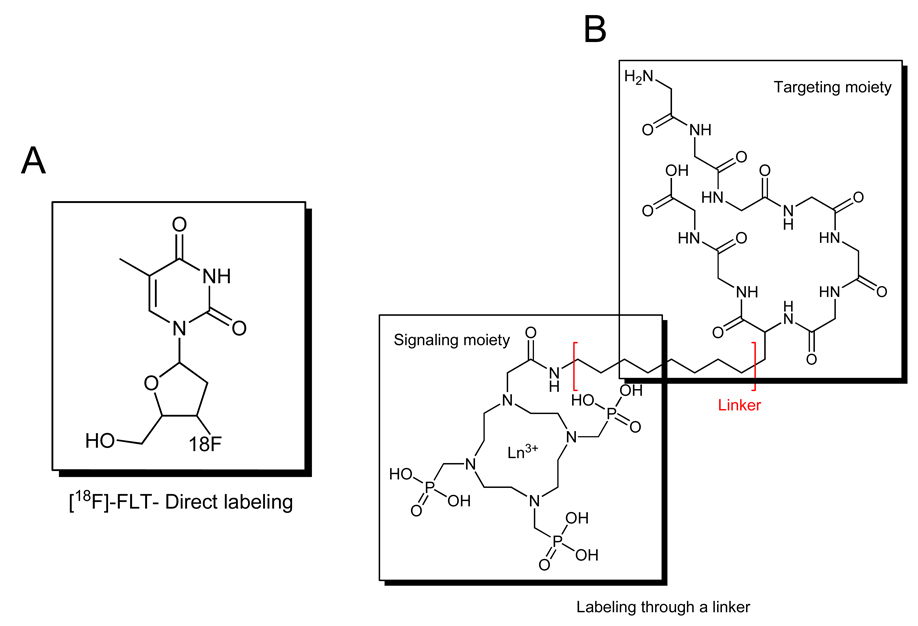
In the most general sense, all molecular imaging agents consist of the same basic features regardless of the imaging modality with which they are used. By definition, a molecular imaging agent targets a specific molecular entity or process and therefore must contain a targeting moiety or carrier. This is the portion of the molecular imaging agent that is responsible for directing the probe to the proper target. Additionally, all molecular imaging agents contain a signaling moiety or sensor. The signaling moiety is the actual species that is responsible for producing the signal that is detected by the imaging system. Common signaling moieties include radionuclides such as [18F] or [99mTc], fluorochromes, and microbubbles. In some cases, the targeting moiety can be directly labeled with signaling moieties, such as [18F] labeling of FLT (Fig. 1A). In other cases, it may be advantageous to separate the signaling moiety from the targeting moiety using a synthetic spacer or linker (Fig. 1B). This may be particularly important if labeling of the targeting species may in some way influence the binding of the imaging probe to the target. In either case, significant synthetic chemistry efforts are generally required to optimize the biological and physical properties of a molecule intended to serve as a molecular imaging agent.
Figure 1.
Examples of synthetic approaches to prepare molecular imaging agents. Direct labeling (A) and labeling through a linker (B) approaches illustrated.
It is important to emphasize that a specific targeting vector can be prepared by labeling it with any one of a number of different signaling moieties, or possibly more than one signaling moiety (11), creating the option of imaging the same target with different modalities. A good example of this are imaging probes based on Annexin V, which is commonly labeled with 99mTc for SPECT imaging or near infrared fluorochromes for optical imaging, but can also be easily labeled with 68Ga or 64Cu for PET imaging. Flexibility in probe chemistry is very attractive because it enables the users to easily tailor the imaging experiment and modality for specific purposes.
Biological processes measurable with molecular imaging agents
As noted above, advancement of the molecular imaging field is driven by 1) the development of improved imaging hardware for use in the preclinical and clinical settings, 2) identification and validation of new, biologically relevant imaging targets, and 3) the development of improved imaging probes derived from novel chemistries. Of these three essential facets which comprise a majority of current molecular imaging research, hardware development and novel target discovery significantly outpace the development and clinical advancement of new molecular imaging probes, particularly with respect to cancer imaging.
To date, a number of imaging probes have been described that aim to measure fundamental biological processes known to be disregulated in diseased tissues, such as tumors and other diseases, including glucose utilization, proliferation, apoptosis, hypoxia, and angiogenesis (12). Many of these imaging probes have been utilized fairly extensively in the preclinical setting and some to a lesser extent clinically, but in reality, the vast majority of molecular imaging in the clinic consists of assessment of glucose utilization using [18F]-fluorodeoxyglucose (FDG)-PET. Since regulation of glycolysis is a complicated process involving a number of biological factors capable of influencing the overall glycolytic pathway, FDG-PET studies must be interpreted with some caution because imaging results can vary significantly depending on the extent and type of disease as well as cellular responses to a therapeutic intervention. As noted above, given the current emphasis placed upon the development of sophisticated molecularly targeted and individualized therapeutic regimens for the treatment of cancer, expanding the oncologist’s imaging repertoire to include probes capable of reporting more specific molecular events and relevant downstream cellular physiology may be of considerable clinical importance. For these reasons, there is significant interest in the development and validation of additional novel imaging probes that have potential to contribute insights into the molecular biology of disease and therapeutic response that may extend beyond what can be learned with currently existing tracers such as FDG. We will focus our discussion of contemporary molecular imaging probes around several facets of relevant biology.
METABOLISM
By far, the most commonly used PET tracer for clinical molecular imaging is [18F]2-fluoro-2-deoxy-D-glucose (FDG) in oncology. FDG-PET exploits the typically increased glucose metabolism of tumor tissues compared to surrounding normal tissues. FDG is transported into tumor cells via glucose transport proteins (such as GLUT1) which tend to be upregulated in tumor cells. Once FDG is internalized, the tracer is phosphorylated to FDG-6-phosphate by an enzyme known as hexokinase. Unlike glucose-6 phosphate, FDG-6-phosphate does not enter glycolysis due to the presence of Fluorine substitution at the 2- position, and the tracer becomes metabolically trapped. Subsequent accumulation of FDG in metabolically active cells leads to imaging contrast. Some key drawbacks of FDG include non-specific accumulation in inflammation, as well as high background accumulation in highly metabolic tissues such as muscle and normal brain.
As an alternative to assessing glucose metabolism with FDG, amino acid-based tracers can be used to measure protein metabolism. Tracers such as O-(2-18F-fluoroethyl)-L-tyrosine [18F]-FET have been used with particular success in brain tumors (13) because unlike FDG these agents have little uptake in normal brain tissues. Amino acid-based tracers typically show little uptake in inflammatory lesions, making them an attractive alternative to FDG in some cases as well. A drawback of amino acid tracers is rapid metabolism and attendant radioactive metabolites present in blood and tissues, which can confound interpretation of imaging data.
PROLIFERATION
Noninvasive imaging approaches designed to longitudinally assess cellular proliferation potentially offer considerable advantages over invasive approaches that rely upon serial biopsy. For this reason, investigation into suitable methodologies for imaging proliferation has been undertaken by a number of groups. Historically, several PET tracers that are precursors for DNA synthesis have been explored and include 11C and 18F labeled nucleosides and structural analogues (14–16). One of the most promising nucleoside-based imaging probes described thus far has been 3’-deoxy-3’[18F]-fluorothymidine, [18F]-FLT (17–23). Theoretically, [18F]-FLT and other nucleoside-based tracers serve as surrogate markers of proliferation by reporting the activity of the thymidine salvage pathway, a cellular mechanism that utilizes uptake of deoxyribonucleosides from the extracellular environment to provide dividing cells with DNA precursors. Like other nucleosides such as thymidine, cytidine, and guanosine, [18F]-FLT is thought to be transported across the cell membrane by facilitated diffusion via low-affinity, non-concentrative nucleoside carrier proteins that are conserved across nearly all animal cells (24). Upon cellular internalization, [18F]-FLT is monophosphorylated in a reaction catalyzed by the cytosolic enzyme thymidine kinase 1 (TK1). Unlike thymidine, [18F]-FLT is not readily incorporated into DNA (25), yet phosphorylation to [18F]-FLT monophosphate results in intracellular trapping and subsequent accumulation. In many tissues, TK1 activity is regulated at transcriptional, translational, and post-translational levels (26) and activity tends to be closely correlated with the DNA synthesis phase of proliferating cells (typically late G1 through S). However, TK1 activity is typically diminished in quiescent, non-proliferating cells (17, 18, 27, 28). Many pre-clinical and clinical studies have been published since the late 1990’s exploring the utility of [18F]-FLT PET imaging as a quantitative metric to assess cellular proliferation in various species, tumor types, and organ sites (18, 20–22, 27, 29) and, although it is not yet FDA approved, use of this tracer is becoming more common clinically.
Cellular proliferation can also be estimated by measuring phospholipid metabolism with [11C]-choline. Choline imaging has been shown to be particularly useful in brain cancer and prostate cancer imaging where the increased activities of choline transporters and choline kinase are associated with increased cell membrane synthesis and proliferation. Similarly, [11C]-acetate, a precursor of fatty acid synthesis, can be used as a surrogate marker of metabolism and proliferation and has shown utility in prostate cancer and other diseases.
HYPOXIA
Solid tumors larger than a few millimeters in diameter rapidly outgrow their nutrient and oxygen supply. Poorly oxygenated tumor tissues rapidly become hypoxic. Hypoxia is known to play a role in angiogenesis, metastasis, and resistance to therapy. For this reason, molecular imaging assessment of hypoxia potentially could be of considerable importance. Two PET tracers, [18F]-fluoromisonidazole (FMISO) (30) and 64Cu-ATSM (31), have been evaluated rather extensively in various solid tumors. Both tracers have shown utility for measuring hypoxia, though additional validation studies are required and are underway at a number of institutions.
APOPTOSIS
In normal cells, programmed cell death or apoptosis, is a tightly regulated intracellular suicide program that is widely employed as a method for shedding redundant and/or dysfunctional cells. Cellular regulation of apoptosis proceeds via a complex cascade of intracellular signaling machinery that is conserved across a majority of animal cells. Specific molecular defects in apoptosis regulation are closely associated with numerous diseases including neurodegenerative disease and cancer. Considerable research has been undertaken to more closely understand apoptosis, particularly with respect to the adaptive mechanisms that many tumor cells utilize to maximize survival. Indeed, an impaired apoptosis program is a feature of many types of malignant tumor cells. Though the term ‘apoptosis’ itself was coined nearly 40 years ago by Kerr and colleagues (32) and an awareness of the general phenomenon was known for many years prior to that (33), numerous biological factors affecting the molecular regulation of apoptosis still require further characterization at the basic science level.
Significant efforts have gone into the development of non-invasive imaging methods to longitudinally assess apoptosis. Many of these efforts have focused on the use of Annexin-V, an endogenous, 36 kDa human protein that binds phosphatidyl serine (PS) with nanomolar affinity. Under the majority of normal circumstances, PS is restricted to the inner leaflet of the cell membrane lipid-bilayer. However, the early execution phase of apoptosis is closely associated with the redistribution and externalization of PS to the cell surface. This fact forms the basis of targeting PS with imaging probes as a metric to assess apoptosis.
Numerous clinical studies have demonstrated promising results with SPECT imaging of 99mTc-labeled Annexin-V to assess apoptosis in patients with cancer (34–39), myocardial infarction (40, 41), ischemic preconditioning (42), vulnerable atherosclerotic plaques (43), acute stroke (44, 45), and Alzheimer’s dementia (46). Additionally, fluorophore-labeled versions of Annexin-V have been utilized for flow cytometry and for imaging response to therapy in animal models (47, 48). For example, we recently reported use of a near-infrared dye labeled Annexin-V derivative to assess response to cetuximab therapy in mouse models of colorectal cancer (49). A limitation of measuring apoptosis as a treatment response outcome is that there are a number of other non-apoptotic forms of cell-death such as necrosis, autophagy, and mitotic catastrophe. Each of these non-apoptotic cell death mechanisms is inducible by therapeutics, with the consequence that imaging of apoptosis does not necessarily provide a complete picture of the effectiveness of a therapy in inducing cell death.
More exploratory methods to image apoptosis that may become more common in the future include assessment of caspase activity using peptides or small molecules as well as targeting the activity of cell surface death receptors.
RECEPTORS
A key area of molecular imaging research has historically been and continues to be based on imaging agents that bind to cell surface receptors with high specificity. PET imaging, with its high sensitivity and quantitative capability, is particularly powerful for such studies, especially those performed in the brain. Neuroreceptor imaging plays an important role in the diagnosis and study of neurodegenerative disorders such as Parkinson’s disease. Another major use of neuroreceptor imaging involves quantitative occupancy studies central to drug discovery for the treatment of psychiatric disorders. Additionally, many studies are conducted to evaluate the effects of drugs, including drugs of abuse such as methamphetamine and cocaine, on brain function (50). One of the more common targets of such neuroreceptor imaging studies has been the dopaminergic system, and numerous tracers such as [18F]-Fallypride (51) and [11C]-raclopride (52) exist for this purpose.
Molecular imaging of receptor expression is also important in oncology. In breast cancer, [18F]-Fluoroestrodiol (FES) has been shown to predict those patients who will respond to endocrine therapy (3, 53). Additionally, imaging probes targeting EGFR and HER-2 have been prepared and shown to have potential for the evaluation of receptor status in tumors (49, 54). [111In]-DTPA-D-Phe-octreotide is a routine clinical tool for evaluation of somatostatin receptor in neuroendocrine tumors, yet PET analogs bearing either 68Ga or 64Cu are also being evaluated to extend somatostatin receptor imaging to PET where sensitivity is somewhat better.
ANGIOGENESIS
Angiogenesis, or the formation and recruitment of new vasculature, is a highly orchestrated biological process that in healthy individuals is primarily confined to wound healing and reproduction. Dysregulated angiogenesis is a pathological condition and characteristic of a number of common diseases including diabetes, psoriasis, rheumatoid arthritis, and cancer (55). Angiogenesis plays a central role in the development and progression of tumors, as neovascularization is required to supply oxygen and nutrients to rapidly growing tumor cells and in turn facilitates the spread of metastases (56). Tumor induced angiogenesis is predominately driven by paracrine vascular endothelial growth factor (VEGF) signaling between tumor and/or stromal cells, which can secrete a variety of soluble VEGF ligands, and endothelial cells, which express tyrosine kinase VEGF receptors (57–59).
The VEGF family of receptors is an attractive class of imaging targets as they are primarily expressed on the surface of endothelial cells (60) which enables facile delivery of imaging compounds throughout the bloodstream. PET and SPECT, as well as optical imaging methods, such as fluorescence and bioluminescence techniques, possess the requisite sensitivity and are suitable modalities for studying angiogenesis (61). To this end, various VEGF receptor ligands have been labeled for PET/SPECT (62–70) and near-infrared (NIR) fluorescence imaging (71). Alternative approaches to angiogenesis imaging include the use of VEGF receptor targeted monoclonal antibodies (72) and RGD peptides labeled with radioisotopes and/or NIR dyes, which selectively bind integrins on tumor-associated endothelial cells.
SMART IMAGING AGENTS
An interesting option available to non-nuclear molecular imaging is the use of so-called ‘smart’ probes, also known as activatable probes. Smart molecular imaging agents can be synthesized such that their signaling properties respond to a variety of relevant tissue-based parameters, including pH, enzyme activity, and metal ion gradients. Of these, preclinical optical molecular imaging agents that become fluorescent upon ‘activation’ by a target enzyme are among the most prevalent (73, 74). The development of smart imaging probes for MRI has also been a fruitful area of research. Several prototypical compounds have been reported where portions of the Gd(III) complex serve as substrates for enzyme activity, and the resultant processing of the probe alters the relaxation efficiency of hydrogen protons. The most useful imaging probes demonstrate increased relaxivity upon enzymatic processing, and this is usually accomplished by improving water access to the paramagnetic center (75, 76). An alternative approach was recently suggested where probes were prepared such that following specific enzymatic hydrolysis, the agent was able to bind serum proteins, resulting in an in situ macromolecular agent with increased relaxivity (77).
MULTIPLE IMAGING READOUTS: POTENTIAL FOR IMPROVED CHARACTERIZATION
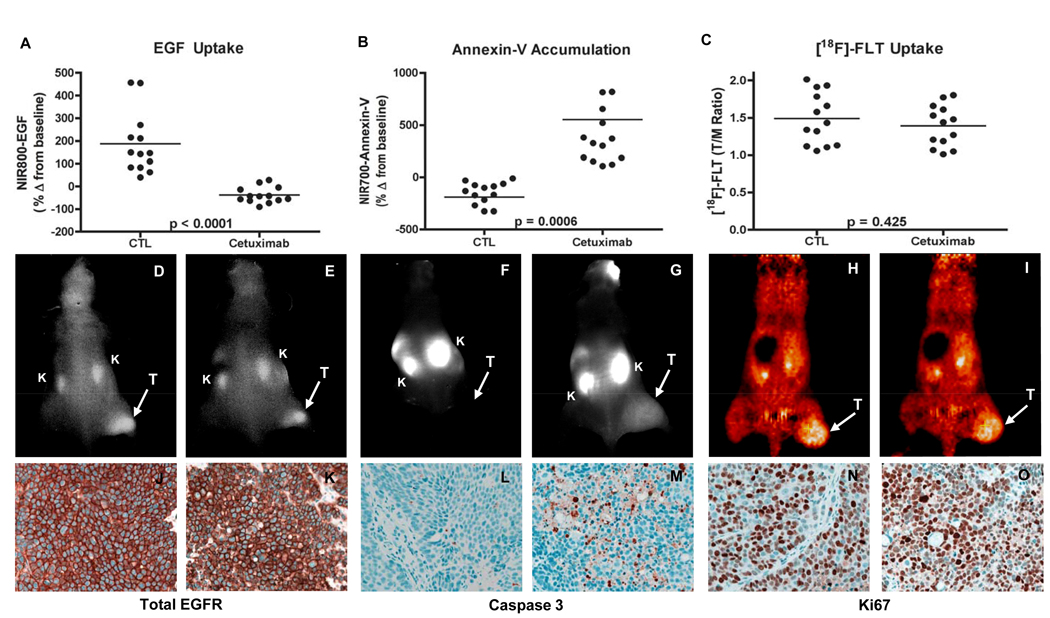
Most notably in the preclinical setting where optical imaging can be combined with PET and/or SPECT imaging, it is quite feasible to image more than one molecular event simultaneously (or nearly so). This capability has considerable potential to improve characterization of diseased tissues longitudinally in a single animal. This strategy is also highly useful for modeling complicated clinical dosing regimens in preclinical animal models. In the case of the latter, one could in theory discover, test, and optimize novel therapeutic regimens that affect cellular and molecular processes such as tumor-cell proliferation, angiogenesis, and apoptosis. Additionally, during the course of such studies, the most suitable imaging biomarkers can be advanced into the clinic together with the therapeutic regimen if so desired. For example, we recently demonstrated the utility of concomitant use of three molecular imaging metrics as potential biomarkers of treatment response to a molecularly targeted therapy employed in metastatic colorectal cancer (CRC) (49) and breast cancer (78). Each of the employed imaging metrics was selected to assess a unique and important aspect of anti-tumor therapeutic response. Specifically, we synthesized and validated an optical imaging probe to assess the molecular targeting and tumor-cell EGF receptor occupancy by cetuximab in vivo (NIR800-EGF), as well as a spectroscopically distinct optical imaging probe to assess the ensuing treatment-induced apoptosis (NIR700-Annexin-V). Additionally, changes in tumor proliferation occurring in response to cetuximab were assessed in vivo by [18F]-FLT PET imaging. Importantly, the combined non-invasive imaging data illustrate the potential to collect multiple relevant physiological readouts simultaneously in individual animals. This analysis paradigm revealed that cetuximab, in contrast to its effect on both apoptosis and proliferation in cetuximab-sensitive CRC cells in vitro, induced significant levels of tumor-cell apoptosis but was surprisingly ineffective at reducing tumor-cell proliferation in vivo (Fig. 2) (49). Our in vivo assessment of both cetuximab-sensitive (DiFi) and cetuximab-resistant (HCT-116) cell lines demonstrated that complementary molecular imaging techniques can provide important and accurate information on the biological effect of a therapy on the tumor. The tight correlation of these imaging measures with direct measurement of EGF binding, proliferation and apoptosis in the tumor tissue, the ability to detect these changes soon after and throughout dosing and the ability to obtain this information on a longitudinal, non-invasive basis represent very attractive features of molecular imaging.
Figure 2. Non-invasive imaging assessment of response to EGFR-blockade with cetuximab in colorectal cancer xenograft-bearing mice.
Treated and untreated cohorts bearing DiFi xenograft tumors were simultaneously imaged with NIR800-EGF, NIR700-Annexin-V and [18F]-FLT PET. Following cetuximab treatment, DiFi tumors exhibited significantly reduced NIR800-EGF uptake (A) and increased NIR700-Annexin-V uptake (B) compared to untreated controls (CTL). No statistical difference in [18F]-FLT uptake was observed between treated and untreated mice (C). Representative NIR800-EGF, NIR700-Annexin-V, and [18F]-FLT PET images collected from an individual control (D, F, and H) and treated (E, G, and I) mouse. “T” = tumor, “K” = kidney. Strong agreement between the imaging metrics of response and standard immunohistochemistry was observed. Tumors from control (J) and treated (K) animals exhibited similar levels of total EGFR. Treated animals (M) exhibited elevated caspase 3 staining compared to untreated cohorts (L). No discernible difference in Ki67 staining was observed between tumors from control (N) and treated cohorts (O). Figure taken from Clinical Cancer Research 14, 7413–7422, November 15, 2008, with permission.
Such multi-probe approaches currently can be applied clinically via sequential scanning of each molecular imaging agent individually. Dual-isotope SPECT offers the potential to conduct such studies simultaneously via the use of radionuclides with gamma-ray emissions whose energies can be resolved by the gamma camera. 99mTc and 123I are appealing choices for such dual-isotope studies, although the energy resolution of current NaI-based gamma cameras along with down-scatter of 159 keV 123I photons into the 99mTc energy window complicates these measurements. The development of PET/MR hybrid systems coupled with continued improvement of MRI molecular imaging agents may provide another option for true simultaneous readout of multiple molecular imaging probes in humans.
MOLECULAR IMAGING’S IMPACT ON CLINICAL PRACTICE
While most nuclear medicine departments, whether or not they were aware of it, have been engaging in molecular imaging already, the buzz surrounding molecular imaging stems from the paradigm shift that will accompany its full implementation into routine clinical practice. Realization of the full potential of molecular imaging will involve more than simply moving beyond FDG-PET to the incorporation of more PET and SPECT radiotracers. There will be a greater range of purposes served by imaging studies, extending beyond diagnosis to include stratification of patients into the most promising treatment regimen for them individually, as well as early monitoring of response to these personalized therapeutic regimens. The combination of increased specificity of molecular imaging agents and the desire to characterize pathologies to the fullest extent is expected to result in even greater importance for hybrid and multi-modality imaging approaches.
It remains to be seen what role non-nuclear methods of molecular imaging will play in clinical practice. PET and SPECT have definite advantages in sensitivity over competing approaches, but imaging modalities that do not involve ionizing radiation may be preferable in scenarios where multiple scans are performed, such as probing treatment response via comparison of pre- and post-treatment images. Cost, safety, and impact on clinical outcomes will all play a role in the determination of which molecular imaging agents and techniques enter into clinical use. It is also an open question whether the costs associated with the increased scanning implied by full utilization of the molecular imaging arsenal will lead to an overall reduction in healthcare costs through better utilization of all healthcare resources.
CONCLUSION
Molecular imaging already is having a profound impact on basic research and drug development that will influence clinical care independent of the translation of these molecular imaging approaches themselves into the clinic. Although molecular imaging appears to provide tools perfectly suited to personalized medicine, there remains some uncertainty as to which molecular imaging agents and techniques may become a standard part of clinical practice. In addition to the scientific aspect of the question, regulatory and reimbursement issues also are important factors influencing the evolution of molecular imaging. SNM has committed itself to “advancing molecular imaging and therapy”, and its recent creation of a Clinical Trials Network is intended to facilitate the incorporation of molecular imaging into the clinical drug trial process. A similar effort may also be needed to provide a framework through which molecular imaging agents themselves might find a path to FDA approval.
The MICoE has created a web site (www.molecularimagingcenter.org) that provides information and resources for imaging professionals, referring physicians, and patients. The interested reader will find there an extensive bibliography on molecular imaging including both review articles and the latest research breakthroughs.
ACKNOWLEDGMENTS
This work was supported in part by funding from U24 CA126588 (South-Easter Center for Small-Animal Imaging), 1R01 CA140628, K25 CA127349, and 1P50 CA128323. The work of Todd E. Peterson, Ph.D. is supported in part by a Career Award at the Scientific Interface from the Burroughs Wellcome Fund.
REFERENCES
- 1.Mankoff DA. A definition of molecular imaging. J Nucl Med. 2007 Jun;48(6):18N–21N. [PubMed] [Google Scholar]
- 2.Tolmachev V. Imaging of HER-2 overexpression in tumors for guiding therapy. Current pharmaceutical design. 2008;14(28):2999–3019. doi: 10.2174/138161208786404290. [DOI] [PubMed] [Google Scholar]
- 3.Peterson LM, Mankoff DA, Lawton T, et al. Quantitative imaging of estrogen receptor expression in breast cancer with PET and 18F-fluoroestradiol. J Nucl Med. 2008 Mar;49(3):367–374. doi: 10.2967/jnumed.107.047506. [DOI] [PubMed] [Google Scholar]
- 4.Dehdashti F, Mortimer JE, Trinkaus K, et al. PET-based estradiol challenge as a predictive biomarker of response to endocrine therapy in women with estrogen-receptor-positive breast cancer. Breast cancer research and treatment. 2009 Feb;113(3):509–517. doi: 10.1007/s10549-008-9953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Preul MC, Caramanos Z, Collins DL, et al. Accurate, noninvasive diagnosis of human brain tumors by using proton magnetic resonance spectroscopy. Nature medicine. 1996 Mar;2(3):323–325. doi: 10.1038/nm0396-323. [DOI] [PubMed] [Google Scholar]
- 6.Kohler SJ, Yen Y, Wolber J, et al. In vivo 13 carbon metabolic imaging at 3T with hyperpolarized 13C-1-pyruvate. Magn Reson Med. 2007 Jul;58(1):65–69. doi: 10.1002/mrm.21253. [DOI] [PubMed] [Google Scholar]
- 7.Wilson SR, Burns PN. Microbubble contrast for radiological imaging: 2. Applications. Ultrasound quarterly. 2006 Mar;22(1):15–18. [PubMed] [Google Scholar]
- 8.Lindner JR. Molecular imaging with contrast ultrasound and targeted microbubbles. J Nucl Cardiol. 2004 Mar–Apr;11(2):215–221. doi: 10.1016/j.nuclcard.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Park SY, Wang X, Chen Z, et al. Optical imaging of pancreatic beta cells in living mice expressing a mouse insulin I promoter-firefly luciferase transgene. Genesis. 2005 Oct;43(2):80–86. doi: 10.1002/gene.20157. [DOI] [PubMed] [Google Scholar]
- 10.Hsiung PL, Hardy J, Friedland S, et al. Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nature medicine. 2008 Apr;14(4):454–458. doi: 10.1038/nm1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sampath L, Kwon S, Ke S, et al. Dual-labeled trastuzumab-based imaging agent for the detection of human epidermal growth factor receptor 2 overexpression in breast cancer. J Nucl Med. 2007 Sep;48(9):1501–1510. doi: 10.2967/jnumed.107.042234. [DOI] [PubMed] [Google Scholar]
- 12.Wester HJ. Nuclear imaging probes: from bench to bedside. Clin Cancer Res. 2007 Jun 15;13(12):3470–3481. doi: 10.1158/1078-0432.CCR-07-0264. [DOI] [PubMed] [Google Scholar]
- 13.Stadlbauer A, Prante O, Nimsky C, et al. Metabolic imaging of cerebral gliomas: spatial correlation of changes in O-(2-18F-fluoroethyl)-L-tyrosine PET and proton magnetic resonance spectroscopic imaging. J Nucl Med. 2008 May;49(5):721–729. doi: 10.2967/jnumed.107.049213. [DOI] [PubMed] [Google Scholar]
- 14.Reinhardt MJ, Kubota K, Yamada S, Iwata R, Yaegashi H. Assessment of cancer recurrence in residual tumors after fractionated radiotherapy: a comparison of fluorodeoxyglucose, L-methionine and thymidine. J Nucl Med. 1997 Feb;38(2):280–287. [PubMed] [Google Scholar]
- 15.Shields AF, Mankoff D, Graham MM, et al. Analysis of 2-carbon-11-thymidine blood metabolites in PET imaging. J Nucl Med. 1996 Feb;37(2):290–296. [PubMed] [Google Scholar]
- 16.Shields AF, Mankoff DA, Link JM, et al. Carbon-11-thymidine and FDG to measure therapy response. J Nucl Med. 1998 Oct;39(10):1757–1762. [PubMed] [Google Scholar]
- 17.Been LB, Suurmeijer AJ, Cobben DC, Jager PL, Hoekstra HJ, Elsinga PH. [18F]FLT-PET in oncology: current status and opportunities. European journal of nuclear medicine and molecular imaging. 2004 Dec;31(12):1659–1672. doi: 10.1007/s00259-004-1687-6. [DOI] [PubMed] [Google Scholar]
- 18.Chen W, Cloughesy T, Kamdar N, et al. Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. J Nucl Med. 2005 Jun;46(6):945–952. [PubMed] [Google Scholar]
- 19.Choi SJ, Kim JS, Kim JH, et al. [F-18]3 '-deoxy-3 '-fluorothymidine PET for the diagnosis and grading of brain tumors. European journal of nuclear medicine and molecular imaging. 2005 JUN;32(6):653–659. doi: 10.1007/s00259-004-1742-3. [DOI] [PubMed] [Google Scholar]
- 20.Cobben DCP, Elsinga PH, van Waarde A, Jager PL, et al. Correspondence re: H. Barthel et al., 3 '-deoxy-3 '-[F-18]fluorothymidine as a new marker for monitoring tumor response to antiproliferative therapy in vivo with positron emission tomography. Cancer Res., 63: 3791–3798,2003. Cancer research. 2003 DEC 1;63(23):8558–8559. [PubMed] [Google Scholar]
- 21.Dittmann H, Dohmen BM, Paulsen F, et al. [18F]FLT PET for diagnosis and staging of thoracic tumours. European journal of nuclear medicine and molecular imaging. 2003 Oct;30(10):1407–1412. doi: 10.1007/s00259-003-1257-3. [DOI] [PubMed] [Google Scholar]
- 22.Grierson JR, Schwartz JL, Muzi M, Jordan R, Krohn KA. Metabolism of 3 '-deoxy-3 '-[F-18]fluorothymidine in proliferating A549 cells: validations for positron emission tomography. Nuclear medicine and biology. 2004 OCT;31(7):829–837. doi: 10.1016/j.nucmedbio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 23.machulla HJ, Blocher A, Wei R, Whrlichmann W, Kuntzsch M, Solbach C, Dohmen BM, Reischl G. Procedure for routine synthesis of [18F]FLT in high activities. Journal of Nuclear Medicine. 2001;42(5):257p. [Google Scholar]
- 24.Plagemann PG, Wohlhueter RM, Woffendin C. Nucleoside and nucleobase transport in animal cells. Biochimica et biophysica acta. 1988 Oct 11;947(3):405–443. doi: 10.1016/0304-4157(88)90002-0. [DOI] [PubMed] [Google Scholar]
- 25.Toyohara J, Waki A, Takamatsu S, Yonekura Y, Magata Y, Fujibayashi Y. Basis of FLT as a cell proliferation marker: comparative uptake studies with [3H]thymidine and [3H]arabinothymidine, and cell-analysis in 22 asynchronously growing tumor cell lines. Nuclear medicine and biology. 2002 Apr;29(3):281–287. doi: 10.1016/s0969-8051(02)00286-x. [DOI] [PubMed] [Google Scholar]
- 26.Arner ES, Eriksson S. Mammalian deoxyribonucleoside kinases. Pharmacology & therapeutics. 1995;67(2):155–186. doi: 10.1016/0163-7258(95)00015-9. [DOI] [PubMed] [Google Scholar]
- 27.Wagner M, Seitz U, Buck A, et al. 3 '-[F-18]fluoro-3 '-deoxythymidine ([F-18]-FLT) as positron emission tomography tracer for imaging proliferation in a murine B-cell lymphoma model and in the human disease. Cancer research. 2003 MAY 15;63(10):2681–2687. [PubMed] [Google Scholar]
- 28.Waldherr C, Mellinghoff IK, Tran C, et al. Monitoring antiproliferative responses to kinase inhibitor therapy in mice with 3'-deoxy-3'-18F-fluorothymidine PET. J Nucl Med. 2005 Jan;46(1):114–120. [PubMed] [Google Scholar]
- 29.Choi SJ, Kim JS, Kim JH, et al. [18F]3'-deoxy-3'-fluorothymidine PET for the diagnosis and grading of brain tumors. European journal of nuclear medicine and molecular imaging. 2005 Jun;32(6):653–659. doi: 10.1007/s00259-004-1742-3. [DOI] [PubMed] [Google Scholar]
- 30.Rajendran JG, Mankoff DA, O'Sullivan F, et al. Hypoxia and glucose metabolism in malignant tumors: evaluation by [18F]fluoromisonidazole and [18F]fluorodeoxyglucose positron emission tomography imaging. Clin Cancer Res. 2004 Apr 1;10(7):2245–2252. doi: 10.1158/1078-0432.ccr-0688-3. [DOI] [PubMed] [Google Scholar]
- 31.Lewis JS, McCarthy DW, McCarthy TJ, Fujibayashi Y, Welch MJ. Evaluation of 64Cu-ATSM in vitro and in vivo in a hypoxic tumor model. J Nucl Med. 1999 Jan;40(1):177–183. [PubMed] [Google Scholar]
- 32.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. British journal of cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annual review of biochemistry. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 34.Blankenberg FG. Molecular imaging: The latest generation of contrast agents and tissue characterization techniques. Journal of cellular biochemistry. 2003 Oct 15;90(3):443–453. doi: 10.1002/jcb.10635. [DOI] [PubMed] [Google Scholar]
- 35.Haas RL, de Jong D, Valdes Olmos RA, et al. In vivo imaging of radiation-induced apoptosis in follicular lymphoma patients. International journal of radiation oncology, biology, physics. 2004 Jul 1;59(3):782–787. doi: 10.1016/j.ijrobp.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 36.Kartachova M, Haas RL, Olmos RA, Hoebers FJ, van Zandwijk N, Verheij M. In vivo imaging of apoptosis by 99mTc-Annexin V scintigraphy: visual analysis in relation to treatment response. Radiother Oncol. 2004 Sep;72(3):333–339. doi: 10.1016/j.radonc.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Loose D, Vermeersch H, De Vos F, Deron P, Slegers G, Van de Wiele C. Prognostic value of (99m)Tc-HYNIC Annexin-V imaging in squamous cell carcinoma of the head and neck. European journal of nuclear medicine and molecular imaging. 2008 Jan;35(1):47–52. doi: 10.1007/s00259-007-0577-0. [DOI] [PubMed] [Google Scholar]
- 38.Rottey S, Loose D, Vakaet L, et al. 99mTc-HYNIC Annexin-V imaging of tumors and its relationship to response to radiotherapy and/or chemotherapy. Q J Nucl Med Mol Imaging. 2007 Jun;51(2):182–188. [PubMed] [Google Scholar]
- 39.Vermeersch H, Ham H, Rottey S, et al. Intraobserver, interobserver, and day-to-day reproducibility of quantitative 99mTc-HYNIC annexin-V imaging in head and neck carcinoma. Cancer biotherapy & radiopharmaceuticals. 2004 Apr;19(2):205–210. doi: 10.1089/108497804323071986. [DOI] [PubMed] [Google Scholar]
- 40.Kietselaer BL, Reutelingsperger CP, Boersma HH, et al. Noninvasive detection of programmed cell loss with 99mTc-labeled annexin A5 in heart failure. J Nucl Med. 2007 Apr;48(4):562–567. doi: 10.2967/jnumed.106.039453. [DOI] [PubMed] [Google Scholar]
- 41.Thimister PW, Hofstra L, Liem IH, et al. In vivo detection of cell death in the area at risk in acute myocardial infarction. J Nucl Med. 2003 Mar;44(3):391–396. [PubMed] [Google Scholar]
- 42.Rongen GA, Oyen WJ, Ramakers BP, et al. Annexin A5 scintigraphy of forearm as a novel in vivo model of skeletal muscle preconditioning in humans. Circulation. 2005 Jan 18;111(2):173–178. doi: 10.1161/01.CIR.0000151612.02223.F2. [DOI] [PubMed] [Google Scholar]
- 43.Kietselaer BL, Reutelingsperger CP, Heidendal GA, et al. Noninvasive detection of plaque instability with use of radiolabeled annexin A5 in patients with carotid-artery atherosclerosis. The New England journal of medicine. 2004 Apr 1;350(14):1472–1473. doi: 10.1056/NEJM200404013501425. [DOI] [PubMed] [Google Scholar]
- 44.Blankenberg FG, Kalinyak J, Liu L, et al. 99mTc-HYNIC-annexin V SPECT imaging of acute stroke and its response to neuroprotective therapy with anti-Fas ligand antibody. European journal of nuclear medicine and molecular imaging. 2006 May;33(5):566–574. doi: 10.1007/s00259-005-0046-6. [DOI] [PubMed] [Google Scholar]
- 45.Lorberboym M, Blankenberg FG, Sadeh M, Lampl Y. In vivo imaging of apoptosis in patients with acute stroke: correlation with blood-brain barrier permeability. Brain research. 2006 Aug 4;1103(1):13–19. doi: 10.1016/j.brainres.2006.05.073. [DOI] [PubMed] [Google Scholar]
- 46.Lampl Y, Lorberboym M, Blankenberg FG, Sadeh M, Gilad R, Annexin V. SPECT imaging of phosphatidylserine expression in patients with dementia. Neurology. 2006 Apr 25;66(8):1253–1254. doi: 10.1212/01.wnl.0000208436.75615.8c. [DOI] [PubMed] [Google Scholar]
- 47.Ohnishi S, Vanderheyden JL, Tanaka E, et al. Intraoperative detection of cell injury and cell death with an 800 nm near-infrared fluorescent annexin V derivative. Am J Transplant. 2006 Oct;6(10):2321–2331. doi: 10.1111/j.1600-6143.2006.01469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schellenberger EA, Weissleder R, Josephson L. Optimal modification of annexin V with fluorescent dyes. Chembiochem. 2004 MAR 5;5(3):271–274. doi: 10.1002/cbic.200300741. [DOI] [PubMed] [Google Scholar]
- 49.Manning HC, Merchant NB, Foutch AC, et al. Molecular imaging of therapeutic response to epidermal growth factor receptor blockade in colorectal cancer. Clin Cancer Res. 2008 Nov 15;14(22):7413–7422. doi: 10.1158/1078-0432.CCR-08-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Volkow ND, Wang GJ, Telang F, et al. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. NeuroImage. 2008 Feb 1;39(3):1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mukherjee J, Christian BT, Dunigan KA, et al. Brain imaging of 18F-fallypride in normal volunteers: blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse (New York, NY. 2002 Dec 1;46(3):170–188. doi: 10.1002/syn.10128. [DOI] [PubMed] [Google Scholar]
- 52.Farde L, Pauli S, Hall H, et al. Stereoselective binding of 11C-raclopride in living human brain--a search for extrastriatal central D2-dopamine receptors by PET. Psychopharmacology. 1988;94(4):471–478. doi: 10.1007/BF00212840. [DOI] [PubMed] [Google Scholar]
- 53.Linden HM, Stekhova SA, Link JM, et al. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. J Clin Oncol. 2006 Jun 20;24(18):2793–2799. doi: 10.1200/JCO.2005.04.3810. [DOI] [PubMed] [Google Scholar]
- 54.Eiblmaier M, Meyer LA, Watson MA, Fracasso PM, Pike LJ, Anderson CJ. Correlating EGFR expression with receptor-binding properties and internalization of 64Cu-DOTA-cetuximab in 5 cervical cancer cell lines. J Nucl Med. 2008 Sep;49(9):1472–1479. doi: 10.2967/jnumed.108.052316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995 Jan;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 56.Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989 May 4;339(6219):58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 57.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jun 15;161(2):851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 58.Folkman J, Merler E, Abernathy C, Williams G. Isolation of a tumor factor responsible for angiogenesis. J Exp Med. 1971 Feb 1;133(2):275–288. doi: 10.1084/jem.133.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989 Dec 8;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 60.Vaisman V, Gospodarowicz D, Neufeld G. Characterization of the receptors for vascular endothelial growth factor. J Biol Chem. 1990 Nov 15;265(32):19461–19466. [PubMed] [Google Scholar]
- 61.Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003 Mar 1;17(5):545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 62.Blankenberg FG, Backer MV, Levashova Z, Patel V, Backer JM. In vivo tumor angiogenesis imaging with site-specific labeled (99m)Tc-HYNIC-VEGF. European journal of nuclear medicine and molecular imaging. 2006 Jul;33(7):841–848. doi: 10.1007/s00259-006-0099-1. [DOI] [PubMed] [Google Scholar]
- 63.Blankenberg FG, Mandl S, Cao YA, et al. Tumor imaging using a standardized radiolabeled adapter protein docked to vascular endothelial growth factor. J Nucl Med. 2004 Aug;45(8):1373–1380. [PubMed] [Google Scholar]
- 64.Cai W, Chen K, Mohamedali KA, et al. PET of vascular endothelial growth factor receptor expression. J Nucl Med. 2006 Dec;47(12):2048–2056. [PubMed] [Google Scholar]
- 65.Chan C, Sandhu J, Guha A, et al. A human transferrin-vascular endothelial growth factor (hnTf-VEGF) fusion protein containing an integrated binding site for (111)In for imaging tumor angiogenesis. J Nucl Med. 2005 Oct;46(10):1745–1752. [PubMed] [Google Scholar]
- 66.Collingridge DR, Carroll VA, Glaser M, et al. The development of [(124)I]iodinated-VG76e: a novel tracer for imaging vascular endothelial growth factor in vivo using positron emission tomography. Cancer Res. 2002 Oct 15;62(20):5912–5919. [PubMed] [Google Scholar]
- 67.Cornelissen B, Oltenfreiter R, Kersemans V, et al. In vitro and in vivo evaluation of [123I]-VEGF165 as a potential tumor marker. Nuclear medicine and biology. 2005 Jul;32(5):431–436. doi: 10.1016/j.nucmedbio.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 68.Li S, Peck-Radosavljevic M, Kienast O, et al. Iodine-123-vascular endothelial growth factor-165 (123I-VEGF165). Biodistribution, safety and radiation dosimetry in patients with pancreatic carcinoma. Q J Nucl Med Mol Imaging. 2004 Sep;48(3):198–206. [PubMed] [Google Scholar]
- 69.Lu E, Wagner WR, Schellenberger U, et al. Targeted in vivo labeling of receptors for vascular endothelial growth factor: approach to identification of ischemic tissue. Circulation. 2003 Jul 8;108(1):97–103. doi: 10.1161/01.CIR.0000079100.38176.83. [DOI] [PubMed] [Google Scholar]
- 70.Yoshimoto M, Kinuya S, Kawashima A, Nishii R, Yokoyama K, Kawai K. Radioiodinated VEGF to image tumor angiogenesis in a LS180 tumor xenograft model. Nuclear medicine and biology. 2006 Nov;33(8):963–969. doi: 10.1016/j.nucmedbio.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 71.Backer MV, Levashova Z, Patel V, et al. Molecular imaging of VEGF receptors in angiogenic vasculature with single-chain VEGF-based probes. Nat Med. 2007 Apr;13(4):504–509. doi: 10.1038/nm1522. [DOI] [PubMed] [Google Scholar]
- 72.Virostko J, Xie J, Hallahan DE, Arteaga CL, Gore JC, Manning HC. A Molecular Imaging Paradigm to Rapidly Profile Response to Angiogenesis-directed Therapy in Small Animals. Mol Imaging Biol. 2009 Jan 7; doi: 10.1007/s11307-008-0193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McIntyre JO, Fingleton B, Wells KS, et al. Development of a novel fluorogenic proteolytic beacon for in vivo detection and imaging of tumour-associated matrix metalloproteinase-7 activity. Biochemical Journal. 2004 FEB 1;377:617–628. doi: 10.1042/BJ20030582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McIntyre JO, Matrisian LM. Molecular imaging of proteolytic activity in cancer. Journal of cellular biochemistry. 2003 DEC 15;90(6):1087–1097. doi: 10.1002/jcb.10713. [DOI] [PubMed] [Google Scholar]
- 75.Louie AY, Huber MM, Ahrens ET, et al. In vivo visualization of gene expression using magnetic resonance imaging. Nature Biotechnology. 2000 MAR;18(3):321–325. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]
- 76.Moats RA, Fraser SE, Meade TJ. A "smart" magnetic resonance imaging agent that reports on specific enzymatic activity. Angewandte Chemie-International Edition in English. 1997 APR 18;36(7):726–728. [Google Scholar]
- 77.Nivorozhkin AL, Kolodziej AF, Caravan P, Greenfield MT, Lauffer RB, McMurry TJ. Enzyme-Activated Gd(3+) Magnetic Resonance Imaging Contrast Agents with a Prominent Receptor-Induced Magnetization Enhancement We thank Dr. Shrikumar Nair for helpful discussions. Angewandte Chemie (International ed. 2001 Aug 3;40(15):2903–2906. [PubMed] [Google Scholar]
- 78.Shah C, Miller TW, Wyatt SK, et al. Imaging biomarkers predict response to anti-HER2 (ErbB2) therapy in preclinical models of breast cancer. Clin Cancer Res. 2009 Jul 15;15(14):4712–4721. doi: 10.1158/1078-0432.CCR-08-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]