Abstract
Herein a water-soluble ‘click’ modified coumarin-based fluorescent probe for hydrogen peroxide is reported. This probe shows significant intensity increases (up to 5 fold) in near-green fluorescence upon reaction with hydrogen peroxide, and good selectivity over other reactive oxygen species.
Keywords: Hydrogen peroxide, reactive oxygen species (ROS), coumarin, click chemistry, fluorescence
As a relatively stable reactive oxygen species (ROS), hydrogen peroxide (H2O2) generation in cellular environments has been correlated with various pathophysiological conditions.1,2 Hydrogen peroxide is generated in response to various stimuli, including cytokines and growth factors, and is involved in cellular “redox” signaling in regulating many severe diseases, such as cancer and Parkinson’s and Alzheimer’s syndromes.3–5 Ample evidence also demonstrates that hydrogen peroxide generated by mitochondrial respiration is a potent inducer of oxidative damage and mediator of aging.6 Moreover, recent studies also indicated that hydrogen peroxide could mediate rapid wound detection in zebrafish.7 Although a number of reports have been published on the subject, the dual characters of adverse and beneficial effects of hydrogen peroxide generation in biological system are still poorly understood because of the limited availability of detection methods.8
So far several types of chemical probes based on fluorescein,9 coumarin,10 Amplex Red,11 and luciferin12, have been reported for the selective detection of hydrogen peroxide and some have been used for the detection of intracellular hydrogen peroxide levels.13 A recent review has summarized the current state of this field very well.14 Each of the reported probes has its own advantages and limitations in terms of issues such as membrane permeability, photostability, wavelength, ease of synthesis, synthetic modularity or lack of, and solubility. An ideal probe should remain non-fluorescent until interactions (chemical reaction or physical binding) with its target, have the appropriate physicochemical properties to allow for ready permeation across membrane barriers, are stable and have certain solubility in water. In addition, ease of synthesis and synthetic modularity are also important factors as the latter would allow for easy preparation of analogs with different chemical and spectroscopic properties and for incorporation of additional recognition moieties. Based on the ability for hydrogen peroxide to oxidatively convert arylboronates into phenols,15,16 we had previously reported a umbelliferone-based fluorescent probe for the detection of hydrogen peroxide.10 In this case, oxidative cleavage of the boronate moiety converts a non-fluorescent coumarin derivative to fluorescent umbelliferone, which signals hydrogen peroxide detection. The small size, high permeability, and easy synthetic manipulation of coumarin compounds make them very attractive as probes for ROS detection.17 However, this umbelliferone probe has a short excitation wavelength at 332 nm, which could result in photo-damage to labeled biomolecules and/or cells. Therefore, we were interested in the design and synthesis of new coumarin-based probes with a longer excitation wavelength. In addition, we were also interested in designing a system that allows for synthetic modularity and ligation of additional functional groups that could be used to manipulate the physicochemical properties, permeability, and spectroscopic properties of the system, and for tethering of a recognition moiety for site-specific delivery of the hydrogen peroxide probe. Recently, the labs of Wang and Fahrni reported that “click” modifications of coumarin could be used as a way to manipulate the spectroscopic properties of this fluorophore.18–20 In the work from the Wang lab, it was reported that 3-azido-7-hydroxy-coumarin was not fluorescent. However, “click” modification by reaction with an alkyne converts this compound to a very fluorescent product. By taking advantage of the electronic and spectroscopic changes associated with triazole ring formation after Cu(I)-catalyzed azide-alkyne click reaction,21–23 we designed compound 1, which showed improved spectroscopic properties with an increase of excitation wavelength by about 70 nm compared with the umbelliferone-based probe and afforded the possibility of tethering additional functional groups for targeted delivery and/or improved physicochemical properties.
Synthesis of the designed probe (1) started from 3-azido-7-hydroxycoumarin18 (3) as shown in Scheme 2. By reacting with phenylacetylene in the presence of Cu(I), triazole-conjugated coumarin (2) was obtained in over 70% yield.23 After conversion to the corresponding triflate (4) in over 80% yield palladium-mediated borylation using pinacol-protected diborate gave the final probe (1) in about 5% yield.24
Scheme 2.

Synthesis of compound 1
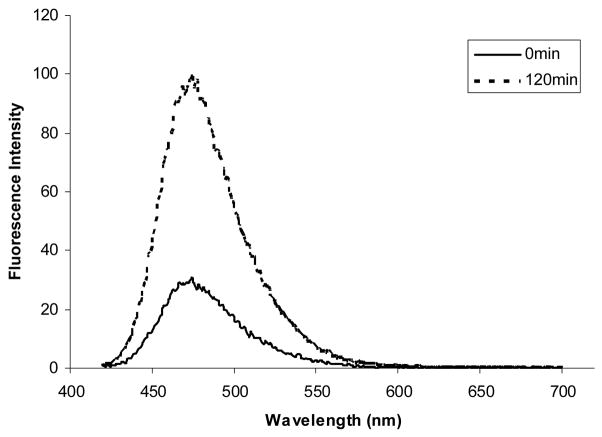
Compound 1 was evaluated for its ability to detect hydrogen peroxide under near physiological conditions (0.1 M phosphate buffer, pH 7.4). The probe itself (1) displays almost no background fluorescence. Addition of hydrogen peroxide triggered a very significant fluorescence intensity increase (around 5-fold) at 475 nm (Figure 1). The excitation wavelength (400 nm) was about 70 nm higher than that of the umbelliferone-based hydrogen peroxide probe reported earlier from our lab.10 NMR and MS experiments confirmed that hydroxylcoumarin (2) was the product after reaction of 1 with hydrogen peroxide.
Figure 1.
Fluorescence response of 5 μM compound 1 to 100 μM H2O2 after 120 min. The top and bottom spectra were recorded before and after H2O2 addition, respectively. Spectra were acquired in 0.1 M phosphate buffer, pH 7.4 (λex = 400 nm).
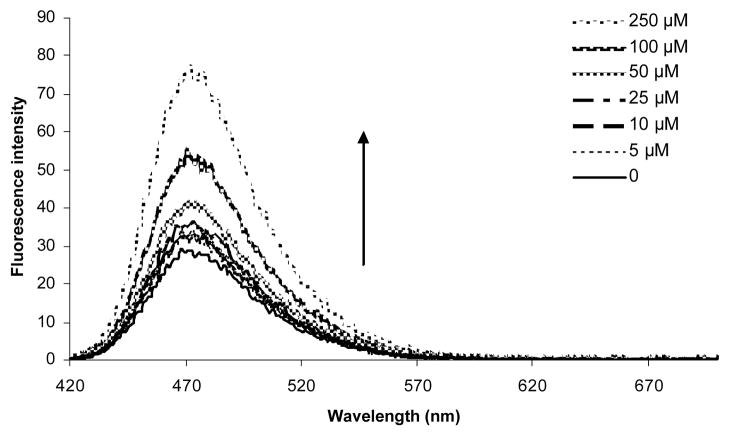
Concentration-dependent fluorescence response of compound 1 was also examined at 30 min after the addition of hydrogen peroxide (Figure 2). The fluorescence intensity of compound 1 increased significantly as a function of hydrogen peroxide concentration.
Figure 2.
Concentration-dependent emission intensity changes of compound 1 at room temperature: Experiments were conducted in 0.1 M phosphate buffer, pH 7.4 with excitation at 400 nm. The emission spectra were obtained at 30 min after the addition of hydrogen peroxide to a 5 μM solution of compound 1
Compound 1’s specificity in fluorescence response for hydrogen peroxide was examined.9,25–27 Figure 3 compares the relative reactivity of compound 1 towards various kinds of ROS at several time points over 120 min. Compound 1 exhibited a 2–4-fold higher responses to hydrogen peroxide over other ROS species (Figure 3). Specifically, the selectivity of this probe for hydrogen peroxide is more than 3-fold over hydroxyl radical (·OH), 2-fold over superoxide (O2−), 2-fold over hypochlorite (OCl−), and 3-fold over tert-butyl hydroperoxide (TBHP) and tert-butoxy radical (t-BuO·). It is important to note that hydroxyl radical (·OH), tert-butyl hydroperoxide (TBHP) and tert-butoxy radical (t-BuO·) did not induce concentration-dependent fluorescence intensity changes, suggesting that it might be impurities in these reagents that induced the low level response observed.
Figure 3.
Fluorescence response of compound 1 (5 μM) to various reactive oxygen species (ROS). Data shown are for 100 μM of all ROS reagents. Bar graph from left to right are for 0, 30, 60, 90, and 120 min data points. Hydrogen peroxide (H2O2), tert-butyl hydroperoxide (TBHP), and hypochlorite (OCl−) were diluted from 30%, 70% and 13% aqueous solutions, respectively. Superoxide (O2−) was added as solid KO2. Hydroxyl radical (·OH) and tert-butoxy radical (t-BuO·) were generated by reaction of 1 mM Fe2+ with 100 μM H2O2 or 100 μM TBHP, respectively. Spectra were acquired in 0.1M phosphate buffer, pH 7.4, and all data were obtained after incubation with the appropriate ROS ar room temperature. Emission intensity was collected at 475 nm (λex= 400 nm).
In conclusion, we have designed and synthesized a new water-soluble ‘click’ modified coumarin-based fluorescent probe, which shows large increases in fluorescent intensity upon reaction with hydrogen peroxide (around 5 fold) at 5 μM. The introduction of the triazole ring increased the excitation wavelength by about 70 nm. The probe (1) also shows good selectivity for hydrogen peroxide over other ROS. In addition, the probe has the advantage of easy synthesis from readily available inexpensive starting materials and can be adapted to include other functional groups through click chemistry for structural and spectroscopic diversity.
Supplementary Material
Scheme 1.
Reaction of compound 1 with hydrogen peroxide
Acknowledgments
Financial support from the NIH (CA123329, CA113917, GM086925, and GM084933), Georgia Cancer Coalition, Georgia Research Alliance and the Molecular Basis of Diseases program at GSU is gratefully acknowledged.
Footnotes
Supplementary Data: Experimental detail for 1. The supplementary data are available online with the paper in Science Direct.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Rhee SG. Science. 2006;312:1882–3. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 2.Veal EA, Day AM, Morgan BA. Mol Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 3.Krohn K, Maier J, Paschke R. Nat Clin Pract Endocrinol Metab. 2007;3:713–20. doi: 10.1038/ncpendmet0621. [DOI] [PubMed] [Google Scholar]
- 4.Galaris D, Skiada V, Barbouti A. Cancer Lett. 2008;266:21–9. doi: 10.1016/j.canlet.2008.02.038. [DOI] [PubMed] [Google Scholar]
- 5.Lin MT, Beal MF. Nature. 2006;443:787–95. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 6.Giorgio M, Trinei M, Migliaccio E, Pelicci PG. Nat Rev Mol Cell Biol. 2007;8:722–8. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- 7.Niethammer P, Grabher C, Look AT, Mitchison TJ. Nature. 2009;459:996–9. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao W. Angew Chem Int Ed. 2009;48:3022–4. doi: 10.1002/anie.200805651. [DOI] [PubMed] [Google Scholar]
- 9.Miller EW, Albers AE, Pralle A, Isacoff EY, Chang CJ. J Am Chem Soc. 2005;127:16652–9. doi: 10.1021/ja054474f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du L, Li M, Zheng S, Wang B. Tetrahedron Lett. 2008;49:3045–3048. doi: 10.1016/j.tetlet.2008.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller EW, Tulyathan O, Isacoff EY, Chang CJ. Nat Chem Biol. 2007;3:263–7. doi: 10.1038/nchembio871. [DOI] [PubMed] [Google Scholar]
- 12.Sekiya M, Umezawa K, Sato A, Citterio D, Suzuki K. Chem Commun. 2009:3047–9. doi: 10.1039/b903751a. [DOI] [PubMed] [Google Scholar]
- 13.Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S. Nat Methods. 2006;3:281–6. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- 14.Soh N. Anal Bioanal Chem. 2006;386:532–43. doi: 10.1007/s00216-006-0366-9. [DOI] [PubMed] [Google Scholar]
- 15.Kuivila HG, Wiles RA. J Am Chem Soc. 1955;77:4830–4834. [Google Scholar]
- 16.Kuivila HG, Armour AG. J Am Chem Soc. 1957;79:5659–5662. [Google Scholar]
- 17.Katerinopoulos HE. Curr Pharm Des. 2004;10:3835–52. doi: 10.2174/1381612043382666. [DOI] [PubMed] [Google Scholar]
- 18.Sivakumar K, Xie F, Cash BM, Long S, Barnhill HN, Wang Q. Org Lett. 2004;6:4603–6. doi: 10.1021/ol047955x. [DOI] [PubMed] [Google Scholar]
- 19.Xie F, Sivakumar K, Cash BM, Wang Q. Abstracts of Papers of the 229th ACS Meeting; San Diego, CA: American Chemical Society; 2005. pp. U358–U358. [Google Scholar]
- 20.Zhou Z, Fahrni CJ. J Am Chem Soc. 2004;126:8862–3. doi: 10.1021/ja049684r. [DOI] [PubMed] [Google Scholar]
- 21.Tornoe CW, Christensen C, Meldal M. J Org Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 22.Kolb HC, Finn MG, Sharpless KB. Angew Chem Int Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 23.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem Int Ed. 2002;41:2596–9. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Ishiyama T, Itoh Y, Kitano T, Miyaura N. Tetrahedron Lett. 1997;38:3447–3450. [Google Scholar]
- 25.Soh N, Sakawaki O, Makihara K, Odo Y, Fukaminato T, Kawai T, Irie M, Imato T. Bio org Med Chem. 2005;13:1131–9. doi: 10.1016/j.bmc.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 26.Chang MC, Pralle A, Isacoff EY, Chang CJ. J Am Chem Soc. 2004;126:15392–3. doi: 10.1021/ja0441716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dickinson BC, Chang CJ. J Am Chem Soc. 2008;130:9638–9. doi: 10.1021/ja802355u. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.