Abstract
Primary objective
To investigate the occurrence of post-concussive symptoms (PCS) and symptoms of post-traumatic stress disorder (PTSD) in children following mild traumatic brain injuries (TBI).
Research design
Longitudinal study comparing the outcomes of mild TBI and orthopaedic injuries (OI) in children aged 8–15.
Methods and procedures
One hundred and eighty-six children with mild TBI and 99 with OI were recruited prospectively. Parents rated children's PCS and symptoms of PTSD at 2 weeks, 3 months and 12 months post-injury. One hundred and sixty-seven with mild TBI and 84 with OI completed all assessments.
Main outcomes and results
Controlling for symptoms of PTSD, the mild TBI group demonstrated more PCS than the OI group, although the magnitude of group differences diminished with time. Controlling for PCS, the OI group displayed more symptoms of PTSD than the mild TBI group at baseline, but not thereafter. Symptoms of PTSD and PCS were correlated significantly, but more highly in the OI group than the mild TBI group.
Conclusions
Although PCS and symptoms of PTSD are correlated, children with mild TBI are more distinguishable from children with OI based on PCS than on symptoms of PTSD. The latter symptoms, moreover, do not account for increased PCS following mild TBI in children.
Keywords: Pediatric, closed head injury, outcome
Introduction
Traumatic brain injury (TBI) in children results in high rates of mortality and morbidity and therefore is a major public health concern [1]. The majority of TBI are mild in nature, but the long-term outcomes of mild TBI in children are still poorly understood. Most children recover successfully, but some suffer from a range of post-concussive symptoms (PCS) [2], which in some cases persist for months post injury [3, 4]. PCS include cognitive, somatic and emotional symptoms, such as impaired concentration and memory, headaches, sleep disturbances, irritability and anxiety [5]. Depending on the circumstances of the injury, children with mild TBI may also suffer from symptoms of post-traumatic stress disorder (PTSD). These symptoms include reports of re-experiencing the trauma event, increased arousal and avoidance of trauma-related stimuli [6]. PCS and symptoms of PTSD often overlap and their co-occurrence following mild TBI is controversial, although evidence suggests the two can occur simultaneously [7].
Research suggests that ~10% of adults suffer from persistent PCS following mild TBI, although the vast majority of individuals experience only transient symptoms [8]. The outcomes of mild TBI in children are less well documented and have historically been controversial, with researchers debating whether or not PCS occur in children [3, 9]. Studies indicate that children with mild TBI demonstrate higher levels of PCS compared to uninjured siblings or children with injuries not involving the head [9, 10]. PCS are more likely in the days immediately following the injury and decrease with time [4, 11]. In a sub-group of children, PCS may persist and this cluster of symptoms is thought to represent a coherent syndrome. Post-concussion syndrome is included in the International Classification of Diseases [12] and research criteria for post-concussional disorder is included in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) [6].
Evidence concerning the origin and maintenance of PCS is still inconclusive. Some researchers have proposed that mild TBI may be sufficient to cause neurological dysfunction that initially engenders PCS, but that persistent PCS are more likely to be maintained by a variety of non-neurological factors [3, 14]. Influential factors in the origin and maintenance of PCS may include injury severity, history of prior head trauma, children's pre-morbid functioning, family functioning or other life stressors [14, 25].
Studies have yielded mixed results regarding the occurrence of symptoms of PTSD following mild TBI. Some studies have found little evidence of these symptoms following mild TBI in adults, but others have found prevalence rates ranging from 20–40% [16]. Complicating matters, post-concussional disorder and PTSD share overlapping symptoms, including anxiety, depression, poor concentration, sleep disturbance and irritability [16]. The possible simultaneous occurrence of the two disorders following mild TBI is controversial [15, 17]. In adults, Bryant and Harvey [7] found that PCS were more common in patients with mild TBI who displayed concurrent PTSD symptoms than in those without PTSD symptoms. The results suggest a possible relationship between PCS and PTSD symptoms following TBI [7].
Research on the relationship between symptoms of PTSD and TBI in children is still lacking. Existing studies have primarily examined TBI of mixed or unspecified severity or moderate-to-severe TBI rather than mild TBI. Previous studies suggested that symptoms of PTSD could not occur in individuals who experienced amnesia, loss of consciousness or other symptoms of more severe TBI [18]. However, contemporary studies indicate that such symptoms occur in patients with and without loss of consciousness or amnesia [17, 19–23]. In a group of 95 children with severe TBI and amnesia for the event, Gerring et al. [19] documented PTSD in 12 (13%) of these children. Moreover, Max et al. [23] examined 50 children following mild-to-severe TBI and found that children commonly experienced PTSD symptoms despite amnesia for the event [23]. Most research suggests that children rarely meet full DSM-IV diagnostic criteria for PTSD following TBI, but the onset of a number of the symptoms of PTSD is fairly common [23]. The symptoms are more likely to appear shortly after TBI and diminish over time.
Efforts have been made to identify possible psychological and physical risk factors for symptoms of PTSD following TBI in children. Levi et al. [24] reported that injury severity may be an influential factor in the onset of symptoms of PTSD. They examined parent- and child-rated symptoms of PTSD in children following TBI and found that children with severe TBI reported higher rates of PTSD symptoms than children with moderate TBI or orthopaedic injuries (OI) [24]. Group differences emerged at 6 and 12 months based on parent ratings and at 12 months based on child ratings. The results suggest that children continue to experience PTSD symptoms at least 1 year post-injury. Gerring et al. [19] examined additional risk factors and found that pre-morbid psychopathology, female gender, psychosocial adversity and injury severity were related to an increased vulnerability to develop PTSD symptoms in children following TBI.
To the authors’ knowledge, no previous research has examined both PCS and symptoms of PTSD in a large sample of children following mild TBI. Therefore, the primary goal of the current study was to examine PCS and symptoms of PTSD in children with mild TBI, as compared to children with mild orthopaedic injuries (OI) not involving the head. As part of the larger study, it was previously reported that children with mild TBI displayed more somatic and cognitive PCS compared to children with OI [4, 25]. It was hypothesized that children with mild TBI also would exhibit more symptoms of PTSD than children with OI. This result was anticipated based on research examining symptoms of PTSD in children with more severe TBI [24]. The secondary goal of the study was to examine the overlap and relationship between symptoms of PTSD and PCS in these two groups of children. The secondary hypothesis, based on symptom overlap, was that measures of PCS and symptoms of PTSD would demonstrate significant positive correlations. This study also sought to determine whether group differences in PCS would be accounted for by those in symptoms of PTSD and vice versa.
Method
Participants
Participants were part of a larger prospective study examining the neurobehavioural outcomes of mild TBI in children and adolescents [3–5]. Children were prospectively recruited from the Emergency Departments at Nationwide Children's Hospital (Columbus, OH) and Rainbow Babies and Children's Hospital (Cleveland, OH). They were eligible if they were 8–15 years of age at the time of their injury and met pre-specified injury criteria. General exclusion criteria for both groups included: injury-related surgery; hypoxia or shock following the injury; previous head injury requiring hospitalization; pre-morbid neurological disorders; severe psychiatric disorder requiring hospitalization; associated injuries with an Abbreviated Injury Scale [26] greater than 3; injuries that would hinder neuropsychological assessment; and injuries related to child abuse or drug/alcohol use. A history of previous traumatic events was not an exclusion criterion.
In the mild TBI group, children were eligible if they demonstrated an observed loss of consciousness, a Glasgow Coma Scale (GCS) score of 13–14 or at least two symptoms of concussion as reported by Emergency Department physicians. Symptoms of concussion include persistent post-traumatic amnesia, transient neurological deficits, vomiting, nausea, headache, diplopia, dizziness, disorientation and other mental status changes. Children were excluded from the mild TBI group if they experienced a loss of consciousness over 30 minutes or received a GCS score below 13. Children were not excluded if they demonstrated intracranial lesions or skull fractures on acute computerized tomography or if they were hospitalized.
Children were eligible for the OI group if they demonstrated a fracture with an Abbreviated Injury Scale (AIS) score of 3 or less [26]. Children were excluded from the OI group if they had any evidence of head injury, including external trauma or symptoms of concussion. A comparison group of children with injuries not involving the head was selected to control for differences in children's pre-morbid functioning or environment that may predispose them to being injured, as well as for the experience of an acute medical trauma [3, 27].
A total of 393 children were determined to be eligible for the mild TBI group and 286 for the OI group and invited to participate in the study. From these, 48% in the mild TBI group and 35% in the OI group agreed to participate. Parents frequently cited scheduling difficulties, distance from the hospital or disinterest in the study as reasons for declining to participate. Participants and non-participants did not significantly differ in terms of age, gender, race/ethnicity or in census tract measures of socioeconomic status (i.e. mean family income, percentage of minority heads of household and percentage of households below the poverty line). The final sample included 186 children with mild TBI and 99 children with mild OI not involving the head. The mild TBI group did not differ from the OI group on demographic factors such as age, gender, race/ethnicity and socioeconomic status (SES). SES was measured by averaging sample z-scores for years of maternal education, median family income for census tract and the Duncan Socioeconomic Index, a measure of occupational prestige [28]. Children in both groups were also similar in terms of mechanism of injury, although transportation-related injuries were significantly more common among the mild TBI group.
Procedure and attrition
Institutional review board approval and informed parent consent and child assent were obtained prior to participation. During the larger study from which the current data were derived, eligible children and their parents were assessed four times during the first year post-injury. The first evaluation occurred no later than 3 weeks post-injury, with 80% completed between 1 and 2 weeks post-injury (M = 11.35 days, SD = 3.42). The initial assessment included magnetic resonance imaging (MRI) of the brain for children with mild TBI and retrospective assessments of child, parent and family pre-injury functioning, as well as concurrent assessments of the child's acute post-injury cognitive functioning, PCS and symptoms of PTSD. The measures of PCS were administered again by phone 1 month post-injury, but data from this assessment were not used in the current analyses because PTSD data were not collected at that time. Complete follow-up assessments that included measures of both types of symptoms occurred at 3 and 12 months following the injury.
A total of 285 children completed the initial assessment, 268 (94%) completed the 3 month assessment (178 or 96% with mild TBI, 90 or 91% with OI) and 253 (89%) completed the 12 month assessment (169 or 91% with mild TBI, 84 or 85% with OI). Attrition did not differ by group. Children who completed all assessments did not differ from those who did not do so in age, sex, pre-injury symptoms or early post-injury post-concussive symptoms, but were less likely to be of minority status and had higher socioeconomic status. The analyses presented here are based on the 251 children who completed all follow-up assessments (167 with mild TBI, 84 with orthopaedic injury). Among children with complete data, the TBI and OI groups did not differ on demographic factors such as age, gender, race/ethnicity and SES (see Table I).
Table I.
Demographic characteristics of the mild TBI and OI groups.
| Group |
||
|---|---|---|
| Demographics | Mild TBI | OI |
| n | 167 | 84 |
| % male | 71 | 63 |
| % white, non-Hispanic | 74 | 66 |
| Age | 11.90 ± 2.19 | 11.66 ± 2.22 |
| Socioeconomic status | 0.07 ± 0.92 | –0.05 ± 1.21 |
Measures
Post-concussive symptoms
PCS were assessed using two measures, the Health and Behaviour Inventory (HBI) and the Post-Concussive Symptom Interview (PCS-I) [9, 10]. The HBI consists of 50 items covering somatic, cognitive, affective and behavioural symptoms. The frequency of each PCS is rated on a 4-point scale that ranges from ‘never’ to ‘often’. Previous factor analyses revealed two underlying dimensions of the HBI, representing cognitive and somatic symptoms, that are robust across raters and time [5]. Scores for the two dimensions were used in analyses for this study. Parent ratings on both dimensions showed high internal consistency (Cronbach's α ≥ 0.95 at all assessments for cognitive symptoms and ≥0.85 at all assessments for somatic symptoms).
The PCS-I includes 15 cognitive, somatic and emotional symptoms. The PCS-I is administered orally; parents are asked to report the presence or absence of each symptom. The total score on the PCS-I equals the number of symptoms endorsed by parents. For this study, children were classified as displaying post-concussional disorder at each post-injury assessment if they demonstrated at least three new PCS compared to pre-morbid levels. This classification is consistent with the DSM-IV research criteria requiring three PCS to meet the diagnosis for post-concussional disorder. The PCS-I has shown satisfactory reliability and validity in previous studies [10]. In the current study, the PCS-I demonstrated satisfactory internal consistency (Cronbach's α ≥ 0.78 at all assessments).
Post-traumatic stress symptoms
The PTSD Checklist for Children/Parent Report (PCL-C/PR) was used to assess children's symptoms of PTSD [29]. This measure is based on parental perceptions of their child's symptoms. Specifically, parents are asked to rate the extent to which their child displays 17 post-traumatic symptoms. The rating scale ranges from ‘not at all’ (1) to ‘extremely’ (5). The PCL-C/PR has items tapping the three major symptom dimensions of PTSD (i.e. re-experiencing, avoidance/numbing and hyperarousal). In previous studies, the measure has shown satisfactory internal consistency and reliability [29]. Total scores were used in the analyses and can range from 17–85. For this study, children were classified as meeting symptom criteria for PTSD if they had at least one symptom of re-experiencing, at least three symptoms of avoidance/numbing and at least two symptoms of hyperarousal. This classification is consistent with the DSM-IV diagnostic criteria for PTSD. The PCL-C/PR demonstrated high internal consistency for the total score across all three assessments (Cronbach's α ≥ 0.80). The sub-scales of the PCL-C/PR also demonstrated acceptable levels of internal consistency.
Data analysis
Multivariate repeated measures analyses were used to examine symptoms of PTSD and PCS in the groups longitudinally. The total number of symptoms of PTSD (total score on the PCL-C/PR) in the mild TBI group was compared to that in the OI group at the initial, and 3 and 12 monhs post-injury assessments. Then, the total number of PCS (total score on the PCS-I) in the mild TBI group was compared to that in the OI group at these three time points. After examining total symptom scores, similar analyses examined the specific dimensions of PCS and symptoms of PTSD to determine which types of symptoms differed between groups. Separate analyses were conducted for each of the sub-scales on the PCL-C/PR (i.e. re-experiencing, avoidance and hyperarousal) and the cognitive and somatic sub-scales from the HBI. Race and SES were treated as covariates in all analyses. Pre-morbid PCS were controlled in the analyses of the PCS-I and HBI by using the appropriate retrospective parent ratings obtained at the initial assessment as a covariate. Finally, the proportions of children in the mild TBI and OI groups meeting symptom criteria for PTSD were compared using chi squares analyses at each occasion. Similar analyses were conducted to compare the proportion of children in each group meeting symptom criteria for post-concussional disorder.
The correlations between the measures of PCS and symptoms of PTSD were examined in the combined sample and in the mild TBI and OI groups separately. Specifically, correlations among the PCL-C/PR (total score and sub-scales), the PCS-I (total score) and the HBI (sub-scales) were examined at all three assessment occasions. It was also determined if group differences in PCS could be accounted for by increased levels of symptoms of PTSD and vice versa. Multivariate repeated measures analyses were conducted again separately for total number of symptoms of PTSD and number of PCS symptoms, as well as for the sub-scales of the PCL-C/PR and HBI. The baseline PCS-I total score was used as a covariate for the analysis of symptoms of PTSD. In turn, the baseline PCL-C/PR total score was treated as a covariate for the analysis of PCS. Race and SES were treated as covariates as well, as was pre-morbid PCS in the analyses of post-injury PCS.
Results
Group differences in PCS and symptoms of PTSD
We have previously reported that the mild TBI group showed more PCS at baseline than the OI group, but the difference diminished with time [25]. In summary, both groups showed a similar decreasing trend over time, but the decline was faster in the mild TBI group. Analyses of cognitive and somatic PCS also revealed group differences in PCS based on symptom type. Compared to the OI group, children with mild TBI displayed higher levels of somatic PCS at baseline, but group differences were not apparent by 12 months. Conversely, significant group differences in cognitive PCS were apparent at all three assessments, with children in the mild TBI group demonstrating higher symptom levels than children in the OI group.
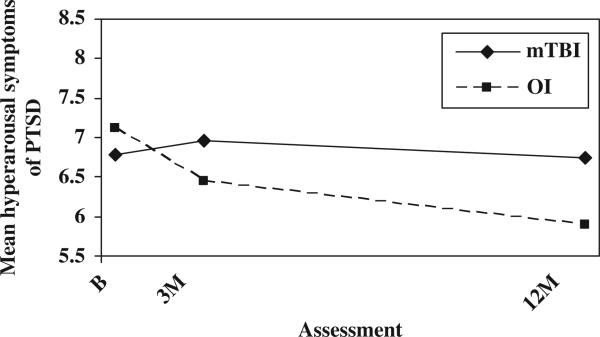
The analysis of the symptoms of PTSD total score did not reveal a significant main effect for group or group-by-time interaction. Analyses also did not reveal any significant group main effects or group-by-time interactions for the re-experiencing or avoidance sub-scales. The analysis of the hyperarousal sub-scale indicated a significant group × time interaction, F(2, 247) = 6.11, p = 0.002. As shown in Figure 1, the mild TBI group displayed a stable level of hyperarousal across the first year post-injury, whereas the OI groups showed a gradual decline. According to follow-up tests, the mild TBI group was slightly but significantly elevated compared to the OI group at 12 months. In contrast, the proportion of children meeting symptom criteria for PTSD was significantly higher in the OI group than in the mild TBI group at 12 months post-injury, but not prior to that (see Table II).
Figure 1.
Mean hyperarousal symptoms of PTSD by group and assessment.
Table II.
Number and percentage of children meeting DSM-IV symptom criteria for PTSD or post-concussional disorder.
| Assessment |
|||
|---|---|---|---|
| Baseline | 3 Months | 12 Months | |
| PTSD | |||
| Mild TBI | 15 (8%) | 15 (8%) | 3 (2%)* |
| OI | 7 (7%) | 6 (7%) | 6 (7%)* |
| Post-concussional disorder | |||
| Mild TBI | 94 (51%)* | 48 (27%) | 32 (19%) |
| OI | 29 (30%)* | 19 (21%) | 16 (19%) |
Group difference significant, p < .05
Relationships between PCS and symptoms of PTSD
Significant positive correlations between PCS and symptoms of PTSD were found at all three occasions when examining total scores on the PCL-C/PR and PCS-I, both across and within groups. Correlations between the PCL-C/PR total score and the HBI cognitive and somatic sub-scales were lower but still significant. The sub-scales of the PCL-C/PR were also significantly positively correlated with the HBI sub-scales at all three occasions. The magnitude of the correlations was often significantly higher in the OI group than the mild TBI group, suggesting less overlap between the two constructs for children with mild TBI (see Tables III and IV).
Table III.
Correlations between symptoms of PTSD (PCL-C/PR) and PCS scales (HBI) for the mild TBI and OI groups separately.
| Assessment |
||||||
|---|---|---|---|---|---|---|
| Baseline |
3 Months |
12 Months |
||||
| Mild TBI | OI | Mild TBI | OI | Mild TBI | OI | |
| Cognitive PCS | ||||||
| Total PTSD symptoms | 0.32* | 0.45* | 0.45* | 0.60* | 0.41* | 0.42* |
| Re-experiencing symptoms | 0.16 | 0.20* | 0.40* | 0.35* | 0.21* | 0.44* |
| Avoidance symptoms | 0.20* | 0.23* | 0.27* | 0.49* | 0.19 | 0.39* |
| Hyperarousal symptoms | 0.38* | 0.36* | 0.49* | 0.61* | 0.46* | 0.32* |
| Somatic PCS | ||||||
| Total PTSD symptoms | 0.46* | 0.43* | 0.29* | 0.48* | 0.24* | 0.56* |
| Re-experiencing symptoms | 0.23* | 0.29* | 0.30* | 0.32* | 0.13 | 0.57* |
| Avoidance symptoms | 0.25* | 0.28* | 0.15 | 0.33* | 0.15 | 0.52* |
| Hyperarousal symptoms | 0.29* | 0.31* | 0.30* | 0.56* | 0.24* | 0.48* |
p < .05; italics indicate significant differences between groups, p < .05
Table IV.
Correlations between symptoms of PTSD (PCL-C/PR) and PCS (PCS-I) for the mild TBI and OI groups.
| Assessment |
||||||
|---|---|---|---|---|---|---|
| Baseline |
3 Months |
12 Months |
||||
| PCS-I total score | Mild TBI | OI | Mild TBI | OI | Mild TBI | OI |
| Total symptoms of PTSD | 0.62* | 0.72* | 0.55* | 0.63* | 0.55* | 0.58* |
| Re-experiencing symptoms | 0.36* | 0.57* | 0.49* | 0.44* | 0.35* | 0.58* |
| Avoidance symptoms | 0.43* | 0.62* | 0.38* | 0.50* | 0.26* | 0.52* |
| Hyperarousal symptoms | 0.61* | 0.73* | 0.56* | 0.67* | 0.59* | 0.51* |
p < .05; italics indicate significant differences between groups, p < .05
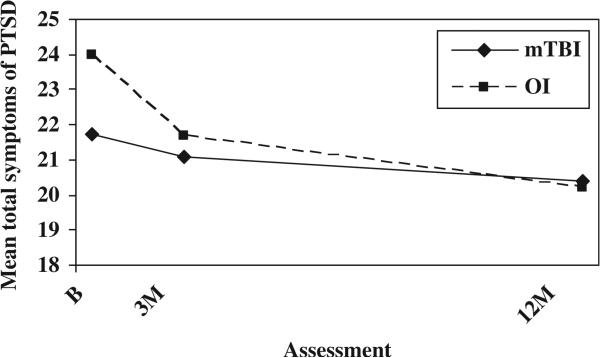
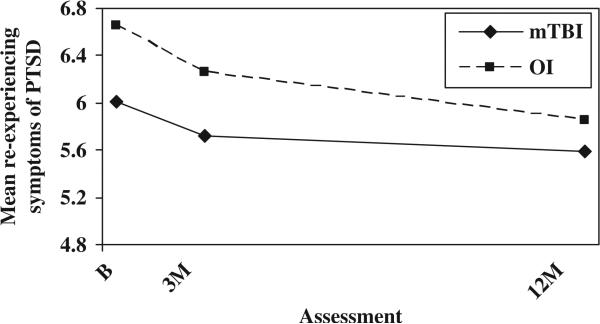
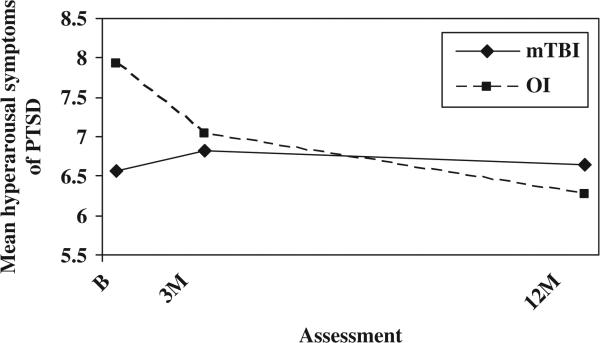
The relationship between PCS and symptoms of PTSD was further analysed to determine whether group differences in PCS could be accounted for by increased levels of symptoms of PTSD or vice versa. After controlling for baseline PCS, the OI group displayed higher total scores on the PCL-C/PR than the mild TBI group at the initial assessment, but group differences were minimal thereafter (see Figure 2). The group-by-time interaction was significant, F(2, 247) = 5.52, p = 0.004. The OI group showed higher scores on the re-experiencing sub-scale at all occasions, as reflected in a significant group main effect, F(1, 246) = 5.39, p = 0.021 (see Figure 3). The OI group displayed more hyperarousal symptoms than the mild TBI group at the initial assessment, but group differences were minimal thereafter; the group-by-time interaction was significant, F(2, 246) = 13.085, p = 0.000 (see Figure 4). Neither the group main effect nor the group-by-time interaction was significant on the avoidance sub-scale.
Figure 2.
Mean symptoms of PTSD total score by group and assessment (controlling for baseline PCS).
Figure 3.
Mean re-experiencing symptoms of PTSD by group and assessment (controlling for baseline PCS).
Figure 4.
Mean hyperarousal symptoms of PTSD by group and assessment (controlling for baseline PCS).
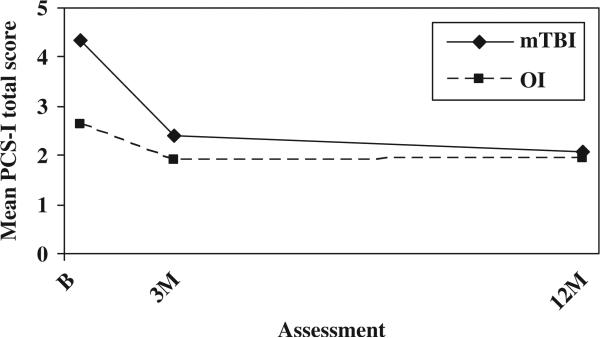
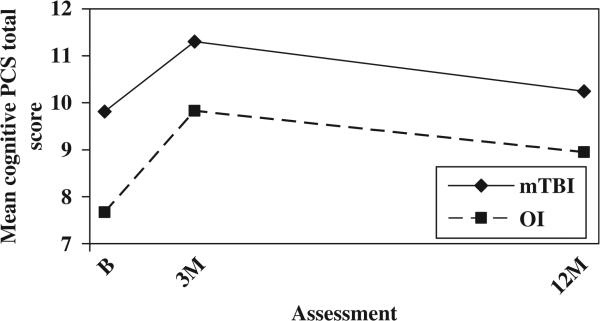
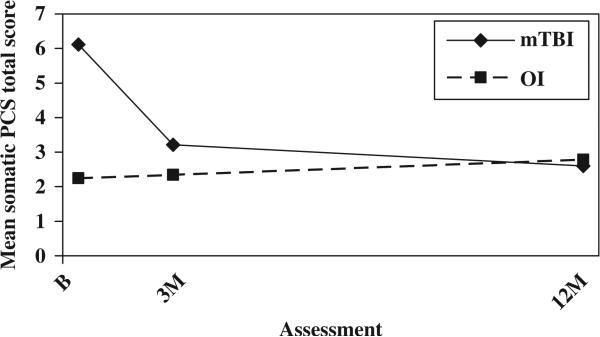
The mild TBI group continued to demonstrate higher total scores on the PCS-I after controlling for baseline symptoms of PTSD; both the group main effect, F(1, 246) = 8.188, p = 0.005, the group-by-time interaction, F(2, 246) = 8.682, p = 0.000, remained significant (see Figure 5). The mild TBI group also showed higher scores on the HBI cognitive sub-scale at all three occasions after controlling for baseline symptoms of PTSD, as reflected in a significant group main effect, F(1, 245) = 7.909, p = 0.005 (see Figure 6). As before, scores on the HBI somatic sub-scale were higher in the mild TBI group than the OI group at the initial assessment, but the difference decreased rapidly over time. Both the main effect for group, F(1, 245) = 18.219, p = 0.000, and group-by-time interaction F(2, 245) = 21.180, p = 0.000, were significant (see Figure 7).
Figure 5.
Mean PCS by group and assessment (controlling for baseline symptoms of PTSD).
Figure 6.
Mean cognitive PCS by group and assessment (controlling for baseline symptoms of PTSD).
Figure 7.
Mean somatic PCS (HBI) by group and assessment (controlling for baseline symptoms of PTSD).
Discussion
The primary hypothesis of the study was that children with mild TBI would exhibit more symptoms of PTSD than children with OI, but that the elevation in PTSD symptoms would not fully account for group differences in PCS between children with mild TBI and OI. This study did not find consistent evidence of group differences in symptoms of PTSD, with results failing to reveal differences on the total PCL-C/PR score or on the re-experiencing or avoidance sub-scales of this measure. The mild TBI group did demonstrate higher reported levels of hyperarousal at 3 and 12 months post-injury, but not when controlling for baseline PCS. Moreover, the OI group displayed higher levels of PTSD symptoms than the mild TBI group when controlling for baseline PCS and was more likely to meet symptom criteria for DSM-IV PTSD at 12 months compared to the mild TBI group. Higher rates of symptoms of PTSD were predicted in the mild TBI group compared to the OI group because of previous research demonstrating a higher incidence of symptoms of PTSD in children following severe TBI compared to OI [24]. However, the results suggest this trend may not be apparent in children with mild TBI.
The reasons that the OI group displayed higher rates of PTSD symptoms when controlling for PCS are unclear. Research has shown that children can experience psychological distress and PTSD symptoms following motor vehicle accidents [30–32]. However, transportation-related injuries accounted for significantly more mild TBI (17%) than OI (3%); thus, mechanism of injury is unlikely to be related to higher levels of PTSD symptoms in the OI group. Other factors, such as pain resulting from the injury or parental anxiety, may be related to increased levels of PTSD symptoms in children following traumatic injuries [33, 34]. These factors may have differentially affected the OI and mild TBI groups; however, they were not assessed in the current study, so further research is needed to delineate the reasons for elevated PTSD symptoms in children with mild OI.
As previously reported, this study found group differences in PCS, with higher symptom ratings reported in the mild TBI group than the OI group [5, 25]. Additional studies with both adults and children have consistently documented higher levels of PCS in individuals in the days and weeks immediately following mild TBI [9, 10, 14]. The current study also found that the mild TBI group exhibited significantly higher rates of post-concussional disorder at baseline compared to the OI group, but not thereafter. Previous research has shown that PCS are most common immediately following mild TBI, but diminish with time, in both children and adults [4, 9, 11, 13, 27].
The secondary hypothesis of the study was that measures of PCS and symptoms of PTSD would demonstrate significant positive correlations. This study examined the correlations between the two types of symptoms and determined whether increased rates of PCS can be accounted for by increased rates of symptoms of PTSD, or vice versa. Significant positive correlations were found between the two types of symptoms. Notably, however, the correlations tended to be significantly higher in the OI group than the mild TBI group. This suggests that PCS and symptoms of PTSD may be more distinct in the mild TBI group compared to the OI group. This could potentially reflect a closer link between PCS and neurological dysfunction in the mild TBI group. In any case, the findings are consistent with previous research demonstrating an association between high rates of PCS and PTSD in adults following mild TBI [7].
Among the sub-scales on the PCL-C/PR, the hyperarousal scale was most strongly correlated with the total PCS-I score. This may not be surprising, given that many of the symptoms of hyperarousal that define PTSD are also characteristic of post-concussive disorder [7]. On the PCL-C/PR, hyperarousal symptoms include ‘trouble falling or staying asleep’, ‘difficulty concentrating’, ‘being “super-alert” or watchful or on guard’, ‘feeling jumpy or easily startled’. The PCS-I measures similar symptoms, such as ‘has your child had trouble paying attention?’, ‘has it been hard for your child to think?’ and ‘has your child had trouble sleeping?’ Despite overlap between symptoms of PTSD and PCS, analyses indicated that symptoms of PTSD cannot account for the increased level of PCS in children following mild TBI. In contrast, group differences in symptoms of PTSD following mild TBI may be accounted for by differences in PCS. After controlling for baseline PCS, the OI group actually displayed higher scores on the PCL-C/PR, as well as more re-experiencing symptoms, at baseline and 3 months, compared to the mild TBI group.
Researchers have suggested that symptoms of PTSD not only overlap with PCS but also may exacerbate PCS, such that PCS are more common in individuals who experience symptoms of PTSD [7, 35]. Hypothetically, discomfort related to the latter symptoms may further deplete the already limited cognitive resources that individuals have available following mild TBI [7, 15, 35]. Similarly, PCS may prolong symptoms of PTSD because PCS may render an individual unable to adequately cope with traumatic events [24]. The results of the current study suggest that, following mild TBI in children, PCS help to account for symptoms of PTSD, but the latter symptoms do not influence the rates of PCS. This study is one of the first to examine the co-occurrence and overlap of symptoms of PTSD and PCS in a large group of children following mild TBI and OI. Methodological strengths of the study include clear criteria for defining mild TBI, the use of a control group with other injuries and the prospective, longitudinal design.
Future research should include both child and parent symptom reports, as children were not assessed directly in the current study. Structured clinical interviews have traditionally been the ‘gold standard’ in assessing PTSD in children [36–38]. However, clinical interviews are time-consuming and difficult to conduct with large samples of children and limited resources [36, 39]. Because satisfactory youth self-reports were unavailable at the time the study was designed, it relied on the PCL-C/PR. However, the recent development of youth self-report measures offers an efficient and effective alternative to structured interviews in a research setting [36, 39]. Furthermore, future studies are needed to investigate the effect of various child characteristics on the occurrence and overlap of PCS and PTSD. Previous research demonstrates that factors such as age, gender, pre-existing psychopathology, SES and injury characteristics may affect PCS and PTSD symptom ratings [25, 36, 39]. For instance, the inclusion of younger children and older adolescents in future research would be important.
More practically, researchers and clinicians should work to develop clinical management techniques for children following mild TBI. Assessment of psychological and cognitive functioning is needed, especially in the weeks and months immediately following mild TBI, to identify the sub-group of children with persistent PCS and other problems [11]. Interventions for this sub-group of children are critical and should be individually tailored to each child's specific needs [11]. Current interventions for PCS may need to be modified for children experiencing symptoms of PTSD, as the two kinds of symptoms may exacerbate one another [7]. Combining effective treatment components for PCS and PTSD may have potential for reducing the occurrence of both types of symptoms.
Acknowledgements
The research reported here served as the basis for a thesis submitted by the first author to The Ohio State University in partial fulfilment of the requirements for a master's degree. Portions of the research were presented at the annual meeting of the International Neuropsychological Society, Waikoloa, Hawaii, February 2008 and the annual meeting of the Society for Developmental and Behavioral Pediatrics, Cincinnati, Ohio, October 2008. The larger study on which the research was based was supported by grants R01 HD39834 and K02 HD44099 from the National Institute of Child Health and Human Development and the National Center for Medical Rehabilitation Research to Keith Owen Yeates. The authors wish to acknowledge the contributions of Lauren Ayr, Anne Birnbaum, Amy Clemens, Taryn Fay, Kalaichelvi Ganesalingham, Amanda Lininger, Melissa Ginn, Katie Pestro, Elizabeth Roth, Elizabeth Shaver and Heidi Walker in conducting the study.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Yeates KO. Closed-head injury. In: Yeates KO, Ris MD, Taylor HG, editors. Pediatric neuropsychology: Research, theory, and practice. Guilford; New York: 2000. pp. 92–116. [Google Scholar]
- 2.Satz P, Zaucha K, McCleary C, Light R, Asarnow R, Becker D. Mild head injury in children and adolescents: A review of studies (1970–1995). Psychological Bulletin. 1997;122:107–131. doi: 10.1037/0033-2909.122.2.107. [DOI] [PubMed] [Google Scholar]
- 3.Yeates KO, Taylor HG. Neurobehavioral outcomes of mild head injury in children and adolescents. Pediatric Rehabilitation. 2005;8:5–16. doi: 10.1080/13638490400011199. [DOI] [PubMed] [Google Scholar]
- 4.Yeates KO, Taylor HG, Rusin J, Bangert B, Dietrich A, Nuss K, Wright M, Nagin DS, Jones BL. Longitudinal trajectories of postconcussive symptoms in children with mild traumatic brain injuries and their relationship to acute clinical status. Pediatrics. 2009;123:735–743. doi: 10.1542/peds.2008-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayr LK, Yeates KO, Taylor HG, Browne M. Dimensions of post-concussive symptoms (PCS) in children with mild closed head injuries. Journal of the International Neuropsychological Society. 2009;15:19–30. doi: 10.1017/S1355617708090188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th edn. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 7.Bryant RA, Harvey AG. Postconcussive symptoms and posttraumatic stress disorder after mild traumatic brain injury. The Journal of Nervous and Mental Disease. 1999;187:302–305. doi: 10.1097/00005053-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Bigler ED. Neuropsychology and clinical neuroscience of persistent post-concussive syndrome. Journal of the International Neuropsychological Society. 2008;14:1–22. doi: 10.1017/S135561770808017X. [DOI] [PubMed] [Google Scholar]
- 9.Yeates KO, Luria J, Bartkowski H, Rusin J, Martin L, Bigler ED. Postconcussive symptoms in children with mild closed head injuries. Journal of Head Trauma Rehabilitation. 1999;14:337–350. doi: 10.1097/00001199-199908000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Mittenberg W, Wittner MS, Miller LJ. Postconcussion syndrome occurs in children. Neuropsychology. 1997;11:447–452. doi: 10.1037//0894-4105.11.3.447. [DOI] [PubMed] [Google Scholar]
- 11.Kirkwood MW, Yeates KO, Taylor HG, Randolph C, McCrea M, Anderson VA. Management of pediatric mild traumatic brain injury: A neuropsychological review from injury through recovery. The Clinical Neuropsychologist. 2007;24:1–32. doi: 10.1080/13854040701543700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Medical Association . International Classification of Diseases. 9th edn. American Medical Association; Chicago, IL: 1997. [Google Scholar]
- 13.Alexander MP. Mild traumatic brain injury: Pathophysiology, natural history, and clinical management. Neurology. 1995;45:1253–1260. doi: 10.1212/wnl.45.7.1253. [DOI] [PubMed] [Google Scholar]
- 14.Ponsford J, Wilmott C, Rothwell A, Cameron P, Ayton G, Nelms R, Curran C, Ng KT. Cognitive and behavioral outcome following mild traumatic head injury in children. Journal of Head Trauma Rehabilitation. 1999;14:360–372. doi: 10.1097/00001199-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Bryant RA. Posttraumatic stress disorder and traumatic brain injury: Can they co-exist? Clinical Psychology Review. 2001;21:931–948. doi: 10.1016/s0272-7358(00)00074-x. [DOI] [PubMed] [Google Scholar]
- 16.Harvey AG, Brewin CR, Jones C, Kopelman MD. Coexistence of posttraumatic stress disorder and traumatic brain injury: Towards a resolution of the paradox. Journal of the International Neuropsychological Society. 2003;9:663–676. doi: 10.1017/S1355617703940069. [DOI] [PubMed] [Google Scholar]
- 17.Sojka P, Stalnacke BM, Bjornstig U, Karlsson K. One-year follow-up of patients with mild traumatic brain injury: Occurrence of post-traumatic stress-related symptoms at follow-up and serum levels of cortisol, S-100B and neuron-specific enolase in acute phase. Brain Injury. 2006;20:613–620. doi: 10.1080/02699050600676982. [DOI] [PubMed] [Google Scholar]
- 18.Mayou R, Bryant B, Duthie R. Psychiatric consequences of road traffic accidents. British Medical Journal. 1993;307:647–651. doi: 10.1136/bmj.307.6905.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerring JP, Slomine B, Vasa RA, Grados M, Chen A, Rising W, Christensen JR, Denckla MB, Ernst M. Clinical predictors of posttraumatic stress disorder after closed head injury in children. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:157–165. doi: 10.1097/00004583-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Creamer M, O'Donnell ML, Pattison P. Amnesia, traumatic brain injury, and posttraumatic stress disorder: A methodological inquiry. Behaviour Research and Therapy. 2005;43:1383–1389. doi: 10.1016/j.brat.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Hickling EJ, Gillen R, Blanchard EB, Buckley T, Taylor A. Traumatic brain injury and posttraumatic stress disorder: A preliminary investigation of neuropsychological test results in PTSD secondary to motor vehicle accidents. Brain Injury. 1998;12:265–274. doi: 10.1080/026990598122566. [DOI] [PubMed] [Google Scholar]
- 22.Mayou RA, Black J, Bryant B. Unconsciousness, amnesia, and psychiatric symptoms following road accident injury. British Journal of Psychiatry. 2000;177:540–545. doi: 10.1192/bjp.177.6.540. [DOI] [PubMed] [Google Scholar]
- 23.Max JE, Castillo CS, Robin DA, Lindgren SD, Smith WL, Sato Y, Stephan A. Posttraumatic stress symptomatology after childhood traumatic brain injury. The Journal of Nervous and Mental Disease. 1998;186:589–596. doi: 10.1097/00005053-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Levi RB, Drotar D, Yeates KO, Taylor HG. Posttraumatic stress symptoms in children following orthopedic or traumatic brain injury. Journal of Clinical Child Psychology. 1999;28:232–243. doi: 10.1207/s15374424jccp2802_10. [DOI] [PubMed] [Google Scholar]
- 25.Taylor HG, Dietrich A, Nuss K, Wright M, Rusin J, Bangert B, Minich N, Yeates KO. Post-concussive symptoms in children with mild traumatic brain injury. Neuropsychology. doi: 10.1037/a0018112. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Association for Automotive Medicine . The abbreviated injury scale (AIS)-1990 revision. American Association for Automotive Medicine; Des Plaines, IL: 1990. [Google Scholar]
- 27.Satz P, Alfano MS, Light R, Morgenstern H, Zaucha K, Asarnow RF, Newton S. Persistent post-concussive syndrome: A proposed methodology and literature review to determine the effects, if any, of mild head and other bodily injury. Journal of Clinical and Experimental Neuropsychology. 1999;21:620–628. doi: 10.1076/jcen.21.5.620.870. [DOI] [PubMed] [Google Scholar]
- 28.Stevens G, Cho JH. Socioeconomic indexes and the new 1980 census occupational classification scheme. Social Science Research. 1985;14:142–168. [Google Scholar]
- 29.Daviss WB, Mooney D, Racusin R, Ford JD, Fleischer A, McHugo GJ. Predicting posttraumatic stress after hospitalization for pediatric injury. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:576–583. doi: 10.1097/00004583-200005000-00011. [DOI] [PubMed] [Google Scholar]
- 30.de Vries AP, Kassam-Adams N, Cnaan A, Sherman-Slate E, Gallager PR, Winston FK. Looking beyond the physical injury: Posttraumatic stress disorder in children and parents after pediatric injury. Pediatrics. 1999;104:1293–1299. doi: 10.1542/peds.104.6.1293. [DOI] [PubMed] [Google Scholar]
- 31.Gallo Di, Barton A, Parry-Jones J, WL Road traffic accidents: Early psychological consequences in children and adolescents. British Journal of Psychiatry. 1997;170:358–362. doi: 10.1192/bjp.170.4.358. [DOI] [PubMed] [Google Scholar]
- 32.Keppel-Benson JM, Ollendick TH, Benson MJ. Post-traumatic stress in children following motor vehicle accidents. Journal of Child Psychology and Psychiatry. 2002;43:203–212. doi: 10.1111/1469-7610.00013. [DOI] [PubMed] [Google Scholar]
- 33.Aaron J, Zaglul H, Emery RE. Posttraumatic stress in children following acute physical injury. Journal of Pediatric Psychology. 1999;42:335–343. doi: 10.1093/jpepsy/24.4.335. [DOI] [PubMed] [Google Scholar]
- 34.Landolt MA, Vollrath M, Ribi K, Gnehm HE, Sennhauser FH. Incidence and associations of parental and child posttraumatic stress symptoms in pediatric patients. Journal of Child Psychology and Psychiatry. 2003;44:1199–1207. doi: 10.1111/1469-7610.00201. [DOI] [PubMed] [Google Scholar]
- 35.Bryant RA, Harvey AG. The influence of traumatic brain injury on acute stress disorder and post-traumatic stress disorder following motor vehicle accidents. Brain Injury. 1999;13:15–22. doi: 10.1080/026990599121836. [DOI] [PubMed] [Google Scholar]
- 36.Davis L, Siegel L. Posttraumatic stress disorder in children and adolescents: A review and analysis. Clinical Child and Family Psychology Review. 2000;3:135–154. doi: 10.1023/a:1009564724720. [DOI] [PubMed] [Google Scholar]
- 37.Foa EB, Johnson KM, Feeny NC, Treadwell KRH. The child PTSD symptom scale: A preliminary examination of its psychometric properties. Journal of Clinical Child Psychology. 2001;30:376–384. doi: 10.1207/S15374424JCCP3003_9. [DOI] [PubMed] [Google Scholar]
- 38.McNally RJ. Assessment of posttraumatic stress disorder in children and adolescents. Journal of School Psychology. 1996;34:147–161. [Google Scholar]
- 39.Strand VC, Sarmiento TL, Pasquale LE. Assessment and screening tools for trauma in children and adolescents. Trauma, Violence, & Abuse. 2005;6:55–78. doi: 10.1177/1524838004272559. [DOI] [PubMed] [Google Scholar]