Abstract
Self-assembling biomaterials are promising as cell-interactive matrices because they can be constructed in a modular fashion, which enables the independent and simultaneous tuning of several of their physicochemical and biological properties. Such modularity facilitates the optimization of multi-component matrices for use in complex biological environments such as 3-D cell culture or scaffolds for regenerative medicine. This Highlight will discuss recent strategies for producing modular self-assembling biomaterials, with a particular focus on how ligand presentation and matrix mechanics can be controlled in modular ways. In addition, it will discuss key hurdles that remain for employing these materials as cell-interactive scaffolds in biomedical applications, particularly those that relate to how they may interface with the immune system.
Introduction
A cell’s behavior is regulated by the complex milieu of signals in which it resides. Whether a cell finds itself in culture, in a native tissue, in an engineered tissue, or in suspension, its decision to apoptose, proliferate, differentiate, migrate, or subtly change its phenotype will always be made by integrating all of the available signals at hand.1 When designing cell-interactive biomaterial scaffolds for applications such as defined 3-D culture or tissue engineering, a long and growing list of such signals may be relevant, including the density and spatial positioning of different ligands, the mechanics of the matrix, the time course of matrix degradation, the release of soluble signaling factors, and others. In cell-material interactions, relationships between these parameters may be additive, synergistic, or antagonistic, and they depend on the context of all signals present. Moreover, these relationships almost always vary with time. This incredibly large, convoluted, and time-dependent parameter space presents a challenge for engineering biomaterials that can predictably and controllably interface with biology.
Self-assembling biomaterials can be constructed in a modular fashion, which enables the independent and simultaneous tuning of many of these physicochemical and biological factors. The term modularity can take on different meanings in different contexts and fields; in the context of self-assembling biomaterials, it indicates both a multi-component segmental construction and a capability to orthogonally adjust many of these components at once (Figure 1). These features promise to facilitate more systematic explorations of the multidimensional parameter space of cell-material interactions than have been previously possible. Modularity arises from both the non-covalent architecture of self-assembling biomaterials as well as their chemical definition, enabling the precise integration of different components simply by mixing and inducing assembly. In this way, combinations of parameters may be systematically fine-tuned while observing biological outcome (Fig. 1). This optimization is more difficult in other biomaterials such as tissue-derived biopolymers or covalent polymer networks, which can be more polydisperse, incompletely defined especially in the case of biologically derived materials, and which tend to confound multiple physicochemical properties with each other. Given the exceedingly multifunctional and complex microenvironment that determines cell behavior, the orthogonal modularity exhibited by self-assembling systems is advantageous.
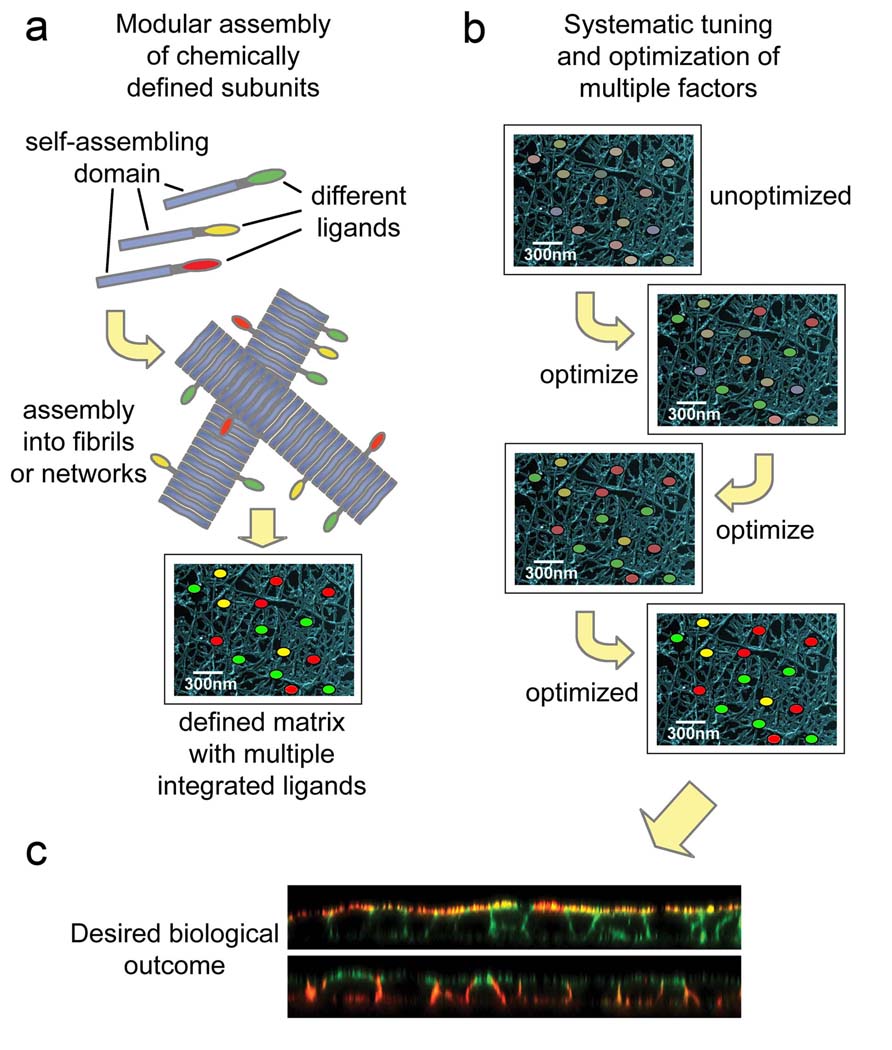
Figure 1.
Self-assembly enables a modular approach to biomaterials construction. The co-assembly of chemically defined molecular elements (a) promises to facilitate more systematic optimization and efficient exploration of the large parameter space of cell-biomaterials interactions (b) in order to experimentally identify those combinations of parameters that most effectively drive a desired biological response, for example the formation of a polarized epithelium (c). In panel (c), polarized MDCK epithelial cells are shown with confocal microscopy. The top image shows apical staining (red, gp135), and the bottom image shows basolateral staining (red, E-cadherin). In both images, F-actin is counterstained with phalloidin (green).
This brief Highlight is an account of recent advancements in self-assembling biomaterials designed to promote specific cellular responses, both in vitro and in vivo, and it emphasizes work that extends these materials’ modularity. It will also discuss a few areas of challenge and opportunity that exist as self-assembling biomaterials move closer to biotechnological and biomedical applications, including how these materials may interface with the immune system. For more detailed discussions of other aspects of self-assembling biomaterials, including stimulus-responsiveness,2 protein delivery from self-assembled scaffolds,3 polypeptide-based materials,4, 5 biomaterials for controlling stem cell phenotype,6 nanofibrous biomaterials,5, 7, 8 peptide-amphiphiles,8, 9 and the cell-surface interface,10 the reader is referred to other recent reviews. In addition, for more extensive discussions of modularity in supramolecular biomaterials, please see the comprehensive reviews published recently by Dankers and Meijer11 and Weck and coworkers.12
Modular ligand presentation in self-assembled biomaterials
Although modularity can take on many dimensions, several recent approaches to construct self-assembling biomaterials have particularly emphasized the modular integration of various ligands for cell binding. For example, Meijer and co-workers designed ureido-pyrimidinone (UPy)-functionalized polymers able to incorporate a variety of peptide ligands.13, 14 Backbone polymer networks of UPy-functionalized polyesters provided sites where UPy-functionalized peptides could dock via four precise hydrogen bonds. The usefulness of this approach lies in its simplicity, as one can envision a nearly endless combination of UPy-functionalized peptides immobilized simply by mixing them together and applying them to the polymer matrix. A variety of UPy-functionalized peptides have already been produced, demonstrating the potential breadth of this approach.13 An analogous strategy has also been utilized to decorate collagen matrices with peptides able to co-assemble non-covalently into the collagen triple helix.15 Other recent advances in modular self-assemblies have sought not to decorate a base scaffold but to construct the entire material from self-assembling small molecular weight components. For example, Stupp and coworkers recently extended their previous work with peptide-amphiphile biomaterials16–18 by investigating the assembly and bioactivity of new peptide-amphiphiles capable of presenting additional ligands such as biotin,9, 19 RGD, 20, 21 and others.22 In these systems, the ligands are effectively displayed and bioactive, enabling future work where complex combinations of them are tuned. Other recent work with β-sheet fibrils has demonstrated that such peptides can also be functionalized with a variety of pendant peptide sequences so as to integrate different ligands into a gel network of peptide fibrils.23–26 Expressed α-helical coiled coil systems have also been designed where cell-binding domains may be integrated into backgrounds of non-functionalized protein gels in a modular way.27 It is expected that the continuing refinement of modular strategies for ligand incorporation such as these will enable increasingly systematic fine-tuning of these scaffolds for applications ranging from 3-D cell culture scaffolds to tissue engineering. Encouraging successes have already been reported using ligand-presenting self-assembling biomaterials to influence a variety of cell behaviors. Representative examples include the differentiation of neural progenitor cells,16 the adhesion and spreading of fibroblasts,21 the proliferation and protein expression of endothelial cells,26, 28 the proliferation, differentiation, and migration of osteoblasts,24 and the intracellular uptake of the material itself.29 However, the modularity of these materials has not yet been fully exploited, as many simultaneously co-assembled factors have yet to be systematically tuned and optimized.
Orthogonal modularity: Matrix mechanics and ligand presentation
The concept of modularity can be extended beyond the incorporation of various different ligands to include other physicochemical aspects such as matrix viscoelasticity.28 It is increasingly appreciated that the mechanical environment surrounding a cell profoundly influences its behavior30 (for recent reviews, see 31). Given that self-assembled biomaterials are constructed predominantly from non-covalent interactions, recent work has focused on ways to adjust these materials’ covalent connectivity and therefore their viscoelasticity. For example, we sought to develop a means for controlling the mechanical properties of β-sheet fibrillar matrices in a way that would be modular, chemically specific, and independent of ligands co-assembled within the matrix. We employed native chemical ligation32 as a chemoselective way of accomplishing this by designing short peptide thioesters capable of self-assembling and then oligomerizing into covalently stabilized networks (Figure 2).28 Other laboratories have also recently utilized chemical ligation in combination with peptide self-assembly to produce long collagen-mimetic33 or α-helical fibrils.34 In the case of β-sheet fibrillar gels studied in our laboratory, native chemical ligation led to as much as a six-fold stiffening of the gels without significantly altering the morphology of the self-assembled fibrils.28 Ligation could be performed in a range of peptide concentrations, providing a way of decoupling matrix stiffness from peptide concentration, and therefore presumably from porosity. The chemistry was also cytocompatible in cultures of primary human endothelial cells, and matrix stiffening by ligation led to a significant increase in cell proliferation over non-ligated gels (Figure 2c). In addition, co-assembling an RGD-functionalized peptide into the matrix could specifically enhance proliferation further. This latter example demonstrated the modularity of this approach, in which mechanical properties and cell binding could be controlled in separate, orthogonal, easily combined ways. Matrix mechanics in self-assemblies of peptides or peptide-amphiphiles have also been controlled through the formation of disulfide bonds,18 through primary sequence variations,35 by ion complexation,36 and by adjusting the concentration of the self-assembled species,37 though the latter example is less modular because cell attachment points, porosity, and other biophysical aspects may change with varying peptide concentration. It is expected that advancing sophistication in the ability to regulate the mechanical environments surrounding cells will continue to improve the ability to direct desirable cell and tissue responses. Those approaches that enable the modular tuning of viscoelasticity may be particularly useful for systematically mapping how combinations of mechanical and biological stimuli promote specific biological outcomes.
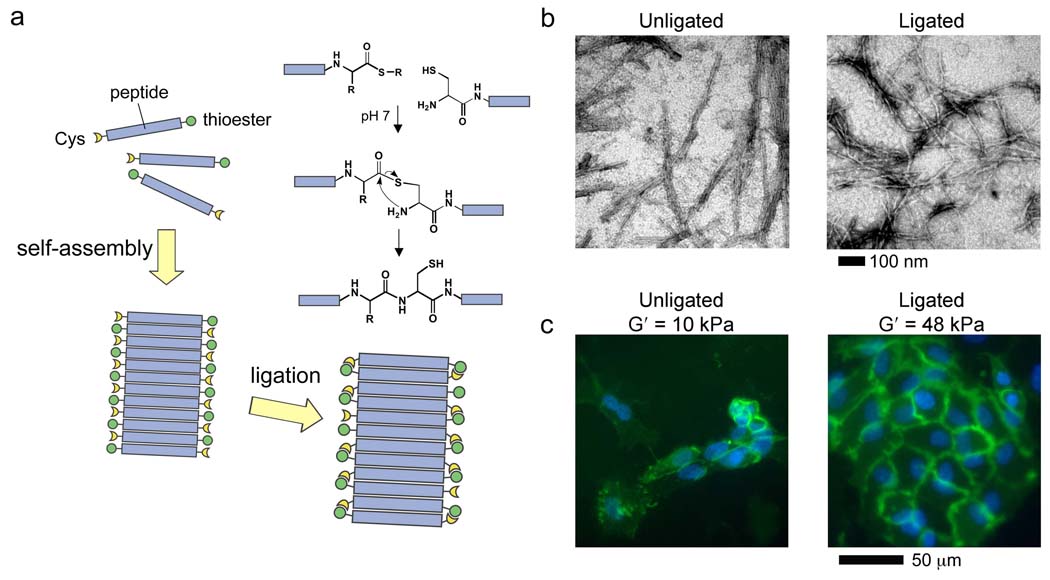
Figure 2.
Matrix mechanics as a modular aspect of biomaterials. β-sheet forming peptide-thioesters self-assemble into fibrils and undergo native chemical ligation, leading to matrix stiffening (a). Ligated peptides retain the ability to fibrillize (b), and stiffening leads to significantly improved proliferation of human endothelial cells over un-stiffened matrices (c, blue DAPI, green CD31). Reprinted with permission from Jung et. al, Biomaterials, 2008.28
Challenges and opportunities for implementation in biotechnology and biomedicine
The full implementation of modular self-assembling biomaterials in vitro and in vivo faces a number of challenges arising from biology’s ability to remodel such scaffolds. However carefully or cleverly a self-assembled biomaterial is constructed, as soon as it is placed in a biological environment, its molecular features will start to be remodeled by proteolytic degradation, soluble protein adsorption, the deposition of new extracellular matrix, and in the case of in vivo environments, possible inflammatory or immune responses (Fig. 3). All implanted biomaterials must appropriately interface with the natural inflammatory processes that result from implantation,38 but in the case of self-assembling biomaterials constructed from peptides or proteins, the possibility of immunogenicity must also be considered. In fact, several recent approaches for producing synthetic vaccines resemble many of the approaches described above for producing modular biomaterials, indicating a potential for self-assembled biomaterials to interact with the immune system for good or for bad. For example, a peptide-lipid was recently reported that forms spherical self-assemblies, and the repetitive display of a peptide sequence on the surface of the assemblies produced an immunogen capable of producing high antibody titers without even the presence of any adjuvant.39 Spatial repetition of antigens has long been recognized as a means for enhancing immune responses.40 One mechanism by which this occurs is through the clustering and cross-linking of B cell receptors by multivalent ligands, which can stimulate IgM production without the help of T cells (Figure 3e). It is also known that fibrillization and aggregation can significantly increase the immunogenicity of commercially available protein therapeutics even for fully human proteins.41 Such studies indicate that in the case of self-assembling biomaterials, which also display multivalent and repetitive ligands, care must be taken to ensure that the chosen ligands are exceptionally nonimmunogenic while at the same time capable of exerting their desired biological effects (e.g. integrin binding). However, even the most common and popular biomaterials modifications such as the RGD motif have been found to raise the immunogenicity of other peptides in some cases,42 and biomaterials surfaces themselves are capable of adjuvanticity,43 further adding to the difficulty of predicting how a particular self-assembled biomaterial will be tolerated. In addition, many self-assembling biomaterials exhibit some degree of heterogeneity in lateral aggregation, potentially making it difficult to draw connections between self-assembled structure and immunoreactivity. Recent advancements in controlling lateral aggregation44 or network topology45 may prove helpful in this regard. It is also encouraging that many self-assembling biomaterials have not demonstrated evidence of cytotoxicity24, 25, 28, 46, 47 or elicited obvious inflammatory responses in immunocompetent animals.16, 47–49 However, with a few exceptions,16, 49 most in vivo studies to date have been performed with non-functionalized self-assemblies, so the range of possible ligands that will be tolerated is not yet clear.
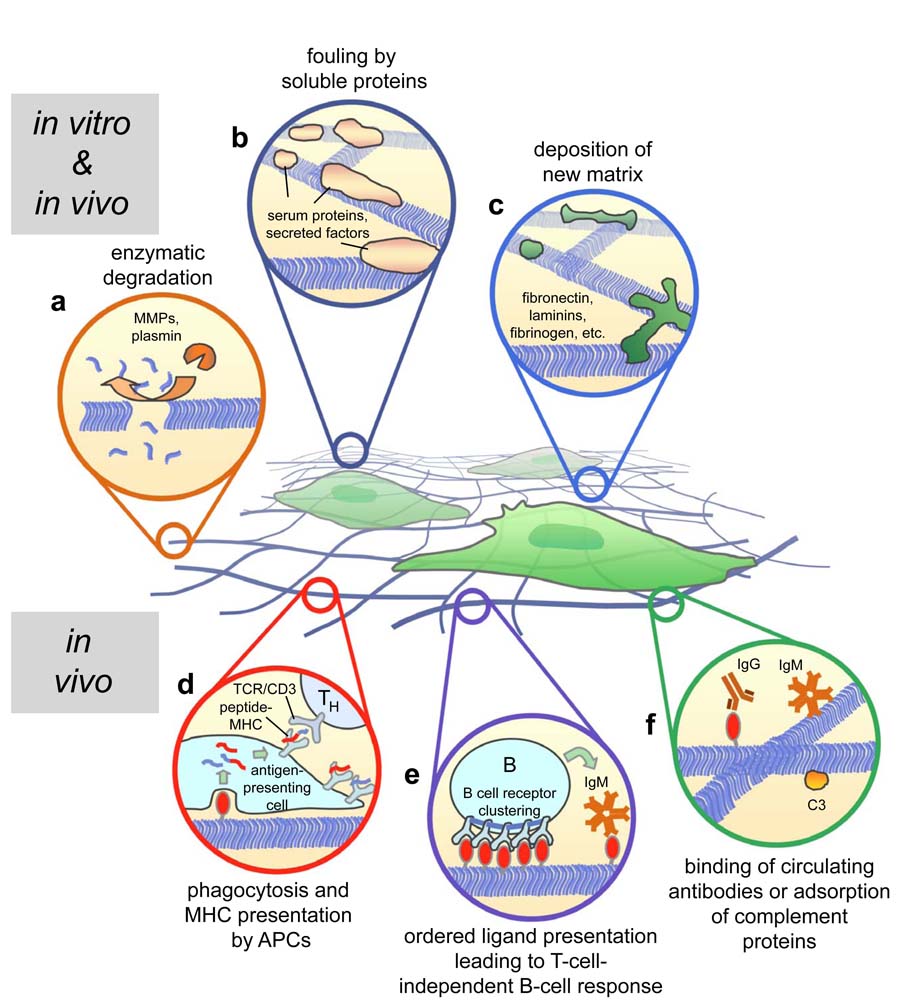
Figure 3.
Biological mechanisms that can modify self-assembling biomaterials construction in vitro (a–c) and in vivo (a–f). Processes at work in both environments include enzymatic degradation (a), fouling by serum-derived or other soluble proteins (b), and the deposition of new extracellular matrix by cells in contact with the material (c). Processes at work in vivo include phagocytosis of the matrix by antigen-presenting cells (APCs) and presentation in the MHC to potentially stimulate T-cell-dependent immune responses (d, note, many accessory and co-stimulatory proteins are not shown for simplicity), T-independent IgM responses induced by multivalent ligand display and B cell receptor clustering (e), and the binding of low-affinity circulating antibodies or complement proteins (f).
Questions about how materials interact with the immune system are not unique to supramolecular structures, as a great deal of current research in the field of Biomaterials as a whole is concerned with the careful spatial positioning of molecular features (peptides, engineered proteins, growth factors, protein-polymers, biofunctionalized supports) that could also be recognized immunologically to various degrees. Perhaps self-assembling biomaterials, with their precise and modular control over multiple aspects of the material, will prove to be useful platforms for systematically intervening in these responses so that relationships between ligand antigenicity, spacing, co-presentation, matrix stiffness, and other factors may be more clearly understood. Given that the immune and inflammatory systems are intimately involved in tissue remodeling and healing, it is possible that elucidating these relationships may help provide novel strategies for regulating regenerative processes in tissue engineering.
Conclusions
This Highlight has attempted to provide a brief discussion of self-assembling biomaterials and how orthogonal modularity is an essential and useful characteristic of them. Modularity is being exploited to greater and greater degrees, particularly with different cell-binding ligands and matrix viscoelasticity. It is expected that such modular approaches will facilitate more rapid and systematic explorations of the complex parameter space of cell-materials interactions. The potential for these materials to engage immune responses presents a challenge for fully implementing them in biomedical applications, but if such interactions can be understood and controlled in the coming years, these materials may prove highly advantageous in regenerative medicine.
Acknowledgements
Research in our laboratory is supported by a National Science Foundation CAREER award to JHC (CHE-0802286), the National Institutes of Health (NIDCR, grant no. DE017703; NIBIB, grant no. EB007335), and the American Heart Association (grant no. 0665218B). I also thank Bill Murphy, Mirjam Zegers, Martin ter Beest, Kathy Goss, and Jai Rudra for helpful comments on the manuscript.
References
- 1.Lutolf MP, Hubbell JA. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 2.Mart RJ, Osborne RD, Stevens MM, Ulijn RV. Soft Matter. 2006;2:822–835. doi: 10.1039/b607706d. [DOI] [PubMed] [Google Scholar]
- 3.Segers VFM, Lee RT. Drug Discovery Today. 2007;12:561–568. doi: 10.1016/j.drudis.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Deming TJ. Progress in Polymer Science. 2007;32:858–875. [Google Scholar]; MacPhee CE, Woolfson DN. Current Opinion in Solid State & Materials Science. 2004;8:141–149. [Google Scholar]; Hamley IW. Angewandte Chemie-International Edition. 2007;46:8128–8147. doi: 10.1002/anie.200700861. [DOI] [PubMed] [Google Scholar]
- 5.Channon K, MacPhee CE. Soft Matter. 2008;4:647–652. doi: 10.1039/b713013a. [DOI] [PubMed] [Google Scholar]
- 6.Saha K, Pollock JF, Schaffer DV, Healy KE. Current Opinion in Chemical Biology. 2007;11:381–387. doi: 10.1016/j.cbpa.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim YB, Lee M. Journal of Materials Chemistry. 2008;18:723–727. [Google Scholar]; Toh Y-C, Ng S, Khong YM, Zhang X, Zhu Y, Lin P-C, Te C-M, Sun W, Yu H. Nano Today. 2006;1:34–43. [Google Scholar]
- 8.Jun HW, Paramonov SE, Hartgerink JD. Soft Matter. 2006;2:177–181. doi: 10.1039/b516805h. [DOI] [PubMed] [Google Scholar]
- 9.Guler MO, Soukasene S, Hulvat JF, Stupp SI. Nano Letters. 2005;5:249–252. doi: 10.1021/nl048238z. [DOI] [PubMed] [Google Scholar]
- 10.Stevens MM, George JH. Science. 2005;310:1135–1138. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 11.Dankers PYW, Meijer EW. Bulletin of the Chemical Society of Japan. 2007;80:2047–2073. [Google Scholar]
- 12.South CR, Burd C, Weck M. Accounts of Chemical Research. 2007;40:63–74. doi: 10.1021/ar0500160. [DOI] [PubMed] [Google Scholar]
- 13.Dankers PYW, Adams PJHM, Lowik DWPM, van Hest JCM, Meijer EW. European Journal of Organic Chemistry. 2007:3622–3632. [Google Scholar]
- 14.Dankers PYW, Harmsen MC, Brouwer LA, Van Luyn MJA, Meijer EW. Nat Mater. 2005;4:568–574. doi: 10.1038/nmat1418. [DOI] [PubMed] [Google Scholar]
- 15.Wang AY, Mo X, Chen CS, Yu SM. Journal of the American Chemical Society. 2005;127:4130–4131. doi: 10.1021/ja0431915. [DOI] [PubMed] [Google Scholar]
- 16.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Science. 2004;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 17.Hartgerink JD, Beniash E, Stupp SI. Science. 2001;294:1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 18.Hartgerink JD, Beniash E, Stupp SI. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:5133–5138. doi: 10.1073/pnas.072699999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guler MO, Soukasene S, Hulvat JF, Stupp SI. Nano Lett. 2005;5:249–252. doi: 10.1021/nl048238z. [DOI] [PubMed] [Google Scholar]
- 20.Harrington DA, Cheng EY, Guler MO, Lee LK, Donovan JL, Claussen RC, Stupp SI. Journal of Biomedical Materials Research Part A. 2006;78A:157–167. doi: 10.1002/jbm.a.30718. [DOI] [PubMed] [Google Scholar]; Guler MO, Hsu L, Soukasene S, Harrington DA, Hulvat JF, Stupp SI. Biomacromolecules. 2006;7:1855–1863. doi: 10.1021/bm060161g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Storrie H, Guler MO, Abu-Amara SN, Volberg T, Rao M, Geiger B, Stupp SI. Biomaterials. 2007;28:4608–4618. doi: 10.1016/j.biomaterials.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 22.Niece KL, Hartgerink JD, Donners JJ, Stupp SI. J Am Chem Soc. 2003;125:7146–7147. doi: 10.1021/ja028215r. [DOI] [PubMed] [Google Scholar]
- 23.Gras SL, Tickler AK, Squires AM, Devlin GL, Horton MA, Dobson CM, MacPhee CE. Biomaterials. 2008;29:1553–1562. doi: 10.1016/j.biomaterials.2007.11.028. [DOI] [PubMed] [Google Scholar]; Kasai S, Ohga Y, Mochizuki M, Nishi N, Kadoya Y, Nomizu M. Biopolymers. 2004;76:27–33. doi: 10.1002/bip.10565. [DOI] [PubMed] [Google Scholar]
- 24.Horii A, Wang X, Gelain F, Zhang S. PLoS ONE. 2007;2:e190. doi: 10.1371/journal.pone.0000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gelain F, Bottai D, Vescovi A, Zhang S. PLoS ONE. 2006;1:e119. doi: 10.1371/journal.pone.0000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Genove E, Shen C, Zhang S, Semino CE. Biomaterials. 2005;26:3341–3351. doi: 10.1016/j.biomaterials.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Fischer SE, Liu XY, Mao HQ, Harden JL. Biomaterials. 2007;28:3325–3337. doi: 10.1016/j.biomaterials.2007.03.026. [DOI] [PubMed] [Google Scholar]; Mi L, Fischer S, Chung B, Sundelacruz S, Harden JL. Biomacromolecules. 2006;7:38–47. doi: 10.1021/bm050157p. [DOI] [PubMed] [Google Scholar]
- 28.Jung JP, Jones JL, Cronier SA, Collier JH. Biomaterials. 2008;29:2143–2151. doi: 10.1016/j.biomaterials.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim YB, Lee E, Lee M. Angewandte Chemie-International Edition. 2007;46:9011–9014. doi: 10.1002/anie.200702732. [DOI] [PubMed] [Google Scholar]
- 30.Engler AJ, Sen S, Sweeney HL, Discher DE. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]; Pelham RJ, Wang YL. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Discher DE, Janmey P, Wang YL. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]; Levental I, Georges PC, Janmey PA. Soft Matter. 2007;3:299–306. doi: 10.1039/b610522j. [DOI] [PubMed] [Google Scholar]
- 32.Dawson PE, Muir TW, Clarklewis I, Kent SBH. Science. 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 33.Paramonov SE, Gauba V, Hartgerink JD. Macromolecules. 2005;38:7555–7561. [Google Scholar]
- 34.Ryadnov MG, Woolfson DN. Journal of the American Chemical Society. 2007;129:14074–14081. doi: 10.1021/ja072960s. [DOI] [PubMed] [Google Scholar]
- 35.Caplan MR, Schwartzfarb EM, Zhang SG, Kamm RD, Lauffenburger DA. Journal of Biomaterials Science-Polymer Edition. 2002;13:225–236. doi: 10.1163/156856202320176493. [DOI] [PubMed] [Google Scholar]
- 36.Ozbas B, Rajagopal K, Haines-Butterick L, Schneider JP, Pochan DJ. Journal of Physical Chemistry B. 2007;111:13901–13908. doi: 10.1021/jp075117p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sieminski AL, Was AS, Kim G, Gong H, Kamm RD. Cell Biochemistry and Biophysics. 2007;49:73–83. doi: 10.1007/s12013-007-0046-1. [DOI] [PubMed] [Google Scholar]
- 38.Lumelsky NL. Tissue Engineering. 2007;13:1393–1398. doi: 10.1089/ten.2007.0100. [DOI] [PubMed] [Google Scholar]
- 39.Boato F, Thomas RM, Ghasparian A, Freund-Renard A, Moehle K, Robinson JA. Angewandte Chemie-International Edition. 2007;46:9015–9018. doi: 10.1002/anie.200702805. [DOI] [PubMed] [Google Scholar]
- 40.Fehr T, Bachmann MF, Bucher E, Kalinke U, DiPadova F, Lang AB, Hengartner H, Zinkernagel RM. Journal of Experimental Medicine. 1997;185:1785–1792. doi: 10.1084/jem.185.10.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bachmann MF, Rohrer UH, Kundig TM, Burki K, Hengartner H, Zinkernagel RM. Science. 1993;262:1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]; Rosenberg AS. Aaps Journal. 2006;8:E501–E507. doi: 10.1208/aapsj080359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maas C, Hermeling S, Bouma B, Jiskoot W, Gebbink MFBG. Journal of Biological Chemistry. 2007;282:2229–2236. doi: 10.1074/jbc.M605984200. [DOI] [PubMed] [Google Scholar]
- 42.Yano A, Onozuka A, Asahi-Ozaki Y, Imai S, Hanada N, Miwa Y, Nisizawa T. Vaccine. 2005;23:2322–2326. doi: 10.1016/j.vaccine.2005.01.031. [DOI] [PubMed] [Google Scholar]; Yano A, Onozuka A, Matin K, Imai S, Hanada N, Nisizawa T. Vaccine. 2003;22:237–243. doi: 10.1016/s0264-410x(03)00561-9. [DOI] [PubMed] [Google Scholar]
- 43.Babensee JE, Yoshida M, Stein M, Coulter WH. Cell Transplantation. 2003;12:159–159. [Google Scholar]; Lester EA, Babensee JE. Journal of Biomedical Materials Research Part A. 2003;64A:397–410. doi: 10.1002/jbm.a.10378. [DOI] [PubMed] [Google Scholar]; Matzelle MM, Babensee JE. Biomaterials. 2004;25:295–304. doi: 10.1016/s0142-9612(03)00531-3. [DOI] [PubMed] [Google Scholar]
- 44.Collier JH, Messersmith PB. Advanced Materials. 2004;16:907–910. [Google Scholar]; Lamm MS, Sharma N, Rajagopal K, Beyer FL, Schneider JP, Pochan DJ. Advanced Materials. 2008;20:447. [Google Scholar]; Lamm MS, Rajagopal K, Schneider JP, Pochan DJ. Journal of the American Chemical Society. 2005;127:16692–16700. doi: 10.1021/ja054721f. [DOI] [PubMed] [Google Scholar]; Rajagopal K, Ozbas B, Pochan DJ, Schneider JP. 2006;35:162–169. doi: 10.1007/s00249-005-0017-7. [DOI] [PubMed] [Google Scholar]; Rosler A, Klok HA, Hamley IW, Castelletto V, Mykhaylyk OO. Biomacromolecules. 2003;4:859–863. doi: 10.1021/bm034058s. [DOI] [PubMed] [Google Scholar]; Burkoth TS, Benzinger TLS, Jones DNM, Hallenga K, Meredith SC, Lynn DG. Journal of the American Chemical Society. 1998;120:7655–7656. [Google Scholar]; Burkoth TS, Benzinger TLS, Urban V, Lynn DG, Meredith SC, Thiyagarajan P. Journal of the American Chemical Society. 1999;121:7429–7430. [Google Scholar]; Konig HM, Kilbinger AFM. Angewandte Chemie-International Edition. 2007;46:8334–8340. doi: 10.1002/anie.200701167. [DOI] [PubMed] [Google Scholar]
- 45.Shen W, Zhang KC, Kornfield JA, Tirrell DA. Nature Materials. 2006;5:153–158. doi: 10.1038/nmat1573. [DOI] [PubMed] [Google Scholar]; Xu C, Breedveld V, Kopecek J. Biomacromolecules. 2005;6:1739–1749. doi: 10.1021/bm050017f. [DOI] [PubMed] [Google Scholar]; Shen W, Lammertink RGH, Sakata JK, Kornfield JA, Tirrell DA. Macromolecules. 2005;38:3909–3916. [Google Scholar]
- 46.Kretsinger JK, Haines LA, Ozbas B, Pochan DJ, Schneider JP. Biomaterials. 2005;26:5177–5186. doi: 10.1016/j.biomaterials.2005.01.029. [DOI] [PubMed] [Google Scholar]; Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, Grodzinsky AJ. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9996–10001. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zhang S, Holmes TC, DiPersio CM, Hynes RO, Su X, Rich A. Biomaterials. 1995;16:1385–1393. doi: 10.1016/0142-9612(95)96874-y. [DOI] [PubMed] [Google Scholar]
- 47.Davis ME, Motion JP, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, Zhang S, Lee RT. Circulation. 2005;111:442–450. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holmes TC, de Lacalle S, Su X, Liu G, Rich A, Zhang S. Proc Natl Acad Sci U S A. 2000;97:6728–6733. doi: 10.1073/pnas.97.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]; Engel FB, Hsieh PCH, Lee RT, Keating MT. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15546–15551. doi: 10.1073/pnas.0607382103. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hsieh PCH, MacGillivray C, Gannon J, Cruz FU, Lee RT. Circulation. 2006;114:637–644. doi: 10.1161/CIRCULATIONAHA.106.639831. [DOI] [PubMed] [Google Scholar]; Hsieh PCH, Davis ME, Gannon J, MacGillivray C, Lee RT. Journal of Clinical Investigation. 2006;116:237–248. doi: 10.1172/JCI25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davis ME, Hsieh PCH, Takahashi T, Song Q, Zhang SG, Kamm RD, Grodzinsky AJ, Anversa P, Lee RT. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8155–8160. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kapadia MR, Chow LW, Tsihlis ND, Ahanchi SS, Eng JW, Murar J, Martinez J, Popowich DA, Jiang Q, Hrabie JA, Saavedra JE, Keefer LK, Hulvat JF, Stupp SI, Kibbe MR. J Vasc Surg. 2008;47:173–182. doi: 10.1016/j.jvs.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]