Abstract
We report two cases of myocarditis complicating acute schistosomiasis in returning travelers. Treatment with corticosteroids led to full recovery in both cases. Although the pathophysiology of this complication remains unclear, we recommend treating such patients with corticosteroids rather than praziquantel, which can be associated with clinical deterioration.
Introduction
Schistosomiasis is a helminthic disease of major importance on a worldwide scale. Acute (or invasive) schistosomiasis (AS) is especially observed in nonimmune travelers returning from endemic areas.1 It is usually characterized by a benign clinical course but life-threatening events such as neurological complications can occur.2,3 Cardiac events have been rarely reported during AS but may also be potentially lethal. We report two cases of myocarditis complicating AS.
Case Reports
The first patient was a 16-year-old French tourist who had been traveling for 1 month in West Africa, Burkina Faso, and Mali. During his journey, he bathed once in a waterfall in the Dogon area of Mali. Four weeks later, he was hospitalized in Paris for fever (39–39.2°C) and arthromyalgias lasting for 10 days. He presented with urticarial rash, cough, dyspnea, face edema, and oppressive retrosternal chest pain for 2 days. These symptoms appeared 26 days after the exposure. His eosinophil count was 520/mm3 (12%), troponin level was 1.5 µg/L (normal < 0.04 µg/L), aspartate and alanine aminotransferase levels were high, 162 IU/L and 219 IU/L, respectively (normal < 40 IU/L). Chest x-ray was normal. Stool and urine samples were negative for parasite eggs, larvae, and adult forms of schistosomes. Serodiagnosis of schistosomiasis was positive (1/800) by indirect immunofluorescence (S. mansoni in-house prepared antigen; Laboratoire de Parasitologie-Mycologie, Groupe Hospitalier Pitié-Salpêtrière, Paris, France4,5) and negative by hemagglutination (Cellognost Schistosomiasis; Chiron-Behring, Marburg, Germany). AS was suspected. Electrocardiography showed nonspecific repolarization disorders in apico-lateral territory: negative T-wave in lead V1 and depression of the S-T segment in lead V4, V5, V6 and DII (Figure 1). He was thus admitted in the Cardiological Intensive Care Unit. Results of echocardiography showed segmentary hypokinesia (apical and anterior medium third). Troponin levels rose to 4.5 µg/L. The patient received intravenous corticosteroids (prednisone 1 mg/kg/d) and acebutolol associated with ramipril for acute myocarditis. The cardiac magnetic resonance imaging (MRI) was normal. Corticosteroid therapy was gradually reduced. Three months later, he received praziquantel (Biltricide®, Bayer Santé, Loos, France) (40 mg/kg). In the following months, clinical examinations, eosinophil count, and troponin remained normal. One year later, the patient was clinically well.
Figure 1.
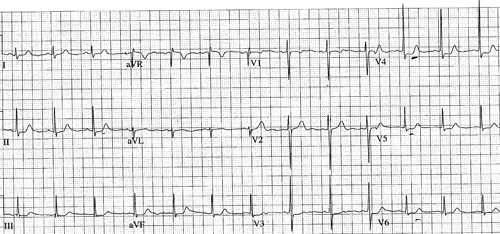
Electrocardiogram of patient number 1: negative T-Wave in lead VI and depression of the S-T segment in lead V4, V5, V6 and DII.
The second patient was a 21-year-old French traveler who was hospitalized for persistent fever (38–39°C), myalgias, headache, dry cough, and loss of appetite. One month earlier, he had developed a maculopapular rash just after bathing once, with other members of a tourist group, in a lake in the Dogon area of Mali where he had been traveling for one month. The first clinical sign was a self-limited right palpebral angioedema that appeared 27 days after the exposure, as well as swimmer's itch. His eosinophil count was 2,000/mm3 (22%), and an acute schistosomiasis was suspected. Electrocardiogram showed nonspecific repolarisation disorders: inverted ST-waves in lead V4 (Figure 1). The troponin level was 13.9 µg/L (normal < 0.2 µg/L) and the creatine kinase-MB form (CK-MB) level was 6.6 µg/L (normal < 5 µg/L). Results of echocardiography were normal. Aspartate and alanine aminotransferase levels were 115 IU/L and 225 IU/L, respectively. Multiple stool and urine samples were negative for parasite eggs, larvae, and adult forms of schistosomiasis. Serodiagnosis of schistosomiasis, initially negative, became positive by indirect immunofluorescence (1/800) (S. mansoni in-house prepared antigen; Laboratoire de Parasitologie-Mycologie, Groupe Hospitalier Pitié-Salpêtrière, Paris, France) and by hemagglutination (1/512) (Cellognost Schistosomiasis; Chiron-Berhing, Marburg, Germany). The patient received praziquantel (40 mg/kg). Two days later, he abruptly developed mental confusion, anosognosia, and splinter hemorrhages under his nails. His temperature was normal. Cerebral MRI showed multiple bilateral brain infarcts suggestive of cerebral vasculitis. Electrocardiography showed persistent nonspecific repolarisation disorders. Heart computed tomography was normal, with slight myocardium and dry pericardium. Corticosteroid therapy (prednisone 1 mg/kg/d) was started, and the symptoms disappeared within 48 hours. Eosinophil count fell down to 300/mm3 and troponin level became negative 2 weeks later. Cardiac MRI was performed 2 month later and revealed a discrete delayed myocardial perfusion of the septum with subendocardial enhancement compatible with endocardial fibrosis. Four months later, S. haematobium eggs were found by direct microscopic examination of centrifugated urine samples from the patient and five of his friends who had bathed in the same lake. In the next 2 years, clinical examinations, eosinophil count, and troponin levels were still normal.
Discussion
Acute schistosomiasis typically occurs in nonimmune persons 3 to 6 weeks after exposure during a bath in an endemic area. It is considered as a systemic hypersensitivity reaction against the migrating schistosomulae in tissue.1,6,7 It's usually self-limited and subsides over a few weeks, although life-threatening complications have been described that were mainly neurological.2,8,9 In our two cases, the first signs of AS appeared 26 and 27 days after the exposure, respectively. Our two cases show that cardiac complications can be added to the clinical spectrum of AS. These cardiac signs differ from those observed during the chronic phase of schistosomiasis.10
Our two patients presented with acute myocarditis during AS. In the first case, chest pain occurred at the same time of troponin level elevation and electrocardiogram abnormalities despite negative MRI. In the second case, the patient did not present any cardiac signs but the diagnosis of myocarditis relied on high troponin level and electrocardiogram repolarisation abnormalities. In both cases the patients were nonimmune travelers presenting with hypersensitivity symptoms and eosinophilia characteristics of AS.1,6,11 The involved species is S. haematobium according to the results of urinalysis in the second patient; the first patient bathed in the same area of Mali.
The involvement of the heart is barely described during AS.2,3 The first description was made during World War II, as a result of military operations on the island of Leyte in the Philippines, where more than 1500 American soldiers contracted AS related to S. japonicum.12 An analysis was made of 315 electrocardiograms and found varied repolarisation abnormalities: anomalies of T-waves (99%) or S-T segments (52%). These changes were attributed to the side-effects of antimony, the anti-schistosomiasis drugs used at that moment. According to our knowledge, only one study described cardiac features during an S. mansoni epidemic in Brazil.13 Among the 31 patients who had contracted the disease after bathing in a contaminated lake, 12 people (38.7%) had chest pain and six (19%) had pericarditis diagnosed with ultrasounds. Besides pericarditis, one case of endomyocardial fibrosis has been described in association with central nervous system disorders in a 25-year-old patient infected by Schistosoma mansoni.3 Thus S. mansoni, S. haematobium, as well as S. japonicum have been involved in heart involvement during AS.
Acute myocarditis during AS may be related to the immuno-allergic phenomena caused by the presence of schistosomulae in the blood circulation and tissue.6 Eosinophils play an essential role in the regulation of allergy and in the protection against helminthiasis with the aid of proteins contained in their granules such as the MBP (Major Basic protein) and the ECP (Eosinophilic Cationic Protein).14 Besides their beneficial role, eosinophils may be toxic for the host and especially for the heart. MBP and ECP are particularly important in those phenomena because of the cytopathogenic effect on endothelial cells and on cardiomyocytes, myocardial interstitial fibrosis, development of parietal thrombi, and then fibrosis of the endocardium and myocardium.15 A study performed in Nigeria found that 4 out of 89 patients treated for a endomyocardial fibrosis had presented initial symptoms compatible with acute myopericarditis, without formal evidence.16 In this study, endomyocardial fibrosis was more likely to be due to filariasis. Some authors suggest an auto-immune mechanism, not only eosinophils toxicity, to explain the organs involvement during AS. An immunological study of patients from two Brazilian outbreaks of AS (S. mansoni) demonstrated that immunological phenomena independent of eosinophilia played a role during acute schistosomiasis.13 Thus the symptoms, especially cardiac involvement, could also be mediated by the deposit of immune-complexes in the organs and pro-inflammatory cytokines as IL-2 and IFN-γ. This hypothesis supports the clinical presentation of our two cases, mainly in the second patient where the cerebral signs and splinter hemorrhages under nails are compatible with a diagnosis of vasculitis induced by immune-complexes and eosinophilia toxicity.2
Regardless of its pathophysiology, the treatment of AS remains a real challenge. Praziquantel is not effective during AS and, as described in our second patient, is associated with worsening of signs and symptoms in 40% of cases.17 In addition, praziquantel does not prevent the chronic phase of the disease.17,18 Instead, corticosteroids are the recommended treatment of severe complications occurring during AS.19 Obviously corticosteroids might attenuate the cardiac and neurologic toxicity of eosinophils and immune complexes and also the hypersensitivity reaction to parasite toxins and surface antigens.15,20 Furthermore, the optimal time for administration of specific antiparasitic treatment is not clearly defined. Praziquantel, the available drug to treat schistosomiasis, is ineffective against schistosomulae.21,22 Its administration should therefore be delayed until ova are detected in stools or urine, or, by default, several weeks after the acute stage.19
These two cases are the first describing acute myocarditis complicating AS. Current knowledge does not allow good explanations for the immuno-allergic phenomena that lead to myocarditis. However, we recommend routine screening with EKG and troponin assays in patients with acute schistosomiasis to identify asymptomatic myocarditis. In order to avoid potentially more dramatic events such as acute necrotizing myocarditis, cardiac rhythm abnormalities need appropriate management. Myocarditis during AS should be treated with corticosteroid but not praziquantel, which should be postponed until after cardiac recovery.
Footnotes
Authors' addresses: Loïc Epelboin, Service de Médecine, Centre Hospitalier de Mayotte, Mayotte Island, France, E-mail: epelboincrh@hotmail.fr. Stéphane Jauréguiberry, Martin Danis, François Bricaire, and Eric Caumes, Département des Maladies Infectieuses, Tropicales et Parasitaires, Assistance Publique des Hôpitaux de Paris, Paris, France, E-mails: stephane.jaureguiberry@psl.aphp.fr, martin.danis@psl.aphp.fr, francois.bricaire@psl.aphp.fr, and eric.caumes@psl.aphp.fr. Jean-Baptiste Estève and Michel Komajda, Département de Cardiologie, Assistance Publique des Hôpitaux de Paris, Paris, France, E-mails: jb_esteve@hotmail.com and michel.komajda@psl.aphp.fr.
References
- 1.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 2.Jauréguiberry S, Ansart S, Perez L, Danis M, Bricaire F, Caumes E. Acute neuroschistosomiasis: two cases associated with cerebral vasculitis. Am J Trop Med Hyg. 2007;76:964–966. [PubMed] [Google Scholar]
- 3.Sarazin M, Caumes E, Cohen A, Amarenco P. Multiple microembolic borderzone brain infarctions and endomyocardial fibrosis in idiopathic hypereosinophilic syndrome and in Schistosoma mansoni infestation. J Neurol Neurosurg Psychiatry. 2004;75:305–307. [PMC free article] [PubMed] [Google Scholar]
- 4.Niel G, Rosin G, Danis M, Gentilini M. Topographie particulière du marquage fluorescent au cours des bilharzioses à Schistosoma mansoni. Intérêt dans le diagnostic précoce. Pathol Biol (Paris) 1976;24:277–282. [in French] [PubMed] [Google Scholar]
- 5.Niel G, Sainte-Laudy J, Carme B, Boiteau A, Ben-Ismail R, Benveniste J, Gentilini M. The comparative value of the human basophil degranulation test and classical serology (immunofluorescence, haemagglutination, electrosyneresis, Vogel and Minning's Test) in the diagnosis of bilharzioses due to Schistosoma mansoni and Schistosoma haematobium. Ann Biol Clin (Paris) 1982;40:573–577. [PubMed] [Google Scholar]
- 6.Jauréguiberry S, Perez L, Paris L, Bricaire F, Danis M, Caumes E. Bilharzioses invasives. Presse Med. 2005;34:1641–1645. doi: 10.1016/s0755-4982(05)84241-1. [DOI] [PubMed] [Google Scholar]
- 7.Ross AG, Bartley PB, Sleigh AC, Olds GR, Li Y, Williams GM, McManus DP. Schistosomiasis. N Engl J Med. 2002;346:1212–1220. doi: 10.1056/NEJMra012396. [DOI] [PubMed] [Google Scholar]
- 8.Kane CA, Most H. Schistosomiasis of the central nervous system: experiences in World War II and a review of the literature. Arch Neurol Psychiatry. 1948;59:141–183. doi: 10.1001/archneurpsyc.1948.02300370003001. [DOI] [PubMed] [Google Scholar]
- 9.Pittella JE. Neuroschistosomiasis. Brain Pathol. 1997;7:649–662. doi: 10.1111/j.1750-3639.1997.tb01080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franco-Paredes C, Rouphael N, Mendez J, Folch E, Rodriguez-Morales AJ, Santos JI, Hurst JW. Cardiac manifestations of parasitic infections part 3: pericardial and miscellaneous cardiopulmonary manifestations. Clin Cardiol. 2007;30:277–280. doi: 10.1002/clc.20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross AG, Vickers D, Olds GR, Shah SM, McManus DP. Katayama syndrome. Lancet Infect Dis. 2007;7:218–224. doi: 10.1016/S1473-3099(07)70053-1. [DOI] [PubMed] [Google Scholar]
- 12.Most H, Kane CA, Lavietes PH, Schroeder EF, Behm A, Blum L, Katzin B, Hayman JM., Jr Schistosomiasis japonica in American military personnel; clinical studies of 600 cases during the first year after infection. Am J Trop Med Hyg. 1950;30:239–299. doi: 10.4269/ajtmh.1950.s1-30.239. [DOI] [PubMed] [Google Scholar]
- 13.de Jesus AR, Silva A, Santana LB, Magalhaes A, de Jesus AA, de Almeida RP, Rego MA, Burattini MN, Pearce EJ, Carvalho EM. Clinical and immunologic evaluation of 31 patients with acute schistosomiasis mansoni. J Infect Dis. 2002;185:98–105. doi: 10.1086/324668. [DOI] [PubMed] [Google Scholar]
- 14.Touze JE, Fourcade L, Heno P, Mafart B, Mourot S. Le coeur et l'éosinophile. Med Trop (mars) 1998;58:459–464. [PubMed] [Google Scholar]
- 15.Brito-Babapulle F. The eosinophilias, including the idiopathic hypereosinophilic syndrome. Br J Haematol. 2003;121:203–223. doi: 10.1046/j.1365-2141.2003.04195.x. [DOI] [PubMed] [Google Scholar]
- 16.Andy JJ, Ogunowo PO, Akpan NA, Odigwe CO, Ekanem IA, Esin RA. Helminth associated hypereosinophilia and tropical endomyocardial fibrosis (EMF) in Nigeria. Acta Trop. 1998;69:127–140. doi: 10.1016/s0001-706x(97)00125-3. [DOI] [PubMed] [Google Scholar]
- 17.Grandiere-Perez L, Ansart S, Paris L, Faussart A, Jaureguiberry S, Grivois JP, Klement E, Bricaire F, Danis M, Caumes E. Efficacy of praziquantel during the incubation and invasive phase of Schistosoma haematobium schistosomiasis in 18 travelers. Am J Trop Med Hyg. 2006;74:814–818. [PubMed] [Google Scholar]
- 18.Meltzer E, Artom G, Marva E, Assous MV, Rahav G, Schwartzt E. Schistosomiasis among travelers: new aspects of an old disease. Emerg Infect Dis. 2006;12:1696–1700. doi: 10.3201/eid1211.060340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jauréguiberry S, Caumes E. Neurological involvement during Katayama syndrome. Lancet Infect Dis. 2008;8:9–10. doi: 10.1016/S1473-3099(07)70299-2. [DOI] [PubMed] [Google Scholar]
- 20.Kirchhoff LV, Weiss LM, Wittner M, Tanowitz HB. Parasitic diseases of the heart. Front Biosci. 2004;9:706–723. doi: 10.2741/1255. [DOI] [PubMed] [Google Scholar]
- 21.Xiao SH, Booth M, Tanner M. The prophylactic effects of artemether against Schistosoma japonicum infections. Parasitol Today. 2000;16:122–126. doi: 10.1016/s0169-4758(99)01601-4. [DOI] [PubMed] [Google Scholar]
- 22.Brindley PJ, Sher A. The chemotherapeutic effect of praziquantel against Schistosoma mansoni is dependent on host antibody response. J Immunol. 1987;139:215–220. [PubMed] [Google Scholar]


