Abstract
Onchocerciasis control is currently based on mass ivermectin treatment. Unfortunately, this drug can induce serious adverse events (SAEs) in persons with high levels of Loa loa microfilaremia (> 30,000 microfilaria/mL). A means of preventing SAEs would be to treat at risk populations with a drug that would progressively reduce the microfilarial loads before administering ivermectin. Antimalarial drugs are a potential solution because they have shown some activity against various filarial species. A controlled trial was conducted to assess the effect of standard doses of quinine, chloroquine, amodiaquine, and artesunate on L. loa microfilaremia. Ninety-eight patients were randomly allocated into five groups (one for each drug and a control group) after stratification on microfilarial load. Loa loa microfilaremia was monitored on days 0, 3, 7, 15, 30, 60, and 90. No significant change in the loads was recorded in any of the treatment groups. A comprehensive review of the effects of antimalarial drugs against filariae is also provided.
Introduction
Community-directed treatment with ivermectin is the most cost-effective and sustainable strategy to control onchocerciasis.1 However, the participation of persons in distribution campaigns is limited in some areas because of the occurrence of rare but sometimes fatal serious adverse events (SAEs). The worst presentation of these events involves encephalopathy with neurologic disorders and coma.2 The main risk factor for these SAEs is the presence of a high Loa loa microfilaremia.3 Loiasis is highly endemic in rainforest areas of central Africa and its main clinical manifestations include migrating angioedema known as Calabar swelling, pruritus, and passage of the adult worm under the conjunctiva. Although the pathophysiology of the SAEs is not fully understood, it is known that ivermectin has a steady effect on L. loa microfilaremia, and clinical observations suggest that the neurologic disorders may be caused by obstruction of brain capillaries by paralyzed L. loa microfilaria (mf), or penetration into the central nervous system of mf trying to escape the effects of ivermectin.4,5 The risk of post-ivermectin encephalopathy is significantly increased when the L. loa count exceeds 30,000 mf/mL, and then increases exponentially with microfilaremia.3
One way of preventing SAEs would be to progressively reduce the L. loa microfilaremia below the risk level before treating with ivermectin. To achieve this reduction, a treatment that eliminates L. loa mf and that can easily be distributed on a large scale needs to be identified. Albendazole given at a dose of 200 mg twice a day for 21 days may reduce L. loa microfilaremia by 80% after 6 months,6 but this regimen is logistically challenging for mass treatment programs. Unfortunately, when this drug is given in a single dose of 600 mg, 400 mg once a day for 3 days, or 400 mg twice a day for 3 days, it has little or no effect on L. loa microfilaremia.7–9 Use of ivermectin at low single doses (25–50 µg/kg) do not give significantly different results from that observed with a standard dose (150 µg/kg).10,11
A number of studies have been conducted to evaluate the effects of antimalarial drugs on filariae. We have undertaken an exhaustive literature review on the topic, the results for which are presented in the form of detailed supplementary material (Supplementary Tables 1–6, available at www.ajtmh.org). The drugs tested included aryl-amino-alcohols (quinine, mefloquine), 4-aminoquinolines (chloroquine, amodiaquine, quinacrine), and primaquine. Different drugs showed variable efficacies against different filarial species (see Supplementary Tables 1–6 and Discussion for details). Results of in vivo trials of chloroquine and amodiaquine are of particular interest. Chloroquine resulted in a marked decrease in Onchocerca volvulus microfilarial loads, and had a macrofilaricidal effect when injected into the onchocercal nodules, but not when administered orally.12–14 Amodiaquine showed a macrofilaricidal effect on Wuchereria bancrofti in one patient treated with a high dose (2,400 mg over 4 days).15
Artemisinins are effective against Plasmodium spp. and various trematodes such as Schistosoma mansoni and Fasciola hepatica.16 To the best of our knowledge, these drugs have been tested only once against filariae. Artemisinin, when given at a dose of 100 mg/kg/day for 5 days in the rodent Meriones unguiculatus transplanted with adult Acanthocheilonema vitae and Brugia malayi, appeared to have little effect on the parasites.17 However, it is worth further exploring antifilarial activity of artemisinins because their mode of action against sensitive helminths is still not well known.18,19
Because of results of trials of chloroquine12–14 and amodiaquine15 on filariae, we selected these two compounds as good candidates for the present trial. Quinine has never been tested in vivo against filariae, but was selected because of its moderate effect in vitro on B. pahangi.20 Artesunate was chosen for the reason given in the preceding paragraph.
Safety considerations were also taken into account to select the drugs to be tested. This criterion was most important because should a compound show a significant effect on L. loa, then mass treatment using this drug may be used to reduce the L. loa microfilaremias in the population before distributing ivermectin. The main adverse reactions after quinine treatment is a complex of symptoms, known as cinchonism, which resolve with discontinuation of treatment.21 Quinine may induce serious adverse events such as blackwater fever or hypoglycemia. However, blackwater fever occurs mainly in persons with no or low immunity against malaria, which is exceptional in persons from malaria-endemic areas,22 and hypoglycemia occurs in patients with severe malaria23 or after high doses of quinine.24 Standard doses of chloroquine are generally well tolerated.21 Extrapyramidal symptoms and psychiatric reactions have been reported during chloroquine treatment but are exceptional.25 A study conducted in the United Kingdom indicated that amodiaquine may induce agranulocytosis and hepatitis with fatality rates of 1/31,300 and 1/15,650, respectively.26,27 However, because the combination amodiaquine plus artesunate is a first-line therapy for treatment of patients with uncomplicated malaria in Cameroon, we decided to keep amodiaquine in our trial. Lastly, artesunate, as are other derivatives of artemisinin, is remarkably safe and well tolerated.21 Following the above considerations, we decided to assess the effect of quinine, chloroquine, amodiaquine, and artesunate on L. loa microfilaremia.
Patients and Methods
Study area and patient selection.
The trial was conducted in six villages 40–80 km east of Yaoundé, in the Mefou and Afamba Division, Center Region, Cameroon. Because loiasis is highly endemic and onchocerciasis is hypoendemic in the area,11 no mass ivermectin distribution has ever been implemented in this region.
The first phase of the trial consisted of identification and recruitment of microfilaremic persons. Information on the objectives and procedures of the survey was given to the population of each selected village. Persons ≥ 15 years of age who volunteered to participate in the study underwent a parasitologic examination. Blood was collected between 11:00 am and 3:00 pm by fingerprick and a calibrated thick blood film (CTBF) of 50 µL was prepared for each volunteer. Slides were stained using Giemsa and L. loa mf were counted under a microscope. Subjects harboring ≥ 200 L. loa mf/mL and who signed an informed consent form underwent a clinical examination and were interviewed regarding their medical history. Those persons who had received antifilarial treatment during the previous year, persons with epilepsy, and pregnant or breastfeeding women were excluded from the trial. All remaining persons, when in good health, were enrolled in the trial.
Study design, randomization, treatment, and follow-up.
This was an open trial. All patients enrolled were stratified according to their L. loa microfilaremia by using the following classes: 200–7,999, 8,000–30,000, and > 30,000 mf/mL). Persons were then randomly allocated, for each stratum, into five treatment groups: one for each of the four antimalarial drugs tested and a control group.
On the first day (D0), a CTBF was prepared for every patient by using the same procedure as in the preliminary survey to re-assess their baseline L. loa microfilaremia immediately before treatment. Patients treated with quinine received 600 mg twice a day for 5 days, those treated with chloroquine received 600 mg once a day for 3 days, those treated with amodiaquine received 800 mg once a day for 3 days, and those treated with artesunate received 200 mg once a day for 3 days. We used generic quinine and chloroquine tablets and Falcimon kits® (Cipla, Mumbai, India) containing separate amodiaquine and artesunate tablets. Patients in the control group received a single dose of iron-folic acid (four tablets each containing 65 mg of iron and 0.25 mg of folic acid). Treatments were administered under the direct observation of investigators. Surveillance of potential adverse events was conducted on a daily basis during the treatment period and then at each subsequent visit.
Loa loa microfilaremia was monitored by examination of CTBF on days 3, 7, 15, 30, 60, and 90. To reduce the effect of daytime variability in microfilaremia,28 the samples were collected from each patient at approximately the same time as it was collected on D0. The slides were examined independently by two microscopists in a blind manner relative to treatment, and the microfilaremia counts were considered valid when the two results differed by less than 10%. The average value was used for statistical analyses.
Statistical analyses.
Microfilaremia was compared between the five groups at each examination round by using the Kruskal-Wallis test. To compare the longitudinal trend in microfilaremia between treatments, we performed a regression analysis using a linear mixed-effects model, which adequately handles cross-sectional measures taken repeatedly on the same persons. The outcome variable was the L. loa microfilaremia. The regressors were the treatment group and the day of sampling, which were entered in the model as main factors and as interaction terms. To account for non-normality of microfilarial load distribution, these loads were logarithmically transformed. Intra-individual correlation between consecutive measures and potential inter-individual variation around the average trend were accounted for by a random effect set at the subject level. Analyses were performed using Stata software version 10.0 (Stata Corp., College Station, TX).
Ethical agreement.
The trial was reviewed and approved by the National Ethics Committee of Cameroon. Administrative authorization was provided by the Cameroon Ministry of Public Health. All patients benefited from general health checks during the follow-up.
Results
Overall, 901 persons were screened during the preliminary survey. The prevalence of L. loa microfilaremia was 27.1%. A total of 142 persons were eligible for the trial (microfilaremia ≥ 200 mf/mL) and allocated into the five groups. Only 98 patients (50 female patients and 48 male patients) were present on D0 and finally enrolled in the trial. The mean age of the study population was 52.6 years (range = 14–80 years).
All patients included in the trial received the full treatment, and no serious adverse events were reported. All events recorded were mild, and related mostly to amodiaquine, chloroquine, and quinine. In the amodiaquine group, six patients had asthenia, one had a headache, one had anorexia and one had ocular itching. Among persons treated with quinine, three reported asthenia, one reported dizziness, and one reported tinnitus. In the chloroquine group, three patients had itching, one had asthenia, one had tinnitus, and one had insomnia. Among the persons treated with artesunate, two reported asthenia and one reported itching. All adverse events spontaneously regressed within 24–48 hours after the last dose of drug.
At D0, microfilaremia levels were similar in the different treatment groups. No statistically significant difference was observed between the groups at any of the subsequent examinations (Table 1). Individual changes with time in each treatment group are shown in Figure 1. No significant decrease in microfilaremia was observed in any of the groups during the 90-day follow-up. This finding was confirmed by results of the mixed-effects linear model (Table 2).
Table 1.
Loa loa microfilaremia in the five treatment groups, at different times of follow-up*
| Treatment group† | Day 0 | Day 3 | Day 7 | Day 15 | Day 30 | Day 60 | Day 90 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | GM (range) | No. | GM (range) | No. | GM (range) | No. | GM (range) | No. | GM (range) | No. | GM (range) | No. | GM (range) | |
| Quinine | 17 | 6,540 (860–140,400) | 17 | 6,104 (500–154,800) | 16 | 6,359 (400–111,760) | 15 | 5,696 (740–100,400) | 17 | 5,010 (480–140,200) | 13 | 4,513 (720–34,540) | 11 | 4,897 (540–147,360) |
| Chloroquine | 25 | 4,062 (220–87,640) | 24 | 3,933 (100–120,620) | 19 | 4,245 (180–39,280) | 23 | 3,385 (80–93,860) | 25 | 3,379 (100–80,500) | 19 | 1,979 (60–29,980) | 18 | 3,448 (140–53,640) |
| Artesunate | 23 | 6,266 (600–24,540) | 21 | 5,684 (580–24,180) | 18 | 7,051 (780–31,420) | 23 | 6,659 (620–33,800) | 23 | 6,546 (320–31,100) | 15 | 6,320 (340–31,000) | 13 | 5,359 (500–30,760) |
| Amodiaquine | 18 | 5,980 (200–30,400) | 16 | 5,731 (1,900–26,320) | 16 | 5,560 (200–41,480) | 16 | 4,776 (180–29,060) | 18 | 5,130 (380–29,700) | 11 | 7,229 (320–28,460) | 7 | 4,641 (520–16,460) |
| Control | 15 | 3,911 (500–154,800) | 14 | 3,574 (280–21,260) | 12 | 4,235 (340–26,760) | 14 | 3,689 (80–24,800) | 15 | 3,145 (120–15,300) | 12 | 3,659 (260–16,060) | 10 | 4,136 (480–18,820) |
| P‡ | 0.68 | 0.79 | 0.73 | 0.72 | 0.51 | 0.15 | 0.95 | |||||||
No. = number of patients; GM = geometric mean of Loa microfilaremia (microfilariae per milliliter).
Quinine = 1,200 mg/day for 5 days; chloroquine = 600 mg/day for 3 days; artesunate = 200 mg/day for 3 days; amodiaquine = 800 mg/day for 3 days; control = iron-folic acid tablets.
Results of Kruskal-Wallis test for the time point.
Figure 1.
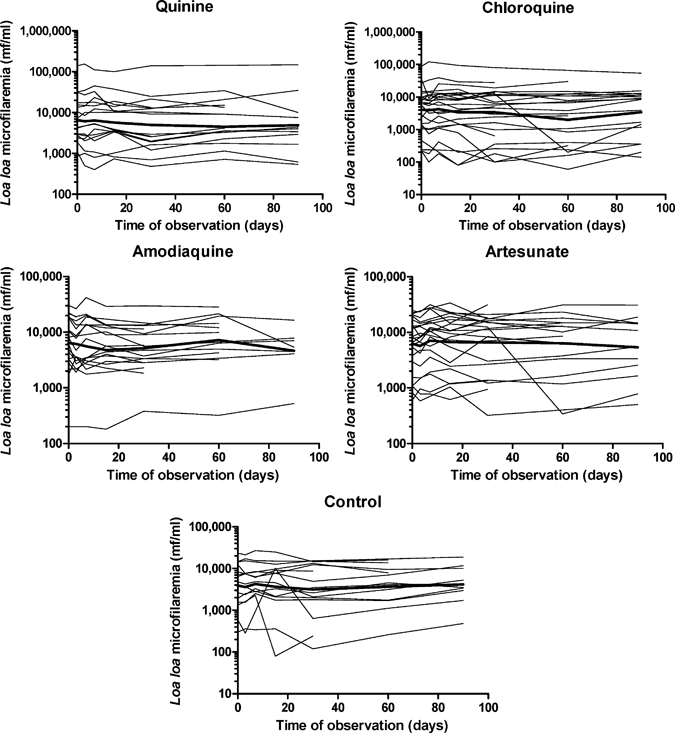
Individual follow-up of Loa loa microfilaremia in the five treatment groups (quinine, 1,200 mg/day for 5 days; chloroquine, 600 mg/day for 3 days; amodiaquine, 800 mg/day for 3 days; artesunate, 200 mg/day for 3 days; control group, persons receiving iron-folic acid tablets). Thick lines represent the geometric mean numbers of Loa loa microfilaria per milliliter of blood.
Table 2.
Results of the multivariate mixed-effect regression model of microfilaremia by treatment group and time
| Variables | Estimates | P | 95% confidence interval |
|---|---|---|---|
| Treatment | |||
| Quinine | 0.4810 | 0.321 | –0.4684 to 10.4305 |
| Chloroquine | –0.0029 | 0.995 | –0.8784 to 0.8727 |
| Amodiaquine | 0.3564 | 0.456 | –0.5811 to 10.2939 |
| Artesunate | 0.5466 | 0.229 | –0.3432 to 10.4364 |
| Time, in days | 0.0007 | 0.771 | –0.0039 to 0.0052 |
| Interaction terms | |||
| Quinine × time | –0.0013 | 0.676 | –0.0076 to 0.0049 |
| Chloroquine × time | –0.0027 | 0.357 | –0.0085 to 0.0031 |
| Amodiaquine × time | –0.0001 | 0.981 | –0.0066 to 0.0064 |
| Artesunate × time | –0.0017 | 0.575 | –0.0077 to 0.0043 |
| Constant term | 50.2484 | 0.0001 | 40.5562 to 50.9406 |
| Random effects parameters | |||
| Time | 0.0068 | – | 0.0053 to 0.0088 |
| Intercept | 10.3520 | – | 10.1677 to 10.5654 |
| Residual | 0.3753 | – | 0.3503 to 0.4021 |
Discussion
To the best of our knowledge, the present trial is the first ever conducted to evaluate the effect of antimalarial drugs on L. loa. We included patients harboring a wide range of L. loa microfilaremias, including some who had less than 8,000 mf/mL, and thus would not be at risk for developing marked or serious adverse events after ivermectin treatment. Because of the initial stratification procedure, the geometric mean L. loa microfilaremia did not differ significantly between the groups before treatment.
The drug regimens we used were not the exact recommendations for the treatment of patients with malaria but were adapted so as to be applicable (in terms of number of doses received per day and numbers of days) as part of a mass treatment regimen. The time points of follow-up were chosen so as to be able to detect either a rapid decrease in the microfilarial count (from D1 to D15), as recorded in the trials of chloroquine on O. volvulus,12,13 or a more progressive effect (on D30 and D90), as observed in one patient infected with W. bancrofti treated with amodiaquine.15 The examinations on D90 would have enabled us to detect an effect limited to the adult worms, whether a macrofilaricidal effect or an interruption of the release of new mf. Knowing that the lifespan of Loa mf is approximately 6–12 months,29 the microfilarial load on D90 would be reduced by 25–50%.
In light of our objective (to bring about a slow decrease in the L. loa microfilaremia), even a moderate or delayed effect would have been desirable. Unfortunately, our results show that none of the four antimalarial regimens tested has a significant effect on L. loa microfilaremia. The fact that no decrease was recorded at any time point suggests that the drugs at the doses administered were inactive on mf and adult worms.
Given the promising results previously obtained with chloroquine, its inefficacy against L. loa is particularly disappointing. Chloroquine was first tested in vitro against mf and adults of O. volvulus and O. gutturosa,20,30–35 and then in vivo in onchocerciasis patients.12–14 This drug, when given per os, appeared to bring about a marked decrease in microfilarial loads and had a macrofilaricidal effect when injected into the onchocercal nodules but not when administered orally. In addition, it was shown that after oral treatment, adult O. volvulus accumulated the drug to high concentrations.36 Chloroquine was also tested in vitro against B. pahangi, B. patei, Dirofilaria immitis and A. vitae,20,33,34,37 and in vivo against D. immitis.38 It was less effective or less active on these species than on Onchocerca spp.
The discrepancy in the results obtained for O. volvulus and L. loa may be caused by differences in the localization of parasites, and thus in the level of exposure to the drug. Onchocerca volvulus mf live in the dermis, whereas L. loa mf circulate in the bloodstream. With regard to the adult stages, O. volvulus macrofilaria are located in subcutaneous or deep nodules, whereas those of L. loa are found in the connective tissue under the skin and the fascial layers overlying the somatic muscles. Because chloroquine concentrations observed in the skin are much higher than those in plasma,36,39 one may assume that for a given oral dose of chloroquine mf of O. volvulus are more exposed to the drug than those of L. loa. With regard to adult worms, it has been shown that after oral treatment, O. volvulus accumulates chloroquine to concentrations more than 100 times higher than that measured in the skin or the nodular tissue around the adult worms.36 Despite this finding, no macrofilaricidal effect was observed after oral chloroquine treatment,13 and adult O. volvulus were killed only when the drug was injected into the nodule.14 Thus, high concentrations are required to obtain a macrofilaricidal effect, which may also be true for L. loa, whose adults are often located in poorly vascularized tissues.
The lack of effect of amodiaquine on L. loa is also disappointing. The possible antifilarial activity of amodiaquine has also been studied in vitro against B. pahangi20 and Breinlia booliati40 and in vivo against O. volvulus,41 Wuchereria bancrofti,15,42 and Litomosoides carinii.43–45 A macrofilaricidal effect was reported for all species tested, except for O. volvulus (see supplementary material for details). With regard to W. bancrofti, a progressive decrease in microfilaremia (from 114 mf/mL on D0 to 16 mf/mL on D90) was observed in a patient treated with a dose (2,400 mg over 4 days) similar to that administered as part of the present trial.15 We have no explanation for the absence of an effect of amodiaquine on L. loa.
The lack of an effect obtained with artesunate confirms previous observations in animal models, which showed little antifilarial effect for this drug.17 This finding may be related to the fact that artesunate has a shorter life-time (less than one hour) than chloroquine or amodiquine.
Other antimalarial compounds that had been tested against filariae were not used in the present trial because of safety reasons or because they were not available commercially. Quinacrine showed no effect against O. volvulus mf in vivo,46 but had a moderate effect against B. pahangi adult females in vitro.20 Attempts to increase the antifilarial activity of 4-aminoquinolines were made by synthesizing derivatives,40,47,48 but the results were disappointing. The activity of mefloquine was evaluated in vitro against B. patei mf and against adult stages of O. gutturosa,49 B. malayi, and B. patei.37 The drug seemed to have a paralyzing effect but for the latter two species this effect disappeared when fetal calf serum and human serum were added to the medium. Lastly, primaquine was shown to have a marked effect against B. pahangi adult worms in vitro20 and a delayed effect on W. bancrofti microfilaremia in humans.50
Despite the disappointing results of the present trial, it would be worth investigating the modes of action of antimalarial drugs against filariae. The detailed modes of action of quinoline-containing drugs on Plasmodium spp. seem to differ according to the compounds, but in each case they rely on the mechanisms related to hemoglobin digestion.51,52 Interference with hemozoin formation also explains at least partly the action of antimalarial drugs on Schistosoma spp.53–57 However, what occurs in blood-feeding helminths such as Schistosoma spp. may not be true for filariae. Even if iron was found in the intestinal epithelial cells of adult Onchocerca sp.,58–60 and blood feeding has been observed in young adult L. sigmodontis,61 it is thought that filariae feed mainly through the body wall.62 Thus, the antifilarial activity of quinoline drugs probably results from mechanisms different from those occurring in other sensitive parasites. To our knowledge, this activity has been investigated only by VandeWaa and others,63 who evaluated the effects of intermediary metabolites and electron transport inhibitors on the paralyzing effects of chloroquine on B. pahangi and O. volvulus. Their results suggested that chloroquine may inhibit aerobic energy metabolism in the filariae, possibly at the level of electron transport.63 Unfortunately, these studies were not continued.
We also think that despite the lack of effect obtained in the present study with artesunate, it would also be worth considering artemisinin-derived compounds for future trials against filariae. This suggestion is justified because artemisinin reacts with translationally controlled tumor protein (TCTP) in Plasmodium spp.,64 and homologs of TCTP are also present in S. mansoni,65 B. malayi, and W. bancrofti.66
As mass distribution of ivermectin extends progressively to cover all areas endemic for onchocerciasis, the probability of observing post-ivermectin SAEs is decreasing because most people living in these foci and at risk would have already taken this drug at least once. Nonetheless, ivermectin is soon to be distributed outside these areas as part of the lymphatic filariasis elimination program. Even if the geographic distribution of lymphatic filariasis in central Africa is not well known, it is likely that many areas are co-endemic with loiasis. For this reason, research should continue to identify a drug able to progressively reduce high L. loa microfilarial loads.
Supplementary Material
Acknowledgements
We thank the population of the Awae Health District who participated in this trial and J. Bopda and S. Bickmen Tchana who examined the CBS.
Note: Supplemental tables appear at www.ajtmh.org.
Footnotes
Financial support: The present study was funded by Mectizan Donation Program as part of the activities of the Filariasis Research Center sponsored by MDP.
Authors' addresses: Joseph Kamgno and Patrick Nguipdop Djomo, Filariasis Research Centre and Faculty of Medicine and Biomedical Sciences, University of Yaounde I, Yaounde, Cameroon, E-mails: jkamgno@yahoo.fr and ndpatrick@yahoo.fr. Sébastien D. Pion and Michel Boussinesq, Unité Mixte de Recherche 145, Institut de Recherche pour le Développement and Université Montpellier 1, Montpellier, France, E-mails: sebastien.pion@ird.fr and michel.boussinesq@ird.fr. Björn Thylefors, Mectizan Donation Program, Decatur, GA, E-mail: bthylefors@gmail.com.
References
- 1.Sékétéli A, Adeoye G, Eyamba A, Nnoruka E, Drameh P, Amazigo UV, Noma M, Agboton F, Aholou Y, Kale OO, Dadzie KY. The achievements and challenges of the African Programme for Onchocerciasis Control (APOC) Ann Trop Med Parasitol. 2002;96(Suppl 1):S15–S28. doi: 10.1179/000349802125000628. [DOI] [PubMed] [Google Scholar]
- 2.Boussinesq M, Gardon J, Gardon-Wendel N, Chippaux JP. Clinical picture, epidemiology and outcome of Loa-associated serious adverse events related to mass ivermectin treatment of onchocerciasis in Cameroon. Filaria J. 2003;2(Suppl 1):S4. doi: 10.1186/1475-2883-2-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gardon J, Gardon-Wendel N, Demanga-Ngangue, Kamgno J, Chippaux JP, Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet. 1997;350:18–22. doi: 10.1016/S0140-6736(96)11094-1. [DOI] [PubMed] [Google Scholar]
- 4.Fobi G, Gardon J, Santiago M, Ngangue D, Gardon-Wendel N, Boussinesq M. Ocular findings after ivermectin treatment of patients with high Loa loa microfilaremia. Ophthalmic Epidemiol. 2000;7:27–39. [PubMed] [Google Scholar]
- 5.Boussinesq M, Kamgno J, Pion SD, Gardon J. What are the mechanisms associated with post-ivermectin serious adverse events? Trends Parasitol. 2006;22:244–246. doi: 10.1016/j.pt.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Klion AD, Massougbodji A, Horton J, Ekoué S, Lanmasso T, Ahouissou NL, Nutman TB. Albendazole in human loiasis: results of a double-blind, placebo-controlled trial. J Infect Dis. 1993;168:202–206. doi: 10.1093/infdis/168.1.202. [DOI] [PubMed] [Google Scholar]
- 7.Kamgno J, Boussinesq M. Effect of a single dose (600 mg) of albendazole on Loa loa microfilaraemia. Parasite. 2002;9:59–63. doi: 10.1051/parasite/200209159. [DOI] [PubMed] [Google Scholar]
- 8.Tabi TE, Befidi-Mengue R, Nutman TB, Horton J, Folefack A, Pensia E, Fualem R, Fogako J, Gwanmesia P, Quakyi I, Leke R. Human loiasis in a Cameroonian village: a double-blind, placebo-controlled, crossover clinical trial of a three-day albendazole regimen. Am J Trop Med Hyg. 2004;71:211–215. [PubMed] [Google Scholar]
- 9.Tsague-Dongmo L, Kamgno J, Pion SD, Moyou-Somo R, Boussinesq M. Effects of a 3-day regimen of albendazole (800 mg daily) on Loa loa microfilaraemia. Ann Trop Med Parasitol. 2002;96:707–715. doi: 10.1179/000349802125001933. [DOI] [PubMed] [Google Scholar]
- 10.Kamgno J, Gardon J, Boussinesq M. Essai de prévention des encéphalopathies à Loa loa post-ivermectine par l'administration d'une faible dose initiale. Med Trop (Mars) 2000;60:275–277. [PubMed] [Google Scholar]
- 11.Kamgno J, Pion SD, Tejiokem MC, Twum-Danso NA, Thylefors B, Boussinesq M. Randomized, controlled, double-blind trial with ivermectin on Loa loa microfilaraemia: efficacy of a low dose (~25 µg/kg) versus current standard dose (150 µg/kg) Trans R Soc Trop Med Hyg. 2007;101:777–785. doi: 10.1016/j.trstmh.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Burnham G, Harries A, Macheso A, Wirima J, Molyneux M. Chloroquine-induced pruritus in Malawi: lack of association with onchocerciasis. Trans R Soc Trop Med Hyg. 1989;83:527–528. doi: 10.1016/0035-9203(89)90278-2. [DOI] [PubMed] [Google Scholar]
- 13.Guderian RH, Anselmi M, Beck BJ, Mackenzie CD, Williams JF, Proaño S, Jr, Cooper PJ. The effect of antimalarial chloroquine therapy and prophylaxis on concurrent infection with Onchocerca volvulus in Ecuador. Trans R Soc Trop Med Hyg. 1991;85:634–638. doi: 10.1016/0035-9203(91)90372-6. [DOI] [PubMed] [Google Scholar]
- 14.Guderian RH, Anselmi M, Cooper PJ, Chico ME. Macrofilaricidal effects of chloroquine on adult Onchocerca volvulus by local infiltration of palpable onchocercal nodules. Rev Soc Bras Med Trop. 1997;30:469–473. doi: 10.1590/s0037-86821997000600005. [DOI] [PubMed] [Google Scholar]
- 15.McMahon JE. Preliminary screening of antifilarial activity of levamisole and amodiaquine on Wuchereria bancrofti. Ann Trop Med Parasitol. 1979;73:465–472. doi: 10.1080/00034983.1979.11687286. [DOI] [PubMed] [Google Scholar]
- 16.Keiser J, Utzinger J. Artemisinins and synthetic trioxolanes in the treatment of helminth infections. Curr Opin Infect Dis. 2007;20:605–612. doi: 10.1097/QCO.0b013e3282f19ec4. [DOI] [PubMed] [Google Scholar]
- 17.Kinnamon KE, Klayman DL, Poon BT, McCall JW, Dzimianski MT, Rowan SJ. Filariasis testing in a jird model: new drug leads from some old standbys. Am J Trop Med Hyg. 1994;51:791–796. doi: 10.4269/ajtmh.1994.51.791. [DOI] [PubMed] [Google Scholar]
- 18.Xiao SH. Development of antischistosomal drugs in China, with particular consideration to praziquantel and the artemisinins. Acta Trop. 2005;96:153–167. doi: 10.1016/j.actatropica.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Glanfield A, McManus DP, Anderson GJ, Jones MK. Pumping iron: a potential target for novel therapeutics against schistosomes. Trends Parasitol. 2007;23:583–588. doi: 10.1016/j.pt.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.VandeWaa EA, Bennett JL, Williams JF, Satti MZ, Geary TG. Anti-filarial effects of nine quinoline-containing drugs on adult filariae in vitro. J Parasitol. 1989;75:367–372. [PubMed] [Google Scholar]
- 21.World Health Organization . Guidelines for the Treatment of Malaria. WHO/HTM/MAL/2006.1108. Geneva: World Health Organization; 2006. [Google Scholar]
- 22.Rogier C, Imbert P, Tall A, Sokhna C, Spiegel A, Trape JF. Epidemiological and clinical aspects of blackwater fever among African children suffering frequent malaria attacks. Trans R Soc Trop Med Hyg. 2003;97:193–197. doi: 10.1016/s0035-9203(03)90116-7. [DOI] [PubMed] [Google Scholar]
- 23.White NJ, Warrell DA, Chanthavanich P, Looareesuwan S, Warrell MJ, Krishna S, Williamson DH, Turner RC. Severe hypoglycemia and hyperinsulinemia in falciparum malaria. N Engl J Med. 1983;309:61–66. doi: 10.1056/NEJM198307143090201. [DOI] [PubMed] [Google Scholar]
- 24.Limburg PJ, Katz H, Grant CS, Service FJ. Quinine-induced hypoglycemia. Ann Intern Med. 1993;119:218–219. doi: 10.7326/0003-4819-119-3-199308010-00007. [DOI] [PubMed] [Google Scholar]
- 25.Phillips-Howard PA, ter Kuile FO. CNS adverse events associated with antimalarial agents. Fact or fiction? Drug Saf. 1995;12:370–383. doi: 10.2165/00002018-199512060-00003. [DOI] [PubMed] [Google Scholar]
- 26.Phillips-Howard PA, West LJ. Serious adverse drug reactions to pyrimethamine-sulphadoxine, pyrimethamine-dapsone and to amodiaquine in Britain. J R Soc Med. 1990;83:82–85. doi: 10.1177/014107689008300208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor WR, White NJ. Antimalarial drug toxicity: a review. Drug Saf. 2004;27:25–61. doi: 10.2165/00002018-200427010-00003. [DOI] [PubMed] [Google Scholar]
- 28.Kamgno J, Pion SD, Mackenzie CD, Thylefors B, Boussinesq M. Loa loa microfilarial periodicity in ivermectin treated patients: comparison between those developing, and those free of, serious adverse events. Am J Trop Med Hyg. 2009;81:1056–1061. doi: 10.4269/ajtmh.2009.09-0356. [DOI] [PubMed] [Google Scholar]
- 29.Duke BL. Studies on loiasis in monkeys. III. The pathology of the spleen in drills (Mandrillus leucophaeus) infected with Loa. Ann Trop Med Parasitol. 1960;54:141–146. [PubMed] [Google Scholar]
- 30.Salazar Mallén M, González Barranco D. Investigación del efecto filaricida sobre microfilarias de Onchocerca volvulus de diferentes medicamentos. Rev Invest Salud Publica. 1966;26:297–300. [PubMed] [Google Scholar]
- 31.Guderian RH, Williams JF, Mackenzie CD. Chloroquine and onchocerciasis. Lancet. 1986;1:807–808. doi: 10.1016/s0140-6736(86)91823-4. [DOI] [PubMed] [Google Scholar]
- 32.Townson S, Connelly C, Dobinson A, Muller R. Drug activity against Onchocerca gutturosa males in vitro: a model for chemotherapeutic research on onchocerciasis. J Helminthol. 1987;61:271–281. doi: 10.1017/s0022149x00010178. [DOI] [PubMed] [Google Scholar]
- 33.Satti MZ, VandeWaa EA, Bennett JL, Williams JF, Conder GA, McCall JW. Comparative effects of anthelmintics on motility in vitro of Onchocerca gutturosa, Brugia pahangi and Acanthocheilonema vitae. Trop Med Parasitol. 1988;39:480–483. [PubMed] [Google Scholar]
- 34.VandeWaa EA, Williams JF, Geary TG. pH-dependent uptake and macrofilaricidal effects of chloroquine on adult filarial parasites in vitro. Exp Parasitol. 1989;68:31–39. doi: 10.1016/0014-4894(89)90005-2. [DOI] [PubMed] [Google Scholar]
- 35.Ukaga CN, Ezigbo JC, Onyeka PI, Anosike JC, Nwoke BE. The effect of chloroquine on the male worms of Onchocerca volvulus in vitro. Nigerian J Parasitol. 2003;24:71–76. [Google Scholar]
- 36.Mahmoud BM, Vandewaa EA, Geary TG, Guderian R, Williams JF. Uptake of chloroquine by Onchocerca volvulus in vivo and in vitro. Ann Trop Med Parasitol. 1991;85:523–528. doi: 10.1080/00034983.1991.11812603. [DOI] [PubMed] [Google Scholar]
- 37.Walter RD, Wittich RM, Kuhlow F. Filaricidal effect of mefloquine on adults and microfilariae of Brugia patei and Brugia malayi. Trop Med Parasitol. 1987;38:55–56. [PubMed] [Google Scholar]
- 38.Desowitz RS, Palumbo NE, Perri SF. The occurrence of an adverse reaction to chloroquine in Dirofilaria immitis-infected dogs. Trop Med Parasitol. 1983;34:27–29. [PubMed] [Google Scholar]
- 39.Olatunde IA. Chloroquine concentrations in the skin of rabbits and man. Br J Pharmacol. 1971;43:335–340. [PMC free article] [PubMed] [Google Scholar]
- 40.Go ML, Ngiam TL, Wan AS. Synthesis of some novel amodiaquine analogues as potential antimalarial and antifilarial compounds. J Med Chem. 1981;24:1471–1475. doi: 10.1021/jm00144a020. [DOI] [PubMed] [Google Scholar]
- 41.Kale OO. Clinical trials of amodiaquine in onchocerciasis. Bull World Health Organ. 1982;60:929–932. [PMC free article] [PubMed] [Google Scholar]
- 42.Chakravertty RK, Rai RN, Chand P, Dey KP, Rao CK. Amodiaquine as a filaricide. J Commun Dis. 1982;14:306–308. [PubMed] [Google Scholar]
- 43.Thompson PE, Boche L, Blair LS. Effects of amodiaquine against Litomosoides carinii in gerbils and cotton rats. J Parasitol. 1968;54:834–837. [Google Scholar]
- 44.Lämmler G, Herzog H, Schütze HR. Chemotherapeutic studies on Litomosoides carinii infection of Mastomys natalensis. 3. The activity of drugs against adult parasites. Bull World Health Organ. 1971;44:765–770. [PMC free article] [PubMed] [Google Scholar]
- 45.Lämmler G. Experimental chemotherapy and chemoprophylaxis of filariasis. Pestic Sci. 1977;8:543–576. [Google Scholar]
- 46.Kale OO. Mepacrine—ineffective in onchocerciasis. Trop Med Parasitol. 1980;31:365–366. [PubMed] [Google Scholar]
- 47.Elslager EF, Perricone SC, Tendick FH. Antifilarial agents. I. Effects of 4-[(7-chloro-4-quinolyl)amino]-alpha-(mono- and dialkylamino)-o-cresols and related compounds against Litomosoides carinii in gerbils. J Med Chem. 1969;12:965–969. doi: 10.1021/jm00306a001. [DOI] [PubMed] [Google Scholar]
- 48.Tewari S, Chauhan PM, Bhaduri AP, Fatima N, Chatterjee RK. Syntheses and antifilarial profile of 7-chloro-4-(substituted amino) quinolines: a new class of antifilarial agents. Bioorg Med Chem Lett. 2000;10:1409–1412. doi: 10.1016/s0960-894x(00)00255-9. [DOI] [PubMed] [Google Scholar]
- 49.Townson S, Dobinson AR, Townsend J, Siemienska J, Zea-Flores G. The effects of ivermectin used in combination with other known anti-parasitic drugs on adult Onchocerca gutturosa and O. volvulus in vitro. Trans R Soc Trop Med Hyg. 1990;84:411–416. doi: 10.1016/0035-9203(90)90340-k. [DOI] [PubMed] [Google Scholar]
- 50.Ravindranathan TC, Roychowdhury SP, Russel S, Rao CK. Filaricidal effect of primaquine. J Commun Dis. 1982;14:88–89. [PubMed] [Google Scholar]
- 51.Famin O, Ginsburg H. Differential effects of 4-aminoquinoline-containing antimalarial drugs on hemoglobin digestion in Plasmodium falciparum-infected erythrocytes. Biochem Pharmacol. 2002;63:393–398. doi: 10.1016/s0006-2952(01)00878-4. [DOI] [PubMed] [Google Scholar]
- 52.Roberts L, Egan TJ, Joiner KA, Hoppe HC. Differential effects of quinoline antimalarials on endocytosis in Plasmodium falciparum. Antimicrob Agents Chemother. 2008;52:1840–1842. doi: 10.1128/AAC.01478-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oliveira MF, d'Avila JCP, Tempone AJ, Corrêa Soares JB, Rumjanek FD, Ferreira-Pereira A, Ferreira ST, Oliveira PL. Inhibition of heme aggregation by chloroquine reduces Schistosoma mansoni infection. J Infect Dis. 2004;190:843–852. doi: 10.1086/422759. [DOI] [PubMed] [Google Scholar]
- 54.Keiser J, Chollet J, Xiao SH, Mei JY, Jiao PY, Utzinger J, Tanner M. Mefloquine—an aminoalcohol with promising antischistosomal properties in mice. PLoS Negl Trop Dis. 2009;3:e350. doi: 10.1371/journal.pntd.0000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corrêa Soares JB, Menezes D, Vannier-Santos MA, Ferreira-Pereira A, Almeida GT, Venancio TM, Verjovski-Almeida S, Zishiri VK, Kuter D, Hunter R, Egan TJ, Oliveira MF. Interference with hemozoin formation represents an important mechanism of schistosomicidal action of antimalarial quinoline methanols. PLoS Negl Trop Dis. 2009;3:e477. doi: 10.1371/journal.pntd.0000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang CW, Xiao SH, Utzinger J, Chollet J, Keiser J, Tanner M. Histopathological changes in adult Schistosoma japonicum harbored in mice treated with a single dose of mefloquine. Parasitol Res. 2009;104:1407–1416. doi: 10.1007/s00436-009-1341-0. [DOI] [PubMed] [Google Scholar]
- 57.Xiao SH, Chollet J, Utzinger J, Mei JY, Jiao PY, Keiser J, Tanner M. Effect of single-dose oral mefloquine on the morphology of adult Schistosoma japonicum in mice. Parasitol Res. 2009;105:853–861. doi: 10.1007/s00436-009-1471-4. [DOI] [PubMed] [Google Scholar]
- 58.Franz M, Büttner DW. The fine structure of adult Onchocerca volvulus. V. The digestive tract and the reproductive system of the female worm. Trop Med Parasitol. 1983;34:155–161. [PubMed] [Google Scholar]
- 59.Franz M, Copeman DB. The fine structure of male and female Onchocerca gibsoni. Trop Med Parasitol. 1988;37:466–468. [PubMed] [Google Scholar]
- 60.Determan A, Mehlhorn H, Ghaffar FA. Electron microscope observations on Onchocerca ochengi and O. fasciata (Nematoda: Filarioidea) Parasitol Res. 1997;83:591–603. doi: 10.1007/s004360050303. [DOI] [PubMed] [Google Scholar]
- 61.Attout T, Babayan S, Hoerauf A, Taylor DW, Kozek WJ, Martin C, Bain O. Blood-feeding in the young adult filarial worms Litomosoides sigmodontis. Parasitology. 2005;130:421–428. doi: 10.1017/s0031182004006651. [DOI] [PubMed] [Google Scholar]
- 62.Howells RE, Chen SN. Brugia pahangi: feeding and nutrient uptake in vitro and in vivo. Exp Parasitol. 1981;51:42–58. doi: 10.1016/0014-4894(81)90041-2. [DOI] [PubMed] [Google Scholar]
- 63.VandeWaa EA, Williams JF, Geary TG. Effects of intermediary metabolites and electron transport inhibitors on action of chloroquine on Brugia pahangi and Onchocerca volvulus. Biochem Pharmacol. 1989;38:4327–4332. doi: 10.1016/0006-2952(89)90533-9. [DOI] [PubMed] [Google Scholar]
- 64.Bhisutthibhan J, Pan XQ, Hossler PA, Walker DJ, Yowell CA, Carlton J, Dame JB, Meshnick SR. The Plasmodium falciparum translationally controlled tumor protein homolog and its reaction with the antimalarial drug artemisinin. J Biol Chem. 1998;273:16192–16198. doi: 10.1074/jbc.273.26.16192. [DOI] [PubMed] [Google Scholar]
- 65.Rao KV, Chen L, Gnanasekar M, Ramaswamy K. Cloning and characterization of a calcium-binding, histamine-releasing protein from Schistosoma mansoni. J Biol Chem. 2002;277:31207–31213. doi: 10.1074/jbc.M204114200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gnanasekar M, Rao KV, Chen L, Narayanan RB, Geetha M, Scott AL, Ramaswamy K, Kaliraj P. Molecular characterization of a calcium binding translationally controlled tumor protein homologue from the filarial parasites Brugia malayi and Wuchereria bancrofti. Mol Biochem Parasitol. 2002;121:107–118. doi: 10.1016/s0166-6851(02)00027-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


