Abstract
Trypanosoma cruzi II is associated with Chagas disease in the southern part of South America. We analyzed T. cruzi variants in field-collected triatomines and congenitally infected infants living in the same disease-endemic region in Paraguay. Results of polymerase chain reactions for T. cruzi kinetoplast DNA and satellite DNA were positive in 83 triatomine feces samples and 58 infant blood samples. However, lineages were detected in 33 and 38 samples, respectively. Trypanosoma cruzi genotypes were determined in 56 (97%) blood samples after hybridization by using specific probes. The Tc I genotype was not detected. The prevalent sublineage was Tc IId in triatomines (27 of 33) and infant blood (36 of 58) as assessed by amplification of the 24Sα ribosomal RNA and the mini-exon region genes. The Tc IIc genotype was detected in 20 infant blood samples and in 1 triatomine. This study shows T. cruzi II is the predominant lineage circulating in triatomines and humans in endemic areas of eastern region of Paraguay.
Introduction
Chagas disease is caused by the protozoa parasite Trypanosoma cruzi. In the Southern Cone countries, Triatoma infestans is the main domiciliated vector for Chagas disease and over the last 10 years, Uruguay, Chile, Brazil and the Oriental Region of Paraguay (14 of 17 departments) have been certified as being free from disease transmission by T. infestans. The epidemiologic and sociocultural aspects encountered during disease management are 1) difficulty of eradication caused by the large number of animal reservoirs that perpetuate the presence of infectious sources; 2) the absence of available drugs that can be used on a large scale for treatment of patients in the chronic phase of the disease; 3) the lack of vaccines for protection of susceptible persons; and 4) the low social demand for medical attention because of chronicity of the infection.1 It is estimated that approximately 160,000 persons are infected with T. cruzi in Paraguay (population of approximately six million); seroprevalence among persons 15–45 years of age is 10–12% from-endemic regions.2 Extrapolating from this estimation, it is likely that 600 congenitally infected newborns are born per year, yielding a frequency of vertical transmission of 7%.2
Trypanosoma cruzi is genetically classified into two major evolutionary lineages, T. cruzi I and T. cruzi II, with a significant genetic distance between the two lineages. Linkage between molecular markers, such as the divergent domain of the 24Sα ribosomal RNA (rRNA) and the intergenic region of mini-exon genes, have been extensively analyzed, and clearly demonstrate the division of T. cruzi into two distinct phylogenetic lineages.3–5 The major lineage, Tc II is not homogenous and it can itself be divided into five sublineages on the basis of genotypic and phenotypic properties.6,7 According to clinical, epidemiologic, biological, biochemical and immunologic findings, it is assumed by some investigators that Chagas disease is caused by infection with T. cruzi II strains.8–12 However, isolation and classification of T. cruzi I from the myocardial tissue of a chronic chagasic patient with end-stage heart failure has been described.13 Epidemiologic studies suggest that T. cruzi IIb, IId, and IIe are related to anthroponotic environments and chronic Chagas disease patients, T. cruzi lineages IIa and IIc to sylvatic environments, and T. cruzi lineage I to both environments.14,15
In this study, T. cruzi lineages were typed from blood samples obtained from congenitally infected infants identified through a previously described locally sustainable system of prenatal diagnosis2 and in T. infestans collected within the same disease-endemic region under entomologic surveillance.16 Genetic studies of T. cruzi are important to clarify the intraspecies heterogeneity of the parasite. Studies of host-parasite relationships are also crucial to understand the impact of the genetic diversity of the parasite on factors such as pathogenicity and virulence.
Materials and Methods
Epidemiology of Chagas disease in study areas.
Congenitally infected infants who were born to chronically infected mothers in an area free of domiciliary vector transmission were identified through a locally sustainable system of prenatal screening of T. cruzi infection implemented in 37 rural health care centers of two Chagas disease-endemic departments of the eastern region of Paraguay (Cordillera and Paraguarí). The study area is free of domiciliary transmission of T. infestans because of integral vector control conducted during 1999–2000, and an established entomologic surveillance system based on community participation implemented since 2001. A description of vertical transmission and laboratory techniques used for analyses have been reported.2
The human populations within these regions are stable, of low density, and low migration, and 87% of the population in these two areas were born there and lived there continuously since birth. The seroprevalence of T. cruzi infection in pregnant women in these areas range from 10% to 12%. The baseline infestation rates detected in 1999 and 2000 at the departmental level were 1.4% for Cordillera and 2.5% for Paraguarí.16 A total of 1,921 triatomine samples were collected during 2001–2006 during the entomologic surveillance established to impede reinfestation in both areas (37 districts and approximately 95,000 dwellings). We determined that 69% of these triatomines were captured during the first two years of the surveillance system (2001–2002) because of residual foci if we take into account the colonization index. These triatomines were recorded and analyzed for T. cruzi infection in the Entomology Department of Servicio Nacional de Erradicación del Paludismo SENEPA in Asunción. Most (1,446 [75%] of 1,921) triatomines were T. infestans; the remaining 25% were autochthonous species such as T. sordida, T. guasayana, and T. guasu. With regard to the location of T. infestans species, 688 (48%) were captured from indoor living areas (domiciliary) and 739 (51%) were captured in the outdoor environment immediately surrounding living areas (peridomiciliary).
Samples, DNA extraction, and polymerase chain reaction with species-specific primers.
Seventy-six blood samples of T. cruzi-infected infants (age range = < 1–5 years) who were born to T. cruzi-infected mothers after 2001 were included in this study. These congenitally infected infants were confirmed as infected in follow-up studies during 2001–2006 by direct microscopic observation and polymerase chain reaction (PCR) or after 8 months of age by serologic techniques (enzyme-linked immunosorbent assay and immunofluorescent antibody assay). All infected children were treated with benznidazole (total daily dose of 5–7.5 mg/kg/day), which was given in two doses at regular intervals over a 60-day period.2
Of 1,921 triatomine specimens captured, 1,020 (53%) were kept at −20°C at the Molecular Biology Laboratory at the Instituto de Investigaciones en Ciencias de la Salud, Universidad Nacional de Asunción for further analyses. Fecal samples of 320 T. infestans representing 145 infested households detected during 2001–2006 during integrated entomologic surveillance were analyzed; 191 were captured in domiciliary areas and 129 were captured in peridomiciliary areas (Table 1). Triatoma infestans samples that were subsequently analyzed by PCR were selected to represent 80% all capture sites. DNA extraction from blood samples was performed by using the QIAamp Blood Kit (Qiagen, Valencia, CA), and DNA from triatomines feces was extracted by using the phenol-chloroform method.17
Table 1.
Distribution by habitat of Triatoma infestans positive for Trypanosoma cruzi
| Habitat | Total samples | No. (%) T. cruzi positive* | Total no. identified lineages† | Total | |
|---|---|---|---|---|---|
| Cordillera | Paraguari | ||||
| Domicile | 191 | 67 (35) | 7 | 23 | 30 |
| Peri-domicile | 129 | 16 (12) | 0 | 3 | 3 |
| Total | 320 | 83 (26) | 7 | 26 | 33 |
T. cruzi-positive samples identified by using species-specific primers TCZ 1–TCZ 2 and 121–122.
Lineage-positive samples identified by using lineage-specific primers for mini-exon and 24Sα ribosomal RNA genes.
The PCR assay used for detection of T. cruzi involved the amplification of a fragment of 330 basepairs from the variable regions of the kinetoplast minicircles DNA by using primers 121–122 and TCZ 1–TCZ 2.18–21 DNA extracted from cultured parasites was included as a positive control in PCRs, and a sample from a confirmed non-infected person was included as a negative control. All PCRs were performed in a PTC-100 Thermal Cycler (MJ Research Inc., Waltham, MA).
PCR-based identification of T. cruzi phylogenetic lineages.
To discriminate between Tc I and Tc IIa–IIe lineages from blood samples positive for T. cruzi by PCR and triatomine feces, two parasite genomic sequences were amplified, the D7 domain of the 24Sα rRNA genes and the mini-exon region.3,22 The combination of both markers distinguishes the lineages and sublineages. The expected PCR products for sublineages are TcIIa: 120, 125, or 130 basepairs for the rRNA genes and no product for mini-exon or a product of 300 basepairs observed with low intensity in combination with 120 basepairs for rRNA; TcIIb: 125 basepairs for rRNA and 300 basepairs for mini-exon; TcIIc: 110 basepairs for rRNA and no product for mini-exon or a product of 300 basepairs observed with low intensity; TcIId: 110 basepairs for rRNA and 300 basepairs for mini-exon; a low-intensity product of 125 basepairs can be also observed; and TcIIe: 125 basepairs for rRNA and 300 basepairs for mini-exon.6
The PCR amplification of the 24Sα rRNA genes was performed under the following cycling conditions: 4 minutes at 94°C; 35 cycles for 1 minute at 94°C, 1 minute at 55°C, and 1 minute at 72°C; and a final extension step for 5 minutes at 72°C. Primers TC-1 (T. cruzi I), TC-2 (T. cruzi II), and TC (common to T. cruzi I and II) were used to amplify the mini-exon gene. The following thermal profile was used: 1 minute at 94°C; 35 cycles for 30 seconds at 94°C, 30 seconds at 65°C, and 30 seconds at 72°C; and a final denaturation step for 10 minutes at 72°C. Amplification products were 300 basepairs (T. cruzi II) and 350 basepairs (T. cruzi I).3 The PCR products were separated by electrophoresis on 2% agarose gels, stained with ethidium bromide, and visualized under ultraviolet light. This system did not differentiate between Tc IIb and Tc IIe sublineages.6
Hybridization.
Blood samples that were species-specific positive by PCR, but which had no detectable lineage-specific PCR products by agarose gel electrophoresis, were subjected to hybridization. The PCR amplification products of DNA extracted from these samples, using the 24Sα rRNA gene and the min-exon gene as targets, were subjected to 2% agarose gel electrophoresis and transferred to a nylon Hybond-N+ membrane (Amersham Biosciences, Little Chalfont, United Kingdom). Probes were prepared with the PCR amplification products of the 24Sα rRNA and the mini-exon genes from reference strains (MN cl2, clon 39 for T. cruzi II and X10 cl11, clon 20 for T. cruzi I).6 The Enhanced Chemiluminescence Direct Nucleic Acid Labeling and Detection System Kit (Amersham Biosciences) was used for detection.
Results
PCR with species-specific primers.
Trypanosoma cruzi DNA was detected in 58 (76%) of 76 blood samples from congenitally infected infants by using primers TCZ 1–TCZ 2 and 121–122. The sensitivity of PCR in detecting infected infants was higher in children less than two years of age old (71–82%), and four infants positive by primers TCZ 1–TCZ 2 were also positive by primers 121–122. The intensity of the PCR products observed in a 2% agarose gel, and species-specific primers TCZ 1–TCZ 2 was evaluated as weak, average, and strong (Table 2). This variation was used to compare results of lineage and sublineage detection in the same samples. A total of 98% of blood samples had an average and strong intensity of T. cruzi PCR products with the species-specific primers TCZ 1–TCZ 2.
Table 2.
Distribution by age of 76 infants congenitally infected with Trypanosoma cruzi analyzed by PCR with species-specific primers
| Age, months | Total | No. (%) T. cruzi positive by PCR* | Intensity of PCR products by primers TCZ 1–TCZ 2† | No. PCR positive by primers 121–122‡ | ||
|---|---|---|---|---|---|---|
| Weak | Average | Strong | ||||
| 0–12 | 45 | 37 (82) | 0 | 17 | 18 | 2 |
| 13–24 | 14 | 10 (71) | 1 | 4 | 4 | 1 |
| 25–36 | 6 | 4 (67) | 0 | 3 | 1 | 0 |
| 37–48 | 6 | 4 (67) | 0 | 1 | 2 | 1 |
| 49–60 | 5 | 3 (60) | 0 | 3 | 0 | 0 |
| Total | 76 | 58 (100) | 1 | 28 | 25 | 4 |
T. cruzi-positive samples identified by using species-specific primers TCZ 1–TCZ 2 and 121–122.
Intensity of PCR products observed by 2% agarose gel electrophoresis and staining with ethidium bromide.
T. cruzi DNA was detected in these samples with primers 121–122; they were not detectable with primers TCZ 1–TCZ 2 primers.
Amplified products of T. cruzi DNA were detected in 83 (26%) of 320 T. infestans fecal specimens sampled from 80% of the capture sites. Detection of T. cruzi DNA was more frequent in the fecal specimens collected from domiciliary T. infestans than from peridomiciliary captures (67 [35%] of 191 versus 16 [12%] of 129, respectively; Table 1).
Polymerase chain reaction with 24 Sα rRNA and mini-exon primers.
DNA dilutions containing 10, 100, and 1000 parasites (Ypsilon strain, T. cruzi II) were used to determine the sensitivity of primers specific for 24Sα rRNA and mini-exon. Both genetic markers were detected in a minimum of 100 parasites. The lineages and sublineages were determined through combination of results using the two molecular markers.6
The distribution by region and habitat of T. infestans in comparison with T. cruzi is shown in Table 1. The PCR sensitivity was low for T. cruzi lineage determination when DNA extracted from the feces of triatomines was used. Only 33 (40%) of 83 T. cruzi-positive samples could be amplified by the lineage-specific primers, and 30 of 33 lineages detected were from domicile habitats. Hybridization was not performed for triatomine samples.
Trypanosoma cruzi genotypes were determined for 56 (97%) of 58 blood samples from PCR-positive infants (Table 3). Of these 56 blood samples, 38 (68%) were directly detected by agarose gel electrophoresis and 18 were detected after hybridization (all PCR products were 110 basepairs for the 24Sα rRNA gene) (Table 4 and Figures 1 and 2).
Table 3.
Distribution of Trypanosoma cruzi II subgroups detected in infant blood samples and triatomine feces from Cordillera and Paraguarí, Paraguay
| Department | T. cruzi lineages in infant blood samples | T. cruzi lineages in Triatoma infestans | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | IIc | IId | ND | Total | IIb/e | IIc | IId | ND | |
| Cordillera | 36 | 14 | 21 | 1* | 7 | 0 | 0 | 7 | 0 |
| Paraguari | 22 | 6 | 15 | 1* | 26 | 2 | 1 | 20 | (2*, 1†) |
| Total | 58 | 20 | 36 | 2 | 33 | 2 | 1 | 27 | 3 |
ND = not described. Atypical pattern not described previously.
Samples with a PCR amplification product of 300 basepairs with mini-exon primers and no amplification with 24Sα ribosomal RNA primers.
Sample with PCR amplification products of 300 basepairs and 350 basepairs with mini-exon primers and 24Sα ribosmal RNA primers, respectively.
Table 4.
Hybridization and PCR results in infected blood
| PCR Intensity of TCZ amplicons | Total PCR positive | T. cruzi-PCR positive by lineage-specific primers | Hybridization of Trypanosoma cruzi PCR mini-exon and 24Sα rRNA probes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 24Sα rRNA | Intensity of mini-exon amplicons | n = 24 | n = 20 | |||||||
| No. (%) positive | Strong | Average | Weak | Total, no. (%) | Mini-exon positive | Mini-exon negative | 24Sα rRNA positive | 24Sα rRNA negative | ||
| Weak | 1 | 1 (100) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Average | 28 | 21 (75) | 1 | 10 | 3 | 14 (50) | 1 | 12 | 6 | 1 |
| Strong | 25 | 12 (48) | 4 | 12 | 2 | 18 (72) | 3 | 6 | 12 | 1 |
| TCZ/121–122δ | 4 | 4 (100) | 0 | 2 | 0 | 2 (50) | 0 | 2 | 0 | 0 |
| Total | 58 | 38 (65) | 5 | 24 | 5 | 34 (59) | 4 | 20 | 18 | 2 |
Hybridization by primers TCZ1–TCZ2 was not detectable and hybridization by primers 121–122 was detectable.
Figure 1.
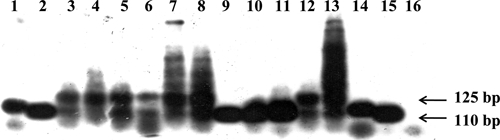
Hybridization of blood samples from infants in Paraguay by using Trypanosoma cruzi I clone 20 strain and 24Sα ribosomal RNA gene as probe. Lanes 1and 14, Mn cl2 positive controls (clone 39 T. cruzi II); lanes 2 and 15, X10 cl1 positive controls (clone 20 T. cruzi I); lane 3, infant blood sample showing weak amplification by PCR; lane 4, blood from an uninfected human sample used as a negative control, showing a non-specific band (130 basepairs) amplification; lanes 5–8: infant blood samples; lanes 9–11, T cruzi II positive controls (extracted from triatomines in which non-specific amplification is not observed); lanes 12 and 13, infant blood sample, lane 16, PCR-negative control.
Figure 2.
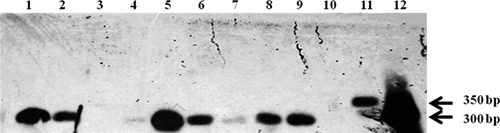
Hybridization of blood samples from infants in Paraguay by using Trypanosoma cruzi II clone 39 strain and mini-exon gene as probe. Use of a hybridization method with blood sample PCR products has resulted in improvement of the sensitivity of the assay. Lanes 1, 8, and 9, infant blood samples (with a weak 300-basepair product on 2% agarose gel electrophoresis); lane 5, infant blood sample with a conspicuous PCR product used as a positive control; lanes 2, 3, 4, 6 and 7, infant blood samples that did not show PCR products on by agarose gel electrophoresis but were visualized by hybridization in lanes 2, 6, and 7; lane 10, PCR-negative control; lanes 11 and 12, positive controls, T. cruzi I (X10 cl1, clone 20) and T. cruzi II, (MN cl2, clone 39) respectively. WP = weak positive.
Distribution of T. cruzi II subgroups detected in infant blood samples and triatomine feces from Cordillera and Paraguarí.
Lineage T. cruzi I was not detected in either area, and the PCR product of 350 basepairs was not observed in any of the biological samples when the mini-exon gene was used. Sublineages Tc IIc and Tc IId were found mainly in the 58 infected infants (97%). The proportion of the sublineages detected in blood samples from infants residing in Cordillera and Paraguarí areas were 39% (14 of 36) and 27% (6 of 22) for Tc IIc and 58% (21 of 36) and 68% (15 of 22) for Tc IIb, respectively. The main sublineage detected among T. infestans captured in both departments was Tc IId; all seven (100%) triatomines from Cordillera and 20 (76%) of 26 from Paraguari (Table 3).
Hybridization of PCR products to increase sublineage detection in infants blood samples.
All samples that were T. cruzi-negative with lineage-specific primers were subjected to hybridization with probes prepared from amplified products of the 24Sα rRNA gene and the mini-exon gene by using reference strains (MN cl2, clon 30 for T. cruzi II and X10 cl11, clon 20 for T. cruzi I). This procedure was conducted to PCR sensitivity and specificity. Twenty-four samples were hybridized with the mini-exon probe and 20 blood samples had no amplification product for the mini-exon gene. Eight of 24 blood samples subjected to hybridization with the 24Sα rRNA this probe were positive (Table 4).
The sizes of the 24 Sα rRNA PCR amplification products with the primers D71-D72 are 110 basepairs and 125 basepairs for T. cruzi II and 110 basepairs for T. cruzi I.22 Although a 130-basepair non-specific PCR product was amplified in human blood samples, it was distinguishable from the 125-basepair and 110-basepair T. cruzi-specific DNA bands. A blood sample from an uninfected person was used as negative control to show that the non-specific band (130 basepair) amplified with this primer pair (Figure 1).
Discussion
We describe T. cruzi lineages and sublineages circulating in congenitally infected infants and triatomines collected within the same endemic region in Paraguay under entomologic surveillance during 2001–2006. Amplification of T. cruzi DNA directly from biological samples without previous in vitro isolation by culture enabled us to make inferences regarding lineages and sublineages circulating in the human host and in vectors within the same geographic region.15 The stability of the population in the region studied virtually eliminated migration as a confounding factor in our investigation.
Because typing lineages with genetic markers directly from human blood samples of chronically ill patients is difficult because of the low levels of parasites present in blood, lineages circulating in congenitally infected infants were selected for this study. Trypanosoma cruzi DNA was detected in 58 (76%) of 76 blood samples from infected infants. The PCR sensitivity of primers TCZ 1–TCZ 2 was better than with that of primers 121–122, although T. cruzi DNA detection was not successful in four blood samples with the primers TCZ 1–TCZ 2. Such results might be related to the small amount of blood samples obtained from infants (1–3 mL) and an incomplete dispersion of the kinetoplast minicircle present in the blood samples previously boiled but not treated with guanidine-HCl.21 The PCR sensitivity of detection of T. cruzi DNA was higher for blood samples infants less than two years of age. However, when PCR was performed using primers specific for the 24Sα rRNA and mini-exon, the sensitivity was substantially lower; only 38 (66%) of the 58 T. cruzi PCR-positive samples were detected by visualization after agarose gel electrophoresis. When hybridization with the lineage-specific primers for 24Sα rRNA and mini-exon genes was used, the sensitivity increased to 97% (56 of 58).
Results of PCR-based assays for detection of T. cruzi DNA using primers 121–122 and TCZ 1–TCZ 2 primers were positive for 83 triatomines feces, and no differences in sensitivity were observed between primer sets. However, further amplification with lineage-specific primers resulted in the successful detection of sublineages in only 33 (40%) of the 83 samples.
Thirty-eight samples were positive by PCR with the 24Sα rRNA primers, and no difference was observed in the sensitivity of these reactions in relation to the amount of parasites present in blood samples (assessed by the intensity of the TCZ 1–TCZ 2 PCR products). Nevertheless, the mini-exon primers seemed to be more dependant on the amount of parasites present; a 72% (18 of 25) detection level was observed in those samples with strong TCZ 1–TCZ 2 PCR-products. The Tc IIc genotypes detected in 20 blood samples were identified after hybridization with the mini-exon probe (when no PCR products were detected).
Sublineages Tc IIc and Tc IId were the prevalent genotypes among 58 PCR-positive congenitally infected infants, with a distribution of 20 (35%) of 58 for Tc IIc and 36 (62%) of 58 for Tc IId, respectively. Other genotypes had atypical patterns not described previously and are under study. In addition, Tc IId was the prevalent genotype among 27 of 33 PCR-positive infected triatomines.
On the basis of our findings, it can be inferred that sublineages Tc IIc and Tc IId are related to cases of chronic disease in the study area. These sublineages were present in 97% of congenitally infected infants, and it can be inferred the same lineages infected their mothers.23,24 Five samples, three in triatomines and two in human blood, showed PCR-product patterns that have not been described. These variants are under further study to determine their significance.
In 2008, both areas were declared free of domiciliary vector transmission by international experts and intergovernmental resolutions (official documents of the Pan American Health Organization and the World Health Organization). Our study indicates that we detected T. cruzi in T. infestans during the entomologic surveillance system established in 2001 that were focal/residual triatomines that remained after integral vector control with insecticides performed in 1999–2000. This study identified T. cruzi II strains as the predominant lineages circulating in the main endemic areas of the eastern region of Paraguay, as detected in field-collected triatomines and humans living in the same region. Trypanosoma cruzi I was not found in these regions and does not appear to play a significant role in the epidemiology of Chagas disease in Paraguay.
Acknowledgments
We thank the operational staff of the National Control Program of Chagas Disease of the Ministry of Health of Paraguay for their assistance, Dr. Richard Culleton and Dr. Christopher Spitters for critically reading the manuscript.
Footnotes
Financial support: This study was supported by the United Nations Development Program/World Bank/World Health Organization Special Program for Research and Training in Tropical Diseases.
Authors' addresses: Florencia del Puerto, Zunilda Sánchez, Eva Nara, Graciela Meza, and Graciela Russomando, Departamento de Biología Molecular y Genética, Instituto de Investigaciones en Ciencias de la Salud, Universidad Nacional de Asunción, Río de la Plata y Lagerenza, Asunción, Paraguay, E-mail: grusso@rieder.net.py. Berta Paredes and Elizabeth Ferreira, Departamento de Entomología Servicio Nacional de Erradicación del Paludismo, Dominguez c/Brasil, Asunción, Paraguay.
References
- 1.World Health Organization Control of Chagas disease: second report of a WHO expert committee. World Health Organ Tech Rep Ser. 2002;905:1–109. [PubMed] [Google Scholar]
- 2.Russomando G, Almirón M, Candia N, Franco L, Sánchez Z, de Guillen I. Implementation and evaluation of a locally sustainable system of prenatal diagnosis to detect cases of congenital Chagas disease in endemic areas of Paraguay. Rev Soc Bras Med Trop. 2005;2:49–54. [PubMed] [Google Scholar]
- 3.Souto R, Fernandez O, Macedo A, Campbell D, Zingales B. DNA markers define two major phylogenetic lineages of Trypanosoma cruzi. Mol Biochem Parasitol. 1996;83:141–152. doi: 10.1016/s0166-6851(96)02755-7. [DOI] [PubMed] [Google Scholar]
- 4.Nunes LR, de Carvalho MR, Buck GA. Trypanosoma cruzi strains partition into two groupsbased on the structure and function of the spliced leader RNA and rRNA gene promoters. Mol Biochem Parasitol. 1997;2:211–224. doi: 10.1016/s0166-6851(97)02857-0. [DOI] [PubMed] [Google Scholar]
- 5.Zingales B, Souto RP, Mangia RH, Lisboa CV, Campbell DA, Coura JR, Jansen A, Fernandes O. Molecular epidemiology of American trypanosomiasis in Brazil based on dimorphisms of rRNA and mini-exon gene sequences. Int J Parasitol. 1998;1:105–112. doi: 10.1016/s0020-7519(97)00178-1. [DOI] [PubMed] [Google Scholar]
- 6.Brisse S, Verhoef J, Tibayrenc M. Characterisation of large and small subunit rRNA and mini-exon genes further supports the distinction of six Trypanosoma cruzi lineages. Int J Parasitol. 2001;11:1218–1226. doi: 10.1016/s0020-7519(01)00238-7. [DOI] [PubMed] [Google Scholar]
- 7.Brisse S, Henriksson J, Barnabé C, Douzery EJ, Berkvens D, Serrano M, De Carvallo MR, Buck GA, Dujardin JC, Tibayrenc M. Evidence for genetic exchange and hybridization in Trypanosoma cruzi based on nucleotide sequences and molecular karyotype. Infect Genet Evol. 2003;2:173–183. doi: 10.1016/s1567-1348(02)00097-7. [DOI] [PubMed] [Google Scholar]
- 8.Buscaglia CA, Di Noia JM. Trypanosoma cruzi clonal diversity and the epidemiology of Chagas' disease. Microbes Infect. 2003;5:419–427. doi: 10.1016/s1286-4579(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes O, Souto RP, Castro JA, Pereira JB, Fernandes NC, Junqueira AC, Naiff RD, Barrett TV, Degrave W, Zingales B, Campbell DA, Coura JR. Brazilian isolates of Trypanosoma cruzi from humans and triatomines classified into two lineages using mini-exon and ribosomal RNA sequences. Am J Trop Med Hyg. 1998;6:807–811. doi: 10.4269/ajtmh.1998.58.807. [DOI] [PubMed] [Google Scholar]
- 10.Breniere SF, Bosseno MF, Tellería J, Carrasco R, Vargas F, Yasick N, Noireau F, Alcaraz JL, Barnabé C, Wincker P, Tibayrenc M. Different behaviour of two Trypanosoma cruzi major clones: transmission and circulation young Bolivian patients. Exp Parasitol. 1998;89:285–295. doi: 10.1006/expr.1998.4295. [DOI] [PubMed] [Google Scholar]
- 11.Briones MRS, Souto RP, Sotlf BS, Zingales B. The evolution of two Trypanosoma cruzi inferred from rRNA genes can be correlated with the interchange of American mammalian faunas in the Cenozoic and has implications to pathogenicity and host specificity. Mol Biochem Parasitol. 1999;104:219–232. doi: 10.1016/s0166-6851(99)00155-3. [DOI] [PubMed] [Google Scholar]
- 12.Risso MG, Garbarino GB, Mocetti E, Campetella O, Gonzalez Cappa SM, Buscaglia CA, Leguizamon MS. Differential expression of a virulence factor, the trans-sialidase, by the main Trypanosoma cruzi phylogenetic lineages. J Infect Dis. 2004;189:2250–2259. doi: 10.1086/420831. [DOI] [PubMed] [Google Scholar]
- 13.Teixeira MM, da Silva FM, Marcili A, Umezawa ES, Shikanai-Yasuda MA, Cunha-Neto E, Kalil J, Stolf N, Stolf AM. Short communication: Trypanosoma cruzi lineage I in endomyocardial biopsy from a north-eastern Brazilian patient at end-stage chronic Chagasic cardiomyopathy. Trop Med Int Health. 2006;11:294–298. doi: 10.1111/j.1365-3156.2006.01575.x. [DOI] [PubMed] [Google Scholar]
- 14.Yeo M, Acosta N, Llewellyn M, Sanchez H, Adamson S, Miles GA, Lopez E, Gonzalez N, Patterson JS, Gaunt MW, de Arias AR, Miles MA. Origins of Chagas disease: Didelphis species are natural hosts of Trypanosoma cruzi I and armadillos hosts of Trypanosoma cruzi II, including hybrids. Int J Parasitol. 2005;35:225–233. doi: 10.1016/j.ijpara.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 15.Marcet PL, Duffy T, Cardinal MV, Burgos JM, Lauricella MA, Levin MJ, Kitron U, Gürtler RE, Schijman AG. PCR-based screening and lineage identification of Trypanosoma cruzi directly from faecal samples of triatomine bugs from northwestern Argentina. Parasitol. 2006;132:57–65. doi: 10.1017/S0031182005008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rojas de Arias A, Russomando G. El Control de la Enfermedad de Chagas en los Países del Cono Sur de América. Historia de Una Iniciativa Internacional. 1991/2001. Washington, DC: Pan American Health Organization; 2002. pp. 269–300. (El control de la enfermedad de Chagas del Paraguay). [Google Scholar]
- 17.Sambrook J, Russell DW. Molecular Cloning. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2001. pp. 6.23–6.27. [Google Scholar]
- 18.Degrave W, Fragoso SP, Britto C, van Heuverswyn H, Kidane GZ, Cardoso MA, Mueller RU, Simpson L, Morel CM. Peculiar sequence organization of kinetoplast DNA minicircles from Trypanosoma cruzi. Mol Biochem Parasitol. 1988;27:63–70. doi: 10.1016/0166-6851(88)90025-4. [DOI] [PubMed] [Google Scholar]
- 19.Russomando G, Carpinelli de Tomassone MM, Guillen I, Acosta N, Vera N, Almirón M, Candia N, Calcena MF, Figueredo A. Treatment of congenital Chagas' disease diagnosed and followed up by polymerase chain reaction. Am J Trop Med Hyg. 1998;59:487–491. doi: 10.4269/ajtmh.1998.59.487. [DOI] [PubMed] [Google Scholar]
- 20.Moser DR, Kirchhoff LV, Donelson JE. Detection of Trypanosoma cruzi by DNA amplification using the polymerase chain reaction. J Clin Microbiol. 1989;7:1477–1482. doi: 10.1128/jcm.27.7.1477-1482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russomando G, Figueredo A, Almirón M, Sakamoto M, Morita K. Polymerase chain reaction-based detection of Trypanosoma cruzi DNA in serum. J Clin Microbiol. 1992:2864–2868. doi: 10.1128/jcm.30.11.2864-2868.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Souto JR, Zingales B. Sensitive detection and strain classification of Trypanosoma cruzi by amplification of a ribosomal RNA sequence. Mol Biochem Parasitol. 1993;62:45–52. doi: 10.1016/0166-6851(93)90176-x. [DOI] [PubMed] [Google Scholar]
- 23.Virreira M, Truyens C, Alonso-Vega C, Brutus L, Jijena J, Torrico F, Carlier Y, Svoboda M. Comparison of Trypanosoma cruzi lineages and levels of parasitic DNA in infected mothers and their newborns. Am J Trop Med Hyg. 2007;1:102–106. [PubMed] [Google Scholar]
- 24.Burgos JM, Altcheh J, Bisio M, Duffy T, Valadares HM, Seidenstein ME, Piccinali R, Freitas JM, Levin MJ, Macchi L, Macedo AM, Freilij H, Schijman AG. Direct molecular profiling of minicircle signatures and lineages of Trypanosoma cruzi bloodstream populations causing congenital Chagas disease. Int J Parasitol. 2007;12:1319–1327. doi: 10.1016/j.ijpara.2007.04.015. [DOI] [PubMed] [Google Scholar]


