Abstract
Validation of the sensitivity of the SYBR Green I in vitro test against an enzyme-linked immunosorbent assay (ELISA)-based drug sensitivity assay. Our results suggest that the SYBR Green I assay is a fast and inexpensive malaria drug screening assay for laboratory use. However, because of its lack of sensitivity in whole blood samples its usefulness for testing clinical samples may be limited.
Introduction
Fast and reliable in vitro growth assessment of malaria parasites is the key to the assessment of in vitro drug susceptibility of malaria parasites, and to high throughput screening of newly developed drugs and drug combinations. Although there are several highly sensitive assays available today, the time and effort necessary to test a single microtiter plate remains substantial. Our goal was to assess the value of the SYBR Green I dye for drug susceptibility testing in Plasmodium falciparum cultures, both, under controlled, simulated field conditions and under optimal laboratory conditions.
Recently, several new in vitro flourescene assays have been reported.1–4 The assays detect the presence of malaria DNA in infected erythrocytes as a measure of parasite growth and propagation and parasite growth inhibition by antimalarial drugs.
Although fast and relatively inexpensive, growth assessment using nucleic acid stains has inherent limitations because these stains are not specific for malaria DNA. The SYBR Green I binds to any double-stranded DNA, including the DNA inherently present in whole blood samples, thereby resulting in high background readings.
SYBR Green I has been used in molecular biology as a substitute for ethidium bromide for several years. It is an asymmetrical cyanine dye, binding to double stranded DNA, preferring G and C base pairs.2 When intercalated into DNA, it is highly fluorescent, absorbing light at a wavelength between 390 and 505 nm, with a peak at 497 nm and a secondary peak near 254 nm. It emits light at 505 to 615 nm, with a peak at 520 nm.5
Materials and Methods
A series of experiments were conducted to evaluate the usefulness of SYBR Green I for in vitro drug susceptibility testing of malaria parasites, not only at the previously tested higher,4 but also at lower parasite densities. For all tests 20 µL of a 10× concentration of SYBR Green I plus 80 µL either undiluted or, if post-incubation parasitemia exceeded ~3.5% infected red blood cells (IRBC), diluted (1:2) sample was used. The dye was obtained as 10,000X stock in DMSO (Sigma-Aldrich GesmbH, Vienna, Austria) and diluted in sterilized distilled water. The enzyme-linked immunosorbent assay (ELISA) assays were done after dilution of the samples in distilled water as previously described.6 Background fluorescence of the medium was analyzed by comparing regular RPMI 1640 medium containing HEPES, gentamycin, and phenol red to RPMI medium with HEPES and gentamycine, but without phenol red. The latter medium was then measured with and without addition of gentamycine.
Following initial testing the phenol red-free medium containing HEPES and gentamycine was used as standard medium. To assess the influence of uninfected erythrocytes on the measurements, these were tested against the standard medium at different hematocrit levels. In addition, whole blood collected in heparinized tubes and fresh erythrocyte concentrate, both washed three times in RPMI medium, were analyzed. Two-fold serial dilutions of infected blood, with and without leukocytes at a hematocrit of 1.5% in RPMI medium starting with either 1% or 2% IRBC, were produced. In addition, drug-coated microtiter plates (IWAKI 3860-096; IWAKI Co. Ltd., Tokyo, Japan) were incubated for 72 hours at 37°C with parasitized samples at starting parasitemias of 0.05%, 0.2%, and 1% IRBCs in either whole blood or erythrocyte concentrate (at 1.5% hematocrit in RPMI medium with Albumax II (Gibco, Invitrogen GmbH, Lofer, Austria) and a total volume of 200 µL/well). These two sets of experiments were the basis for a comparison of the sensitivity of the SYBR Green I assay to the HRP2 assay. The drug-coated microtiter plates featured serial dilutions of four different drugs: dihydroartemisinin (DHA; 0.01–10 ng/mL), mefloquine (MEF; 0.34–250 ng/mL), quinine (QNN; 3.34–2,500 ng/mL), and chloroquine (CHL; 3.34–2,500 ng/mL), as well as several control wells. All tests were done in duplicate. All drugs were dissolved in 70% ethanol. A 1 mg/mL stock solution was diluted in sterile distilled water and transferred to the plates and dried. Two sets of microtiter plates with serial dilutions of the 3D7 P. falciparum clone and one set of microtiter plates with dilutions of the DD2 clone were produced. Each plate contained a triplicate of serial dilutions with and without leukocytes. Each set was freeze-thawed and transferred to fresh culture plates for the SYBR Green I assay or to antibody-coated ELISA plates (IWAKI 3801-096) for the HRP2 assay. Fluorescence was measured twice in a Victor2 plate reader (Perkin Elmer, Salem, MA) at an excitation wavelength of 485 nm and an emission wavelength of 535 nm, and once with the HRP2 ELISA in a GENios (Tecan Austria GmbH, Grödig, Austria) reader. For analysis of the fluorescence readings, the geometric mean of the readings of the first and second pass was used to compensate for measurement errors. The limit of detection (LOD) was defined as the mean background in each column plus its 2-fold standard deviation.
Results
Background.
When mixed with SYBR Green I, RPMI medium provided a stable high background essentially regardless of additives like gentamycine or phenol red. Relative to RPMI, distilled water gave a much lower reading of only 13.92% (95% CI: 12.97–14, 89%, N = 16). After initial tests with non-hemolyzed blood, hemolyzed samples proved to have not just a higher signal-to-noise ratio (SNR) but also to be much more practical in handling. Possibly because of signal quench caused by the hemoglobin, samples with uninfected erythrocytes showed a significantly lower background than plain RPMI. An optimal SNR was achieved when using a hematocrit of 6.5%. The SNR did not drop significantly when lowering the hematocrit to a value of 1.5%, as typically used for drug sensitivity testing. Staining times longer than 10 minutes also did not seem to improve the SNR.
Level of detection.
In 2-fold serial dilutions (SD) of both 3D7 and DD2, the ELISA showed a significantly higher sensitivity (P < 0.001) than the SYBR Green I-based assay. This effect was seen to a high degree when testing with whole blood (P < 0.001) (Figure 1), but could also be observed in erythrocyte only samples. For SYBR Green I added to white blood cell (WBC)-free cell medium mixture (CMM) the mean background reading, calculated as the arithmetic mean of all background readings in the individual serial dilutions, was 593.67 (95% CI: 452.01–735.33, N = 18) equivalent to 45.42% (95% CI: 42.60–48.25) of the highest reading, with a coefficient of variation (CV) of 6.2%. The resulting LOD was calculated as 0.20% (95% CI: 0.16–0.24) IRBC. For whole blood a significantly (P = 0.011) higher LOD of 0.55% (95% CI: 0.38–0.71) IRBC was found, based on an almost 5 times higher background reading of 2913.09 (95%CI: 2651.55–3174.63, N = 18) corresponding to 68.60% (95% CI: 64.79–72.40, N = 18) of the highest reading with a CV of 9.1% (Figure 1). Even though the CV was similar for both testing conditions, the higher absolute value measured for the background in whole blood cultures results in a significantly reduced sensitivity of the assay in the presence of WBCs (P = 0.011). For the HRP2 ELISA the equivalent LOD values were 0.013% (95% CI: 0.003–0.023) in WBC-free culture based on a mean background optical density (OD) reading of only 0.017 (95% CI: 0.013–0.021, N = 9), equivalent to 1.83% (95% CI: 1.24–2.41, N = 9) of the highest reading, with a CV of 5.5%, and a LOD of 0.022% (95%CI: 0.001–0.042) IRBC in whole blood with a CV of 5.9% for a background reading of 0.017 (95%CI: 0.013–0.020, N = 9) corresponding to 2.326% (95%CI: 1.493–3.160, N = 9) of the highest reading, suggesting little influence of the presence of WBCs on the LOD in the ELISA-based assay (P = 0.48) (Figure 1).
Figure 1.
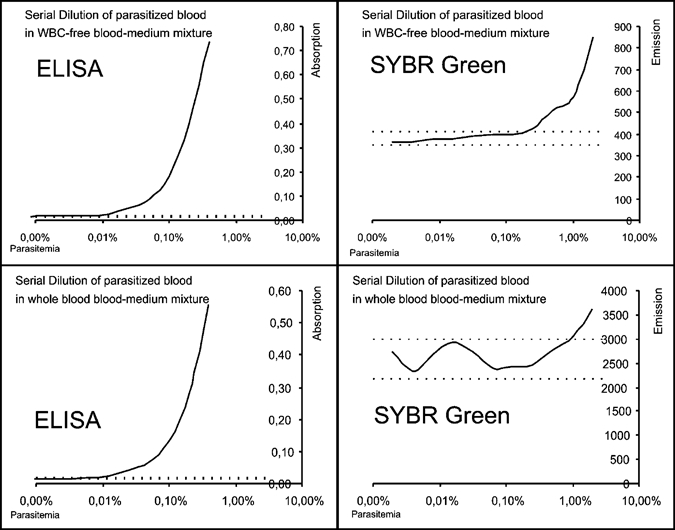
Two-fold serial dilution of 3D7 in white blood cell free and whole blood medium mixture. Exemplary display of a single sample. Initial parasitemia: 2%. Analyzed with HRP2 ELISA and SYBR Green I. When analyzed with the HRP2 ELISA diluted 1:5 in H20. Dotted lines indicate limit of detection.
Drug coated plates.
Even under optimal growth conditions, which are rarely attainable under field conditions, the SYBR Green I readings of samples in whole blood on drug-coated microtiter plates showed major variations and gave results that are hardly interpretable due to poor SNR for pre-culture parasitemias of 0.05%. At an initial IRBC proportion of 0.2% (which is still considerably below the LOD) dose response curves provide reasonably interpretable results. However, even with this parsitemia the curves remain flat and difficult to interpret. Good results were obtained with starting parasitemias of 1% (Figure 2), comparable to the results earlier reported for assays based on the measurement of the enzymatic activity of lactate dehydrogenase.7
Figure 2.
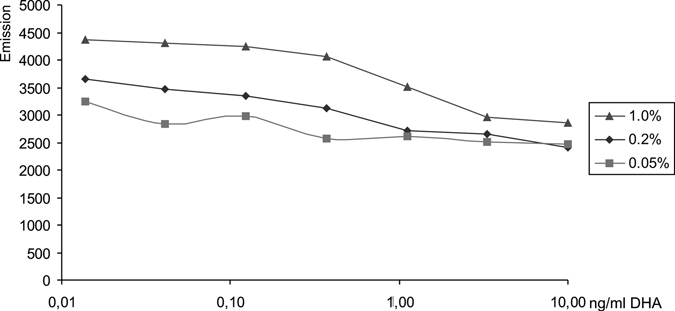
Unmodified SYBR Green I emission values of samples incubated on dihydroartemisinin (DHA)-coated microtiter plates for 72 hours in whole blood. Pre-incubation parasitemia: 0.05%, 0.2%, and 1%.
Discussion
We validated the SYBR Green I in vitro test against an ELISA-based drug sensitivity assay for its use in drug screening and field drug sensitivity testing (i.e., where parasitized blood samples are obtained directly from patients without being able to raise parasite densities by preculturing the samples before testing). Our experiments do not necessarily confirm earlier reports based on similar IC50 and IC90 values measured in field samples using the SYBR Green I and the HRP2 assays, suggesting a similar sensitivity for both assays.8 The high background readings obtained with whole blood seem to considerably increase the variation of the background noise thereby significantly reducing the sensitivity of the SYBR Green I assay. Although high starting parasitemias around 0.75% as used in Ref. 4 are likely to give similar IC values with the SYBR Green I assay as compared with other assays, geometric mean parasite densities as high as this are rarely encountered under field conditions in malaria-endemic countries, e.g., Ref. 9.
Recently, Rason and others10 compared the results of the new SYBR Green I assay with the isotopic assay originally developed in the late 1970s, suggesting that the SYBR Green I assay may be a valid replacement for isotopic methods, which are commonly performed at 0.25–0.5% initial parasite density.11 With LODs of 0.20% in WBC-free culture and 0.55% in whole blood, our findings support their assumption that the new assay may have an application range roughly comparable to that of the isotopic assay. However, study inclusion criteria in this range could potentially lead to the exclusion of a considerable proportion of malaria-positive cases in most endemic regions and could introduce a major selection bias.12 The results obtained by Bacon and others, who used a mean starting parasitemia of 0.75% for their comparison of the SYBR Green I and the HRP2 assay, confirm the good performance of the SYBR Green I assay at higher parasite densities.8 Particularly under field conditions, where parasite densities tend to be low, a lack in sensitivity can preclude the testing of samples that may otherwise provide valuable information.
SYBR Green I is a highly sensitive indicator of DNA. However, its indiscriminate detection of parasitical and non-parasitical DNA, such as WBCs, make it a very unspecific assay, and highly susceptible to contamination of the sample with WBCs and microorganisms. The high LOD threshold and SNR in such samples seem to limit the application of the SYBR Green I assay for field drug sensitivity testing. We did not manage to reduce the background by adjusting the composition of the medium, especially when measuring samples containing WBCs, because the background attributable to the medium seems to have a rather limited impact on the SNR in the presence of WBCs, which produce a much stronger background signal. A complete removal of WBCs from whole blood samples, though possible, is either difficult under field conditions (e.g., using Whatman columns), or, in the case of commercially available leukocyte filters (e.g., Plasmodipur filters; Euro-Diagnostica BV, Arnhem, The Netherlands), may cancel out the cost effectiveness of the SYBR Green I assay because of the high material costs per test. Thus, for field use ELISA-based assays currently seem to be the logical choice because of their high specificity and sensitivity thereby eliminating the need for a preselection of high parasite density samples. However, the SYBR Green I assay shows great potential for application in a controlled laboratory environment (e.g., for drug screening or quality control of plates) because of its high speed and relatively low cost.
Conclusion
Considering that all tests were done under optimal laboratory conditions and that parasite growth was better than what can generally be expected in the field, we conclude that the SYBR Green I assay may not show the sensitivity we would expect from a field assay. However, this assay seems to show great potential for rapid drug screening in a controlled laboratory environment when WBC-free cultures can easily be maintained.
Footnotes
Authors' addresses: Matthias G. Vossen and Harald Noedl, Department of Specific Prophylaxis and Tropical Medicine, Medical University of Vienna, Kinderspitalgasse 15, A-1090 Vienna, Austria, E-mails: matthias.vossen@meduniwien.ac.at and harald.noedl@meduniwien.ac.at. Sandra Pferschy, Department for Medical Genetics, Medical University of Vienna, Währingerstr. 10, A-1090 Vienna, Austria, E-mail: sandra.pferschy@meduniwien.ac.at. Peter Chiba, Department of Medical Chemistry, Medical University of Vienna, Währingerstr. 10, A-1090 Vienna, Austria, E-mail: peter.chiba@meduniwien.ac.at.
Reprint requests: Harald Noedl, Department of Specific Prophylaxis and Tropical Medicine, Medical University of Vienna, Kinderspitalgasse 15, A-1090 Vienna, Austria, Tel: +43-1-4277-64882, Fax: +43-1-4277-64899, E-mail: harald.noedl@meduniwien.ac.at.
References
- 1.Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother. 2004;48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett TN, Paguio M, Gligorijevic B, Seudieu C, Kosar AD, Davidson E, Roepe PD. Novel, rapid, and inexpensive cell-based quantification of antimalarial drug efficacy. Antimicrob Agents Chemother. 2004;48:1807–1810. doi: 10.1128/AAC.48.5.1807-1810.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baniecki ML, Wirth DF, Clardy J. High-throughput Plasmodium falciparum growth assay for malaria drug discovery. Antimicrob Agents Chemother. 2007;51:716–723. doi: 10.1128/AAC.01144-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corbett Y, Herrera L, Gonzalez J, Cubilla L, Capson TL, Coley PD, Kursar TA, Romero LI, Ortega-Barria E. A novel DNA-based microfluorimetric method to evaluate antimalarial drug activity. Am J Trop Med Hyg. 2004;70:119–124. [PubMed] [Google Scholar]
- 5.Sigma Aldrich SYBR Green I product information. 2008. http://www.sigmaaldrich.com/sigma-aldrich/datasheet/s9430dat.pdf Available at. Accessed July 17.
- 6.Noedl H, Bronnert J, Yingyuen K, Attlmayr B, Kollaritsch H, Fukuda M. Simple histidine-rich protein 2 double-site sandwich enzyme-linked immunosorbent assay for use in malaria drug sensitivity testing. Antimicrob Agents Chemother. 2005;49:3575–3577. doi: 10.1128/AAC.49.8.3575-3577.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basco LK, Marquet F, Makler MM, Le Bras J. Plasmodium falciparum and Plasmodium vivax: lactate dehydrogenase activity and its application for in vitro drug susceptibility assay. Exp Parasitol. 1995;80:260–271. doi: 10.1006/expr.1995.1032. [DOI] [PubMed] [Google Scholar]
- 8.Bacon DJ, Latour C, Lucas C, Colina O, Ringwald P, Picot S. Comparison of a SYBR green I-based assay with a histidine-rich protein II enzyme-linked immunosorbent assay for in vitro antimalarial drug efficacy testing and application to clinical isolates. Antimicrob Agents Chemother. 2007;51:1172–1178. doi: 10.1128/AAC.01313-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noedl H, Krudsood S, Leowattana W, Tangpukdee N, Thanachartwet W, Looareesuwan S, Miller RS, Fukuda M, Jongsakul K, Yingyuen K, Sriwichai S, Ohrt C, Knirsch C. In vitro antimalarial activity of azithromycin, artesunate, and quinine in combination and correlation with clinical outcome. Antimicrob Agents Chemother. 2007;51:651–656. doi: 10.1128/AAC.01023-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rason MA, Randriantsoa T, Andrianantenaina H, Ratsimbasoa A, Menard D. Performance and reliability of the SYBR Green I based assay for the routine monitoring of susceptibility of Plasmodium falciparum clinical isolates. Trans R Soc Trop Med Hyg. 2008;102:346–351. doi: 10.1016/j.trstmh.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16:710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menard D, Djalle D, Manirakiza A, Yapou F, Siadoua V, Sana S, Matsika-Claquin MD, Nestor M, Talarmin A. Drug-resistant malaria in Bangui, Central African Republic: an in vitro assessment. Am J Trop Med Hyg. 2005;73:239–243. [PubMed] [Google Scholar]


