Abstract
Recently, the XE-2100 hematology analyzer was investigated in a rather small patient group; pseudoeosinophilia or abnormal white blood cell (WBC) scattergrams reported by this instrument were considered as significantly valuable diagnostic parameters in detecting vivax malaria. This study was conducted not only to assess the usefulness of pseudoeosinophilia or abnormal WBC scattergram in vivax malaria-endemic areas with large patient groups (N = 1,801) but also to investigate the correlation of parasitemia and platelet count with pseudoeosinophilia and abnormal WBC scattergrams. Of the 1,801 analyzed patients, 413 (22.9%) were found to have malaria by Wright–Giemsa stained blood smears. Overall, either pseudoeosinophilia or abnormal WBC scattergram was detected in 191 of 413 malaria patients and 4 of 1,388 patients without malaria (sensitivity = 46.2%, specificity = 99.7%). We suggest that clinical hematology laboratories using the XE-2100 analyzer should be aware of such specific parameters, even with the absence of a clinical request.
Malaria diagnosis has relied on clinical observations and diagnostic tests, usually microscopic detection of Plasmodium spp. of Giemsa-stained blood smears and malaria-antigen or antibody-detection assay. The disadvantage of these diagnostic tests is that they are performed only if there is a clinical request. However, complete blood count (CBC) is almost always requested without exception as part of the routine investigation in febrile patients. There are some reports using routine automated hematology analyzers for presumptive diagnosis of malaria infection. Most reports are studies regarding abnormal depolarizing patterns of the Cell-Dyn hematology analyzer (Abbott Diagnostics, Santa Clara, CA).1–6 Recent studies from South Korea investigated pseudoeosinophilia and abnormal white blood cell (WBC) scattergrams of vivax malaria-infected patients on a Sysmex XE-2100 hematology analyzer (Sysmex Corporation, Kobe, Japan).7,8 Because the sensitivity and specificity of these methods were apparently high, its application to early detection can lead to a significant reduction in patients' morbidity and mortality.2,8
Previous studies had limitations of a rather small patient number and their inaccurate evaluation of parasitemia. The aim of this study was to assess the usefulness of pseudoeosinophilia and abnormal WBC scattergrams for the diagnosis of malaria and to investigate correlation of parasitemia and platelet count with pseudoeosinophilia and abnormal WBC scattergram.
This study was conducted in the department of laboratory medicine of a large teaching hospital (Ilsan Hospital) located at a vivax malaria-endemic area in Korea during 36 months between January 2006 and December 2008. After obtaining informed consent, a total of 1,801 ethylenediaminetetraacetic acid (EDTA)-anticoagulated samples were submitted for both CBC analysis and malaria smears. Real-time polymerase chain reaction (RT-PCR) assay was conducted for determination of parasitemia level. Malaria patient group was composed of 413 patients that were initially diagnosed with Plasmodium vivax infection (276 males and 137 females). A diagnosis of malaria was made by examining Wright–Giemsa stained thick and thin blood smears. The tests were performed no more than 5 hours after blood drawing. This study was reviewed and approved by the Research Ethics Board of the National Health Insurance Corporation at Ilsan Hospital.
All samples were analyzed with the Sysmex XE-2100 hematology analyzer. The Sysmex XE-2100 measures WBCs by flow cytometry using a semiconductor red-diode laser to detect forward- and side-scattered light information. Red-cell lysis was performed using a reagent that selectively suppressed the degranulation of basophils, resulting in their separation from other forms of WBCs. The differential count (DIFF) channel uses side scatter and fluorescence intensity after staining nuclear DNA/RNA with specific dyes, and it is the source of information for counts of neutrophils, lymphocytes, monocytes, and eosinophils. An organic acid reagent binds specifically to the granules of eosinophils and allows them to be discriminated from neutrophils based on their higher side-scatter signal intensities. In addition, in the DIFF channel, a different total WBC count (sum of five-part DIFF) is presented together with the ratio of both WBC counts (WBC-DIFF/WBC-basophil [BASO]). When this ratio is not close to one, results of the differential WBC analyses are not displayed (error message means abnormal WBC scattergram). Manual WBC differential counting was also performed by microscopic examination of Wright–Giemsa-stained smears. Atypical features were categorized as pseudoeosinophilia (a gap of more than 5% in eosinophil counts between the Sysmex XE-2100 analyzer and microscopic examination) and abnormal WBC scattergram.8
Parasitemia was calculated by RT-PCR technology, essentially as described previously.9 A malaria RG PCR kit (QIAGEN, Hamburg, Germany) was used with the Rotor-Gene Q (QIAGEN, Hamburg, Germany). Malaria patients were subgrouped as a low parasitemia group (< 500 copies/μL) and a high parasitemia group (≥ 500 copies/μL).8
Statistical tests were performed using the Statistical Package for the Social Sciences (SPSS) for Windows, Release 12.0 (SPSS Inc., Chicago, IL). Comparisons of parameters were performed with Student's t test, and correlation analyses were performed using Pearson's correlation test. A value of P < 0.05 was considered statistically significant, and values were expressed as mean ± standard deviation (SD).
Of the 1,801 analyzed patients, 413 (22.9%) were found to have malaria by Wright–Giemsa-stained thick and thin blood smears. All patients suffered from P. vivax malaria, of which 130 had low parasitemia and 283 had high parasitemia. Table 1 presents findings of the Sysmex XE-2100 analyzer for 1,801 analyzed patients. Using pseudoeosinophilia for the diagnosis, the sensitivity and specificity were 39.0% and 100%. Using WBC abnormal scattergrams for the diagnosis, the sensitivity and specificity were 15.7% and 99.7%. Overall, either pseudoeosinophilia or WBC abnormal scattergrams were observed in 191 of 413 malaria patients and in 4 of 1,388 patients without malaria (sensitivity = 46.2%, specificity = 99.7%). In the subgroup of malaria patients with parasitemia, either pseudoeosinophilia or abnormal WBC scattergram was observed in 41 of 130 patients with low parasitemia (31.5%) and 150 of 283 patients with high parasitemia (53.0%).
Table 1.
Findings with the Sysmex XE-2100 analyzer for 1,801 patients with or without malaria infection
| Total patients | No. (%) of patients | |||
|---|---|---|---|---|
| Pseudoeosinophilia | WBC abnormal scattergram | 1–2 abnormalities | ||
| P. vivax overall | 413 | 161 (39.0) | 65 (15.7) | 191 (46.2) |
| Low parasitemia | 130 | 35 (27.0) | 14 (10.8) | 41 (31.5) |
| High parasitemia | 283 | 126 (44.5) | 51 (18.0) | 150 (53.0) |
| None | 1,388 | 0 (0) | 4 (0.3) | 4 (0.3) |
| Sensitivity (%) | 39.0 | 15.7 | 46.2 | |
| Specificity (%) | 100 | 99.7 | 99.7 | |
| PPV (%) | 100 | 94.2 | 97.9 | |
| NPV (%) | 84.6 | 79.9 | 86.2 | |
PPV, positive predictive value; NPV, negative predictive value.
In 161 malaria patients with pseudoeosinophilia, the mean neutrophil counts by Sysmex XE-2100 analyzer and microscopic examination were 58.5% and 71.3%, respectively. The mean eosinophil counts by Sysmex XE-2100 analyzer and microscopic examination were 16.7% and 0.8%, respectively (Table 2).
Table 2.
Comparison of differential count between results with the Sysmex XE-2100 analyzer and results with microscopic examination in 161 patients with pseudoeosinophilia
| Differential count [mean ± SD (%)] | |||
|---|---|---|---|
| Sysmex XE-2100 | Microscopic exam | Mean difference | |
| Neutrophil | 58.5 ± 61.1 | 71.3 ± 14.8 | 12.9 |
| Lymphocyte | 22.1 ± 12.7 | 21.4 ± 13.4 | 0.8 |
| Monocyte | 8.2 ± 5.0 | 6.1 ± 4.7 | 2.0 |
| Eosinophil | 16.7 ± 10.2 | 0.8 ± 1.1 | 16.0 |
| Basophil | 0.6 ± 0.8 | 0.1 ± 0.4 | 0.5 |
The patients without pseudoeosinophilia or abnormal WBC scattergrams showed significantly lower parasite counts (2354.3 ± 3542.1 copies/uL versus 10682.3 ± 3458.2 copies/uL) and higher platelet count (71.6 ± 36.3 103/μL versus 51.4 ± 25.6 103/μL) than the patients with pseudoeosinophilia or abnormal WBC scattergram (P < 0.05; Table 3). It was evident that pseudoeosinophilia was significantly correlated with the parasite count (P < 0.001; Figure 1).
Table 3.
Comparison of parasite counts and platelet counts between samples with and without atypical features on the Sysmex XE-2100 analyzer in 413 malaria-infected patients
| Parasite count mean ± SD (copies/μL) | P value | Platelet count mean ± SD (103/μL) | P value | |
|---|---|---|---|---|
| Pseudoeosinophilia | ||||
| Positive (N = 161) | 4,384.5 ± 6,371.2 | 0.003 | 56.2 ± 24.5 | 0.003 |
| Negative (N = 252) | 1,863.4 ± 1,567.6 | 0.003 | 79.4 ± 44.7 | 0.003 |
| WBC abnormal scattergram | ||||
| Positive (N = 65) | 15,735.2 ± 19,635.5 | 0.002 | 42.7 ± 33.3 | 0.000 |
| Negative (N = 348) | 2,578.9 ± 4,506.3 | 0.002 | 73.1 ± 41.7 | 0.000 |
| Either or both abnormalities | ||||
| Positive (N = 191) | 10,682.3 ± 3,458.2 | 0.000 | 51.4 ± 25.6 | 0.001 |
| Negative (N = 222) | 2,354.3 ± 3,542.1 | 0.000 | 71.6 ± 36.3 | 0.001 |
Figure 1.
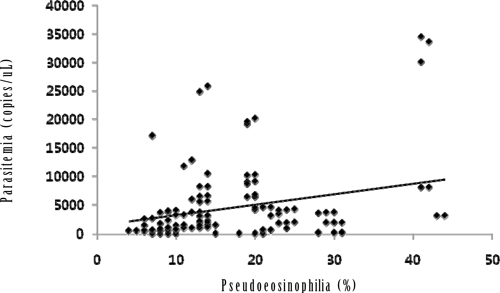
Correlation graph of parasitemia (y) against pseudoeosinophilia as measured by the Sysmex XE-2100 analyzer (x). Regression line was y = 185.4x + 1368 (r = 0.270; P < 0.001; N = 161).
Automated hematology analyzers are capable of detecting specific abnormalities in the blood of patients with malaria. The Cell-Dyn (CD) 3500 (Abbott Diagnostics) is the first automated analyzer to detect malaria hemozoin in monocytes according to their abnormal depolarizing pattern.5,6 In addition, hemozoin-containing neutrophils are probably misidentified as eosinophil, because other features of neutrophils are shared with eosinophils.10 Hemozoin is produced by maturing malarial parasites and is the end product of hemoglobin digestion, consisting of a polymer of heme groups.2,11
Several authors have described pseudoeosinophilia or abnormal WBC scattergrams as a result of hemozoin-containing neutrophils using the Sysmex XE-2100 analyzer.7,8,12 The Sysmex XE-2100 analyzer differentiates eosinophils from neutrophils based on side light-scattering differences. The neutrophils may show considerable side light scattering because of the birefringent character of hemozoin, and therefore, hemozoin-containing neutrophils may be incorrectly classified as eosinophils or two atypical eosinophil populations and two neutrophil populations.7,8 Furthermore, because hemozoin in WBC seems to be a sensitive indicator of prognosis, an automated hematology analyzer may offer a new way to assess disease severity.3 In this study, the mean difference in eosinophil counts between the Sysmex XE-2100 analyzer and microscopic examination was 16.0%, and the mean difference in neutrophil counts was 12.9%. This difference could be explained by the incorrect classification of hemozoin-containing neutrophils as eosinophils in malaria patients.
In this study, the overall sensitivity (46.2%) and specificity (99.7%) of the Sysmex XE-2100 analyzer for the detection of malaria were lower than in the previous study of South Korea (69.4% and 100%, respectively).8 In several studies, the percentage of hemozoin-containing WBCs varied according to populations, age, and disease activity.13–15 A remarkable reduction in sensitivity in this study was probably caused by the presence of younger and fewer circulating parasites, severity of infection, and host immunity factors,16–18 which produced less hemozoin and were below the analyzer detection limits. Our study group was larger (1,801 patients) than that of the previous study of South Korea (463 patients),8 but further evaluation will be needed with larger samples.
Our results showed that samples with pseudoeosinophilia or abnormal WBC scattergrams had significantly higher parasite counts and lower platelet counts than the samples without pseudoeosinophilia or abnormal WBC scattergrams (P < 0.05). Because the half-life (6–7 hours) of neutrophils is short,14 recent parasite counts could be reflected by pseudoeosinophilia or abnormal WBC scattergrams on the Sysmex XE-2100 analyzer. In addition, the Sysmex XE-2100 analyzer could be used as a clinical follow-up parameter.
In conclusion, the Sysmex XE-2100 analyzer is capable of detecting specific abnormalities in the blood of patients with unexpected malaria. Although its overall sensitivity is limited compared with the sensitivities of conventional diagnostic methods such as Giemsa-stained thick blood films, the specificity is high. Therefore, the attention to pseudoeosinophilia and abnormal WBC scattergrams provided by the Sysmex XE-2100 may be useful in the detection of unexpected malaria. Because it is likely that general screening tests like a CBC are always undertaken for patients who present with fever, it can be expected that noticing these abnormalities could prevent delay in the diagnosis of malaria. Clinical hematology laboratories operating the Sysmex XE-2100 analyzer should be aware of these specific parameters, even in the absence of a clinical request. Further studies with XE-2100 analyzer for the detection of unexpected malaria infection are needed in other ethnic groups and patients with non-vivax malaria.
Footnotes
Authors' addresses: Jong-Ha Yoo, Young-Kyu Sun, and Young-Ah Kim, Department of Laboratory Medicine, National Health Insurance Corporation, Ilsan Hospital, Goyang, Korea, E-mails: jhyoo92@nhimc.or.kr, labsun@nhimc.or.kr, and yakim@nhimc.or.kr. Jaewoo Song, Kyung-A Lee, and Jong Rak Choi, Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea, E-mails: labdx@yuhs.ac, kal1119@yuhs.ac, and cjr0606@yuhs.ac. Tae Sung Park, Department of Laboratory Medicine, Kyung Hee University School of Medicine, Seoul, Korea, E-mail: 153jesus@khmc.or.kr.
References
- 1.Hänscheid T, Pinto BG, Cristino JM, Grobusch MP. Malaria diagnosis with the haematology analyser Cell-Dyn 3500: what does the instrument detect? Clin Lab Haematol. 2000;22:259–261. doi: 10.1046/j.1365-2257.2000.00327.x. [DOI] [PubMed] [Google Scholar]
- 2.Scott CS, van Zyl D, Ho E, Meyersfeld D, Ruivo L, Mendelow BV, Coetzer TL. Automated detection of malaria-associated intraleucocytic haemozoin by Cell-Dyn CD4000 depolarization analysis. Clin Lab Haematol. 2003;25:77–86. doi: 10.1046/j.1365-2257.2003.00496.x. [DOI] [PubMed] [Google Scholar]
- 3.Wever PC, Henskens YM, Kager PA, Dankert J, van Gool T. Detection of imported malaria with the Cell-Dyn 4000 hematology analyzer. J Clin Microbiol. 2002;40:4729–4731. doi: 10.1128/JCM.40.12.4729-4731.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hänscheid T, Melo-Cristino J, Pinto BG. Automated detection of malaria pigment in white blood cells for the diagnosis of malaria in Portugal. Am J Trop Med Hyg. 2001;64:290–292. doi: 10.4269/ajtmh.2001.64.290. [DOI] [PubMed] [Google Scholar]
- 5.Hänscheid T, Pinto BG, Pereira I, Cristino JM, Valadas E. Avoiding misdiagnosis of malaria: a novel automated method allows specific diagnosis, even in the absence of clinical suspicion. Emerg Infect Dis. 1999;5:836–838. doi: 10.3201/eid0506.990621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coetzer TL, Scott S, Marshall L, Liebowitz L, Wypkema E, Munster M, Tana M, Nhlangothi P, Lyons C, Mendelow BV. Automated malaria detection by depolarization of laser light. Br J Haematol. 1999;104:499–503. doi: 10.1046/j.1365-2141.1999.01199.x. [DOI] [PubMed] [Google Scholar]
- 7.Huh J, Jung J, Yoon H, Chung W. Pseudoeosinophilia associated with malaria infection determined in the Sysmex XE-2100 hematology analyzer. Ann Hematol. 2005;84:400–402. doi: 10.1007/s00277-004-0987-z. [DOI] [PubMed] [Google Scholar]
- 8.Huh HJ, Oh GY, Huh JW, Chae SL. Malaria detection with the Sysmex XE-2100 hematology analyzer using pseudoeosinophilia and abnormal WBC scattergram. Ann Hematol. 2008;87:755–759. doi: 10.1007/s00277-008-0486-8. [DOI] [PubMed] [Google Scholar]
- 9.Farcas GA, Zhong KJ, Mazzulli T, Kain KC. Evaluation of the RealArt Malaria LC real-time PCR assay for malaria diagnosis. J Clin Microbiol. 2004;42:636–638. doi: 10.1128/JCM.42.2.636-638.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grobusch MP, Valadas E, Hänscheid T. Automated malaria diagnosis using pigment detection. Parasitol Today. 2000;16:549–551. doi: 10.1016/s0169-4758(00)01742-7. [DOI] [PubMed] [Google Scholar]
- 11.Hänscheid T, Egan TJ, Grobusch MP. Haemozoin: from melatonin pigment to drug target, diagnostic tool, and immune modulator. Lancet Infect Dis. 2007;7:675–685. doi: 10.1016/S1473-3099(07)70238-4. [DOI] [PubMed] [Google Scholar]
- 12.Park GB, Cha YJ. Three cases of pseudoeosinophilia associated with malaria determined in the Sysmex XE-2100 Automated Hematology Analyzer. Korean J Lab Med. 2006;26:77–80. doi: 10.3343/kjlm.2006.26.2.77. [DOI] [PubMed] [Google Scholar]
- 13.Gbadegesin RA, Olumese PE, Adeyemo AA, Amodu OK. Intraleucocytic malaria pigment and clinical severity of malaria in children. Trans R Soc Trop Med Hyg. 1998;92:54–56. doi: 10.1016/s0035-9203(98)90952-x. [DOI] [PubMed] [Google Scholar]
- 14.White NJ, Tran TH, Nguyan HP, Bethell DB, Nguyen TH, Ly VC, Pham PL, Dinh XS, Phan TL, Pham TD, Day NP. Clearance kinetics of parasites and pigment-containing leukocytes in severe malaria. Blood. 1996;88:4694–4700. [PubMed] [Google Scholar]
- 15.Kremsner PG, Mordmller BG, Metzger WG. Malaria pigment in leucocytes. Trans R Soc Trop Med Hyg. 1995;89:637–638. doi: 10.1016/0035-9203(95)90423-9. [DOI] [PubMed] [Google Scholar]
- 16.Grobusch MP, Hanscheid T, Kramer B, Neukammer J, May J, Seybold J, Kun JF, Suttorp N. Sensitivity of hemozoin detection by automated flow cytometry in non- and semi-immune malaria patients. Cytometry B Clin Cytom. 2003;55:46–51. doi: 10.1002/cyto.b.10039. [DOI] [PubMed] [Google Scholar]
- 17.Mendelow BV, Lyons C, Nhlangothi P, Tana M, Munster M, Wypkema E, Liebowitz L, Marshall L, Scott S, Coetzer TL. Automated malaria detection by depolarization of laser light. Br J Haematol. 1999;104:499–503. doi: 10.1046/j.1365-2141.1999.01199.x. [DOI] [PubMed] [Google Scholar]
- 18.Padial MM, Subirats M, Puente S, Lago M, Crespo S, Palacios G, Baquero M. Sensitivity of laser light depolarization analysis for detection of malaria in blood samples. J Med Microbiol. 2005;54:449–452. doi: 10.1099/jmm.0.45650-0. [DOI] [PubMed] [Google Scholar]


