Abstract
We used kernel density and scan statistics to examine the spatial distribution of cases of pediatric and adult American cutaneous leishmaniasis in an urban disease-endemic area in Salta Province, Argentina. Spatial analysis was used for the whole population and stratified by women > 14 years of age (n = 159), men > 14 years of age (n = 667), and children < 15 years of age (n = 213). Although kernel density for adults encompassed nearly the entire city, distribution in children was most prevalent in the peripheral areas of the city. Scan statistic analysis for adult males, adult females, and children found 11, 2, and 8 clusters, respectively. Clusters for children had the highest odds ratios (P < 0.05) and were located in proximity of plantations and secondary vegetation. The data from this study provide further evidence of the potential urban transmission of American cutaneous leishmaniasis in northern Argentina.
Introduction
The term leishmaniasis involves different nosologic entities, which can be further grouped into the clinical forms: visceral leishmaniasis and cutaneous leishmaniasis. American cutaneous leishmaniasis (ACL) includes a spectrum of clinical entities comparable to leprosy, ranging from the simple cutaneous forms to the mucocutaneous and the anergic forms, the latter of which includes diffuse cutaneous leishmaniasis.1 American cutaneous leishmaniasis is endemic in nine neighboring provinces of northern Argentina located between 22°21'S, 64°39'W and 28°22'S, 65°21'W, which include the provinces of Salta, Jujuy, Catamarca, Tucumán, Misiones, Corrientes, Chaco, Santiago del Estero, and Formosa.2 The etiologic agents are protozoan parasites of the genus Leishmania, and the predominant species in this area has been identified as Leishmania (Viannia) braziliensis, although Leishmania (Leishmania) amazonensis and Leishmania (V.) guyanensis have also been identified in a few cases.3,4 The sand fly vectors of this disease belong to the family Psychodidae and the subfamily Phlebotominae. In this region, the species that have been previously described are Lutzomyia neivai, Lu. migonei, Lu. cortelezzii, Lu. shannoni, and Lu. punctigeniculata.5–8 Lutzomyia neivai is the unique species of phlebotomus sand fly in the region for which infection with Leishmania sp. has been proved.9
Within the province of Salta, the Orán and San Martin jurisdictions have been affected by ACL outbreaks, and rural zones of Orán have exhibited endemic and epidemic transmission levels.10–12 Furthermore, these localities are characterized by the production of sugar cane, banana, citrus fruits, and green vegetables, which are grown in plantations where rodent plagues are common. These rodents include Akodon sp., Rattus rattus, Olygoryzomys sp., and Oryzomys sp., which have been incriminated as potential reservoirs of Leishmania sp. in other Latin American countries.13–16 Dogs with lesions and dogs that are serologically reactive but without lesions have been found in this area. However, these dogs are considered secondary hosts and not reservoirs.17,18
Work or recreational activities in rural areas or deforestation zones, performed mainly by adult men, have been considered the most relevant risk factor related to infection by Leishmania sp.19,20 However, an important number of cases of ACL in children and women have been observed in different areas of South America.11,19–23 Particularly in Salta, domiciliary and peridomiciliary exposure to the vector appears to be a common event among affected patients in these demographic groups.24 A high rate of ACL cases among women and children has been proposed as an indicator of domiciliary and peridomiciliary transmission.11
Spatial epidemiology, defined as the description and analysis of geographic variations in health, illness, and its determinants (demographic, environmental, behavioral, socioeconomic, genetic, and infectious risk factors), is critical to interpreting entomologic, clinical and epidemiologic data, identifying risk areas, and providing efficient interventions.25 Analysis of the distribution of cases of ACL with geographic information systems can help locate hot spots of transmission, thereby further guiding research and surveillance activities. In the present study, we analyzed the spatial distribution of ACL cases of groups stratified by age and sex to identify risk areas of transmission in an urban region in northern Argentina hyperendemic for ACL.
Materials and Methods
Study area.
San Ramon de la Nueva Orán (Department of Orán) is located at 23°08'S, 64°20'W, 350 meters above sea level. The city has a population of 66,915 persons, of whom approximately 11% live in rural areas.26 Orán is situated in the tropical pedemontana Yungas rainforest, a region affected by agricultural activity, forest exploitation, and the continued appearance of new settlements (Figure 1).27
Figure 1.
Geographic location of San Ramón de la Nueva Orán, Province of Salta, Argentina.
Study population.
The study population included patients diagnosed with ACL at the Instituto de Investigaciones en Enfermedades Tropicales (IIET) at the Universidad Nacional de Salta in Orán, a regional reference center for the diagnosis of leishmaniasis. IIET collaborates with the regional hospital as the diagnostic center; treatment of leishmaniasis is managed through the National Program for Leishmaniasis (National Ministry of Health of Argentina). A database including demographic, laboratory, and clinical information was generated by using information gathered from this population during 1987–2007. Approximately 2,400 cases of ACL are archived in this database.
Inclusion criteria for the analysis consisted of patients with lesions clinically compatible with ACL seen at the IIET during 1995–2007, which were confirmed by visualization of amastigotes of Leishmania sp. in Giemsa-stained smears, a positive Montenegro skin test result, or a positive result in an enzyme-linked immunosorbent assay for homogenate protein of L (V.) braziliensis. Patients' addresses, ages at presentation, and sex were obtained from the database (Figure 2). Exclusion criteria included patients with residence outside Orán at the time of illness, and those with missing address information and/or lack of information about age and/or sex. In addition, a centroid was assigned to each of the 45 neighborhoods comprising San Ramon de la Nueva Orán. Each centroid corresponds to the center of the polygons representing these neighborhoods.
Figure 2.
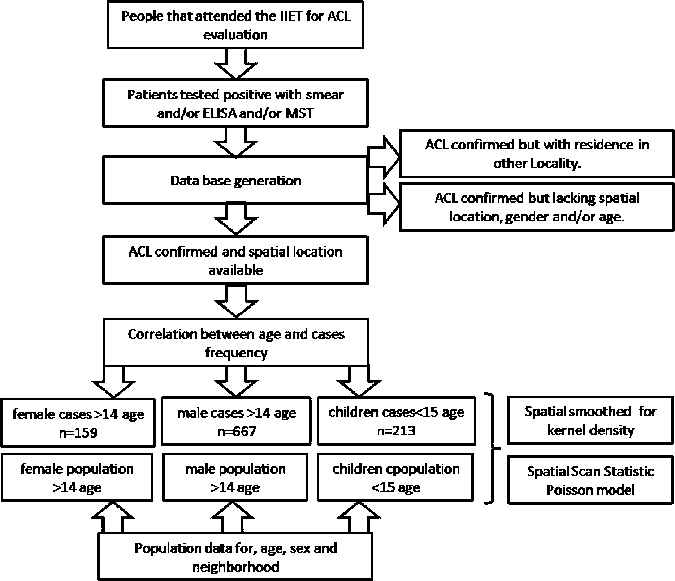
Data from local health system for diagnosis of American cutaneous leishmaniasis, Instituto de Investigaciones en Enfermedades Tropicales, Salta, Argentina.
The demographic and the basic unsatisfied needs index data for each neighborhood were obtained from the 2001 census of Salta Province. This index is used to identify critical lacks in a population and characterize levels of poverty; it uses variables related to four areas of basic needs (housing, sanitary services, basic education, and minimum wage).26 The Spearman correlation was used to study the possible association of ACL prevalence and basic unsatisfied needs score by using a 95% confidence interval.
The study sample was stratified by age and sex. For age, groups were defined taking into account the strength of the association (correlation for R Spearman range test; P < 0.05) between the variable age and the absolute frequency of cases. For this association, we analyzed correlations for sex with data sets from 0 to 8, 0 to 9, and 0 to 10 up to 0 to N years, where N = the maximum age for each sex, to identify the point at which a significant correlations appears. Sex stratification was performed for adult cases to study the spatial tendencies of this variable.
Prevalence analysis.
The ACL prevalence for 1995–2007 was calculated by age, sex, and geographic location by using population data from 2001, the midpoint of the study period.28 Two prevalence analyses were performed, one by neighborhood and one for San Ramon de la Nueva Orán. Prevalences were compared by using the parametric Z test for difference between proportions and EPIDAT version 3.1 software (Xunta de Galicia, Santiago de Compostela, Spain and Pan American Health Organization, Washington, DC).
Kernel density.
Spatial smoothing of the distribution of ACL was estimated by means of the kernel density function using the software ArcGIS 9.1 (Environmental System Research Institute Inc., Redlands, CA). The kernel function is based on the quadratic kernel function described by Silverman.29 This method is an interpolation and smoothing tool used to generalize the position of a point to an area. The kernel function generates smoothing of the magnitude of one variable (prevalence in this study) centered on each point (centroids) using calculations for local neighborhoods. Conceptually and taking into account the framework of the present study, the kernel value is highest at the location of a particular point (centroid) and decreases at increasing distance from that point, reaching zero at the defined search radius from the point. Only a circular neighborhood is possible, and on the overlapping areas among different circular neighborhood, the density at each output raster cell is calculated by adding the values of all the kernel surfaces overlaying on each raster cell, and a smoothed surface of the disease prevalence is generated for the study region.29 The search radius was calculated as the average of the distances among the centroids of each neighborhood with respect to the centroids of the adjacent neighborhoods.30 A cell size of 8 meters2 was used for the output to describe the distribution of ACL prevalence. This cell size was selected because it provided optimal smoothing for the purposes of this analysis.
Scan statistics.
Scan statistics were used to determine spatial aggregation of cases, defined as clusters. Scan statistical analysis sequentially centers circles in each location where information is available and, using a Poisson model, compares the proportion of cases/population for the area inside the circle with the proportion outside the circle.31–33 Therefore, the spatial scan statistical analyses for this study were performed using the data set of the cases and population for each locality (for age and sex) with Sat Scan™ software (http://www.satscan.org). Spatial subanalyses were also performed for three shorter periods (1995–1999, 2000–2003, and 2004–2007).
The size of the spatial window was 30%, and none of the secondary clusters were centered to contain the center of other clusters. A window of 50% was not used because of the short reach of transmission based on vector characteristics; a window of 30% corresponds to approximately 1,000 meters from the edge of town.34 Those clusters with a P < 0.05 obtained through the use of scan statistic analysis were subsequently mapped using the software package SIGEpi 1.0 (http://www.phao.org/Spanish/SHA/shasig.htm). For each spatial cluster, odds ratios (ORs) and 95% confidence intervals were obtained. The cartography corresponding to San Ramón de la Nueva Orán and a projection of the Gauss Krueger model were obtained from the General Office of Statistics and Census of Salta Province, Argentina.
Results
During 1995–2007, 1,910 patients were diagnosed with ACL at the IIET, 22 with unknown locality of residence and 1,274 from Orán; 1,039 fulfilled the criteria for inclusion into the present study. Patients excluded from the analysis included 227 because of missing data on spatial location within Orán, 1 for missing sex data, and 5 for missing age data. There were no statistically significant differences for age and sex between the group that was included in the analysis and those excluded because of lack of spatial location within Orán. For men, the prevalence of ACL was significantly higher for that for persons > 14 years old (P < 0.05). There was no statistically significant difference in disease prevalence for women by age (Figure 3).
Figure 3.
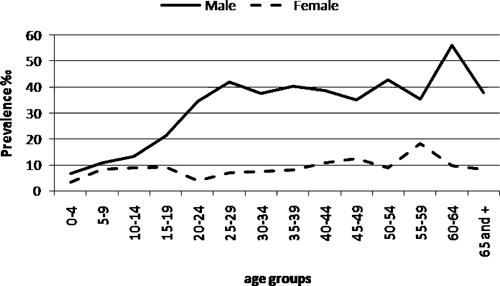
Prevalence of American cutaneous leishmaniasis in San Ramon de la Nueva Oran, Argentina, according to age and sex, 1995–2007.
For men 16–44 years of age, there was a statistically significant correlation between age and prevalence (P < 0.05). No correlation between age and prevalence was found for children less than 15 years of age or women (P > 0.05). The stratification process generated three subgroups: women > 14 years of age old (n = 159), men > 14 years of age (n = 667), and children < 15 years of age (n = 213). Neighborhoods with higher basic unsatisfied needs scores were significantly associated with increased ACL prevalence for children, women, and men (P < 0.05).
When stratified by neighborhood, the average number of observed cases during the study period was 25 (range = 0–142). The obtained search radius was 490 meters and was calculated as an average of the distances between the centroid of each neighborhood and its closest neighborhoods. The prevalence of ACL in children and women were 6.73% and 6.44%, respectively, and did not differ significantly (P > 0.05). In contrast, these groups had a significantly lower prevalence than men (32.89%; P < 0.001). The male:female ratio in children < 15 years of age was 1.51. Likewise, the ACL prevalence was 10.38% in boys < 15 years of age and 6.96% in girls < 15 years of age (P < 0.01).
Spatial smoothness by kernel density was performed for the entire population, men, and women according to neighborhood; it demonstrated a wide distribution of cases. Specifically, 71% of the neighborhoods had cases in San Ramon de la Nueva Orán, with high intensity areas in the periphery, extending from south to north for the entire eastern border and some peripheral nuclei in the western border (Figure 4).
Figure 4.
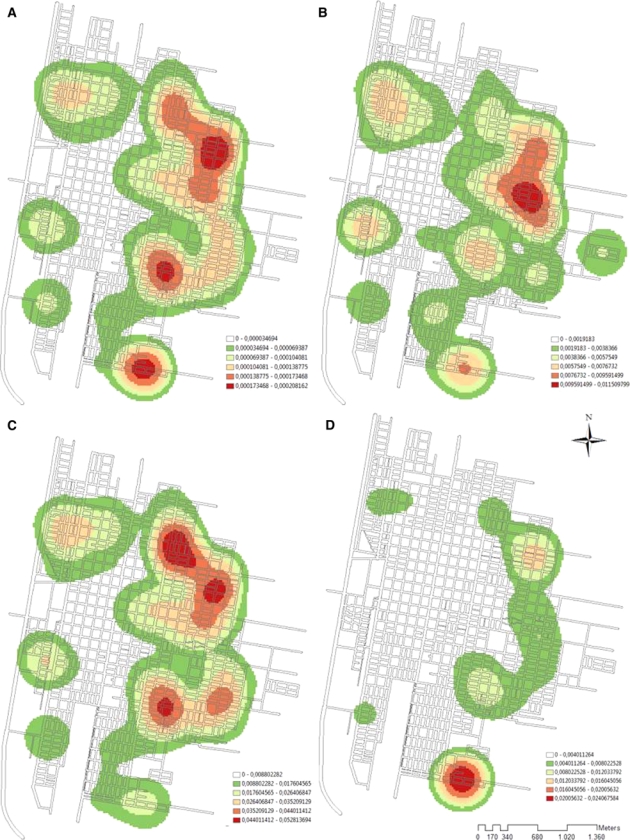
Distribution of cases of American cutaneous leishmaniasis (ATL) in San Ramón de la Nueva Oran, Argentina. Prevalence was disaggregated through Kernel density using A, total prevalence by neighborhood; B, prevalence of ATL in women > 14 years of age; C, prevalence by neighborhood of men > 14 years of age; and D, prevalence by neighborhood of children < 15 years of age. Morbidity and demographic information were assigned to the centroids of every neighborhood (45 centroids). This figure appears in color at www.ajtmh.org.
Regarding smoothness of prevalence in children according to neighborhood, restriction of the disease process was detected towards the eastern border of the city. Other areas of low intensity of ACL were detected in two locations (northwestern and southwestern areas), but disease activity was not detected in the rest of the city for this age group (Figure 4D).
No significant clusters were found in any secondary analyses of shorter periods (1995–1999, 2000–2003, and 2004–2007). During 1995–2007, the spatial scan statistic identified 11 clusters of ACL in men along the eastern border of the city that reached the northern city limit. These clusters had ORs ranging from 1.42 to 3.20, and three had a radius equal to zero (clusters 11–21). Because each identified case uses its respective neighborhood centroid as a reference point, a cluster of zero radius indicates that a specific neighborhood is a cluster in itself. In addition, two clusters were found through scan statistic analysis of cases in women: one in the northeastern part of the city (OR = 2.76) and the other in the eastern part (OR = 2.26) (Figure 5; clusters 9 and 10). Scan statistic analysis of the entire study population detected four isolated clusters, three with a radius equal to zero, and two with ORs > 3 (southern and northeastern areas) (Figure 5; clusters 22–25).
Figure 5.
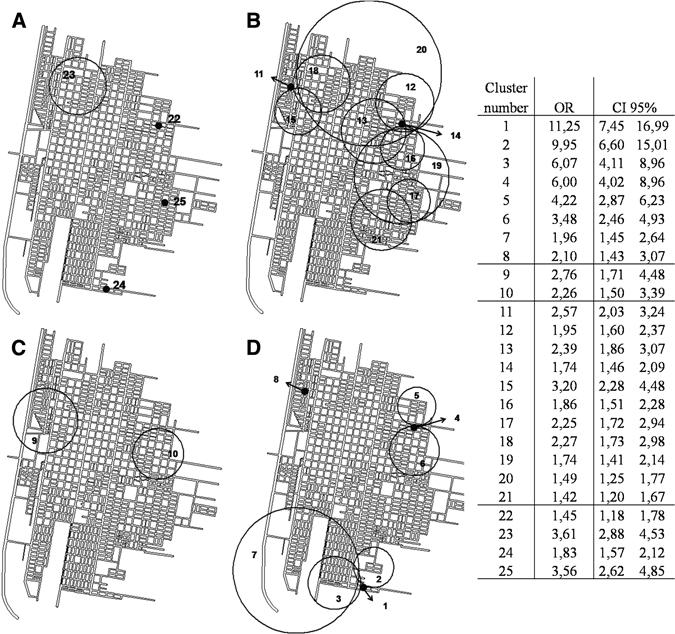
Clusters detected by scan statistic using a Poisson model on case-population data for American cutaneous leishmaniasis in San Ramon de la Nueva Oran, Argentina. A, all cases; B, men > 14 years of age; C, women > 14 years of age; and D, children < 15 years of age. The table on the right shows odds ratios and 95% confidence intervals. The numbers in the map correspond to the clusters included in the table. Only clusters with P values < 0.05 are included.
Analysis of the data set for children < 15 years of age demonstrated seven clusters. Four of these clusters were located in the southern part of the city, three in the northeast part and one with a radius = 0 and an OR = 2.10 (cluster 8), at the city limits to the northwestern part. The ORs of the northeastern clusters ranged between 3.48 and 6.00 (clusters 4–6), and one of these clusters had a radius = 0. The southeastern clusters had ORs higher than those for the other areas of the city (clusters 6.07–11.25) for children < 15 years of age and for all other age and sex groups (Figure 5; clusters 1–3).
The annual variation in ACL frequency demonstrates a similar pattern in children and adults. Increases in frequency were observed in 1998 and 2002 in all subgroups, regardless of whether cases generated clusters per scan statistic analysis. An increase in incidence was observed in children in the southern part of the city during 1996–1997 and was followed by an additional increase in incidence in 2006 in the same area of the city (Figure 6).
Figure 6.
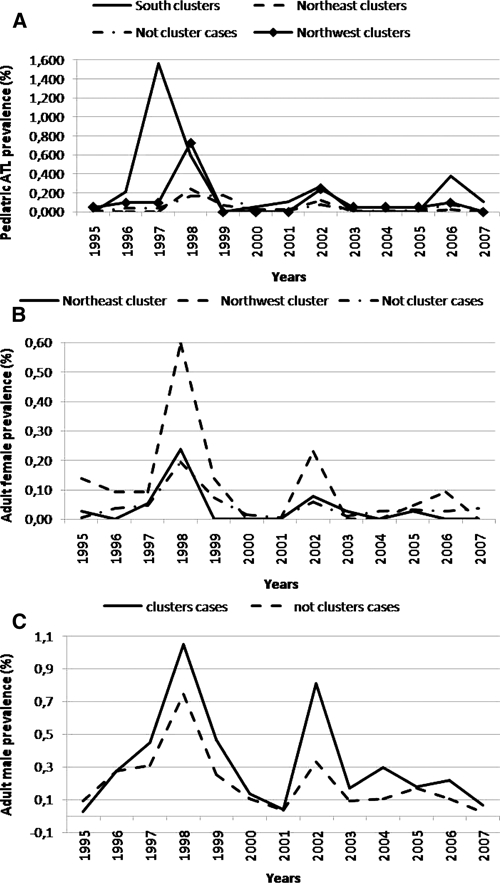
Temporal variation (in years) of prevalence of American cutaneous leishmaniasis during 1995–2007 in San Ramon de la Nueva Oran, Argentina. A, Prevalence per 100 inhabitants in children < 15 years of age; B, prevalence per 100 inhabitants in women; and C, prevalence per 100 inhabitants in men.
Discussion
The results of this spatial analysis study performed in San Ramón de la Nueva Orán, the city with the highest prevalence of cases of ACL in Argentina,6 provides further evidence for the potential occurrence of urban transmission of this infection. In general, use of a patient's home residence as a marker of the place of infection acquisition is not accurate because contact between patient and vector can occur outside the home. However, when applied to children, a subgroup that was analyzed separately in this study, our findings suggest that home residence is an effective marker for these subgroups. A possible explanation for this result is that exposure during work and recreational activities is almost exclusively limited to men > 14 years of age, thereby limiting exposure sites for children to their domiciliary and peridomiciliary environment. This finding is further strengthened by previous epidemiologic studies that suggested domiciliary or peridomiciliary vector exposure in northern Argentina.24 Based on behavioral, socioeconomic and cultural factors, the importance of each type of exposure (domiciliary, workplace, or recreational) variably affects the overall exposure risk for each demographic group. Variations in the incubation period of ACL present an additional difficulty in determining the transmission site.35
Men are the group with greatest vector exposure risk outside the home. In our study, they also had the highest prevalence (32.89%), which was approximately five times higher than that observed in women and children (P < 0.001). Higher disease prevalence among men was also observed in the younger age group, a group without relevant differences in exposure. This difference could be caused by sex-related higher susceptibility to the disease, which has been suggested in animal studies in a hamster model.36 Additionally, a continuous increase in the strength of association between age and prevalence for cases in persons > 15 years of age was also observed. This increase may be related to the inception or intensification of labor and recreational activities (e.g., fishing and hunting) in younger persons.
Kernel density distributions for men and women showed that most neighborhoods in the study site had reported cases for these subgroups. However, the distribution observed for children showed localized distribution, with a high prevalence only on the eastern border (Figure 4). The presence of Lutzomyia spp. has been recently reported in urban and periurban zones in the San Ramón de la Nueva Orán.6 Ecotones, areas of transition between two or more ecosystems that have common characteristics to both ecosystems, are in this case represented by secondary vegetation edges adjacent to urban and rural areas, and appear to be places with the highest potential risk of transmission.37 Moreover, these places are located near neighborhoods with clusters of pediatric cases with high ORs. This finding can be explained by a Lu. neivai metapopulation source-sink pattern, which is characterized by a stable population (source), a location in a favorable habitat for the species (e.g., secondary vegetation patches), and other populations in non-favorable habitats (urban zones).6 The above mentioned vector population would have high mortality rates and would thereby support a relative stability in population levels because of recolonization from the source population.38
The possibility that human populations can serve as a reservoir of ACL is not ruled out in this setting. However, this suggestion is unlikely because it would generate an independence from and synergy with other mammals for establishment of urban transmission. Our results strengthen the hypothesis that in this region of Argentina the reservoir species are mainly wild animals, and not humans or dogs. This hypothesis is based on the absence of high incidence of urban cases with epidemic cycles.6,20
Orán is surrounded by large areas dedicated to agricultural activity (with residual secondary vegetation), which serve as potential foci for rodent plagues, previously reported in other countries as potential reservoirs of Leishmania spp.13,15,16 Different environmental disturbances caused by humans can be associated with increases in ACL incidence.39 In northern Salta, the environmental change and degradation of the ecosystem are secondary to intensification of agricultural practices, and their impact on native forests affects biological diversity and could favor proliferation of species that act as reservoirs or vectors of Leishmania spp.31,40 Likewise, this loss of diversity can increase the incidence of ACL in secondary hosts such as dogs and humans, and can be attributed to the loss of the dilution effect.38,41,42 This hypothesis states that in environments with high diversity, there may be several secondary hosts that can become infected (either symptomatic or asymptomatic) but are not reservoirs. These secondary hosts decrease vector-effective contact with the true reservoir, thus reducing the rate of infection of vectors and humans. When environmental alterations occur, the loss of diversity with maintenance of the reservoir hosts can trigger increased human exposure and infection prevalence.
The high OR values for the clusters corresponding to pediatric cases located in the southern and eastern areas of the city might be related to increases in periurban reservoirs as a source of parasites and their overlap with vector distribution in borders having transitional landscape of urban–rural or urban–residual vegetation (Figure 5).22,43 The association between ACL prevalence and the basic unstatisfied needs index could be related to peripheral location of settlements with impoverished populations, in which geographic proximity to vector exposure is higher. Among those neighborhoods with clusters with radius = 0, these results indicate that those neighborhoods are entities with particular physical, cultural, and/or socioeconomic characteristics that pose a significant risk for infection.
The ACL frequency in all age and sex groups during the study period, including areas with and without clusters, showed a similar pattern (Figure 6). This finding could be secondary to a local increase of risk in urban areas caused by occasional vector and reservoir urbanization, and/or a risk increase in wildnerness, rural, periurban, or urban areas caused by large-scale environmental changes such as deforestation, agricultural and climatic cycles, or other events that influence vector and reservoir dynamic population.21,31,34,44 It has been previously described that the abundance of Lu. neivai in this region is associated with rainfall in the previous year, and that conditions of temperature and relative humidity have an impact on the population densities observed 20 weeks later.7 In Costa Rica, research has shown that cycles of change in the incidence of ACL of three-year periods are associated with changes in temperature. In accordance with these observations, temporal changes in the prevalence of ACL in Orán could be correlated with climatic changes.44
The lack of clusters in the spatial analysis using shorter periods suggests that there is a low level and slow accumulation of cases of an endemic nature in certain areas of the city, which may represent a small but active urban transmission. The median number of annual cases in San Ramón de la Nueva Orán is 53, a fact that would not enable finding significant clusters in scan statistic analysis when shorter periods are considered. Other potential limitations of this study are underreporting and selective referral of patients from particular areas of the city, which would be a potential source of bias. However, this limitation is probably of low magnitude and not significant in the study area because of the centralized and government-based nature of the management of leishmaniasis in Salta Province and the lack of other large specialized laboratories in the area. Conversely, missing data caused by omissions in data capture are randomly distributed across age and sex groups and geographic region.
The population size used for the analysis corresponds to the 2001 census data, the midpoint of the study period, and it was assumed that the birth, mortality, and migration rates varied in the same direction and magnitude in the whole locality and that this variation was not significant. This assumption, a potential limitation of the study, was based on knowledge that no significant political, socioeconomic, or natural events that could affect those rates occurred during the study period in this region.
Besides the uncertainty that can confound the true determinants for establishment of ACL, the compiled evidence shows the need for generation of an entomologic surveillance system in this region and to further study the dynamics of populations of reservoir or potential reservoir species.45 Also, control strategies need to be directed to peridomestic and sylvatic transmission and integrated into surveillance systems that include most of the variables that participate in the transmission cycle of ACL. This information, coupled with geographic information systems, could provide reliable correlations that explain the endemic and/or epidemic patterns of this disease.
In summary, our analysis suggests that urban transmission of ACL occurs in San Ramón de la Nueva Orán. This suggestion is based on areas with an increase ORs in children, a group with exposure restricted to their domiciliary or peridomiciliary environment.
Acknowledgments
We thank Roberto Dib Ashur (Dirección de Estadísticas y Censos de la Provincia de Salta) and Gladis Romero for assistance; Dr. Verónica Rajal (Fogarty International Center–Instituto de Investigaciones para la Industria Química) for providing ArcGIS software; Horacio Castellaro (Ministerio de Educación de la Nación) for technical assistance; and Ravi Kavasery for carefully reviewing the manuscript.
Footnotes
Financial support: This study was supported by the Consejo de Investigación de la Universidad Nacional de Salta and the Secretaria de Ciencia y Técnica de la Nación through PICTo-2006 and the Institut de Recherche pour le Développement, France.
Authors' addresses: José F. Gil, Silvana P. Cajal, Marisa Juarez, and Norma Acosta, Instituto de Investigaciones en Enfermedades Tropicales, Sede Regional Orán, Universidad Nacional de Salta, San Ramón de la Nueva Orán, Salta, Argentina, E-mails: jgil@unsa.edu.ar, spcajal@yahoo.com, marjualu@yahoo.com.ar, and acosta.nor@gmail.com. Julio R. Nasser and Rubén O. Cimino, Cátedra de Química Biológica y Biología Molecular, Facultad de Ciencias Naturales, Universidad Nacional de Salta, Salta, Argentina, E-mails: nasserj@unsa.edu.ar and rcimino@unsa.com.ar. Patricio Diosque, Unidad de Epidemiología Molecular, Instituto de Patología Experimental, Facultad de Ciencias de la Salud, Universidad Nacional de Salta, Salta, Argentina, E-mail: pdiosque@yahoo.com.ar. Alejandro J. Krolewiecki, Área de Investigaciones Clínicas, Fundación Huesped, Buenos Aires, Argentina, E-mail: alekrol@huesped.org.ar.
References
- 1.Grimaldi G, Jr, Tesh RB. Leishmaniases of the New World: current concepts and implications for future research. Clin Microbiol Rev. 1993;6:230–250. doi: 10.1128/cmr.6.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salomon OD, Bogado De Pascual M, Molinari ML, Verri V. Study of a cutaneous leishmaniasis outbreak in General Vedia, Province of Chaco, 1996. Rev Inst Med Trop Sao Paulo. 2001;43:99–104. doi: 10.1590/s0036-46652001000200009. [DOI] [PubMed] [Google Scholar]
- 3.Krolewiecki AJ, Romero HD, Cajal SP, Abraham D, Mimori T, Matsumoto T, Juarez M, Taranto NJ. A randomized clinical trial comparing oral azithromycin and meglumine antimoniate for the treatment of American cutaneous leishmaniasis caused by Leishmania (Viannia) braziliensis. Am J Trop Med Hyg. 2007;77:640–646. [PubMed] [Google Scholar]
- 4.Marco JD, Barroso PA, Calvopina M, Kumazawa H, Furuya M, Korenaga M, Cajal SP, Mora MC, Rea MM, Borda CE, Basombrio MA, Taranto NJ, Hashiguchi Y. Species assignation of Leishmania from human and canine American tegumentary leishmaniasis cases by multilocus enzyme electrophoresis in north Argentina. Am J Trop Med Hyg. 2005;72:606–611. [PubMed] [Google Scholar]
- 5.Barroso PA, Marco JD, Kato H, Tarama R, Rueda P, Cajal SP, Basombrio MA, Korenaga M, Taranto NJ, Hashiguchi Y. The identification of sandfly species, from an area of Argentina with endemic leishmaniasis, by the PCR-based analysis of the gene coding for 18S ribosomal RNA. Ann Trop Med Parasitol. 2007;101:247–253. doi: 10.1179/136485907X156988. [DOI] [PubMed] [Google Scholar]
- 6.Salomon OD, Quintana MG, Zaidenberg M. Urban distribution of Phlebotominae in a cutaneous leishmaniasis focus, Argentina. Mem Inst Oswaldo Cruz. 2008;103:282–287. doi: 10.1590/s0074-02762008005000016. [DOI] [PubMed] [Google Scholar]
- 7.Salomon OD, Wilson ML, Munstermann LE, Travi BL. Spatial and temporal patterns of phlebotomine sand flies (Diptera: Psychodidae) in a cutaneous leishmaniasis focus in northern Argentina. J Med Entomol. 2004;41:33–39. doi: 10.1603/0022-2585-41.1.33. [DOI] [PubMed] [Google Scholar]
- 8.Salomon OD, Zaidenberg M, Burgos R, Heredia VI, Caropresi SL. American cutaneous leishmaniasis outbreak, Tartagal city, province of Salta, Argentina, 1993. Rev Inst Med Trop Sao Paulo. 2001;43:105–108. doi: 10.1590/s0036-46652001000200010. [DOI] [PubMed] [Google Scholar]
- 9.Cordoba-Lanus E, De Grosso ML, Pinero JE, Valladares B, Salomon OD. Natural infection of Lutzomyia neivai with Leishmania spp. in northwestern Argentina. Acta Trop. 2006;98:1–5. doi: 10.1016/j.actatropica.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Salomon OD, Sosa Estani S, Canini L, Cordoba Lanus E. Tegumentary leishmaniasis in area with epidemic levels of transmission, Salta, Argentina, 1998 [in Spanish] Medicina (B Aires) 2001;61:284–290. [PubMed] [Google Scholar]
- 11.Sosa-Estani S, Segura EL, Salomon OD, Gomez A, Peralta M, Coutada V, Ruiz LM. Tegumentary leishmaniasis in northern Argentina: distribution of infection and disease, in three municipalities of Salta, 1990–1992. Rev Soc Bras Med Trop. 2000;33:573–582. doi: 10.1590/s0037-86822000000600009. [DOI] [PubMed] [Google Scholar]
- 12.Taranto NJ, Cajal SP, De Marzi MC, Fernandez MM, Frank FM, Bru AM, Minvielle MC, Basualdo JA, Malchiodi EL. Clinical status and parasitic infection in a Wichi Aboriginal community in Salta, Argentina. Trans R Soc Trop Med Hyg. 2003;97:554–558. doi: 10.1016/s0035-9203(03)80026-3. [DOI] [PubMed] [Google Scholar]
- 13.Zamorano E, Vargas J. La rata negra (Rattus rattus Linneo, 1758) como plaga de los cultivos ibéricos de caña de azúcar. Detección, estima y control de los daños ocasionados. Bol Sanid Veg Plagas. 1988;14:227–240. [Google Scholar]
- 14.Díaz M, Braun J, Mares M, Barquez R. An update of the taxonomy, systematics, and distribution of the mammals of Salta Province, Argentina. Sam Noble Oklahoma Museum of Natural History. 2000;10:1–52. [Google Scholar]
- 15.Kerr SF, Emmons LH, Melby PC, Liu C, Perez LE, Villegas M, Miranda R. Leishmania amazonensis infections in Oryzomys acritus and Oryzomys nitidus from Bolivia. Am J Trop Med Hyg. 2006;75:1069–1073. [PubMed] [Google Scholar]
- 16.Telleria J, Bosseno MF, Tarifa T, Buitrago R, Martinez E, Torrez M, Le Pont F, Breniere SF. Putative reservoirs of Leishmania amazonensis in a sub-Andean focus of Bolivia identified by kDNA-polymerase chain reaction. Mem Inst Oswaldo Cruz. 1999;94:5–6. doi: 10.1590/s0074-02761999000100002. [DOI] [PubMed] [Google Scholar]
- 17.Marco JD, Padilla AM, Diosque P, Fernandez MM, Malchiodi EL, Basombrio MA. Force of infection and evolution of lesions of canine tegumentary leishmaniasis in northwestern Argentina. Mem Inst Oswaldo Cruz. 2001;96:649–652. doi: 10.1590/s0074-02762001000500009. [DOI] [PubMed] [Google Scholar]
- 18.Reithinger R, Davies CR. Is the domestic dog (Canis familiaris) a reservoir host of American cutaneous leishmaniasis? A critical review of the current evidence. Am J Trop Med Hyg. 1999;61:530–541. doi: 10.4269/ajtmh.1999.61.530. [DOI] [PubMed] [Google Scholar]
- 19.Ampuero J, Urdaneta M, Macêdo Vde O. Risk factors for cutaneous leishmaniasis transmission in children aged 0 to 5 years in an endemic area of Leishmania (Viannia) braziliensis. Cad Saude Publica. 2005;21:161–170. doi: 10.1590/s0102-311x2005000100018. [DOI] [PubMed] [Google Scholar]
- 20.Salomon OD, Orellano PW, Quintana MG, Perez S, Sosa Estani S, Acardi S, Lamfri M. Transmission of tegumentary leishmaniasis in Argentina [in Spanish] Medicina (B Aires) 2006;66:211–219. [PubMed] [Google Scholar]
- 21.Campbell-Lendrum D, Dujardin JP, Martinez E, Feliciangeli MD, Perez JE, Silans LN, Desjeux P. Domestic and peridomestic transmission of American cutaneous leishmaniasis: changing epidemiological patterns present new control opportunities. Mem Inst Oswaldo Cruz. 2001;96:159–162. doi: 10.1590/s0074-02762001000200004. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira CC, Lacerda HG, Martins DR, Barbosa JD, Monteiro GR, Queiroz JW, Sousa JM, Ximenes MF, Jeronimo SM. Changing epidemiology of American cutaneous leishmaniasis (ACL) in Brazil: a disease of the urban-rural interface. Acta Trop. 2004;90:155–162. doi: 10.1016/j.actatropica.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Yadon ZE, Rodrigues LC, Davies CR, Quigley MA. Indoor and peridomestic transmission of American cutaneous leishmaniasis in northwestern Argentina: a retrospective case-control study. Am J Trop Med Hyg. 2003;68:519–526. doi: 10.4269/ajtmh.2003.68.519. [DOI] [PubMed] [Google Scholar]
- 24.Sosa-Estani S, Segura EL, Gomez A, Salomon OD, Peralta M, Coutada V, Ruiz LM. Cutaneous leishmaniasis in northern Argentina: identification of risk factors in a case-cohort study of three municipalities in Salta. Rev Soc Bras Med Trop. 2001;34:511–517. doi: 10.1590/s0037-86822001000600003. [DOI] [PubMed] [Google Scholar]
- 25.Elliott P, Wartenberg D. Spatial epidemiology: current approaches and future challenges. Environ Health Perspect. 2004;112:998–1006. doi: 10.1289/ehp.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Instituto Nacional de Estadística y Censos Resultados Provinciales del Censo. Información Seleccionada. 2001. http://www.indec.mecon.ar/webcenso/provincias_2/provincias.asp Available at. Accessed November, 10, 2009.
- 27.Kappelle M, Brown A. Santo Domingo de Heredia, Costa Rica: Instituto Nacional de Biodiversidad Nacional de Biodiversidad. 2001. (Bosque Nublados del Neotrópico). [Google Scholar]
- 28.Pan American Health Organization . Módulos de Principios de Epidemiología para el Control de en Enfermedades PALTEX. Washington, DC: Pan American Health Organization; 2002. [Google Scholar]
- 29.Silverman B. Density Estimation for Statistics and Data Analysis. New York: Chapman and Hall; 1986. [Google Scholar]
- 30.Aramayo CF, Gil JF, Cruz MC, Poma HR, Last MS, Rajal VB. Diarrhea and parasitosis in Salta, Argentina. J Infect Dev Ctries. 2009;3:105–111. doi: 10.3855/jidc.57. [DOI] [PubMed] [Google Scholar]
- 31.Chaves LF, Cohen JM, Pascual M, Wilson ML. Social exclusion modifies climate and deforestation impacts on a vector-borne disease. PLoS Negl Trop Dis. 2008;2:e176. doi: 10.1371/journal.pntd.0000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kulldorff M, Nagarwalla N. Spatial disease clusters: detection and inference. Stat Med. 1995;14:799–810. doi: 10.1002/sim.4780140809. [DOI] [PubMed] [Google Scholar]
- 33.Perez AM, Thurmond MC, Grant PW, Carpenter TE. Use of the scan statistic on disaggregated province-based data: foot-and-mouth disease in Iran. Prev Vet Med. 2005;71:197–207. doi: 10.1016/j.prevetmed.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Aparicio C, Bitencourt MD. Spacial modeling of cutaneous leishmaniasis risk zones. Rev Saude Publica. 2004;38:511–516. doi: 10.1590/s0034-89102004000400005. [DOI] [PubMed] [Google Scholar]
- 35.Melby PC. Experimental leishmaniasis in humans: a review. Rev Infect Dis. 1991;13:1009–1017. doi: 10.1093/clinids/13.5.1009. [DOI] [PubMed] [Google Scholar]
- 36.Travi BL, Osorio Y, Melby PC, Chandrasekar B, Arteaga L, Saravia NG. Gender is a major determinant of the clinical evolution and immune response in hamsters infected with Leishmania spp. Infect Immun. 2002;70:2288–2296. doi: 10.1128/IAI.70.5.2288-2296.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Despommier D, Ellis B, Wilcox B. The role of ecotones in emerging infectious diseases. EcoHealth. 2007;3:281–289. [Google Scholar]
- 38.Chaves LF, Hernandez MJ, Dobson AP, Pascual M. Sources and sinks: revisiting the criteria for identifying reservoirs for American cutaneous leishmaniasis. Trends Parasitol. 2007;23:311–316. doi: 10.1016/j.pt.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Wasserberg G, Abramsky Z, Kotler B, Ostfeld R, Yarom I, Warburg A. Anthropogenic disturbances enhance occurrence of cutaneous leishmaniasis in Israel deserts: patterns and mechanisms. Ecol Appl. 2003;13:868–881. [Google Scholar]
- 40.Kochtcheeva L, Singh A. OMS-UNEP. Geneva: World Health Organization; 1999. (An Assessment of Risks and Threats to Human Health Associated with the Degradation of Ecosystems). [Google Scholar]
- 41.Dobson A. Population dynamics of pathogens with multiple host species. Am Nat. 2004;164(Suppl 5):S64–S78. doi: 10.1086/424681. [DOI] [PubMed] [Google Scholar]
- 42.Keesing F, Holt RD, Ostfeld RS. Effects of species diversity on disease risk. Ecol Lett. 2006;9:485–498. doi: 10.1111/j.1461-0248.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- 43.Fichet-Calvet E, Jomaa I, Zaafouri B, Ashford R, Ben-Ismail R, Delattre P. The spatio-temporal distribution of a rodent reservoir host of cutaneous leishmaniasis. J Appl Ecol. 2000;37:603–615. [Google Scholar]
- 44.Chaves L, Pascual M. Climate cycles and forecasts of cutaneous leishmaniasis, a nonstationary vector-borne disease. PLoS Med. 2006;3:e295. doi: 10.1371/journal.pmed.0030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moragues J, Fernandez A. Uso de la Información Espacial para la Gestión en Salud. Buenos Aires: Comisión Nacional de Actividades Espaciales. 2003:1–17. [Google Scholar]


