Abstract
It is unclear how the prevalence of clinically active trachoma correlates with the prevalence of ocular chlamydial infection at the community level. In 24 villages from a cluster-randomized clinical trial of mass azithromycin distributions in Ethiopia, the correlation between the prevalence of clinical activity (on examination) and chlamydial infection (by polymerase chain reaction) was moderately strong before mass antibiotic treatments (Pearson's correlation coefficient r = 0.75, 95% confidence interval [CI] = 0.52–0.87), but decreased at each time point during four biannual treatments (at 24 months, r = 0.15, 95% CI = −0.14–0.41). One year after the final treatment, the correlation coefficient had increased, but not to the pre-treatment level (r = 0.55, 95% CI = 0.30–0.73). In a region with hyperendemic trachoma, conjunctival examination was a useful indicator of the prevalence of chlamydial infection before treatments, less useful during mass treatments, but regained utility by one year after treatments had stopped.
Introduction
Trachoma is the leading infectious cause of blindness worldwide.1 Ocular strains of Chlamydia are responsible for the clinical manifestations of trachoma, which include repeated episodes of conjunctivitis and conjunctival scarring, with subsequent secondary corneal ulceration, and blindness.2
Chlamydial infection can be detected by nucleic acid amplification tests (NAATs) such as polymerase chain reaction (PCR)–based assays.3,4 Although these tests are highly sensitive for detection of chlamydial infection, they are expensive, and currently not used by most trachoma control programs. Instead, programs rely on clinical activity, as determined by conjunctival examination, as a proxy for chlamydial infection. The clinical examination is performed using the World Health Organization (WHO) simplified trachoma grading system.5 Clinically active trachoma, or clinical activity, is typically defined as the presence of follicular trachomatous inflammation (TF, ≥ 5 follicles in the upper tarsal conjunctiva), and/or intense trachomatous inflammation (TI, pronounced inflammatory thickening of upper tarsal conjunctiva obscuring more than half of the underlying blood vessels). However, clinically active trachoma is not synonymous with PCR-detectable chlamydial infection because many infected persons are not clinically active, whereas many uninfected persons are clinically active.4,6–10 This discrepancy likely exists because clinical signs of trachoma first develop in a person days after infection, and these clinical signs persist for many weeks after the infection has been cleared.11,12
There have been various studies assessing the relationship between clinically active trachoma and chlamydial infection among persons.6–8,13–18 These studies have shown that at the individual level, the association between clinical activity and chlamydial infection varies depending on the clinical setting. In areas with hyper-endemic trachoma, the proportion of persons with clinical activity who are positive for chlamydial infection by NAATs ranges from 63% to 71%.6,7,16,17 However, in areas of hypo-endemic trachoma, only 0–8% of clinically active persons may be positive for chlamydial infection.16,18 Similarly, the proportion of clinically active persons with infection decreases after mass azithromycin treatments. In one report from a severely affected area, 67% of persons with clinically active trachoma had evidence of chlamydial infection before mass antibiotic treatment, but only 10% did so after treatment.17
Although these studies have been important in understanding the discrepancy between clinical activity and chlamydial infection at the level of the individual person, trachoma programs administer treatments to communities, not individuals. Specifically, the WHO recommends three annual mass treatments if the district prevalence of clinically active trachoma in children 1–9 years of age exceeds 10%, and further mass treatments in individual villages until the village prevalence of clinically active trachoma is less than 5%.19 Therefore, it is less relevant to trachoma programs whether clinical activity corresponds with chlamydial infection in a particular person than whether the prevalence of clinical activity corresponds with the prevalence of chlamydial infection in a community. It is possible that even if clinical activity and infection were poorly correlated in individual persons, the prevalence of each in a village could still be correlated. If so, this would be useful for trachoma programs, which would then be justified in using clinical activity to estimate the amount of chlamydial infection in a community.
In this study, we report the correlation between the village prevalence of clinically active trachoma and the village prevalence of PCR-detectable ocular Chlamydia infection before, during, and after mass antibiotic treatments. Such an analysis may better inform trachoma program treatment decisions by better characterizing the relationship between clinical activity and infection at the village level, where such treatment decisions are made.
Materials and Methods
Study design.
This study was a non–prespecified analysis of a cluster-randomized clinical trial conducted in an area of Ethiopia with hyperendemic trachoma during 2003–2006. As part of the clinical trial, 24 villages, each consisting of approximately 85 households, received mass azithromycin treatment every six months. Sixteen study villages were randomly selected from the Enemore district of the Gurage Zone, and received biannual treatment for two years (at months 0, 6, 12, 18). Eight study villages randomly selected from the adjacent district of Goro received biannual treatment for three years (at months 0, 6, 12, 18, 24, 30). During a mass treatment, all eligible members of the village were offered single dose, directly observed oral azithromycin (1 g for adults and 20 mg/kg for children ≥ 1 year of age). Pregnant women and children less than one year of age were offered topical tetracycline to be applied twice a day for six weeks. Conjunctival examination and swabbing for chlamydial PCR preceded each treatment, and were continued every six months until month 42. All study activities were pre-specified and described in detail in a Manual of Operations and Procedures. In this study, the primary outcome was the relationship between the village prevalence of clinically active trachoma and the village prevalence of chlamydial infection based on PCR. Other trachoma interventions, such as promotion of face washing or environmental improvements, were not conducted and did not change throughout the study period. The clinical trial was registered with ClinicalTrials.gov (NCT00221364). Approval for the study was obtained by the Committee for Human Research at the University of California, San Francisco, and by the Ethiopian Science and Technology Commission, and included verbal consent for adult study participants, and verbal consent from guardians of minors. The research adhered to the tenets of the Declaration of Helsinki.
Clinical examination and PCR.
All children 1–5 years of age in each village were offered clinical examination and conjunctival swabbing for chlamydial PCR. Only the right upper tarsal conjunctiva was examined and swabbed. Clinically active trachoma, frequently termed clinical activity in this report, was defined as TF and/or TI, as described by the WHO simplified grading system.5 Medical personnel (nurses, ophthalmic assistants, medical students, residents, and fellows) were trained to grade trachoma by the WHO simplified grading scale. Training consisted of a slide lecture and in-field observation. Persons were allowed to become graders if they achieved in-field agreement of greater than 80% with a gold-standard observer (TL, JPW, or BDG). To validate the graders, we performed photography of the right upper tarsal conjunctiva on a random sample of 230 children and compared the grades from the field with the grades from the photographs, as assessed by a gold standard grader (TL). In general, inter-reader reliability was good, with a mean kappa of 0.61 (95% confidence interval [CI] = 0.45–0.76) among 10 graders.20
The same medical personnel were also trained in conjunctival swabbing technique for PCR. A Dacron swab was passed over the right upper tarsal conjunctiva three times, rotating roughly 120° between each pass. A randomly selected 10% of children had two control swabs performed: one duplicate control swab and one negative air control swab, which consisted of passing the swab 1 inch above the subject's conjunctiva. All samples were stored at 4°C in the field and at −20°C within 6 hours of collection. Samples were shipped at 4°C to San Francisco, where they were stored at −70°C until processed. The AMPLICOR PCR test (Roche Diagnostics, Branchburg, NJ) was used by masked technicians to detect chlamydial DNA. PCR samples at baseline were tested individually, but at all other time points, samples from the same village were pooled into groups of five samples. If two-thirds of the pools were positive, then the individual samples were re-pooled into groups of two samples. If any pool produced an equivocal result, all samples were re-tested individually. As per the AMPLICOR protocol, an internal control was performed for each pool to rule out the presence of PCR inhibitors. Any inhibitory pools were re-tested, and if still inhibitory, the samples were tested individually. Although samples necessarily were diluted in the pooling process, this dilution is not thought to significantly impact the sensitivity of the test.21 The prevalence of Chlamydia in each village was calculated by maximum likelihood estimations, with the number of positive individual samples most likely to have resulted in the observed pooled results being used as the village prevalence for ocular Chlamydia.22
Statistical analysis.
All analyses were performed at the village level by using village prevalence. We report the mean village population at baseline, separately for all persons and for children 1–5 years of age and the mean antibiotic coverage over all time points from 0 to 24 months. We also report the prevalence of clinical activity and chlamydial infection over time for person 1–5 years of age separately for the 16 villages that received two years of biannual treatment, and for the eight villages that received three years of biannual treatments. The 95% CIs of the mean were calculated by bootstrapping because of non-normality of the data (9,999 repetitions). Pearson's correlation coefficient between the prevalence of clinically active trachoma and the prevalence of chlamydial infection by PCR testing was performed at the village level, with 95% confidence intervals calculated by bootstrapping to account for non-normality of the data (bootcor command, 9,999 repetitions, STATA version 10.0; Statacorp, College Station, TX). Analyses using Spearman's correlation coefficient or incorporating the treatment arm as a confounder produced similar results. Using data from all 24 villages, we calculated the village correlation coefficient before treatment (month 0), during treatments (months 6, 12, 18, and 24), and after treatment (months 0, 6, and 12 after the final treatment). Note that to calculate the correlation after mass treatments had ended, we first calculated the time since the final mass treatment of each village, and then computed the correlation coefficient for months 0, 6, and 12 after this final treatment, which were the only time points with complete data for all 24 villages. All statistical tests were performed with STATA version 10.0.
Results
Demographics.
Among all 24 villages at baseline, there were 9,646 persons (mean = 401.9, 95% CI = 319.3–484.5) and 1,200 children 1–5 years of age (mean = 50.0, 95% CI = 38.9–61.1). Antibiotic coverage was high, averaging 93.4% (95% CI = 92.4–94.4%) over all treatments. Village population or coverage did not differ between villages receiving four biannual antibiotic treatments and villages receiving six biannual antibiotic treatments.23,24 The population of children 1–5 years of age who received conjunctival examination and swabbing for chlamydial PCR at each study visit is shown in Table 1. All villages received all scheduled treatments, and no village was lost to follow-up over the length of the study.
Table 1.
Number of children 1–5 years of age receiving conjunctival examination and swabbing at each study visit in villages in Ethiopia treated with biannual mass azithromycin treatments*
| Treatment arm | Months | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 18 | 24 | 30 | 36 | 42 | |
| Four biannual treatments (n = 16 villages) | 808 | 815 | 797 | 770 | 891 | 924 | 995 | 957 |
| Six biannual treatments (n = 8 villages) | 392 | 388 | 409 | 408 | 373 | 468 | 519 | 523 |
Data are shown separately for the 16 villages given 4 biannual treatments and the 8 villages given 6 biannual treatments.
Chlamydial infection.
When all 24 villages were analyzed together, the mean village prevalence of chlamydial infection was 52.9% (95% CI = 44.2–61.3%) at baseline, which decreased during biannual azithromycin treatment to 8.4% (95% CI = 5.4%–11.5%) at 6 months and to 2.0% (95% CI = 1.2–2.9%) at 24 months. In 16 villages treated with 4 biannual treatments, the prevalence of chlamydial infection decreased from 63.5% (95% CI = 55.8–70.8%) at baseline to 2.6% (95% CI = 1.5–3.7%) at 24 months, but increased to 25.2% (95% CI = 15.4–35.9%) by two years after the final treatment (Figure 1). In contrast, eight villages treated with six biannual treatments experienced a decrease in chlamydial infection prevalence from 31.6% (95% CI = 21.5–41.0%) at baseline to 0.9% (95% CI = 0.0–2.1%) at 24 months, and remained < 0.7% (95% CI = 0.0–2.0%) one year after cessation of treatment (Figure 2). Of the 862 negative control samples, 854 showed no evidence of Chlamydia by PCR (99.1%, 95% CI = 98.2–99.6%), and 851 of the 855 duplicate control swabs were concordant (99.5%, 95% CI = 98.8–99.9%).
Figure 1.
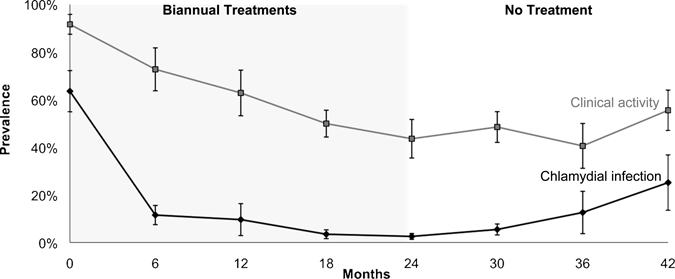
Chlamydial infection and clinical activity in villages in Ethiopia treated with four biannual treatments of azithromycin. Mean prevalence of chlamydial infection and clinically active trachoma are depicted over time for 16 villages that received four biannual mass treatments. Error bars show bootstrapped 95% confidence intervals.
Figure 2.
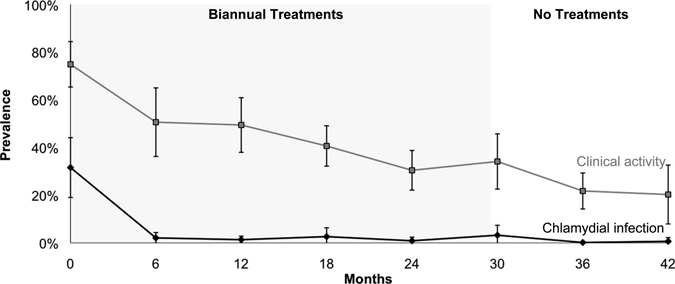
Chlamydial infection and clinical activity in villages in Ethiopia treated with six biannual treatments of azithromycin. Mean prevalence of chlamydial infection and clinically active trachoma are depicted over time for 8 villages that received six biannual mass treatments. Error bars show bootstrapped 95% confidence intervals.
Clinical activity.
The mean prevalence of clinical activity was greater than the mean prevalence of chlamydial infection at all time points. When all 24 villages were analyzed together, the mean village prevalence of clinical activity gradually decreased during biannual azithromycin treatments, from 86.0% (95% CI = 80.9–90.4%) at baseline to 39.2% (95% CI = 33.4–44.9%) at 24 months. In the 16 villages treated with 4 biannual treatments, the prevalence of clinical activity decreased from 91.6% (95% CI = 87.5–95.1%) at baseline to 43.6% (95% CI = 36.4–50.9%) at 24 months, but then increased to 55.5% (95% CI = 47.8–62.9%) at 42 months (two years after the final treatment, Figure 1). In the eight villages receiving two additional biannual treatments, clinical activity prevalence decreased from 74.9% (95% CI = 66.7–80.7%) at baseline to 30.4% (95% CI = 23.7–36.5%) at 24 months and decreased further to 20.3% (95% CI = 11.1–30.2%) at 42 months (one year after the final treatment, Figure 2).
Village correlation coefficient.
As shown in Figure 3A, chlamydial infection and clinically active trachoma correlated moderately well before mass treatments (r = 0.75, 95% CI = 0.52–0.87), but decreased at each time period during azithromycin treatment (r = 0.15 at 24 months, 95% CI = −0.14–0.41). After treatments had ended, the Pearson's correlation coefficient increased from r = 0.18 (95% CI = −0.15–0.46) at the time of the final treatment to r = 0.55 (95% CI = 0.30–0.73) at 12 months after the final treatment (Figure 3B).
Figure 3.
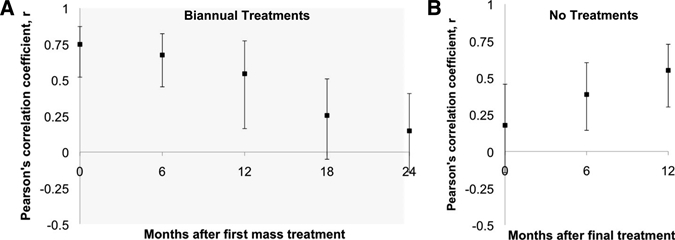
Correlation between prevalence of chlamydial infection and prevalence of clinical activity in villages in Ethiopia treated with repeated mass antibiotic distributions. A, Pearson correlation coefficient before and during mass antibiotic treatments for 24 villages that received biannual treatments at months 0, 6, 12, and 18 (shaded region). Eight of these villages received two additional biannual mass antibiotic treatments at months 24 and 30. B, Pearson correlation coefficient at the final mass treatment and during one year of follow-up for all 24 villages. Error bars show bootstrapped 95% confidence intervals.
Discussion
Most trachoma programs do not use NAATs to determine the prevalence of ocular chlamydial infection. Instead, field workers base treatment decisions on the prevalence of clinically active trachoma, which is used to estimate the amount of chlamydial infection in a village. It is therefore of considerable importance whether clinical activity correlates with chlamydial infection. Although other studies have found a weak correlation between clinical activity and infection, the analysis in these studies was conducted at the individual level.4,6–9 In contrast, our study analyzed the correlation at the village level. Our analysis may therefore be more relevant for trachoma programs, which base treatment on the village prevalence of clinical activity. In our study, there was a moderately strong correlation between clinical activity and chlamydial infection before antibiotic treatments in a region with hyperendemic trachoma. As the prevalence of chlamydial infection detected by PCR decreased during mass antibiotic treatments, the correlation between clinically active trachoma and chlamydial infection also decreased. One year after mass treatments were stopped, the correlation between clinical activity and chlamydial infection increased, although it remained less than the pre-treatment level. This finding suggests that the prevalence of clinically active trachoma may more accurately reflect the infection status of a community before and after mass treatments, but should be interpreted with caution while mass antibiotic treatments are ongoing.
In this area of hyperendemic trachoma, the prevalence of clinical activity greatly overestimated chlamydial infection. For logistical reasons, the eight villages randomized to six biannual treatments came from a separate geographic area than the 16 villages treated with four biannual treatments. The burden of clinically active trachoma and chlamydial infection was lower in those eight villages treated with six biannual treatments, perhaps because these villages were closer to the town of Wolkite and to the highway. Nonetheless, the relationship between chlamydial infection and clinical activity was similar in both groups of villages.
The decrease in correlation between clinical activity and infection after mass treatment is not unexpected. Mass azithromycin treatment results in rapid reduction of chlamydial infection.25 In contrast, resolution of trachomatous clinical activity lags behind resolution of infection, with the clinical signs of trachoma persisting for weeks to months after persons with infections have been adequately treated.11,12,25 Additionally, it has been hypothesized that follicular conjunctivitis may develop in treated persons when they are exposed to other, non-chlamydial stimuli, which may further weaken the correlation between clinically active trachoma and chlamydial infection after mass treatments.16
There are significant cost implications to the relationship between clinical activity and chlamydial infection. Trachoma programs base treatment decisions on clinical activity, which is a low-cost test. However, at least in this population, clinical activity overestimates infection. Use of the clinical examination may therefore result in unnecessary treatments, and thus, unnecessary use of resources. For example, in the eight villages treated with six biannual treatments, the prevalence of clinical activity exceeded 20% three years after chlamydial infection had been brought to a low level. At this level, current guidelines would have recommended continued mass azithromycin distributions.19 However, in these communities, the level of chlamydial infection remained low even without further mass treatments. This finding suggests that any additional mass treatments might have been unnecessary, given the goal of reduction, and not elimination, of trachoma. Other tests, such as NAATs, are more expensive, but may minimize unnecessary treatments. Use of NAATs to determine ocular chlamydial infection is thought to be cost-prohibitive by most trachoma programs. Other tests that are more sensitive and specific than the clinical examination, such as a point-of-care assay, may provide cost savings over NAATs.26 Alternatively, trachoma programs could follow the lead of genital chlamydial research by pooling PCR samples.27,28 Costs for genital Chlamydia screening programs can be cut in half by testing pooled samples and re-analyzing individual samples from positive pools. Cost savings for trachoma programs could be considerably greater because the village prevalence can be estimated from the pooled samples alone, and would therefore not require re-testing of individual samples.22–24,29 In the eight villages treated with six biannual treatments, pooled PCR would have highlighted the lack of infection, which might have been useful for a trachoma program in deciding how to allocate its limited resources. Further studies more formally addressing the cost-effectiveness of different testing modalities would be valuable.
We analyzed the correlation between clinically active trachoma and ocular Chlamydia infection at the village level, which is a major strength of this study. Mass antibiotic treatments are distributed to entire villages, with the desired effect being to lower the prevalence of disease in the village. However, there is inherently variation among villages in their response to mass treatments. Therefore, it is important that studies measure trachoma at the village level using village prevalence and including multiple villages to take into account variation at the village level. In addition, studies conducted on the village level may be more relevant to trachoma programs, which base treatment decisions on the prevalence of clinical activity in a community.
There are several important limitations of this study. We test the correlation coefficient at the village level, which required a group-randomized study, and limited the sample size available for this analysis. We were only able to calculate the correlation coefficient up to 12 months after the final treatment, and therefore cannot comment on times after this. Finally, we studied villages with a high prevalence of ocular Chlamydia infection treated with biannual mass azithromycin treatments and high antibiotic coverage, which may limit generalizability in areas with less severe trachoma, less frequent treatments, or lower antibiotic coverage.
In conclusion, we found a moderately strong correlation between the village prevalence of trachomatous clinical activity and the village prevalence of chlamydial infection before mass antibiotic treatments in a region with hyperendemic trachoma. Our findings suggest that before mass antibiotic distributions have started, clinical examination can be useful for trachoma program administrators in determining which villages to treat with mass antibiotics. During mass antibiotic treatments, the correlation between the prevalence of clinically active trachoma and chlamydial infection becomes weaker, suggesting the clinical examination is not a good proxy for chlamydial infection during this time. By one year after mass treatments are discontinued, the correlation between the village prevalence of clinical activity and chlamydial infection returns, though not to the pre-treatment level. Further research is needed to characterize the correlation between clinical activity and infection at times more than one year after treatments have ended. These results are consistent with current WHO guidelines, which recommend clinical re-evaluation after the completion of three annual mass antibiotic treatments, and may help clarify the relationship between the prevalence of clinical activity and chlamydial infection for trachoma programs.
Acknowledgments
We thank the Ethiopian Ministry of Health and the many health care professionals, including Tadesse Kebede, Berhanu Fikre, Mifta Shifa, and Tadesse Birru, for helping us organize and implement our fieldwork in Ethiopia.
Footnotes
Financial support: This work was supported by the Heed Ophthalmic Foundation, International Trachoma Initiative, the Bernard Osher Foundation, That Man May See, the Peierls Foundation, the Bodri Foundation, the Harper Inglis Trust, the South Asia Research Fund, Research to Prevent Blindness, and grants K23 EY019071 and U10 EY016214 from the National Institutes of Health.
Authors' addresses: Jeremy D. Keenan, Travis C. Porco, Elizabeth Yi, Jenafir I. House, Kathryn J. Ray, Nisha R. Acharya, John P. Whitcher, Bruce D. Gaynor, Zhaoxia Zhou, and Thomas M. Lietman, F. I. Proctor Foundation, University of California, San Francisco, CA. Takele Lakew, Wondu Alemayehu, and Muluken Melese, ORBIS International, Addis Ababa, Ethiopia.
References
- 1.Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 2.Sanford-Smith J. Eye Diseases in Hot Climates. Oxford: Butterworth Heinemann; 1997. pp. 105–120. [Google Scholar]
- 3.Miyashita N, Matsumoto A, Niki Y, Matsushima T. Evaluation of the sensitivity and specificity of a ligase chain reaction test kit for the detection of Chlamydia trachomatis. J Clin Pathol. 1996;49:515–517. doi: 10.1136/jcp.49.6.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey RL, Hampton TJ, Hayes LJ, Ward ME, Whittle HC, Mabey DC. Polymerase chain reaction for the detection of ocular chlamydial infection in trachoma-endemic communities. J Infect Dis. 1994;170:709–712. doi: 10.1093/infdis/170.3.709. [DOI] [PubMed] [Google Scholar]
- 5.Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR. A simple system for the assessment of trachoma and its complications. Bull World Health Organ. 1987;65:477–483. [PMC free article] [PubMed] [Google Scholar]
- 6.Miller K, Schmidt G, Melese M, Alemayehu W, Yi E, Cevallos V, Donnellan C, Olinger L, Fantaye D, Gaynor B, Whitcher JP, Lietman T. How reliable is the clinical exam in detecting ocular chlamydial infection? Ophthalmic Epidemiol. 2004;11:255–262. doi: 10.1080/09286580490514577. [DOI] [PubMed] [Google Scholar]
- 7.Bobo LD, Novak N, Munoz B, Hsieh YH, Quinn TC, West S. Severe disease in children with trachoma is associated with persistent Chlamydia trachomatis infection. J Infect Dis. 1997;176:1524–1530. doi: 10.1086/514151. [DOI] [PubMed] [Google Scholar]
- 8.Bailey RL, Hayes L, Pickett M, Whittle HC, Ward ME, Mabey DC. Molecular epidemiology of trachoma in a Gambian village. Br J Ophthalmol. 1994;78:813–817. doi: 10.1136/bjo.78.11.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burton MJ, Holland MJ, Faal N, Aryee EA, Alexander ND, Bah M, Faal H, West SK, Foster A, Johnson GJ, Mabey DC, Bailey RL. Which members of a community need antibiotics to control trachoma? Conjunctival Chlamydia trachomatis infection load in Gambian villages. Invest Ophthalmol Vis Sci. 2003;44:4215–4222. doi: 10.1167/iovs.03-0107. [DOI] [PubMed] [Google Scholar]
- 10.Hayes LJ, Bailey RL, Mabey DC, Clarke IN, Pickett MA, Watt PJ, Ward ME. Genotyping of Chlamydia trachomatis from a trachoma-endemic village in the Gambia by a nested polymerase chain reaction: identification of strain variants. J Infect Dis. 1992;166:1173–1177. doi: 10.1093/infdis/166.5.1173. [DOI] [PubMed] [Google Scholar]
- 11.Grassly NC, Ward ME, Ferris S, Mabey DC, Bailey RL. The natural history of trachoma infection and disease in a Gambian cohort with frequent follow-up. PLoS Negl Trop Dis. 2008;2:e341. doi: 10.1371/journal.pntd.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor HR, Siler JA, Mkocha HA, Munoz B, West S. The natural history of endemic trachoma: a longitudinal study. Am J Trop Med Hyg. 1992;46:552–559. doi: 10.4269/ajtmh.1992.46.552. [DOI] [PubMed] [Google Scholar]
- 13.Holm SO, Jha HC, Bhatta RC, Chaudhary JS, Thapa BB, Davis D, Pokhrel RP, Yinghui M, Zegans M, Schachter J, Frick KD, Tapert L, Lietman TM. Comparison of two azithromycin distribution strategies for controlling trachoma in Nepal. Bull World Health Organ. 2001;79:194–200. [PMC free article] [PubMed] [Google Scholar]
- 14.Solomon AW, Holland MJ, Alexander ND, Massae PA, Aguirre A, Natividad-Sancho A, Molina S, Safari S, Shao JF, Courtright P, Peeling RW, West SK, Bailey RL, Foster A, Mabey DC. Mass treatment with single-dose azithromycin for trachoma. N Engl J Med. 2004;351:1962–1971. doi: 10.1056/NEJMoa040979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright HR, Taylor HR. Clinical examination and laboratory tests for estimation of trachoma prevalence in a remote setting: what are they really telling us? Lancet Infect Dis. 2005;5:313–320. doi: 10.1016/S1473-3099(05)70116-X. [DOI] [PubMed] [Google Scholar]
- 16.Baral K, Osaki S, Shreshta B, Panta CR, Boulter A, Pang F, Cevallos V, Schachter J, Lietman T. Reliability of clinical diagnosis in identifying infectious trachoma in a low-prevalence area of Nepal. Bull World Health Organ. 1999;77:461–466. [PMC free article] [PubMed] [Google Scholar]
- 17.Bird M, Dawson CR, Schachter JS, Miao Y, Shama A, Osman A, Bassem A, Lietman TM. Does the diagnosis of trachoma adequately identify ocular chlamydial infection in trachoma-endemic areas? J Infect Dis. 2003;187:1669–1673. doi: 10.1086/374743. [DOI] [PubMed] [Google Scholar]
- 18.Thein J, Zhao P, Liu H, Xu J, Jha H, Miao Y, Pizzarello L, Tapert L, Schachter J, Mabon M, Osaki-Holm S, Lietman T, Paxton A. Does clinical diagnosis indicate ocular chlamydial infection in areas with a low prevalence of trachoma? Ophthalmic Epidemiol. 2002;9:263–269. doi: 10.1076/opep.9.4.263.1508. [DOI] [PubMed] [Google Scholar]
- 19.Solomon A, Zondervan M, Kuper H, Buchan JC, Mabey DC, Foster A. Trachoma Control: A Guide for Programme Managers. Geneva: World Health Organization; 2006. [Google Scholar]
- 20.Altman D. Practical Statistics for Medical Research. Chapman and Hall; 1991. London. pp. 403–409. [Google Scholar]
- 21.Morre SA, van Dijk R, Meijer CJ, van den Brule AJ, Kjaer SK, Munk C. Pooling cervical swabs for detection of Chlamydia trachomatis by PCR: sensitivity, dilution, inhibition, and cost-saving aspects. J Clin Microbiol. 2001;39:2375–2376. doi: 10.1128/jcm.39.6.2375-2376.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diamant J, Benis R, Schachter J, Moncada J, Pang F, Jha HC, Bhatta RC, Porco T, Lietman T. Pooling of Chlamydia laboratory tests to determine the prevalence of ocular Chlamydia trachomatis infection. Ophthalmic Epidemiol. 2001;8:109–117. doi: 10.1076/opep.8.2.109.4156. [DOI] [PubMed] [Google Scholar]
- 23.Melese M, Alemayehu W, Lakew T, Yi E, House J, Chidambaram JD, Zhou Z, Cevallos V, Ray K, Hong KC, Porco TC, Phan I, Zaidi A, Gaynor BD, Whitcher JP, Lietman TM. Comparison of annual and biannual mass antibiotic administration for elimination of infectious trachoma. JAMA. 2008;299:778–784. doi: 10.1001/jama.299.7.778. [DOI] [PubMed] [Google Scholar]
- 24.Lakew T, House J, Hong KC, Yi E, Alemayehu W, Melese M, Zhou Z, Ray K, Chin S, Romero E, Keenan J, Whitcher JP, Gaynor BD, Lietman TM. Reduction and return of infectious trachoma in severely affected communities in Ethiopia. PLoS Negl Trop Dis. 2009;3:e376. doi: 10.1371/journal.pntd.0000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bailey RL, Arullendran P, Whittle HC, Mabey DC. Randomised controlled trial of single-dose azithromycin in treatment of trachoma. Lancet. 1993;342:453–456. doi: 10.1016/0140-6736(93)91591-9. [DOI] [PubMed] [Google Scholar]
- 26.Michel CE, Solomon AW, Magbanua JP, Massae PA, Huang L, Mosha J, West SK, Nadala EC, Bailey R, Wisniewski C, Mabey DC, Lee HH. Field evaluation of a rapid point-of-care assay for targeting antibiotic treatment for trachoma control: a comparative study. Lancet. 2006;367:1585–1590. doi: 10.1016/S0140-6736(06)68695-9. [DOI] [PubMed] [Google Scholar]
- 27.Peeling RW, Toye B, Jessamine P, Gemmill I. Pooling of urine specimens for PCR testing: a cost saving strategy for Chlamydia trachomatis control programmes. Sex Transm Infect. 1998;74:66–70. doi: 10.1136/sti.74.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shipitsyna E, Shalepo K, Savicheva A, Unemo M, Domeika M. Pooling samples: the key to sensitive, specific and cost-effective genetic diagnosis of Chlamydia trachomatis in low-resource countries. Acta Derm Venereol. 2007;87:140–143. doi: 10.2340/00015555-0196. [DOI] [PubMed] [Google Scholar]
- 29.Melese M, Chidambaram JD, Alemayehu W, Lee DC, Yi EH, Cevallos V, Zhou Z, Donnellan C, Saidel M, Whitcher JP, Gaynor BD, Lietman TM. Feasibility of eliminating ocular Chlamydia trachomatis with repeat mass antibiotic treatments. JAMA. 2004;292:721–725. doi: 10.1001/jama.292.6.721. [DOI] [PubMed] [Google Scholar]


