Abstract
Sarcopenia, loss of muscle mass and function, is a common feature of aging. Oxidative damage and apoptosis are likely underlying factors. Autophagy, a process for the degradation of cellular constituents, may be a mechanism to combat cell damage and death. We investigated the effect of age on autophagy and apoptosis in plantaris muscle of male Fischer344 rats that were either fed ad libitum, or mild, life-long calorie restricted (CR) alone or combined with life-long voluntary exercise. Upstream autophagy regulatory proteins were either upregulated with age (Beclin-1) or unchanged (Atg7 and 9). LC3 gene and protein expression pattern as well as LAMP-2 gene expression, both downstream regulators of autophagy, however, suggested an age-related decline in autophagic degradation. Atg protein expression and LC3 and LAMP-2 gene expression were improved in CR rats with or without exercise. The age-related increase in oxidative damage and apoptosis were attenuated by the treatments. Both, oxidative damage and apoptosis correlated negatively with autophagy. We conclude that mild CR attenuates the age-related impairment of autophagy in rodent skeletal muscle, which might be one of the mechanisms by which CR attenuates age-related cellular damage and cell death in skeletal muscle in vivo.
Keywords: Autophagy, plantaris, aging, apoptosis, calorie restriction, exercise, wheel running
Introduction
Sarcopenia encompasses the loss of muscle mass and function with age, and occurs in a variety of species (Marzetti and Leeuwenburgh, 2006). The loss of muscle mass at older age has significant ramifications for the individual’s health, and impaired muscle strength has been implicated in increased incidence of falls, disability and all-cause mortality in humans (Metter et al., 2002; Rantanen et al., 1999; Szulc et al., 2005). The etiology of sarcopenia is complex and characterized by the contribution of multiple factors (Marzetti and Leeuwenburgh, 2006), and there is growing evidence for a prominent role of accelerated apoptosis in sarcopenia (Dirks and Leeuwenburgh, 2002; Marzetti et al., 2008a; Marzetti and Leeuwenburgh, 2006; Marzetti et al., 2008b; Pistilli et al., 2006).
Another important factor in the development of muscle loss is the imbalance between protein synthesis and protein degradation (Combaret et al., 2009). Skeletal muscle contains four proteolytic systems: 1) the lysosomal system, 2) the caspase system, 3) the calpain system, and 4) the proteasome. Although evidence suggests that calcium-dependent proteolysis, caspases, and lysosomal degradation contribute to accelerated muscle proteolysis, such as in muscle disuse and denervation, the major pathway responsible for the degradation of contractile proteins in skeletal muscle is orchestrated through the ubiquitin-proteasome system (UPS) (Combaret et al., 2009; Lecker et al., 1999; Mitch and Goldberg, 1996; Sandri, 2008). Lowell et al presented evidence that non-lysosomal proteolysis is responsible for myofibrillar degradation (Lowell et al., 1986), and Wing et al subsequently reported that the ubiquitin-proteasomal pathway is upregulated during atrophy in denervation and starvation regimens (Wing et al., 1995).There are contradictory data and varying interpretations in the literature on the effect of aging on proteasome-mediated protein degradation in skeletal muscle. Attaix, Combaret and colleagues concluded in their reviews of the literature that it currently remains unclear whether proteasome activity and proteasome function (activity relative to amount of proteasome components) are increased or decreased in aged skeletal muscle (Attaix et al., 2005; Combaret et al., 2009). However, there seems to be evidence that the activation and regulation of the UPS may be altered with aging (Combaret et al., 2005) Hepple et al. found no support for an effect of age or calorie restriction (CR) on proteasome function (Hepple et al., 2008). However, CR as an anti-aging intervention protected muscle performance (Hepple et al., 2005), and the authors suggested that CR may act through the optimization of proteasome activation (Hepple et al., 2008).
While the UPS is considered the major protein degradation pathway in skeletal muscle, the lysosomal-autophagy system, on the other hand, has been suggested responsible for the degradation of surface membrane proteins and endocytosed, extracellular proteins (reviewed in (Lecker et al., 1999)). That the UPS and the lysosomal-autophagy system in skeletal muscle are interconnected was suggested by two recent reports by Mammucari et al and Zhao et al (Mammucari et al., 2007; Zhao et al., 2007). Both studies identified FoxO3 as a regulator of both lysosomal and proteasomal pathways in muscle wasting. FoxO3 is a transcriptional regulator of the ubiquitin ligases MuRF1 and MAfbx, both involved in proteasome-dependent muscle atrophy (Lecker, 2003). It has now been linked to expression of autophagy-related genes in skeletal muscle in vivo and in C2C12 myotubes (Mammucari et al., 2007; Zhao et al., 2007).
Autophagy literally means “self-eating” and is a vital cellular process by which intracellular components are degraded within lysosomes (Meijer and Codogno, 2004; Xie and Klionsky, 2007). Three major mechanisms of autophagy have been described: (a) microautophagy, in which lysosomes directly take up cytosol, inclusions and organelles for degradation; (b) chaperone-mediated autophagy, in which soluble proteins with a particular pentapeptide motif are recognized and transported across the lysosomal membrane for degradation; and (c) macroautophagy, in which a portion of cytoplasm including subcellular organelles is sequestered within a double membrane-bound vacuole that ultimately fuses with a lysosome (Wang and Klionsky, 2003). A decline in autophagy during normal aging has been described for invertebrates and higher organisms (Cuervo et al., 2005; Cuervo and Dice, 2000; Donati et al., 2001; Terman, 1995). Inefficient autophagy has been attributed a major role in the apparent age-related accumulation of damaged cellular components, such as undegradable lysosome-bound lipofuscin, protein aggregates, and damaged mitochondria (Terman and Brunk, 2006). Interestingly, the degree of age-related changes in autophagy appears to be organ-specific. While the autophagic activity in liver declines with age (Cuervo and Dice, 2000; Donati et al., 2001), data from our laboratory suggest that autophagy is maintained in the heart of aged rats (Wohlgemuth et al., 2007). However, the mere maintenance of autophagy in the rodent heart might not be sufficient to cope with the magnitude of age-related cellular damage. Importantly, and to the best of our knowledge, changes of autophagy with age and the effect of anti-aging interventions on autophagy in skeletal muscle have not yet been examined.
Two important functions suggest an essential role for macroautophagy (subsequently referred to as autophagy) in skeletal muscle: 1) its activation when the proteasomal system fails to degrade protein aggregates (Pandey et al., 2007); and 2) its unique role as the only mechanisms so far attributed to the degradation of mitochondria (Lemasters, 2005). The autophagic removal of mitochondria becomes crucial in conditions when mitochondria are damaged and dysfunctional. It is widely accepted that mitochondria play a central role in the regulation of skeletal muscle apoptosis and regarded as key players in the pathogenesis of myocyte loss during aging and other atrophying conditions (Jeong and Seol, 2008; Marzetti et al., 2009). Oxidative damage to mitochondrial constituents (as well as extramitochondrial cellular components), impaired respiration and energy production, and altered mitochondrial turnover have been proposed as potential triggering events for mitochondrial apoptotic signaling. Consequently, a deficiency in autophagic housekeeping may lead to an accumulation of damaged mitochondria, which in turn might induce oxidative damage and trigger apoptotic events. Previous data from our lab suggest that in rat skeletal muscle the mitochondrial, caspase-independent apoptotic pathway may play a more prominent role in skeletal muscle loss than caspase-mediated apoptosis (Marzetti et al., 2008b). We therefore hypothesized that the autophagic removal of damaged mitochondria represents one strategy in a healthy cell to prevent or attenuate mitochondria-induced apoptosis and subsequent loss of muscle quality and mass.
Therefore, the aim of this study was to evaluate whether autophagy in skeletal muscle changes with age. We furthermore investigated whether interventions like calorie restriction (CR) and exercise, known to attenuate apoptosis and to successfully mitigate sarcopenia (Dirks and Leeuwenburgh, 2004; Phillips and Leeuwenburgh, 2005; Siu et al., 2004; Song et al., 2006; Xu et al., 2008), exert an effect on autophagy at old age. We chose to investigate the fast-twitch plantaris muscle, predominantly comprised of type II fibers, which are especially prone to sarcopenia (Larsson et al., 1978). We determined changes in the expression of autophagy-regulatory proteins, oxidative damage and apoptosis markers with normal aging, and the anti-aging interventions mild calorie restriction (i.e., 8% calorie intake reduction, CR) and lifelong voluntary exercise combined with 8% CR (CREx). We hypothesized that autophagy in type II muscle of old, ad libitum fed rats would be impaired, and insufficient to remove cellular damage at old age, accompanied by increased apoptosis. We further hypothesized that CR and exercise would ameliorate the autophagic response in old rats, thereby counteracting cell damage and death.
Materials and Methods
Animals
Male Fischer 344 rats were purchased from Harlan (Indianapolis, IN) at 10–11 wk of age and were housed at the University of Florida (Gainesville, FL) until sacrifice at 6 (young) and 24 (old) months of age, respectively. One week after arriving at our facility, rats were randomly assigned to one of four groups: (1) young sedentary ad libitum fed (Y-AL; n = 12), (2) old sedentary ad libitum fed (O-AL; n = 19), (3.) old lifelong 8% calorie restricted (O-CR; n = 20) and (4) old lifelong 8% calorie restricted with lifelong daily voluntary wheel running (O-CREx; n = 20). Mild calorie restriction of 8% was necessary to stimulate voluntary wheel running activity. Rats fed an ad libitum diet tend to decrease their running activity abruptly, but slight food restriction (8-10%) has been shown to prevent this decline (Holloszy and Schechtman, 1991; Holloszy et al., 1985). Food intake for the O-CR and O-CREx groups was therefore restricted by 8% below the ad libitum food intake of a separate group of sedentary, age-matched, male Fischer 344 rats, which were housed in the same facility. Throughout the duration of the study, food intake of these two groups was adjusted each week based on ad libitum food intake from the previous week. All animals were singly housed in a temperature (20° ± 2.5°C) and light-controlled (12:12 h light-dark cycle) environment with unrestricted access to water. All sedentary rats were housed in standard rodent cages supplied by the University of Florida’s Animal Care Services. Wheel running animals had free access to Nalgene Activity Wheels (1.081 meters circumference) obtained from Fisher Scientific (Pittsburgh, PA) (Judge et al., 2005). Each wheel was equipped with a magnetic switch and a counter with liquid crystal display (LCD) to record the daily number of wheel revolutions for each animal (Judge et al., 2005; Seo et al., 2006). Body weights of all rats were recorded weekly. Animals were anesthetized with isoflurane (administered via inhalation using a precision vaporizer at 5%) and sacrificed by heart puncture. All experimental procedures were approved by the University of Florida’s Institute on Animal Care and Use Committee.
Preparation of tissue extracts and subcellular fractions
Upon sacrifice, the plantaris muscle was removed, trimmed of adipose tissue and tendons, rinsed in phosphate-buffered saline solution, blotted dry, weighed and then frozen in liquid nitrogen. For the preparation of tissue extracts, a small piece of frozen tissue was pulverized in liquid nitrogen. The powder was resuspended in ice-cold buffer (pH 8) containing 250 mM sucrose, 1 mM ethylenediaminetetraacetic acid (EDTA), 2% sodium dodecyl sulfate (SDS), 1 mM dithiothreitol (DTT), 10 mM Tris-HCl, and 0.01% protease inhibitor cocktail (Pierce Biotechnology, Inc., Rockford, IL), then vortexed briefly and heated 3 times for 3 minutes at 85°C. After centrifugation at 12,000xg for 10 minutes at room temperature, the supernatant was transferred into a new microcentrifuge tube and kept at 45°C on a heating block. Protein quantification was then performed using a modified Lowry assay, following the manufacturer’s instructions (DC protein assay, BioRad, Hercules, CA). Tissue extracts were aliquoted and stored at −80°C until further analysis.
For subcellular fractionation, the plantaris muscle was homogenized in 1:5 w/v ice-cold isolation buffer (210 mM mannitol, 70 mM sucrose, 5 mM HEPES, 1 mM EDTA, and 0.2% fatty acid-free bovine serum albumin, pH 7.4) on ice in a glass-glass Duall homogenizer. Subsequently, the homogenate was centrifuged for 10 min at 1,000×g at 4°C to pellet cellular debris and nuclei. The supernatant was collected and further centrifuged for 15 min at 14,000×g at 4°C. The resulting supernatant, representing the mitochondria-free cytosolic fraction, was collected, aliquoted and stored at −80°C. The mitochondrial pellet was resuspended in 1.5 mL ice-cold wash buffer (210 mM mannitol, 70 mM sucrose, 5 mM HEPES, 1 mM EDTA, pH 7.4) and centrifuged for 15 min at 14,000×g at 4°C. The supernatant was decanted and the mitochondrial pellet resuspended in 50 μL storage buffer (250 mM sucrose, 1 mM EDTA, pH 7.4) and stored at −80°C.
Western blot analysis
Prior to loading, tissue extracts were boiled at 95°C for 5 minutes in Laemmli buffer (62.5 mM Tris HCl, 2% SDS, 25% Glycerol, 0.01% Bromophenol Blue, pH 6.8; BioRad) with 5% β-mercaptoethanol. Equal protein amounts of tissue extracts were applied to pre-cast Tris-HCl gels (Criterion system, BioRad, Hercules, CA) formulated to 7.5% (Atg9), 10% (Beclin-1, Atg7), or 15% (LC3) acrylamide. After electrophoretic separation, the proteins were transferred to polyvinylidene difluoride (PVDF) membrane (Immobilon P, 0.45 μm, Millipore, Billerica, MA) using a semidry blotter (BioRad). Transfer efficiency was verified by staining the gels with GelCode Blue Stain Reagent (Pierce) and the membranes with Ponceau S (Sigma-Aldrich, St. Louis, MO). Ponceau S staining was also used as a loading control. After transfer, the membranes were blocked in StartingBlock Blocking Buffer in Tris-buffered saline (TBS; pH 7.5) with 0.05% Tween 20 (Pierce) for 1 h at room temperature, washed in TBS, and incubated overnight in the corresponding primary antibody at 4°C. Subsequently, membranes were washed in TBS with 0.05% Tween 20 (TBS-t) and incubated with alkaline phosphatase-conjugated secondary antibody (Sigma-Aldrich), 1:30,000, at room temperature for 1 h. Membranes were then washed in TBS-t, rinsed in TBS, and washed once in Tris HCl (100 mM, pH 9.5). Finally, the DuoLux chemiluminescent/fluorescent substrate for alkaline phosphatase (Vector Laboratories, Burlingame, CA) was applied, and the chemiluminescent signal captured with an Alpha Innotech Fluorchem SP imager (Alpha Innotech, San Leandro, CA). The digital images were analyzed using AlphaEase FC software (Alpha Innotech). Density of the target band was normalized to the most prominent band previously stained with Ponceau S, and expressed in arbitrary optical density units.
Tissues were assayed for expression of macroautophagy-related proteins using the following antibodies and dilutions as described earlier (Wohlgemuth et al., 2007): mouse anti-Beclin (Atg6; BD Biosciences, San Jose, CA; 1:300), rabbit anti-LC3B (microtubule-associated protein 1B light chain 3, Atg8; Cell Signaling; 1:1000), rabbit anti-human Atg7 (courtesy of Dr. W.A. Dunn; 1:2500), and rabbit anti-human Atg9 antibody (courtesy of Dr. W.A. Dunn, prepared against the N-terminal sequence of human Atg9, MAQFDTEYQERLEASYSDSP; 1:200).
Dot blot analysis for 4-HNE modified mitochondrial proteins
For the determination of 4-HNE-adducts, 4 μL mitochondrial fraction was loaded onto a nitrocellulose membrane (BioRad). Membranes were air-dried for 20 min and blocked in 2% casein with 0.05% Tween 20 for 1 h at room temperature. Membranes were subsequently incubated in primary antibody for 30 min at room temperature and washed in TBS-t. A mouse monoclonal anti-4-HNE primary antibody was used (Oxis International, Foster City, CA; 1:500). Secondary antibody incubation was carried out for 30 min at room temperature, using anti-mouse horseradish peroxidase-conjugated secondary antibody (Affinity BioReagents, Golden, Co; 1:8000). Membranes were washed in TBS-t, rinsed in TBS, and washed once in Tris HCl (100 mM, pH 9.5). Generation of the chemiluminescent signal, digital acquisition and densitometry analysis were performed as described above. Spot density of the target band was normalized to total protein concentration as determined by the DC assay and expressed as % of total protein.
Apoptotic markers
Caspase-3 and -9 activities were determined by monitoring fluorescence of cleaved substrate. The substrates Ac-DEVD-AMC, and Ac-LEHD-AFC are cleaved proteolytically by caspase-3 and -9, respectively. Fluorescence was determined using a Spectra Max Fluorescent Microplate Reader (Molecular Devices, Sunnyvale, CA) at excitation, 400 nm and emission, 505 nm, for AFC and at excitation, 380 nm and emission, 460 nm, for AMC.
The extent of apoptotic DNA fragmentation (apoptotic index) was quantified by measuring the amount of cytosolic mono- and oligonucleosomes (180 base pair nucleotides or multiples) using an enzyme-linked immunosorbent assay (ELISA) kit (Cell Death Detection ELISA; Roche Diagnostics, Mannheim, Germany), following the manufacturer’s instructions, as described previously (Marzetti et al., 2008b).
Relative quantification of LC3 and LAMP-2 gene expression in skeletal muscle by quantitative PCR
To determine the relative gene expression of LC3 and LAMP-2 in plantaris muscles, quantitative PCR (Q-PCR) analysis was performed. Total RNA was isolated with TriReagent (Sigma-Aldrich). Briefly, ~50 mg of tissue were homogenized in 1 ml of TriReagent using a motorized mortar and pestle. The homogenate was cleared by centrifugation, and RNA was isolated from the supernatant according to the manufacturer’s instructions. Total RNA was then dissolved in Nuclease-free water and quantified spectrophotometrically. To remove possible contaminating DNA, DNase digestion was performed using the TURBO DNA-free kit from Ambion (Foster City, CA). All steps were performed as per the manufacturer’s directions. After DNase treatment, RNA concentrations and purity were determined spectrophotometrically (i.e., 260 nm-to-280 nm ratio). Synthesis of cDNA was achieved from 2 μg of RNA using the High capacity cDNA reverse transcription kit (ABI, Foster City, CA). Briefly, 2 μL of 10X Buffer, 0.8 μL of 100 mM dNTP’s, 2 μL of 10X random hexamers, 1 μL of 100 mM dNTP mix, 1 μL of 50 units/μL reverse transcriptase, 1 μL of 20 units/μL of RNase inhibitor, and nuclease-free water were mixed and added to total RNA. The resulting mixture was incubated at 25°C for 10 min, followed by 37°C for 120 min. Enzyme activity was terminated by heating to 85°C for 5 min. Q-PCR was performed using the ABI 7500 real-time PCR system (ABI, Foster City, CA). TaqMan Universal PCR Master Mix (2x) (Roche, Branchburg, NJ), as well as 0.2 μM primers and TaqMan probe mix (ABI) were used for each 25-μL reaction. Amplification of LC3 (NM_022867), LAMP-2 (NM_017068), and α-actin (endogenous control, NM_031144) was achieved with the following PCR cycling conditions: enzyme activation at 95°C for 10 min, 40 cycles of denaturation at 95°C for 15 s, and anneal/extend at 60°C for 1 min. All samples were examined in triplicate, with the Y-AL group used as a calibrator. For both genes, negative controls (i.e., no template and no reverse transcriptase) were also included and run in triplicate.
Statistical analysis
All data are reported as mean ± SEM. Statistical analysis was performed using GraphPrism 4.0.3 software (GraphPad Software, Inc., San Diego, CA). Student’s t test was used to compare Y-AL and O-AL. A one-way ANOVA was employed to detect differences among O-AL, O-CR and O-CREx. Pearson’s test was used to explore correlations between variables. For all tests the significance level was set at p<0.05.
Results
In this study we tested whether age and life-long mild calorie restriction (8% CR) or life-long voluntary exercise (with 8% CR) had independent or additive effects on the expression of autophagy and lysosome-related proteins. The moderate use of caloric restriction in the design is essential to stimulate running activity. With this amount of CR animals habituated into active runners throughout their lifespan. The activity levels of the exercised animals have been published previously (Judge et al., 2005). In summary, although peak running activity occurred at 6 months of age (~2500 meters/day), running activity was maintained at an average of 1145 ± 248 meters/day for the remainder of the study. This is in contrast to previous studies that show a continual decline after approximately midlife in the average distance run per day as the animal’s age. In addition, at 10 months of age, daily energy expenditure (DEE) was estimated in the two groups of rats. Runners exhibited a significant higher DEE (202 ± 31 kJ/day for runners vs. 121±22 kJ/day for pair-fed sedentary controls, p < 0.05), expending approximately 70% more energy per day than the sedentary rats.
Body and muscle weight
Mean body weight did not change with age when old ad libitum fed control rats (O-AL) were compared to young ad libitum fed rats (O-AL versus Y-AL, p=0.6; Table 1). Body weight of old calorie restricted rats (O-CR) and old exercised and calorie restricted rats (O-CREx) were not different from old controls (O-AL versus O-CR, p=0.5; O-AL versus O-CREx, p=0.4). However, O-CREx rats weighed less than O-CR rats (p=0.0004). Total plantaris mass (left and right plantaris combined, Table 1) did not change with age, CR or exercise. Similarly, relative plantaris weight (g/kg body weight) did not change with age. However, it was significantly higher in O-CREx compared to O-CR (p=0.0008), and tended to be higher than O-AL (p=0.057).
Table 1.
Body weight (BW; g), plantaris weight (g) and relative plantaris weight (g/kg BW) in young (Y-AL), old (O-AL), old calorie restricted (O-CR) and old calorie restricted and exercised (O-CREx) Fischer 344 rats. Data presented as mean ± SEM; same indices represent significant difference at p<0.05
| Y-AL (n=12) | O-AL (n=9) | O-CR (n=11) | O-CREx (n=11) | |
|---|---|---|---|---|
| Body weight | 374.3 ± 9.9 | 362.2 ± 23.3 | 375.9 ± 5.6 a | 342.0 ± 5.8 a |
| Plantaris weight | 0.653 ± 0.012 | 0.626 ± 0.030 | 0.603 ± 0.0177 | 0.612 ± 0.014 |
| Rel. plantaris weight | 1.748 ± 0.029 | 1.683 ± 0.030 | 1.604 ± 0.027 b | 1.790 ± 0.039 b |
Autophagy
We analyzed the expression levels of key proteins that are involved in the formation of autophagsosomes (Beclin-1, Atg7 and Atg9, LC3) in plantaris tissue extracts. Furthermore, we measured gene expression of LC3, involved in autophagosome formation, and LAMP-2, essential for fusion of the autophagosome with the lysosome.
Atg6/beclin-1
The mammalian homologue of yeast Atg6, Beclin-1, associates with a multimeric complex of macroautophagy regulatory proteins (Atg14, Vps34/class 3 PI3kinase, and Vps15) that is important for induction and formation of a preautophagosome structure (Kihara et al., 2001a; Kihara et al., 2001b; Liang et al., 1998). We found that Beclin-1 expression increased significantly with age (Y-AL versus O-AL; p<0.05; Figure 1A). CR and CR/exercise treatment did not affect protein expression levels compared to the old controls (p=0.8, 0.7, 0.8 for O-AL vs. O-CR and O-CREx, and O-CR vs. O-CREx, respectively).
Figure 1.
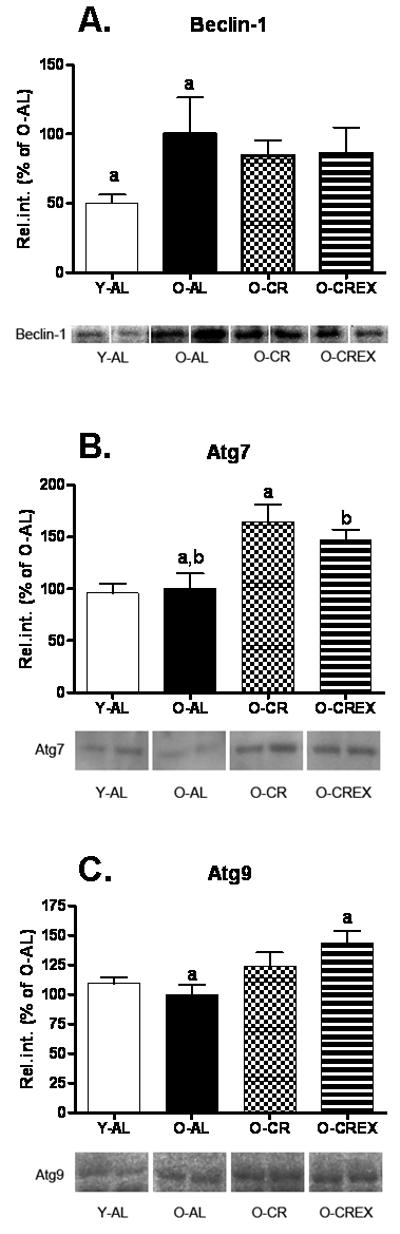
Protein expression of autophagy regulatory proteins Beclin-1 (A.); Atg7 (B.); Atg9 (C.) in tissue extracts (in % of O-AL) of plantaris muscle from young (6 mon), old ad libitum fed rats (24 mon), old, mildly calorie restricted rats (8% CR 24 mon) and old mildly restricted and exercised rats (8% CR+Ex 24 mon). Representative blots are shown below. Data are presented as mean ± standard error of the mean (SEM) and displayed as percent of O-AL. Identical indices indicate significant difference between groups, with significance level set at p<0.05.
Atg7
The protein Atg7 is required for the formation and expansion of the autophagosome by initiating the conjugation of Atg12 to Atg5 and LC3 to phosphatidylethanolamine (Komatsu et al., 2005; Mizushima et al., 1998). We found that Atg7 expression did not change with age (Y-AL versus O-AL, p=0.8; Figure 1B), while its expression was significantly elevated in O-CR and O-CREx compared to O-AL (p=0.02 and 0.05, respectively). Protein expressions in O-CR and O-CREx were not significantly different from each other (p=0.8).
Atg9
The integral membrane protein Atg9 is essential for autophagosome formation (Noda et al., 2000; Reggiori et al., 2005; Yamada et al., 2005) and may function at the preautophagosome structure or at the membrane source of the autophagosome (Reggiori et al., 2004). Atg9 expression did not change with age or CR treatment(Y-AL vs. O-AL, p=0.3; O-AL vs O-CR, p= 0.2; Figure 1C), but was significantly increased in O-CREx compared to O-AL (p=0.022).
LC3 protein expression
The rat microtubule-associated protein light chain, LC3, a mammalian homologue of yeast Atg8, is essential for the expansion of the early autophagosome (Abeliovich et al 2000). Pre-LC3 is processed to its cytosolic form, LC3-I, which is then activated by Atg7, transferred to Atg3 and lipidated to its membrane bound form, LC3-II, localized to the preautophagosome structure and autophagosomes. Protein expression levels of LC3-I and LC3-II were significantly higher in O-AL compared to Y-AL (p=0.04 and 0.0003, respectively; Figure 2B and C). However, in the old rats no significant treatment effect was detected (LC3-I: p= 0.4 for O-AL vs. O-CR, p= 0.3 for O-AL vs. O-CREx, and p=0.8 for O-CR vs. O-CREx; LC3-II: p= 0.4 for O-AL vs. O-CR, p= 0.5 for O-AL vs. O-CREx, and p=0.5 for O-CR vs. O-CREx). Although an increase in cellular level of LC3-II is consistent with activation of autophagy, an increase in the ratio of LC3-II to LC3-I has been suggested as a better biochemical marker to assess ongoing autophagy (Mizushima et al., 2004; Yan et al., 2005). Here we detected no significant effect of age on the LC3-II/I ratio (p=0.1; Figure 2D), although it appeared to be 30% lower in old rats. No effect of CR with or without exercise was detected on old rats (p= 0.4 for O-AL vs. O-CR, p= 0.6 for O-AL vs. O-CREx, and p=0.5 for O-CR vs. O-CREx).
Figure 2.
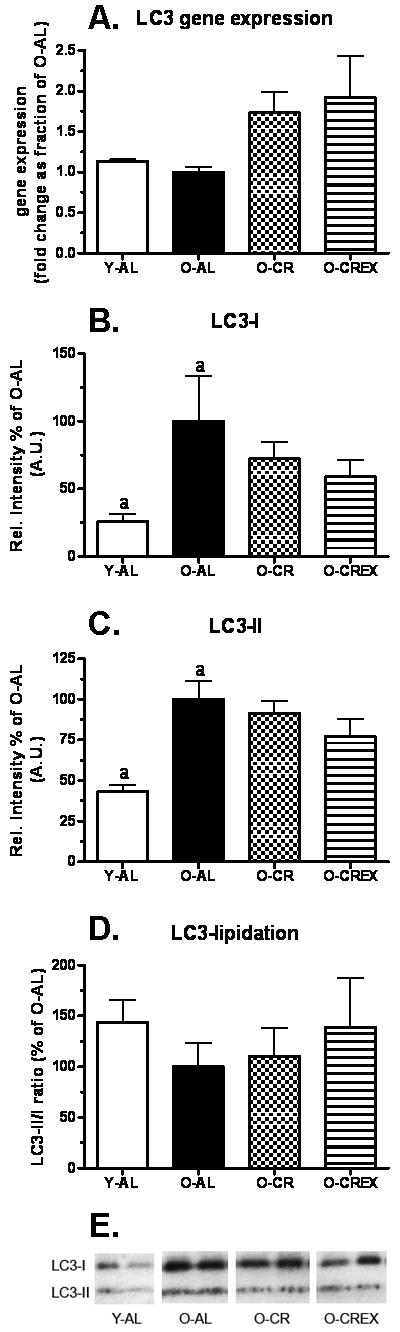
Gene (A) and Protein (B, C, D, E) expression of LC3 in tissue extracts of plantaris muscle from young (6 mon), old ad libitum fed rats (24 mon), old, mildly calorie restricted rats (8% CR 24 mon) and old mildly restricted and exercised rats (8% CR+Ex 24 mon). A. LC3 gene expression (fold-change as fraction of O-AL; see Methods), B. LC3-I; C. LC3-II; D. Ratio of LC3-II and LC3-I protein expression. Representative blots for protein expression are shown below. Data are presented as mean ± standard error of the mean (SEM) and protein expression data displayed as percent of O-AL. Identical indices indicate significant difference between groups, with significance level set at p<0.05.
LC3 gene expression
To evaluate whether increased LC3-I and II protein expression seen in the plantaris from O-AL rats was indicative of an induction of autophagy, we analyzed LC3 gene expression (Figure 2A). We found that the mRNA level of LC3 in plantaris muscle was not changed in O-AL rats compared to young rats, with a tendency to be lower (p=0.086). Compared to O-AL rats LC3 gene expression was 70% and 90% higher in O-CR and O-CREx, respectively, although the difference did not reach significance (p=0.3 for O-AL vs. O-CR and 0.2 for O-AL vs. O-CREx).
LAMP-2 gene expression
LAMP-2 (lysosomal-associated membrane protein 2), a receptor at the lysosomal membrane, is essential for the uptake of the chaperone-substrate complex into the lysosome for degradation during chaperone-mediated autophagy (Cuervo and Dice, 1996). It is furthermore needed for efficient fusion of autophagosomes and lysosomes (Gonzalez-Polo et al., 2005), and thereby crucial for completion of the lysosomal-autophagic degradation process. In plantaris muscle from O-AL rats LAMP-2 mRNA levels were significantly lower compared to Y-AL rats (p=0.0084). In contrast, mRNA levels were 1.8 and 2.3-fold higher in O-CR and O-CREx rats, respectively, compared to O-AL (p= 0.014 for O-AL vs. O-CR, p= 0.001 for O-AL vs. O-CREx).
Oxidative damage and apoptosis
Oxidative damage
Impaired autophagic homeostasis can possibly result in accumulation of oxidative damage with age, and ultimately leads to apoptotic or necrotic cell death in aged post-mitotic tissue. Therefore, oxidative damage and levels of apoptosis in plantaris muscle were determined by measuring mitochondrial 4-HNE-modified proteins, and cytosolic caspase3 and -9 activities together with DNA fragmentation (apoptotic index). We found that 4-HNE modified mitochondrial proteins increased significantly in O-AL rats compared to Y-AL (p=0.003; Figure 4). Mild life-long CR with and without life-long exercise attenuated this increase (p=0.007 and <0.001 for O-AL vs. O-CR and O-CREx, respectively). Furthermore, exercise had an additional beneficial effect compared to CR alone (p=0.02, OCR vs. O-CREx).
Figure 4.
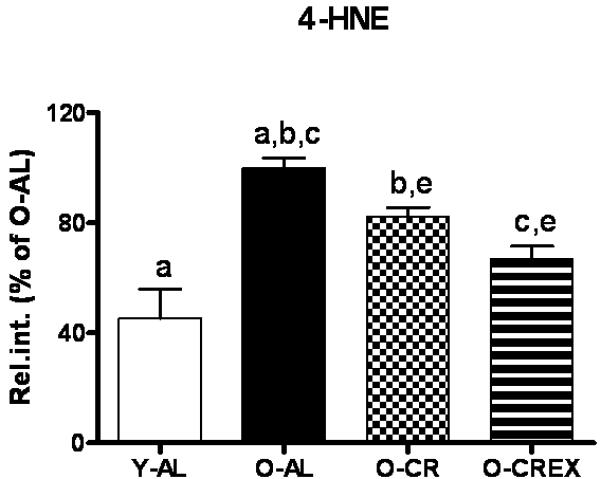
Levels of 4-HNE-modified mitochondrial proteins in the mitochondrial fraction of plantaris muscle from young (6 mon), old ad libitum fed rats (24 mon), old mildly calorie restricted rats (8% CR 24 mon), and old mildly restricted and exercised rats (8% CR+Ex 24 mon) as measured by dot blot analysis. Data are presented as mean ± standard error of the mean (SEM) and displayed as percent of O-AL. Identical indices indicate significant difference between groups, with significance level set at p<0.05.
Apoptosis
Apoptotic DNA fragmentation was increased in plantaris muscle from old control rats (p=0.01, O-AL vs. Y-AL; Figure 5A). This age effect tended to be mitigated by life-long CR with and without life-long exercise (p=0.07 for both O-AL vs. O-CR and O-AL vs. O-CREx). We further evaluated caspase-3 and caspase-9 activities and found that both caspase activities were significantly increased in aged plantaris (p=0.04 for both caspases; Figure 5B and C). However, CR and/or exercise could not mitigate the increased caspase activities in aged muscle (caspase-3: p= 0.4 for O-AL vs. O-CR, p= 0.7 for O-AL vs. O-CREx, and p=0.5 for O-CR vs. O-CREx; caspase-9: p= 0.5 for O-AL vs. O-CR, p= 0.4 for O-AL vs. O-CREx, and p=0.3 for O-CR vs. O-CREx).
Figure 5.
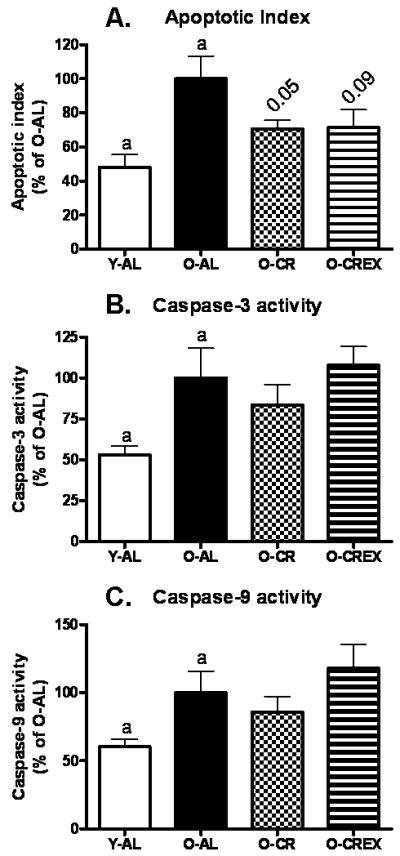
Apoptotic index as determined by quantification of cytosolic mono- and oligonucleosomes (A), and activity of caspase-3 (B) and caspase-9 (C) in tissue extracts of plantaris muscle from young (6 mon), old ad libitum fed rats (24 mon), old mildly calorie restricted rats (8% CR 24 mon), and old mildly restricted and exercised rats (8% CR+Ex 24 mon). Data are presented as mean ± standard error of the mean (SEM) and displayed as percent of O-AL. Identical indices indicate significant difference between groups, with significance level set at p<0.05.
Linear regression analyses
Regression analyses were performed to test the hypothesis that an increase in autophagy is negatively correlated with oxidative stress and apoptosis. For the analysis we included only the data from old animals to evaluate the correlation of autophagy and oxidative damage and apoptosis, respectively, at old age with or without treatment. We found that LAMP-2 gene expression in plantaris muscle was negatively correlated with the level of 4-HNE-modified mitochondrial proteins (Pearson’s r2 = −0.664; p=0.0041; Figure 6A), and with the apoptotic index (Pearson’s r2 = −0.2745; p=0.031; Figure 6B).
Figure 6.
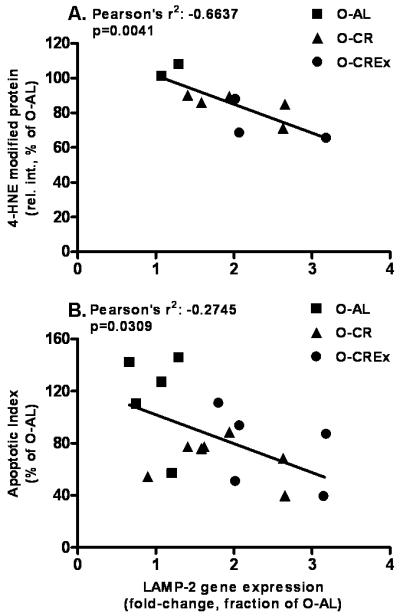
Linear regression analyses between LAMP-2 gene expression (fold-change as fraction of O-AL) and 4-HNE-modified mitochondrial proteins (A), and the apoptotic index (B), respectively. Only data obtained from old animals were included in the regression analysis. Pearson’s r2 and p-values are displayed in the graphs.
Discussion
The main objective of this study was to determine the effect of age on autophagy in rat type II skeletal muscle, and to investigate whether mild, life-long CR (8 %) alone or in combination with life-long voluntary exercise would change the autophagic response in aged rats. We hypothesized that there would be an age-related impairment of autophagy that would be attenuated by those treatments. We further predicted to find a correlation between autophagy and oxidative damage and apoptotic index, based on the fundamental role of autophagic housekeeping. We found that the results are in support of our hypothesis. Although upstream regulators of autophagy were upregulated (Beclin-1) or unchanged (Atg7 and 9) in O-AL compared to Y-AL rats, expression of genes (LAMP-2; LC3) and proteins (LC3-I and II), which regulate the autophagic process further downstream, suggested an impairment of autophagic degradation. Our results show that the mild, 8 % CR treatment increased the expression of upstream as well as downstream regulators of autophagy. However, we did not detect an additive effect of life-long voluntary wheel running when combined with mild CR. Importantly, we found a significant overall negative correlation between autophagy and oxidative damage and apoptosis, respectively (Figure 6, see below). These results indicate for the first time that autophagy in plantaris muscle from Fischer 344 rats declines with age, and that mild CR can attenuate the age-related impairment of autophagic degradation in vivo.
Muscle weight with age
Aging is associated with a progressive decline of muscle mass, strength, and quality, a condition overall known as sarcopenia of aging. Our lab and others have previously reported the occurrence of age-related muscle loss in rats (Hepple et al., 2008; Lushaj et al., 2008; Marzetti et al., 2009; Marzetti et al., 2008b; McKiernan et al., 2004; Pistilli et al., 2006) and its attenuation by CR (Hepple et al., 2008; Marzetti et al., 2009; McKiernan et al., 2004). In the present study we did not detect a decrease in total or relative (g/kg body weight) plantaris weight with age (Table 1), or a significant effect of mild CR and exercise treatment in the old rats. However, Kim and colleagues recently reported a significant decline in fiber cross-sectional area and an increase in extramyocyte space and connective tissue with age in plantaris muscle from the same rats utilized in the present study (Kim et al., 2008). The authors further found that these morphological changes were prevented by mild CR alone or in combination with exercise. We suggest, therefore, that despite an apparent loss of overall plantaris mass in old rats the quality of the muscle tissue has deteriorated, correlating with the impairment of autophagy, and the increase of oxidative damage and apoptosis.
Autophagic markers in aging skeletal muscle and the effect of mild CR and exercise
Autophagy is a highly regulated process for the bulk degradation of cytoplasmic components including protein aggregates and organelles. The involvement of autophagy in life/death decisions of a cell has recently gained growing attention. Autophagy has been implicated in cell death simultaneously or independently of apoptosis (reviewed in (Eisenberg-Lerner et al., 2009; Maiuri et al., 2007)). On the other hand, autophagy as a housekeeping mechanism is essential for the cell’s survival. Regulation of (macro-) autophagy by mTor, PI3K-class III, and other signaling pathways occurs at nucleation and expansion of the membranes that form the early autophagosome. Nucleation from the endoplasmic reticulum is controlled by Beclin-1 (mammalian Atg6) and other proteins, while expansion events modulated by Atg7, Atg8 (LC3), Atg9, and Atg12 control the size of the autophagosome and therefore the amplitude of autophagy. Furthermore, the adjustment of autophagosome size by LC3-mediated tethering and fusion of these membranes is critical to allow sequestration of large mitochondria and protein aggregates. Once the early autophagosome is formed it matures into a late autophagosome by acquiring endosomal components. The final fusion with the lysosome requires, amongst others, LAMP-2, resulting in the formation of the autolysosome, where the contents are degraded and products recycled.
The effect of aging on autophagy has been widely studied in liver cells (Bergamini et al., 2004; Cuervo and Dice, 2000; Del Roso et al., 2003). Recent studies have reported a decline in autophagy with age in postmitotic tissues such as the brain (Keller et al., 2004) and the peripheral nervous system (Rangaraju et al., 2009). However, we are only beginning to understand the importance of autophagy in the development of age-related functional impairment of postmitotic tissues. CR is a potent inducer of autophagy in most species (Bergamini et al., 2003; Del Roso et al., 2003; Mizushima et al., 2004), and we have previously demonstrated that CR stimulates autophagy in heart muscle of young and old rats that were life-long 40 % calorie restricted (Wohlgemuth et al., 2007). The mechanisms by which nutrient availability impacts autophagy likely involves multiple pathways, such as insulin signaling and mTOR pathways (Jia et al., 2006; Yamamoto et al., 2006; Young et al., 2009), or the activation of autophagy proteins through Sirt1 (Lee et al., 2008). On the contrary, little is known regarding the effect of exercise on autophagy. In a recently published study, MacKenzie et al. determined that acute high resistance exercise, resulting in increased protein synthesis and muscle mass, concomitantly caused a proportional increase in protein degradation (Mackenzie et al., 2009). Interestingly, the authors found that the increased degradation was mediated through activated mVps34 (Mackenzie et al., 2009), a PI3 kinase class III, which regulates autophagy by forming a complex with Beclin-1 (Kihara et al., 2001a). However, it is unknown whether chronic exercise would affect autophagy the same way to serve protein turnover and muscle hypertrophy.
Upstream autophagy-regulatory proteins
We report here that protein expression of Beclin-1 (mammalian Atg6) was increased in the aged animals, independent of CR and exercise, which is in agreement with a previous study conducted in rat heart (Wohlgemuth et al., 2007)The role of Beclin-1 is manifold (reviewed in (Cao and Klionsky, 2007; Pattingre et al., 2008)), and it has first been described as a tumor suppressor (Liang et al., 1999). Its specific role in autophagy is defined by direct interactions with proteins that can either promote or inhibit autophagy. For example, binding of the anti-apoptotic protein Bcl-2 inactivates its autophagy-inducing capability, while upon dissociation of Bcl-2 it can interact with PI3K-class III to promote autophagosome formation (reviewed in (Levine et al., 2008). The fact that Beclin-1 can interact with the anti-apoptotic Bcl-2 suggests a role in apoptosis. However, the involvement of Beclin-1 in apoptotic cell death is not clear. Our finding that Beclin-1 increases with age, independent of treatment, is in line with its various functions in the complex and interdependent regulation of autophagy, tumorigenesis and apoptosis (which is discussed below), all of which undergo age-related changes.
The expression levels of Atg7 and Atg9, both involved in the formation and expansion of the autophagosome, did not change with age, but were significantly elevated in O-CR and O-CREx (Atg7) or O-CREx rats only (Atg9). Our results are consistent with our previous study, in which we reported a decrease of Atg7 and Atg9 expression in hearts from old ad libitum fed rats, and an increase when the rats were life-long calorie restricted.
Taken together the expression patterns of those upstream regulatory proteins suggest that the induction, formation and elongation of the autophagic vacuole may not have been significantly impaired at old age and that CR with or without exercise aided improving the availability of those proteins to meet a likely increased, age-related need for cellular housekeeping.
Downstream autophagy-regulatory proteins
In the present study we detected significantly increased levels of both LC3-I and LC3-II in plantaris muscle from O-AL rats. However, the average LC3-II/I ratio was slightly but not significantly lower in this group compared to the young animals. The interpretation of LC3 protein expression is complicated by the fact that LC3 undergoes posttranslational modifications and final autophagic degradation. LC3-I is the cytosolic precursor of the lipidated, autophagosome-membrane-bound LC3-II, which eventually becomes degraded within the autolysosome. The ratio of LC3-II to LC3-I has traditionally been used to evaluate the extent of autophagy, with decreased ratio being interpreted as indicative of diminished autophagy. However, a decreased ratio could be caused by elevated LC3-I levels due to a lack of conversion to LC3-II, or by accelerated autophagic degradation resulting in increased LC3-II turnover. We, therefore, quantified LC3 gene expression. We argued that a reduction of autophagic degradation would cause LC3-I and II accumulation, and, as a feed back, reduce transcriptional activation of LC3 gene. On the contrary, if there was an induction of autophagic flux and an increased need for LC3 protein, then transcription would be stimulated. We found that in old ad libitum fed animals LC3 gene expression was unchanged compared to young animals, suggesting that LC3-I and II processing and final degradation was impaired. On the other hand, O-CR and O-CREx rats had similar LC3 protein levels compared to O-AL rats, but the LC3 mRNA levels were 1.8 and 2.3-fold increased, respectively, although the increase did not reach significance. We concluded that the treatments improved autophagic degradation, indicated by stimulated LC3 transcription.
To support this interpretation, we analyzed gene expression of LAMP-2, which has been demonstrated essential for the fusion event of autophagosome and lysosome. Plantaris muscle from O-AL rats displayed a significantly reduced LAMP-2 mRNA level. CR with and without exercise resulted in markedly increased LAMP-2 mRNA levels in support of our hypothesis that the impairment of autophagic degradation seen in the O-AL rats was alleviated. Our results are consistent with findings reported by Zhang and Cuervo (Zhang and Cuervo, 2008), who showed that induction of LAMP-2 expression in aged transgenic mice carrying an inducible LAMP-2 gene restored macroautophagic pathways. What is more, LAMP-2 expression in these old mice resulted in the improvement of mitochondrial morphology and function, and in reduced levels of oxidized and polyubiquitinated proteins.
Oxidative damage, apoptosis and autophagy in aging skeletal muscle
Oxidative damage to lipids, proteins and DNA, especially in post-mitotic tissue of an aged organism, may be severe and ultimately lead to apoptotic or necrotic cell death. Indeed, we found that the amount of 4-HNE-modified proteins, a marker of lipid peroxidation, was increased in mitochondria from plantaris muscle of aged AL rats. We also observed an age-related increase in apoptotic DNA fragmentation and caspase-3 and -9 activities in the plantaris muscle from O-AL rats compared to young controls. These results are in agreement with data published by our group and others, showing that oxidative damage and apoptosis markers are elevated in skeletal muscle from old rats (Chung and Ng, 2006; Dirks and Leeuwenburgh, 2002; Hepple et al., 2008; Hofer et al., 2008; Leeuwenburgh et al., 1997; Marzetti et al., 2008b; Pistilli et al., 2006; Seo et al., 2008). Consistent with previous reports applying a moderate CR diet (Bevilacqua et al., 2005; Zainal et al., 2000), we observed a decrease in 4-HNE-modified mitochondrial proteins and a trend toward a reduction in the apoptotic index in plantaris muscle from old mildly calorie restricted rats. However, the activity of caspases was not affected by the treatments.
We hypothesized that autophagic housekeeping plays an essential role in skeletal muscle health, and that, consequentially, an impairment of autophagy is associated with an accumulation of cellular damage and cell death. In support of our hypothesis we found a negative correlation between LAMP-2 gene expression and 4-HNE-modified mitochondrial proteins (Figure 6A) and apoptotic index (Figure 6B). These results suggest that autophagic clearance in plantaris muscle (indicated by increased LAMP-2 gene expression) might mitigate the age-related increase in mitochondrial oxidative damage and apoptotic cell death. Further studies will have to investigate whether mitochondrial function and bioenergetics are similarly protected.
Several observations merit further discussion. The correlation between LAMP-2 gene expression and apoptotic index appeared not quite as strong as the correlation with mitochondrial oxidative damage. Mitochondrial damage and, as a consequence, mitochondria-induced apoptosis might have been successfully attenuated by elevated autophagy. However, other apoptotic pathways might not be entirely prevented by increased autophagy. The increase of pro-apoptotic caspase-3 and -9 activities seen with age and the lack of improvement through the treatments applied indicates that activation of alternative, non-mitochondrial pro-apoptotic pathways had occurred. Furthermore, other cellular quality control mechanism in skeletal muscle, such as alternative autophagic pathways, the ubiquitin-proteasome system, or chaperones, have been shown to be impaired with age. Although Hepple and colleagues recently reported that a 40 % reduction in caloric intake optimized the activation of the proteasome in rat plantaris muscle from old rats (Hepple et al., 2008), these pathways may not be similarly affected by the mild CR or exercise regimen employed in this study. Given that these pathways might be quantitatively prevailing compared to autophagy, this would result in the accumulation of cellular damage such as polyubiquitinated proteins, protein aggregates, misfolded proteins even in the presence of a CR regimen.
It needs to be pointed out that the study design did not allow for a direct assessment of autophagic flux and actual protein degradation. The autophagy measures analyzed in this study are therefore indirect measures of autophagy. However, we believe that the results suggest an impairment of autophagy and autophagic degradation with age and at least a partial restoration in animals that underwent the interventions.
Another observation is worth mentioning: we did not observe an additive effect of exercise on improvement of autophagy in plantaris muscle from old rats. However, the necessity to combine exercise with mild CR represents a limitation of our study. Based on our findings and the current literature, we cannot draw definite conclusions on a potential effect of exercise on autophagic cellular quality control that could mediate the anti-apoptotic and muscle morphology preserving benefits of chronic life-long exercise.
Conclusions
The role of autophagosomal degradation in skeletal muscle homeostasis and atrophy is not fully understood. The primary cause for muscle wasting, including proteolysis of major contractile proteins (Clarke et al., 2007), has mainly been attributed to the activity of the ubiquitin-proteasome dependent pathway (Lecker et al., 1999), while the lysosomal pathways have been considered a minor avenue for protein degradation. Two recent studies revealed a mechanistic link between skeletal muscle atrophy and increased autophagy through the activation of the transcription factor FoxO3 and its downstream targets (Mammucari et al., 2007; Zhao et al., 2007). The results of our study suggest that autophagy plays a protective role in aging skeletal muscle from rats that were mildly calorie restricted. Future research needs to investigate whether this effect of autophagy stems from improved mitochondrial function in vivo. What is more, the mild (8 %) CR regimen tested in this study and its positive effect on muscle composition, oxidative stress, cell death and autophagy has the potential to be a more applicable intervention to attenuate sarcopenia of aging in humans.
Figure 3.
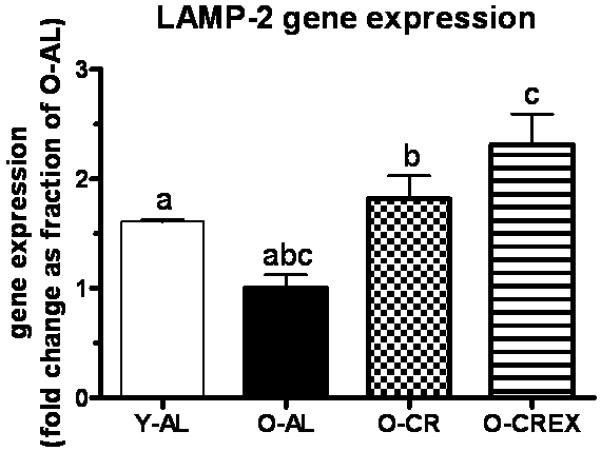
Gene expression of LAMP-2 (fold-change as fraction of O-AL; see Methods) in tissue extracts of plantaris muscle from young (6 mon), old ad libitum fed rats (24 mon), old, mildly calorie restricted rats (8% CR 24 mon) and old mildly restricted and exercised rats (8% CR+Ex 24 mon). Data are presented as mean ± standard error of the mean (SEM). Identical indices indicate significant difference between groups, with significance level set at p<0.05.
Acknowledgements
This research was supported by grants to CL (NIA R01-AG17994 and AG21042). EM and SEW were supported by the University of Florida Institute on Aging and Claude D. Pepper Older Americans Independence Center (1 P30 AG028740). We would like to thank Ms. Debra Akin and Dr. W.A. Dunn from the Department of Anatomy and Cell Biology at the University of Florida for their invaluable help with autophagy measures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Attaix D, Ventadour S, Codran A, Bechet D, Taillandier D, Combaret L. The ubiquitin-proteasome system and skeletal muscle wasting. Essays Biochem. 2005;41:173–86. doi: 10.1042/EB0410173. [DOI] [PubMed] [Google Scholar]
- Bergamini E, Cavallini G, Donati A, Gori Z. The anti-ageing effects of caloric restriction may involve stimulation of macroautophagy and lysosomal degradation, and can be intensified pharmacologically. Biomed Pharmacother. 2003;57:203–8. doi: 10.1016/s0753-3322(03)00048-9. [DOI] [PubMed] [Google Scholar]
- Bergamini E, Cavallini G, Donati A, Gori Z. The role of macroautophagy in the ageing process, anti-ageing intervention and age-associated diseases. Int J Biochem Cell Biol. 2004;36:2392–404. doi: 10.1016/j.biocel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Bevilacqua L, Ramsey JJ, Hagopian K, Weindruch R, Harper ME. Long-term caloric restriction increases UCP3 content but decreases proton leak and reactive oxygen species production in rat skeletal muscle mitochondria. Am J Physiol Endocrinol Metab. 2005;289:E429–38. doi: 10.1152/ajpendo.00435.2004. [DOI] [PubMed] [Google Scholar]
- Cao Y, Klionsky DJ. Physiological functions of Atg6/Beclin 1: a unique autophagy-related protein. Cell Res. 2007;17:839–49. doi: 10.1038/cr.2007.78. [DOI] [PubMed] [Google Scholar]
- Chung L, Ng YC. Age-related alterations in expression of apoptosis regulatory proteins and heat shock proteins in rat skeletal muscle. Biochim Biophys Acta. 2006;1762:103–9. doi: 10.1016/j.bbadis.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, Rakhilin SV, Stitt TN, Patterson C, Latres E, Glass DJ. The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab. 2007;6:376–85. doi: 10.1016/j.cmet.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Combaret L, Dardevet D, Rieu I, Pouch MN, Bechet D, Taillandier D, Grizard J, Attaix D. A leucine-supplemented diet restores the defective postprandial inhibition of proteasome-dependent proteolysis in aged rat skeletal muscle. J Physiol. 2005;569:489–99. doi: 10.1113/jphysiol.2005.098004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combaret L, Dardevet D, Bechet D, Taillandier D, Mosoni L, Attaix D. Skeletal muscle proteolysis in aging. Curr Opin Clin Nutr Metab Care. 2009;12:37–41. doi: 10.1097/MCO.0b013e32831b9c31. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273:501–3. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF. Age-related decline in chaperone-mediated autophagy. J Biol Chem. 2000;275:31505–13. doi: 10.1074/jbc.M002102200. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Bergamini E, Brunk UT, Droge W, Ffrench M, Terman A. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy. 2005;1:131–40. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- Del Roso A, Vittorini S, Cavallini G, Donati A, Gori Z, Masini M, Pollera M, Bergamini E. Ageing-related changes in the in vivo function of rat liver macroautophagy and proteolysis. Exp Gerontol. 2003;38:519–27. doi: 10.1016/s0531-5565(03)00002-0. [DOI] [PubMed] [Google Scholar]
- Dirks A, Leeuwenburgh C. Apoptosis in skeletal muscle with aging. Am J Physiol Regul Integr Comp Physiol. 2002;282:R519–27. doi: 10.1152/ajpregu.00458.2001. [DOI] [PubMed] [Google Scholar]
- Dirks AJ, Leeuwenburgh C. Aging and lifelong calorie restriction result in adaptations of skeletal muscle apoptosis repressor, apoptosis-inducing factor, X-linked inhibitor of apoptosis, caspase-3, and caspase-12. Free Radic Biol Med. 2004;36:27–39. doi: 10.1016/j.freeradbiomed.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Donati A, Cavallini G, Paradiso C, Vittorini S, Pollera M, Gori Z, Bergamini E. Age-related changes in the regulation of autophagic proteolysis in rat isolated hepatocytes. J Gerontol A Biol Sci Med Sci. 2001;56:B288–93. doi: 10.1093/gerona/56.7.b288. [DOI] [PubMed] [Google Scholar]
- Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009;16:966–75. doi: 10.1038/cdd.2009.33. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Polo R-A, Boya P, Pauleau A-L, Jalil A, Larochette N, Souquere S, Eskelinen E-L, Pierron G, Saftig P, Kroemer G. The apoptosis/autophagy paradox: autophagic vacuolization before apoptotic death. J Cell Sci. 2005;118:3091–3102. doi: 10.1242/jcs.02447. [DOI] [PubMed] [Google Scholar]
- Hepple RT, Baker DJ, Kaczor JJ, Krause DJ. Long-term caloric restriction abrogates the age-related decline in skeletal muscle aerobic function. Faseb J. 2005;19:1320–2. doi: 10.1096/fj.04-3535fje. [DOI] [PubMed] [Google Scholar]
- Hepple RT, Qin M, Nakamoto H, Goto S. Caloric restriction optimizes the proteasome pathway with aging in rat plantaris muscle: implications for sarcopenia. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1231–7. doi: 10.1152/ajpregu.90478.2008. [DOI] [PubMed] [Google Scholar]
- Hofer T, Marzetti E, Xu J, Seo AY, Gulec S, Knutson MD, Leeuwenburgh C, Dupont-Versteegden EE. Increased iron content and RNA oxidative damage in skeletal muscle with aging and disuse atrophy. Exp Gerontol. 2008;43:563–70. doi: 10.1016/j.exger.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy JO, Smith EK, Vining M, Adams S. Effect of voluntary exercise on longevity of rats. J Appl Physiol. 1985;59:826–31. doi: 10.1152/jappl.1985.59.3.826. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Schechtman KB. Interaction between exercise and food restriction: effects on longevity of male rats. J Appl Physiol. 1991;70:1529–35. doi: 10.1152/jappl.1991.70.4.1529. [DOI] [PubMed] [Google Scholar]
- Jeong SY, Seol DW. The role of mitochondria in apoptosis. BMB Rep. 2008;41:11–22. doi: 10.5483/bmbrep.2008.41.1.011. [DOI] [PubMed] [Google Scholar]
- Jia G, Cheng G, Gangahar DM, Agrawal DK. Insulin-like growth factor-1 and TNF-[alpha] regulate autophagy through c-jun N-terminal kinase and Akt pathways in human atherosclerotic vascular smooth cells. Immunol Cell Biol. 2006;84:448–454. doi: 10.1111/j.1440-1711.2006.01454.x. [DOI] [PubMed] [Google Scholar]
- Judge S, Jang YM, Smith A, Selman C, Phillips T, Speakman JR, Hagen T, Leeuwenburgh C. Exercise by lifelong voluntary wheel running reduces subsarcolemmal and interfibrillar mitochondrial hydrogen peroxide production in the heart. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1564–72. doi: 10.1152/ajpregu.00396.2005. [DOI] [PubMed] [Google Scholar]
- Keller JN, Dimayuga E, Chen Q, Thorpe J, Gee J, Ding Q. Autophagy, proteasomes, lipofuscin, and oxidative stress in the aging brain. Int J Biochem Cell Biol. 2004;36:2376–91. doi: 10.1016/j.biocel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001a;2:330–5. doi: 10.1093/embo-reports/kve061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara A, Noda T, Ishihara N, Ohsumi Y. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J Cell Biol. 2001b;152:519–30. doi: 10.1083/jcb.152.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kwak HB, Leeuwenburgh C, Lawler JM. Lifelong exercise and mild (8%) caloric restriction attenuate age-induced alterations in plantaris muscle morphology, oxidative stress and IGF-1 in the Fischer-344 rat. Exp Gerontol. 2008;43:317–29. doi: 10.1016/j.exger.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, Kominami E, Tanaka K, Chiba T. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425–34. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L, Sjodin B, Karlsson J. Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22--65 years. Acta Physiol Scand. 1978;103:31–9. doi: 10.1111/j.1748-1716.1978.tb06187.x. [DOI] [PubMed] [Google Scholar]
- Lecker SH, Solomon V, Mitch WE, Goldberg AL. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr. 1999;129:227S–237S. doi: 10.1093/jn/129.1.227S. [DOI] [PubMed] [Google Scholar]
- Lecker SH. Ubiquitin-protein ligases in muscle wasting: multiple parallel pathways? Curr Opin Clin Nutr Metab Care. 2003;6:271–5. doi: 10.1097/01.mco.0000068963.34812.e5. [DOI] [PubMed] [Google Scholar]
- Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proceedings of the National Academy of Sciences. 2008;105:3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeuwenburgh C, Wagner P, Holloszy JO, Sohal RS, Heinecke JW. Caloric restriction attenuates dityrosine cross-linking of cardiac and skeletal muscle proteins in aging mice. Arch Biochem Biophys. 1997;346:74–80. doi: 10.1006/abbi.1997.0297. [DOI] [PubMed] [Google Scholar]
- Lemasters JJ. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 2005;8:3–5. doi: 10.1089/rej.2005.8.3. [DOI] [PubMed] [Google Scholar]
- Levine B, Sinha S, Kroemer G. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy. 2008;4:600–6. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, Herman B, Levine B. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72:8586–96. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–6. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- Lowell BB, Ruderman NB, Goodman MN. Evidence that lysosomes are not involved in the degradation of myofibrillar proteins in rat skeletal muscle. Biochem J. 1986;234:237–40. doi: 10.1042/bj2340237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lushaj EB, Johnson JK, McKenzie D, Aiken JM. Sarcopenia accelerates at advanced ages in Fisher 344xBrown Norway rats. J Gerontol A Biol Sci Med Sci. 2008;63:921–7. doi: 10.1093/gerona/63.9.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie MG, Hamilton DL, Murray JT, Taylor PM, Baar K. mVps34 is activated following high-resistance contractions. J Physiol. 2009;587:253–60. doi: 10.1113/jphysiol.2008.159830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–52. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M. FoxO3 Controls Autophagy in Skeletal Muscle In Vivo. Cell Metab. 2007;6:458–71. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol. 2006;41:1234–8. doi: 10.1016/j.exger.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Groban L, Wohlgemuth SE, Lees HA, Lin M, Jobe H, Giovannini S, Leeuwenburgh C, Carter CS. Effects of short-term GH supplementation and treadmill exercise training on physical performance and skeletal muscle apoptosis in old rats. Am J Physiol Regul Integr Comp Physiol. 2008a;294:R558–67. doi: 10.1152/ajpregu.00620.2007. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Wohlgemuth SE, Lees HA, Chung HY, Giovannini S, Leeuwenburgh C. Age-related activation of mitochondrial caspase-independent apoptotic signaling in rat gastrocnemius muscle. Mech Ageing Dev. 2008b;129:542–9. doi: 10.1016/j.mad.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Hwang JC, Lees HA, Wohlgemuth SE, Dupont-Versteegden EE, Carter CS, Bernabei R, Leeuwenburgh C. Mitochondrial death effectors: Relevance to sarcopenia and disuse muscle atrophy. Biochimica et biophysica acta. 2009 doi: 10.1016/j.bbagen.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan SH, Bua E, McGorray J, Aiken J. Early-onset calorie restriction conserves fiber number in aging rat skeletal muscle. Faseb J. 2004;18:580–1. doi: 10.1096/fj.03-0667fje. [DOI] [PubMed] [Google Scholar]
- Meijer AJ, Codogno P. Regulation and role of autophagy in mammalian cells. Int J Biochem Cell Biol. 2004;36:2445–62. doi: 10.1016/j.biocel.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci. 2002;57:B359–65. doi: 10.1093/gerona/57.10.b359. [DOI] [PubMed] [Google Scholar]
- Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med. 1996;335:1897–905. doi: 10.1056/NEJM199612193352507. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, Klionsky DJ, Ohsumi M, Ohsumi Y. A protein conjugation system essential for autophagy. Nature. 1998;395:395–8. doi: 10.1038/26506. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–11. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda T, Kim J, Huang WP, Baba M, Tokunaga C, Ohsumi Y, Klionsky DJ. Apg9p/Cvt7p is an integral membrane protein required for transport vesicle formation in the Cvt and autophagy pathways. J Cell Biol. 2000;148:465–80. doi: 10.1083/jcb.148.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey UB, Nie Z, Batlevi Y, McCray BA, Ritson GP, Nedelsky NB, Schwartz SL, DiProspero NA, Knight MA, Schuldiner O, Padmanabhan R, Hild M, Berry DL, Garza D, Hubbert CC, Yao TP, Baehrecke EH, Taylor JP. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature. 2007;447:859–63. doi: 10.1038/nature05853. [DOI] [PubMed] [Google Scholar]
- Pattingre S, Espert L, Biard-Piechaczyk M, Codogno P. Regulation of macroautophagy by mTOR and Beclin 1 complexes. Biochimie. 2008;90:313–23. doi: 10.1016/j.biochi.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Phillips T, Leeuwenburgh C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. Faseb J. 2005;19:668–70. doi: 10.1096/fj.04-2870fje. [DOI] [PubMed] [Google Scholar]
- Pistilli EE, Siu PM, Alway SE. Molecular regulation of apoptosis in fast plantaris muscles of aged rats. J Gerontol A Biol Sci Med Sci. 2006;61:245–55. doi: 10.1093/gerona/61.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaraju S, Hankins D, Madorsky I, Madorsky E, Lee WH, Carter CS, Leeuwenburgh C, Notterpek L. Molecular architecture of myelinated peripheral nerves is supported by calorie restriction with aging. Aging Cell. 2009;8:178–91. doi: 10.1111/j.1474-9726.2009.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, White L. Midlife hand grip strength as a predictor of old age disability. Jama. 1999;281:558–60. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- Reggiori F, Tucker KA, Stromhaug PE, Klionsky DJ. The Atg1-Atg13 complex regulates Atg9 and Atg23 retrieval transport from the pre-autophagosomal structure. Dev Cell. 2004;6:79–90. doi: 10.1016/s1534-5807(03)00402-7. [DOI] [PubMed] [Google Scholar]
- Reggiori F, Shintani T, Nair U, JKlionsky DJ. Atg9 cycles between mitochondria and the pre-autophagosomal structure in yeasts. Autophagy. 2005;1:101–109. doi: 10.4161/auto.1.2.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri M. Signaling in Muscle Atrophy and Hypertrophy. Physiology. 2008;23:160–170. doi: 10.1152/physiol.00041.2007. [DOI] [PubMed] [Google Scholar]
- Seo AY, Hofer T, Sung B, Judge S, Chung HY, Leeuwenburgh C. Hepatic oxidative stress during aging: effects of 8% long-term calorie restriction and lifelong exercise. Antioxid Redox Signal. 2006;8:529–38. doi: 10.1089/ars.2006.8.529. [DOI] [PubMed] [Google Scholar]
- Seo AY, Xu J, Servais S, Hofer T, Marzetti E, Wohlgemuth SE, Knutson MD, Chung HY, Leeuwenburgh C. Mitochondrial iron accumulation with age and functional consequences. Aging Cell. 2008;7:706–16. doi: 10.1111/j.1474-9726.2008.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu PM, Bryner RW, Martyn JK, Alway SE. Apoptotic adaptations from exercise training in skeletal and cardiac muscles. Faseb J. 2004;18:1150–2. doi: 10.1096/fj.03-1291fje. [DOI] [PubMed] [Google Scholar]
- Song W, Kwak HB, Lawler JM. Exercise training attenuates age-induced changes in apoptotic signaling in rat skeletal muscle. Antioxid Redox Signal. 2006;8:517–28. doi: 10.1089/ars.2006.8.517. [DOI] [PubMed] [Google Scholar]
- Szulc P, Beck TJ, Marchand F, Delmas PD. Low skeletal muscle mass is associated with poor structural parameters of bone and impaired balance in elderly men--the MINOS study. J Bone Miner Res. 2005;20:721–9. doi: 10.1359/JBMR.041230. [DOI] [PubMed] [Google Scholar]
- Terman A. The effect of age on formation and elimination of autophagic vacuoles in mouse hepatocytes. Gerontology. 1995;41(Suppl 2):319–26. doi: 10.1159/000213753. [DOI] [PubMed] [Google Scholar]
- Terman A, Brunk UT. Oxidative stress, accumulation of biological ‘garbage’, and aging. Antioxid Redox Signal. 2006;8:197–204. doi: 10.1089/ars.2006.8.197. [DOI] [PubMed] [Google Scholar]
- Wang CW, Klionsky DJ. The molecular mechanism of autophagy. Mol Med. 2003;9:65–76. [PMC free article] [PubMed] [Google Scholar]
- Wing SS, Haas AL, Goldberg AL. Increase in ubiquitin-protein conjugates concomitant with the increase in proteolysis in rat skeletal muscle during starvation and atrophy denervation. Biochem J. 1995;307(Pt 3):639–45. doi: 10.1042/bj3070639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlgemuth SE, Julian D, Akin DE, Fried J, Toscano K, Leeuwenburgh C, Dunn WA., Jr. Autophagy in the heart and liver during normal aging and calorie restriction. Rejuvenation Res. 2007;10:281–92. doi: 10.1089/rej.2006.0535. [DOI] [PubMed] [Google Scholar]
- Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–9. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- Xu J, Knutson MD, Carter CS, Leeuwenburgh C. Iron accumulation with age, oxidative stress and functional decline. PLoS ONE. 2008;3:e2865. doi: 10.1371/journal.pone.0002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Carson AR, Caniggia I, Umebayashi K, Yoshimori T, Nakabayashi K, Scherer SW. Endothelial nitric-oxide synthase antisense (NOS3AS) gene encodes an autophagy-related protein (APG9-like2) highly expressed in trophoblast. J Biol Chem. 2005;280:18283–90. doi: 10.1074/jbc.M413957200. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Cremona ML, Rothman JE. Autophagy-mediated clearance of huntingtin aggregates triggered by the insulin-signaling pathway. J. Cell Biol. 2006;172:719–731. doi: 10.1083/jcb.200510065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Vatner DE, Kim SJ, Ge H, Masurekar M, Massover WH, Yang G, Matsui Y, Sadoshima J, Vatner SF. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci U S A. 2005;102:13807–12. doi: 10.1073/pnas.0506843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JE, Martinez RA, La Spada AR. Nutrient Deprivation Induces Neuronal Autophagy and Implicates Reduced Insulin Signaling in Neuroprotective Autophagy Activation. Journal of Biological Chemistry. 2009;284:2363–2373. doi: 10.1074/jbc.M806088200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainal TA, Oberley TD, Allison DB, Szweda LI, Weindruch R. Caloric restriction of rhesus monkeys lowers oxidative damage in skeletal muscle. Faseb J. 2000;14:1825–36. doi: 10.1096/fj.99-0881com. [DOI] [PubMed] [Google Scholar]
- Zhang C, Cuervo AM. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat Med. 2008;14:959–65. doi: 10.1038/nm.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg AL. FoxO3 Coordinately Activates Protein Degradation by the Autophagic/Lysosomal and Proteasomal Pathways in Atrophying Muscle Cells. Cell Metab. 2007;6:472–83. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]


