Abstract
Growing evidence suggests that survivin expression in cancer cell nuclei may represent an important prognostic marker to predict disease outcome for cancer patients. Current reports in this research area, however, are inconsistent and propose opposing conclusions regarding the significance and prognostic value of survivin nuclear expression. The aim of our study is to review and discuss the data reported in the original publications. We have also provided new experimental data to support our view regarding the possible reasons for the observed inconsistencies in the literature. This would alert researchers to pay attention to potential pitfalls in the determination of nuclear or cytoplasmic expression of survivin for the future.
Keywords: survivin expression, cancer, cytoplasmic, nuclear, immunohistochemistry, prognostic marker
Among the 19 publications relevant to survivin localization in nuclei or cytoplasm in various cancer tissues, 9 showed that the expression of survivin in cancer cell nuclei is an unfavorable prognostic marker,1–10 whereas studies from 5 of 19 proposed an opposing notion that the nuclear expression of survivin represented a favorable prognostic marker.10–14 The remaining 5 publications did not focus on studying the significance of nuclear expression of survivin in disease outcome, although these reports pointed out the fact that survivin could be expressed in either cytoplasm or nuclei. 15–19 Two of these 5 studies reported that overall, survivin expression was an unfavorable prognostic factor.16,18 The localization of survivin in nuclei or cytoplasm was determined by immunohistochemistry (IHC) in all 19 reports. One of the 19 publications alternatively carried out Western blots using cell lysates from the fractionated cytoplasm and nuclei for the confirmation of survivin localization.5 In addition, Nakagawa et al.20 reported recently that nuclear localization of survivin, as well as cytoplasmic in some cases, was essentially found in acute lymphocytic leukemia (ALL) cells whereas cytoplasmic expression of survivin was predominantly showed in chronic lymphocytic leukemia (CLL) cells. The significance, however, was not investigated.
Nuclear expression of survivin is an unfavorable prognostic marker
In hepatocellular carcinoma (HCC), studies from Ito et al.1 indicated that 14 of 20 (70%) HCC tissues showed nuclear staining of survivin, whereas non-tumor tissues showed little survivin staining. Nuclear survivin expression strongly correlated with the proliferation index.1 Similarly, studies from Moon et al.4 showed that 22 of 35 (63%) survivin-positive specimens (total = 47) showed punctate nuclear staining in HCC cells. In contrast, nonmalignant hepatocytes showed only cytoplasmic staining. HCC specimens with nuclear survivin expression showed the highest PCNA (proliferating cell nuclear antigen) labeling index and correlated with tumor cell de-differentiation.4 Recently, Fields et al.8 reported that immunohistochemical analyses of 72 hepatocellular carcinoma by tissue microarray indicated 43% frequency of nuclear survivin expression, which correlated with nuclear grade, microvascular invasion, mitotic rate, MIB-1 index, local recurrence and diseasefree survival. These observations are consistent with the previous finding that survivin interacts with Cdk4 in (growing) HCC cell lines,1 which leads to the nuclear translocation of survivin.21 Overexpression of survivin resulted in a decrease in the G0/G1 phase and an increase in the S-phase.1 Blocking nuclear transport suppressed survivin nuclear translocation and S-phase shift.21 These studies suggest that nuclear translocation of survivin might be a prerequisite for cancer cell proliferation whereas the cytoplasmic presence of survivin may be associated with cell survival but not cell proliferation. It should be pointed out that the IHC images provided in the study by Moon and Tarnawski4 for nuclear or cytoplasmic expression of survivin seem to be convincing.
In esophageal squamous cell carcinoma (SCC), nuclear expression of survivin was detected in 67 of 84 (80%) specimens.2 The mean patient survival (28 months) for this group was significantly less than that for patients without survivin expression in the tumor cell nuclei (108 months). In contrast, the tumors with cytoplasmic survivin staining had no prognostic significance.2 Whether the latter conclusion was derived from the 7 specimens with only cytoplasmic staining of survivin or from all the 53 specimens with cytoplasmic staining of survivin (46 of 53 with both) is not clear in the study. We should mention that one is unable to appreciate the nuclear or cytoplasmic staining of survivin because the IHC images are in a black and white format.
In ovarian carcinoma, Cohen et al.3 reported that 36 of 49 (74%) specimens showed nuclear expression of survivin, which correlated significantly with high tumor grade, histologic type and mutant p53, but not with disease-free survival. We should mention that the nuclear staining of survivin provided in the IHC images is convincing. Recently, however, Tringler et al. immunohistochemically analyzed 15 cystadenomas, 15 borderline tumors and 12 cystadenocarcinomas of the ovary. Eighty-seven percent of adenomas/borderline tumors showed survivin-positivity in the nucleus but only 42% for carcinomas, which is statistically significant. This observation suggests that nuclear localization of survivin is more common in benign or borderline tumors than in malignant serous tumors of the ovary.9
In mantle cell lymphoma (MCL), Martinez et al.5 reported that 80 specimens examined showed 5–95% of nuclear survivin expression. Selected MCL cell lines and tumor specimens were used for the confirmation of survivin localization by Western blot as well. The nuclear expression level of survivin was significantly associated with proliferative activity; overall patient survival was significantly shorter in patients with high survivin expression in the cell nucleus.5 It should be emphasized that the results from Martinez et al.5 are not only confirmed by Western blots but the IHC images provided in the study are also convincing.
In gastroenteropancreatic neuroendocrine tumor (GEP-NET), Grabowski et al. (personal communication) found that among 104 GEP-NET patients, 29 patients with localized well-differentiated GEP-NETs without recurrence or tumor-associated deaths, survivin was not expressed in tumor cell nuclei. In contrast, 15 of 60 (25%) patients with advanced metastatic GEP-NETs expressed survivin in cancer cell nuclei (Dr. Hans Scherubl et al., personal communication). Those 15 patients had a statistically significant worse prognosis (survival of 8 vs. 115 months). Their results suggest that nuclear survivin expression was inversely correlated with tumor differentiation and grade. Although 44 of 47 well-differentiated tumors were nuclear survivin-negative, 12 of 13 undifferentiated tumors were nuclear survivin-positive (Dr. Hans Scherubl et al., personal communication).
In non-small cell lung cancer (NSCLC), immunohistochemistry analyses of 48 cancer samples showed 32 cases (67%) with nuclear survivin-positivity, 39 cases (83%) with cytoplasmic survivin-positivity and 19 cases (44%) with survivin-positive staining in both cytoplasm and nucleus. These authors found that nuclear survivin expression was significantly associated with poor survival, whereas cytoplasmic staining of survivin was not.7 They proposed that nuclear presence of survivin might be an important prognostic marker for patients with NSCLC.
In cholangiocarcinoma, Javle et al.6 reported that among 24 consecutive cases of cholangiocarcinoma, cytoplasmic survivin was detectable in 13 cases and nuclear survivin in 11. Among these, strong (>50% of cells positive) cytoplasmic survivin expression was seen in 6 cases and strong (>50% of cells positive) nuclear expression of survivin in 4. Patients with strong nuclear survivin had a median survival of 11 months, which was significantly lower than that for patients with weak nuclear survivin expression (20 months, p = 0.033).6
Nuclear expression of survivin is a favorable prognostic marker
In contrast with the studies described above, several studies indicated that survivin expression in cancer cell nuclei is a favorable prognostic factor for the prediction of disease outcome for cancer patients.
In gastric cancer, nuclear staining of survivin was observed in 109 of 133 (82%) cases.10 Interestingly, although these authors stated that nuclear survivin expression was associated with a favorable prognosis, the IHC results indicated that nuclear expression of survivin was associated with poor differentiation and younger age of the patients.10 In contrast, cytoplasmic localization of survivin in 117 of 133 (88%) cases was associated with older patients.10 It is clear from these data that 93 specimens should have both cytoplasmic and nuclear expression of survivin. The report would be informative if the clinical outcomes of patients in this group had been provided.
In bladder mucosa and transitional cell carcinoma (TCC), nuclear staining of survivin was noted in 26 of 45 cases of TCC and 2 of 14 cases of in situ TCC, but not detected in healthy bladder mucosa.11 The differential nuclear expression of survivin in TCC vs. healthy bladder mucosa and in situ TCC was statistically significant (p < 0.001). These authors indicated, however, that TCC patients with a nuclear survivin staining had longer disease-free survival (27.2 months) compared to TCC patients without nuclear staining of survivin (9.9 months) although the differences were not statistically significant.11 It should be pointed out that the expression of survivin in nuclei or cytoplasm presented in the IHC images in this study seem convincing.
In breast cancer, among the 176 of 293 (60%) cases of invasive primary breast carcinomas with survivin expression, 31% showed exclusive nuclear staining of survivin, 13% showed exclusive cytoplasmic staining and 16% showed both nuclear and cytoplasmic staining.12 These authors indicated that in a multivariate analysis, nuclear expression of survivin was a significant independent prognostic indicator for favorable outcomes of the disease both in relapse-free and overall survival.12 Unfortunately, the black and white IHC images provided in the study do not permit appreciation of the nuclear or cytoplasmic expression of survivin.
In osteosarcoma, 23 of 40 (57.5%) cases showed cytoplasmic expression of survivin, whereas 20 of 40 (50%) cases showed nuclear expression.13 These authors pointed out that nuclear expression of survivin correlated significantly with prolonged survival but cytoplasmic staining showed no correlation with patient outcome.13 It should be pointed out that because we failed to obtain the original reprint, further comments regarding this study are inappropriate.
In the studies of pediatric ependymomas and choroid plexus tumors, it was shown that a strikingly high level of survivin expression is present within normal ependyma and choroid plexus (CP).14 Analysis of corresponding neoplastic tissue in pediatric ependymomas and CP tumors showed that nuclear expression of survivin correlated with morphologic (low) tumor grade, and loss of nuclear expression of survivin was associated with more anaplasia. 14
These authors believed the pattern of survivin expression supports a hypothesis that survivin plays a functional role in normal ependymal growth or neural stem cell differentiation, and abnormally low expression levels of survivin in nuclei may be a marker for more aggressive disease or higher morphologic grade in ependymal and CP tumors.14 On the basis of the IHC images provided in their Figures 1 and 3 in the original publication, however, we feel that the dark brown/blue nuclei in the images of Figure 1A,C may not be truly survivin-positive in nuclei (although these authors indicted in their study that the sc-10811 antisera stains nuclear survivin). This notion is based on: (i) although the images from Figure 1E–H was stained with the NB-500-201K3 antisera, which stain cytoplasmic survivin as indicated in the report, we noted that very dark brown nuclei was obtained in Figure 1E,H, which are similar to that in Figure 1B,D; (ii) our results indicated that the dark brown nuclei in the image of Figure 1B,D are actually not survivin positive (see below). Figure 3B,C did not show nuclear survivin staining (although using sc-10811 antisera), instead they showed cytoplasmic staining; we agree that several nuclei were indeed stained for survivin in Figure 3D with an overall weak survivin staining in cytoplasm. In addition, it is unbelievable that the dark brown nuclei in Figure 3A represent strong nuclear survivin expression although there seems to be cytoplasmic staining of survivin in Figure 3A. We feel that the authors’ interpretation for the nuclear or cytoplasmic expression of survivin in their article requires an alternative approach such as Western blots or a further dilution of the antibodies to confirm their unusual observation. We have to emphasize that this is only our personal view on these IHC images from their original study. Our views may not be entirely correct.
Additional observation
In addition to the reports described above, which presented opposing views on the prognostic value of nuclear expression of survivin in various cancers, 5 additional reports indicated that the expression of survivin can be in nuclei or cytoplasm.15–19 None of these reports, however, was disease outcome correlated with the nuclear or cytoplasmic expression of survivin. Instead, these reports focused on studies of the association of survivin expression (ignoring cytoplasm or nuclei when analyzing their data) in cancer cells with cancer patient outcome.15–19 Below is a summary of these studies.
In the study of survivin expression in cervical squamous tissue (31 normal, 17 low- and 15 high-grade squamous intraepithelial lesions [LSIL, HSIL] and 10 squamous cell carcinomas [SCC]), it was found that nuclear survivin staining was detected in normal mucosa, LSILs and HSILs. Survivin staining intensity increased in cases with morphologic evidence of human papillomavirus (HPV) infection. Cytoplasmic staining was observed in immature squamous metaplasia and in SCC.15 The authors believe that survivin expression in immature metaplastic squamous mucosa may reflect a role for survivin in normal squamous differentiation whereas the histologic correlation between nuclear survivin staining and HPV infection suggests involvement of survivin in HPV-mediated disruption of normal cellular maturation.15 Consistent with the increase of nuclear survivin staining in HPV-infected cells, it was reported previously that the human immunodeficiency virus Type 1 (HIV-1) transcriptionally upregulates survivin expression, which may be actively involved in enhancing cell viability during HIV-1 infection.22 Moreover, it was also reported that survivin require the hepatitis B X-interacting protein (HBXIP), which can interact with the X protein of hepatitis B virus (HBX), as a coactivator to inhibit apoptosis.23 Viral HBX protein interacts with the survivin-HBXIP complex and suppresses caspase activation in a survivin-dependent manner.23 Thus, survivin could be involved in hepatitis B virus-induced hepatocellular carcinogenesis. From this point of view, an alternative potential role for survivin upregulation in HPV-infected cells is to increase the viability of the infected cells to facilitate the production of HPV virus or to facilitate the HPV virus-induced carcinogenesis.
In endometrial carcinomas, studies of 51 specimens (31 endometrial carcinoma and 20 normal endometria) showed that although survivin staining was weakly positive in some normal endometria in the proliferative (0–5.1%) and secretory (0–15.8%) phases, its expression was high in the nucleus or cytoplasm of the endometrial carcinoma cells.16 Scoring on the basis of the percentage of positive cells indicated that survivin expression was significantly associated with PCNA-labeling index, tumor stage, histological grade, the presence of invasion to >1/2 myometrium, and patient survival (p < 0.01, respectively).16 Previously, these authors showed very similar results for survivin expression and the correlation of clinical outcomes using 26 ovarian epithelial carcinomas and 10 benign cystadenomas of ovary.24 We are unable to appreciate the cytoplasmic or nuclear expression of survivin, however, because both reports provided only black and white IHC images.
In the study of 44 NSCLC with Stage I (IA and IB), 31 (24 of 31 is nuclear expression of survivin as well) of 44 (70%) specimens showed cytoplasmic expression of survivin in 10–90% cells, 35 (24 of 35 is cytoplasmic expression of survivin as well) of 44 (80%) showed nuclear immunoreactivity of survivin whereas both were present in 54% (24 of 44). The authors indicated that cytoplasmic immunoreactivity of the 31 specimens (Dr. Falleni, personal communication) correlated with tumor stage.18 These authors indicated that survivin overexpression could be exploited for diagnostic purposes in NSCLC.18
In the study of precancerous and cancerous lesions of the oral cavity, it was shown that survivin was present in 10 of 30 cases (33%) of oral precancerous lesions, and in 15 of 16 cases (94%) of oral precancerous lesions that evolved into full-blown squamous cell carcinoma.19 Therefore, the authors believed that high expression of cytoplasmic or nuclear survivin is an early event during oral carcinogenesis.19 Similarly, in studies of survivin expression in 110 oral squamous cell carcinoma (SCC), one distant and 6 lymph node metastatic lesions, 91 cases (82.7%) of carcinoma and all 7 metastatic cases (100%) were positive for survivin expression. In contrast, normal oral epithelium did not express survivin.25 Patients with low survivin expression had statistically significant longer survival compared to those with high survivin expression (p < 0.05).25 Based on the IHC images provided in these articles, however, the expression of survivin appears primarily in the cytoplasm.
With regard to normal gastric mucosa, survivin expression is seen predominantly in the nuclei of mucosal surface epithelial cells. The authors believe that survivin may play a role in maintaining gastric mucosal integrity and regulating cell renewal in the gastric mucosa.17
Potential pitfalls in determination of nuclear or cytoplasmic localization of survivin by immunohistochemistry
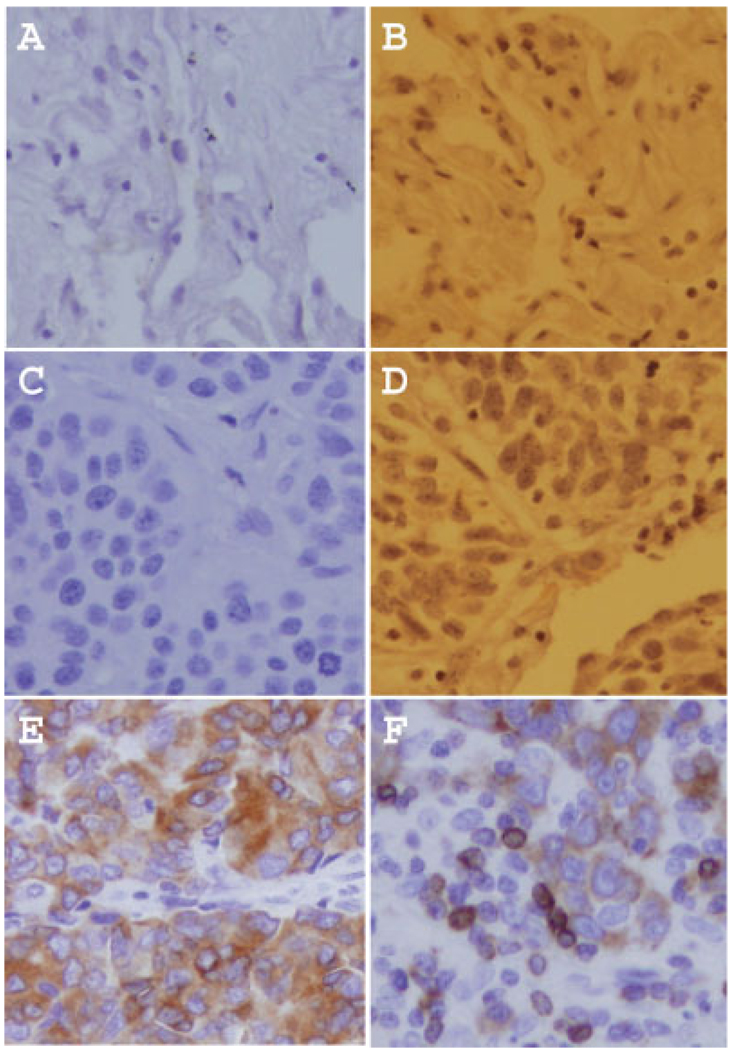
Based on our working experiences with immunohistochemistry (IHC) in the determination of nuclear or cytoplasmic expression of survivin, we feel that IHC results sometimes may lead to misjudgment or misinterpretation of the expression pattern of survivin in normal or cancerous tissues due to the inappropriate processing of tissues or images. We provide representative examples (Fig. 1) to demonstrate that the same tissues without survivin expression (Fig. 1a,b or c,d) could show a dark brown staining (false positive for survivin, Fig. 1b,d) due to inappropriate processing of the tissue or the image. The images in Figure 1a and 1b were from the same non-neoplastic lung tissue without survivin expression. One can easily say “survivin is negative in Fig. 1a” but the brown nuclei in (Fig. 1b) may lead to misjudgment. Similarly, the images in Figure 1c and 1d were from the same neoplastic lung tissue without survivin expression. In contrast, the image in Figure 1e shows the cytoplasmic expression of survivin from one lung neoplastic tissue, and the image in Figure 1f shows both the cytoplasmic and nuclear expression of survivin from another lung neoplastic tissue (Western blots confirmed that lung tissues used for Fig. 1a–d are survivin-negative, and the 2 lung cancerous tissues for Fig. 1e,f are survivin-positive). We feel that researchers should be particularly careful in deriving conclusions from IHC that survivin is expressed in nuclei or cytoplasm in normal or cancerous tissues. This is because, in many tissue-processing conditions for IHC, nuclei could be stained much darker than cytoplasm. We recommend that if a potential problem exists or one is not certain of the IHC results, an alternative approach such as Western blots using selected tissues should be considered for confirmation of the IHC results.
FIGURE 1.
Potential pitfalls in determination of nuclear or cytoplasmic expression of survivin by immunohistochemistry (IHC). (a,b) From the same non-neoplastic lung tissue, show no survivin expression. (c,d) From the same NSCLC tissue, are also negative for survivin. (e) From a NSCLC tissue, shows cytoplasmic expression of survivin. (f) From another NSCLC tissue, displays both nuclear and cytoplasmic expression of survivin.
Conclusion
Survivin seems to exist in 2 subcellular pools (cytoplasmic and nuclear).26 This is consistent with its function in the regulation of both cell viability and cell division.27 One possibility is that the nuclear pool of survivin is involved in promoting cell proliferation in most (if not all) cases whereas the cytoplasmic pool of survivin may participate in controlling cell survival but not cell proliferation. Alternatively, survivin has a number of splicing variants,28,29 which may differ in their subcellular localization and functions with respect to cell survival and cell division. The anti-survivin antibodies available currently recognize them all due to the existence of an identical amino-terminal peptide (73 amino acids) in all survivin variants as well as in survivin. Molecular characterization of the functional mechanism and subcellular localization for survivin and its variants may clarify the function of survivin as well as its variants in cancer cell survival and proliferation. Hopefully, these studies will be done in the coming years.
Acknowledgements
F. Li is currently supported by grants from NIH/NCI (CA109481), Concern Foundation (Beverly Hills, CA), Elsa U. Pardee Foundation (Midland, MI) and Wendy Will Case Cancer Fund, Inc. (Chicago, IL). We would like to thank Dr. H. Scherubl (Universitatsmedizim Berlin, Germany) for sharing data before publication for inclusion in this article.
Grant sponsor: NIH/NCI; Grant number: CA109481; Grant sponsor: Concern Foundation; Grant sponsor: Elsa U. Pardee Foundation; Grant sponsor: Wendy Will Case Cancer Fund, Inc.
References
- 1.Ito T, Shiraki K, Sugimoto K, Yamanaka T, Fujikawa K, Ito M, Takase K, Moriyama M, Kawano H, Hayashida M, Nakano T, Suzuki A. Survivin promotes cell proliferation in human hepatocellular carcinoma. Hepatology. 2000;31:1080–1085. doi: 10.1053/he.2000.6496. [DOI] [PubMed] [Google Scholar]
- 2.Grabowski P, Kuhnel T, Muhr-Wilkenshoff F, Heine B, Stein H, Hopfner M, Germer CT, Scherubl H. Prognostic value of nuclear survivin expression in esophageal squamous cell carcinoma. Br J Cancer. 2003;88:115–119. doi: 10.1038/sj.bjc.6600696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen C, Lohmann CM, Cotsonis G, Lawson D, Santoianni R. Survivin expression in ovarian carcinoma: correlation with apoptotic markers and prognosis. Mod Pathol. 2003;16:574–583. doi: 10.1097/01.MP.0000073868.31297.B0. [DOI] [PubMed] [Google Scholar]
- 4.Moon WS, Tarnawski AS. Nuclear translocation of survivin in hepatocellular carcinoma: a key to cancer cell growth? Hum Pathol. 2003;34:1119–1126. doi: 10.1053/j.humpath.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Martinez A, Bellosillo B, Bosch F, Ferrer A, Marce S, Villamor N, Ott G, Montserrat E, Campo E, Colomer D. Nuclear survivin expression in mantle cell lymphoma is associated with cell proliferation and survival. Am J Pathol. 2004;164:501–510. doi: 10.1016/S0002-9440(10)63140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javle MM, Tan D, Yu J, LeVea CM, Li F, Kuvshinoff BW, Gibbs JF. Nuclear survivin expression predicts poor outcome in cholangiocarcinoma. Hepatogastroenterology. 2004;51:1653–1657. [PubMed] [Google Scholar]
- 7.Lu B, Gonzalez A, Massion PP, Shyr Y, Shaktour B, Carbone DP, Hallahan DE. Nuclear survivin as a biomarker for non-small-cell lung cancer. Br J Cancer. 2004;91:537–540. doi: 10.1038/sj.bjc.6602027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fields AC, Cotsonis G, Sexton D, Santoianni R, Cohen C. Survivin expression in hepatocellular carcinoma: correlation with proliferation, prognostic parameters, and outcome. Mod Pathol. 2004;17:1378–1385. doi: 10.1038/modpathol.3800203. [DOI] [PubMed] [Google Scholar]
- 9.Tringler B, Lehner R, Shroyer AL, Shroyer KR. Immunohistochemical localization of survivin in serous tumors of the ovary. Appl Immunohistochem Mol Morphol. 2004;12:40–43. doi: 10.1097/00129039-200403000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Okada E, Murai Y, Matsui K, Isizawa S, Cheng C, Masuda M, Takano Y. Survivin expression in tumor cell nuclei is predictive of a favorable prognosis in gastric cancer patients. Cancer Lett. 2001;163:109–116. doi: 10.1016/s0304-3835(00)00677-7. [DOI] [PubMed] [Google Scholar]
- 11.Lehner R, Lucia MS, Jarboe EA, Orlicky D, Shroyer AL, McGregor JA, Shroyer KR. Immunohistochemical localization of the IAP protein survivin in bladder mucosa and transitional cell carcinoma. Appl Immunohistochem Mol Morphol. 2002;10:134–138. doi: 10.1097/00129039-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy SM, O’Driscoll L, Purcell R, Fitz-Simons N, McDermott EW, Hill AD, O’Higgins NJ, Parkinson M, Linehan R, Clynes M. Prognostic importance of survivin in breast cancer. Br J Cancer. 2003;88:1077–1083. doi: 10.1038/sj.bjc.6600776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trieb K, Lehner R, Stulnig T, Sulzbacher I, Shroyer KR. Survivin expression in human osteosarcoma is a marker for survival. Eur J Surg Oncol. 2003;29:379–382. doi: 10.1053/ejso.2002.1415. [DOI] [PubMed] [Google Scholar]
- 14.Altura RA, Olshefski RS, Jiang Y, Boue DR. Nuclear expression of Survivin in pediatric ependymomas and choroid plexus tumors correlates with morphologic tumour grade. Br J Cancer. 2003;89:1743–1749. doi: 10.1038/sj.bjc.6601334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frost M, Jarboe EA, Orlicky D, Gianani R, Thompson LC, Enomoto T, Shroyer KR. Immunohistochemical localization of survivin in benign cervical mucosa, cervical dysplasia, and invasive squamous cell carcinoma. Am J Clin Pathol. 2002;117:738–744. doi: 10.1309/6V09-38K3-JQ40-UR50. [DOI] [PubMed] [Google Scholar]
- 16.Takai N, Miyazaki T, Nishida M, Nasu K, Miyakawa I. Survivin expression correlates with clinical stage, histological grade, invasive behavior and survival rate in endometrial carcinoma. Cancer Lett. 2002;184:105–116. doi: 10.1016/s0304-3835(02)00190-8. [DOI] [PubMed] [Google Scholar]
- 17.Chiou SK, Moon WS, Jones MK, Tarnawski AS. Survivin expression in the stomach: implications for mucosal integrity and protection. Biochem Biophys Res Commun. 2003;305:374–379. doi: 10.1016/s0006-291x(03)00724-1. [DOI] [PubMed] [Google Scholar]
- 18.Falleni M, Pellegrini C, Marchetti A, Oprandi B, Buttitta F, Barassi F, Santambrogio L, Coggi G, Bosari S. Survivin gene expression in early-stage non-small cell lung cancer. J Pathol. 2003;200:620–626. doi: 10.1002/path.1388. [DOI] [PubMed] [Google Scholar]
- 19.Lo Muzio L, Pannone G, Leonardi R, Staibano S, Mignogna MD, De Rosa G, Kudo Y, Takata T, Altieri DC. Survivin, a potential early predictor of tumor progression in the oral mucosa. J Dent Res. 2003;82:923–928. doi: 10.1177/154405910308201115. [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa Y, Yamaguchi S, Hasegawa M, Nemoto T, Inoue M, Suzuki K, Hirokawa K, Kitagawa M. Differential expression of survivin in bone marrow cells from patients with acute lymphocytic leukemia and chronic lymphocytic leukemia. Leuk Res. 2004;28:487–494. doi: 10.1016/j.leukres.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki A, Hayashida M, Ito T, Kawano H, Nakano T, Miura M, Akahane K, Shiraki K. Survivin initiates cell cycle entry by the competitive interaction with Cdk4/p16(INK4a) and Cdk2/cyclin E complex activation. Oncogene. 2000;19:3225–3234. doi: 10.1038/sj.onc.1203665. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Y, Roshal M, Li F, Blackett J, Planelles V. Upregulation of survivin by HIV-1 Vpr. Apoptosis. 2003;8:71–79. doi: 10.1023/a:1021653119934. [DOI] [PubMed] [Google Scholar]
- 23.Marusawa H, Matsuzawa S, Welsh K, Zou H, Armstrong R, Tamm I, Reed JC. HBXIP functions as a cofactor of survivin in apoptosis suppression. EMBO J. 2003;22:2729–2740. doi: 10.1093/emboj/cdg263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takai N, Miyazaki T, Nishida M, Nasu K, Miyakawa I. Expression of survivin is associated with malignant potential in epithelial ovarian carcinoma. Int J Mol Med. 2002;10:211–216. [PubMed] [Google Scholar]
- 25.Lo Muzio L, Pannone G, Staibano S, Mignogna MD, Rubini C, Mariggio MA, Procaccini M, Ferrari F, De Rosa G, Altieri DC. Survivin expression in oral squamous cell carcinoma. Br J Cancer. 2003;89:2244–2248. doi: 10.1038/sj.bjc.6601402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fortugno P, Wall NR, Giodini A, O’Connor DS, Plescia J, Padgett KM, Tognin S, Marchisio PC, Altieri DC. Survivin exists in immunochemically distinct subcellular pools and is involved in spindle microtubule function. J Cell Sci. 2002;115:575–585. doi: 10.1242/jcs.115.3.575. [DOI] [PubMed] [Google Scholar]
- 27.Li F. Survivin study: what is the next wave? J Cell Physiol. 2003;197:8–29. doi: 10.1002/jcp.10327. [DOI] [PubMed] [Google Scholar]
- 28.Mahotka C, Wenzel M, Springer E, Gabbert HE, Gerharz CD. Survivin-deltaEx3 and survivin-2B: two novel splice variants of the apoptosis inhibitor survivin with different antiapoptotic properties. Cancer Res. 1999;59:6097–6102. [PubMed] [Google Scholar]
- 29.Badran A, Yoshida A, Ishikawa K, Goi T, Yamaguchi A, Ueda T, Inuzuka M. Identification of a novel splice variant of the human anti-apoptosis gene survivin. Biochem Biophys Res Commun. 2004 doi: 10.1016/j.bbrc.2003.12.178. [DOI] [PubMed] [Google Scholar]