Abstract
This article reviews symptoms and signs of aberrant axon connectivity in humans, and summarizes major human genetic disorders that result, or have been proposed to result, from defective axon guidance. These include corpus callosum agenesis, L1 syndrome, Joubert syndrome and related disorders, horizontal gaze palsy with progressive scoliosis, Kallmann syndrome, albinism, congenital fibrosis of the extraocular muscles type 1, Duane retraction syndrome, and pontine tegmental cap dysplasia. Genes mutated in these disorders can encode axon growth cone ligands and receptors, downstream signaling molecules, and axon transport motors, as well as proteins without currently recognized roles in axon guidance. Advances in neuroimaging and genetic techniques have the potential to rapidly expand this field, and it is feasible that axon guidance disorders will soon be recognized as a new and significant category of human neurodevelopmental disorders.
Mutations in axon guidance receptors, their ligands, and downstream signaling molecules lead to Duane syndrome and various other disorders characterized by errors in muscle innervation.
The human brain is highly organized and contains a myriad of axon tracts that follow precise pathways and make predictable connections. Model organism research has provided tremendous advances in our understanding of the principles and molecules governing axon growth and guidance. Remarkably, however, only a handful of human disorders resulting from primary errors in these processes have been identified.
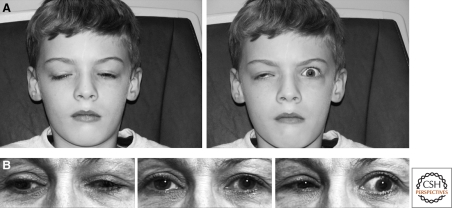
Traditional tools of the physician have limited sensitivity and specificity to detect human disorders of axon guidance. In particular, congenital synkinesis may be the only physical examination finding that has been attributed to such disorders. Synkinesis is the involuntary and pathological contraction of a muscle simultaneously with contraction of the intended muscle, and is typically reported with hand/finger or eye/eyelid movements and confirmed by electrophysiological studies. Mirror movement synkinesis refers to the contraction of homologous hand/finger muscles bilaterally when one attempts to move only one hand (Schott and Wyke 1981). In humans, 75%–90% of corticospinal tract (CST) fibers normally decussate in the lower medulla. Mirror movement synkinesis occurs in several human disorders with pathological, neuroimaging, and/or electrophysiological evidence of reduced CST decussation, including Joubert, Kallmann, and Klippel-Feil syndromes (Vulliemoz et al. 2005; Cincotta and Ziemann 2008). In some individuals with mirror movements, electrophysiological data are also consistent with bilateral engagement of the motor corticies (Leinsinger et al. 1997). Ocular synkinesis refers to aberrant patterns of eye movement and accompanies various congenital cranial dysinnervation disorders (CCDDs) (Gutowski et al. 2003; Engle 2007), including CFEOM, Duane syndrome, and Marcus Gunn jaw-winking phenomenon (Fig. 1). Finger and ocular movements require precise motor control, and errors in innervation of these muscles may be more easily detected than errors in the wiring of larger muscle groups. If true, this suggests that the clinical exam could fail to recognize many guidance errors in both the peripheral and central nervous system.
Figure 1.
Ocular synkinesis. (A) Child with CFEOM1 and Marcus Gunn jaw-winking phenomenon harboring a KIF21A mutation. His superior branch of the oculomotor nerve is hypoplastic/absent, resulting in bilateral ptosis from lack of appropriate innervation of the levator palpebrae superioris (LPS) muscle, and a downward position of each eye from absent innervation of the superior rectus muscle (left). Marcus Gunn phenomenon (right) is seen as the synkinetic elevation of the left eyelid with a subtle change in jaw position associated with a volitional increase in pterygoid muscle tension. This results from aberrant innervation of the LPS by axons from the motor branch of the trigeminal nerve that also innervates the intended ipsilateral pterygoid muscle. (B) Adult with Duane retraction syndrome harboring a CHN1 mutation. Central gaze reveals mild exotropia (middle). On attempted right gaze (left) and left gaze (right), there is limited horizontal excursion with globe retraction and secondary palpebral fissure narrowing of the adducting eye. Globe retraction results from synkinesis of the medial and lateral recti muscles. (A) Modified with permission from Yamada et al. 2005. Copyright © (2005) American Medial Association. All rights reserved. (B) Modified from Demer et al. 2007. Copyright © (2007) Association for Research in Vision and Ophthalmology. All rights reserved.
The physician’s ability to detect disorders of axon guidance has been augmented by classical pathological, radiological, and electrophysiological techniques. Diagnostic radiologic and postmortem neuropathological studies detect overall changes in white matter volume and major abnormalities of axon tracts demarcated from the background such as the corpus callosum, anterior and posterior commissures, optic chiasm, and cerebellar peduncles. Neuropathological studies can also detect absence of axons that normally cross the midline at many points in the brain stem and spinal cord, which are more difficult to visualize by standard magnetic resonance imaging (MRI). Electrophysiological studies such as evoked potentials can reveal aberrant central connections of peripheral sensory or motor nerves.
The genetic disorders with aberrant axon connectivity presented in this article have been defined primarily using traditional approaches described above. Exciting advances in neuroimaging and genetics, however, are revolutionizing the ability to define axon guidance disorders, and it is likely that these syndromes are only the first of an important new category of such human neurodevelopmental disorders. Detailed fiber tract anatomy can now be visualized using noninvasive tractography such as diffusion tensor imaging (DTI) and diffusion spectrum imaging (DSI). These techniques provide tract orientation by determining the anisotropic properties of water diffusion, and can be used to reconstruct the trajectories of fiber systems in three-dimensional space (Tovar-Moll et al. 2007; Wahl et al. 2009). Tractography has successfully confirmed aberrant projections in several of the disorders discussed below (Fig. 2). At the same time, human genetics now provides an unbiased approach to identify the etiologies of disorders with aberrant axon tracts. For some syndromes, animal and in vitro studies have confirmed that the encoded protein has a primary role in axon guidance. For others, such studies reveal a primary role in neuronal specification and/or migration rather than, or in addition to, a role in axon guidance. Finally, some neurodevelopmental disorders without clinical, pathologic, or radiologic evidence of aberrant axon tracts have been found to result from mutations in genes that contribute to axon guidance in animal models.
Figure 2.
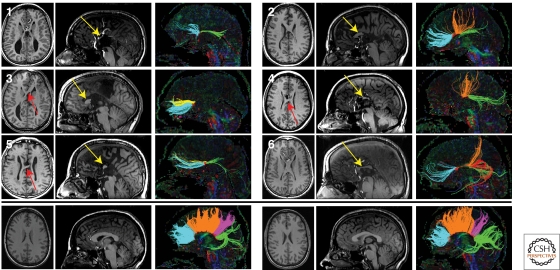
Tractography studies in patients with partial agenesis of the corpus callosum (pACC). T1-weighted anatomic images and DTI tractography of six subjects with pACC (top panels) and two representative controls (bottom panel). Axial (left) and midline sagittal (middle) T1 sections are shown for each subject. Callosal fragments are identified with yellow arrows, and heterotopic fibers visible on T1-weighted images are denoted by red arrows. Midline sagittal DTI color maps are shown with segmented callosal fibers (right). For subjects with pACC, connectivity ranged from anterior frontal connections (subject 3) to only posterior frontal and occipitotemporal connections (subject 4). One individual (subject 5) displayed a discontinuous set of homotopic callosal connections, with anterior frontal and occipitotemporal connectivity without any posterior frontal or parietal connections. Control subjects (bottom panel) display normal callosal morphology and tractography results. Tracts are segmented and colored according to their cortical projections: homotopic anterior frontal, blue; homotopic posterior frontal, orange; homotopic parietal, pink; homotopic occipitotemporal, green; heterotopic left anterior-right posterior, yellow; heterotopic right anterior-left posterior, red. (Reprinted, with permission, from Wahl et al. 2009 [© AJNR].)
The major human genetic disorders that result, or are proposed to result, from defective axon guidance are ordered below from rostral to caudal based on the location of the aberrant axons tracts. These include genetic mutations that alter axon growth cone ligands and receptors, downstream signaling molecules, and axon transport, as well as proteins without currently recognized roles in axon guidance (Fig. 3) (Table 1).
Figure 3.
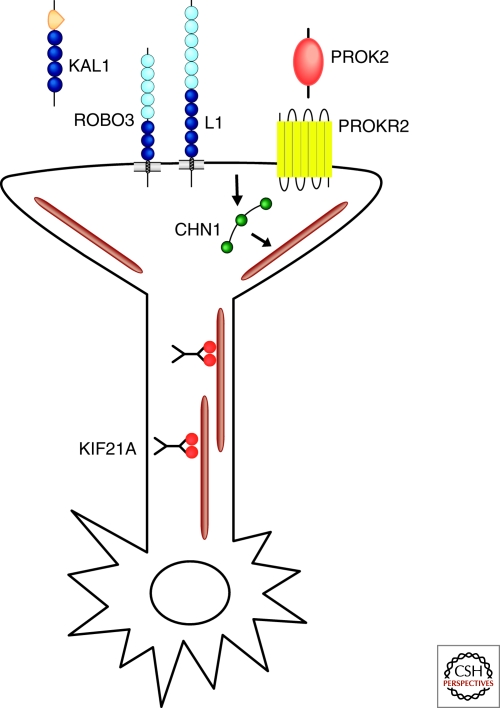
Schematic representation of gene products implicated in human disorders of axon guidance. KAL1 (anosmin) and PROK2 are shown as secreted ligands. ROBO3, L1, and PROKR2 are shown as transmembrane receptors on the growth cone. CHN1 is depicted with 3 green domains (SH2, C1, RacGAP), responding to an unknown activated receptor and altering a microtubule, which is depicted as a brown line. KIF21A dimers are depicted walking down MTs. The OCA/OA and JSRD gene products are not depicted. Note: these gene products are not necessarily expressed in the same neurons or function in the same pathways.
Table 1.
Summary of major human genetic disorders resulting, or hypothesized to result, from errors in axon growth and guidance
| Disorder | L1 | JSRD | HGPPS | KS | Albinism | CFEOM1 | DRS | PTCD |
|---|---|---|---|---|---|---|---|---|
| Inheritance | X-L | AR | AR | X-L, AR | X-L, AR | AD | AD | Sporadic |
| Gene(s) | L1 | AHI1 NPHP1 CEP290 TMEM67 RPGRIP1L ARL13B CC2D2A |
ROBO3 | KAL1 FGFR1 PROKR2 PROK2 CDH7 FGF8 |
TYR OCA2 TYRI1 MATP |
KIF21A | CHN1 | |
| Synkinesis | No | Occurs | No | Occurs (KAL1) | No | Occurs | Occurs | No |
| CC | +/− Thin | Rarely thin | ||||||
| SCP | Thick, Mal-oriented | Small | Mal-oriented | |||||
| SCP-D | Reduced to Absent | Absent | Absent | |||||
| MCP | Small | Small | ||||||
| ICP | Small | Small | ||||||
| CST-P | Flat | Flat | ||||||
| CST-D | +/− Reduced | Reduced to Absent | Absent | Abnormal (KAL1) | ||||
| CPT-D | Reduced | Absent | Absent | |||||
| CN I | Aberrant | |||||||
| CN II | Small | Small | ||||||
| CN II-D | Increased | |||||||
| CN III | Aberrant | +/− Aberrant | ||||||
| CN IV | ||||||||
| CN V | ||||||||
| CN VI | +/− Aberrant | Aberrant | ||||||
| CN VII | Small | |||||||
| CN VIII | Small |
Key: X-L, X-linked; AR, autosomal recessive; AD, autosomal dominant; CC, corpus callosum; SCP, superior cerebellar peduncle; SCP-D, SCP midline decussation; MCP, middle cerebellar peduncle; ICP, inferior cerebellar peduncle; CST-P, corticospinal tract pyramids; CST-D, corticospinal tract midline decussation; CPT-D, central pontine tract decussation; CN I, olfactory nerve; CN II, optic nerve; CN II-D, optic chiasm decussation; CN III, oculomotor nerve; CN VI, abducens nerve; CN VII, facial nerve; CN VIII, vestibulocochlear nerve.
HUMAN GENETIC DISORDERS OF MIDLINE CROSSING
Corpus Callosum Dysgenesis
Corpus callosum (CC) axons normally connect homologous cortical regions in the left and right hemispheres, and are topographically organized along the anteroposterior axis (Hofer and Frahm 2006) (Fig. 2). CC dysgenesis accompanies a multitude of inherited disorders, and results in a clinical spectrum ranging from normal to severe mental retardation. Both complete and partial agenesis (CCA, pCCA) can likely occur secondary to disruption in any one of the multiple steps in callosal development, including primary defects in cell proliferation and migration, axon growth and guidance, and midline glial development (Kamnasaran 2005; Paul et al. 2007). In patients with callosal dysgenesis, axons that fail to cross the midline can form longitudinally oriented bundles of Probst located medial to the lateral ventricles (Probst 1901). Notably, Probst bundles may serve as a relatively specific marker of axon guidance defects, and bundle topography may provide mechanistic insights. Probst bundles are common in patients with CCA without other midline, cortical, or posterior fossa anomalies, and are infrequent in patients with CCA and cortical malformations (Hetts et al. 2006). In some individuals, the bundles maintain a well-organized topography, suggesting that the axons remained responsive to guidance cues despite failure to cross the midline (Utsunomiya et al. 2006; Tovar-Moll et al. 2007). Other individuals have highly variable callosal connectivity, including heterotopic tracts not seen in healthy controls (Fig. 2) (Tovar-Moll et al. 2007; Wahl et al. 2009). It is likely that genetic causes of isolated CCA will be elucidated as imaging advances lead to more precise phenotyping.
L1 Syndrome
The L1 syndrome is a highly variable X-linked neurological disorder resulting from mutations in the L1CAM gene and originally recognized as four distinct entities: X-linked hydrocephalus; MASA (mental retardation, aphasia, shuffling gait, adducted thumbs); X-linked complicated spastic paraplegia type 1; and X-linked corpus callosum agenesis. Based on their genetic homogeneity and phenotypic overlap, these disorders are now considered a single disease entity. Boys with L1 syndrome are mildly to severely affected with a combination of macrocephaly, mental retardation, spastic paraparesis, and thumb flexion deformities. Postmortem and neuroimaging studies may reveal agenesis of the corpus callosum and corticospinal tracts in the absence of cortical malformations (Chow et al. 1985; Halliday et al. 1986; Graf et al. 2000), supporting a defect in axon guidance.
L1 is a transmembrane neural adhesion molecule comprised of six immunoglobulin-like and five fibronectin type III-like extracellular motifs and a short cytoplasmic tail. L1 acts as a short-range axon guidance cue and is highly expressed in developing axons and apical dendrites of cortical neurons, and within migratory axons of the corpus callosum and corticospinal tract (Joosten and Gribnau 1989; Demyanenko et al. 1999). L1 has multiple extracellular binding partners, including β1 integrins, NCAM, TAG-1/axonin-1, contactin, neuropilin-1, and L1 itself, through which it potentiates cell adhesion, provides a mechanical link to the actin cytoskeleton, and serves as a coreceptor to assist in intracellular signal transduction. For example, L1 homophilic binding increases cell adhesion and enhances neuronal migration and neurite outgrowth, whereas binding to neuropilin-1 mediates Sema3A-induced growth cone collapse and axon repulsion (Castellani et al. 2002; Wiencken-Barger et al. 2004; Schmid and Maness 2008). L1 also has multiple intracellular binding partners; L1 links to the actin cytoskeleton through interactions with ankryin or FERM-domain-containing proteins, and the interaction of L1 with AP2 (adaptor protein 2) is required for sorting of L1 to the axonal growth cone (Kamiguchi and Lemmon 1998; Kamiguchi et al. 1998). L1 is also phosphorylated to activate second messenger cascades essential for downstream signaling (Herron et al. 2009).
L1 syndrome results from missense, nonsense, splice site, and frameshift mutations scattered throughout the exons and intron-exon boundaries of the L1CAM gene (Hortsch 1996; De Angelis et al. 2002). The variability of the L1 syndrome phenotype may arise, in part, from differences in how specific mutations disrupt binding of specific partners. Individuals with cytoplasmic mutations or truncations tend to spare extracellular domain activities and have milder phenotypes, whereas extracellular missense mutations and truncations generally correlate with intermediate and severe phenotypes, respectively (Maness and Schachner 2007). Axon guidance defects occur with both extra- and intracellular mutations (Yamasaki et al. 1997; Buhusi et al. 2008). Affected males within a single family can have mild and severe phenotypes, however, highlighting the additional importance of modifying factors (Finckh et al. 2000).
L1 knockout mice recapitulate many aspects of L1 syndrome. These mice can show corpus callosum dysgenesis with Probst bundles, and aberrant retinocollicular, thalamocortical, and corticothalamic projections (Dahme et al. 1997; Cohen et al. 1998; Demyanenko and Maness 2003; Wiencken-Barger et al. 2004). Small uncrossed CST may result from disrupted Sema3A signaling (Castellani et al. 2000; Castellani et al. 2002). Consistent with the human phenotype-genotype correlations, a knockin mouse harboring a human mutation in the L1 cytoplasmic tail has disrupted ankyrin binding and a milder phenotype (Buhusi et al. 2008). Finally, the role of L1CAM in neuronal migration and survival, synaptogenesis, and long-term potentiation may also contribute to the L1 syndrome phenotype (Demyanenko et al. 1999; Dihne et al. 2003; Maness and Schachner 2007; Schmid and Maness 2008).
Joubert Syndrome and Related Disorders (JSRD)
Joubert Syndrome (JS) is an autosomal recessive and genetically heterogeneous trait characterized by combinations of congenital hypotonia, ataxia, abnormal respiratory patterns, mental retardation, social disabilities including autism, and synkinetic mirror movements. JS can also cosegregate with retinopathy, kidney disease, liver disease, polydactyly, obesity, and/or situs inversus. This spectrum is now called Joubert syndrome and related disorders (JSRD) (Joubert et al. 1968; Gleeson et al. 2004; Zaki et al. 2008; Gerdes et al. 2009). Postmortem studies of individuals with genetically undefined JS have revealed severe cerebellar vermian hypoplasia, dysplasia of the deep cerebellar and inferior olivary nuclei, elongation of the caudal midbrain tegmentum, reduction in pontine neurons, and hypoplasia of the solitary, trigeminal, and dorsal column nuclei and tracts. Reduced decussation of the superior cerebellar peduncles (SCP), CST, and central pontine tracts suggests defective axon guidance. In some cases, the CST is split into many small fascicles and the pyramids appeared flat (Joubert et al. 1968; Friede and Boltshauser 1978; Maria et al. 1999; Quisling et al. 1999; Yachnis and Rorke 1999). It is not known whether these crossing defects result from a defect in axon guidance or occur secondary to a defect in cell fate or survival.
In the current era of MRI, the diagnosis of JSRD is dependent on the presence of the “molar tooth” sign, a tooth-like shape on axial images at the level of the midbrain-hindbrain junction that reflects cerebellar vermian hypoplasia, a deepened interpeduncular fossa, and horizontally oriented and thickened SCP (Chance et al. 1999; Maria et al. 1999; Millen and Gleeson 2008). Multiple studies of genetically undefined patients have reported the failure of these mis-oriented SCP fibers to decussate (Padgett et al. 2002; Lee et al. 2005; Widjaja et al. 2006; Spampinato et al. 2008). In one patient with presumed absence of CST decussation, fMRI revealed aberrant bilateral activation of the cerebellar and sensorimotor cortex (Parisi et al. 2004b).
JSRD is genetically heterogeneous, and at least nine loci and seven genes (AHI1, NPHP1, CEP290, TMEM67, RPGRIP1L, ARL13B, and CC2D2A) have been identified to date (Dixon-Salazar et al. 2004; Ferland et al. 2004; Parisi et al. 2004a; Sayer et al. 2006; Arts et al. 2007; Baala et al. 2007; Delous et al. 2007; Cantagrel et al. 2008; Gorden et al. 2008; Noor et al. 2008). Failure of the SCP and CST to decussate has been documented in patients harboring mutations in AHI1, CEP290, and at least two additional JS genes (Poretti et al. 2007).
JS and JSRD are now classified as ciliopathies because the mutated genes encode signal transduction and scaffolding proteins implicated in the function of the primary cilium or its anchoring structure, the basal body (Badano et al. 2006; Gerdes et al. 2009). Several of the proteins interact (Gorden et al. 2008), suggesting that they may all be part of a signaling complex (Millen and Gleeson 2008). Although a role for cilia in axon guidance has not been elucidated, cilia are similar to growth cones in that they sense environmental cues and mediate signals through receptor-dependent pathways such as sonic hedgehog, noncanonical Wnt, and platelet-derived growth factor receptor (Fliegauf et al. 2007; Gerdes et al. 2009). Future studies will determine whether there is a primary axon guidance defect in JSRD and, if so, if this is mediated through ciliary-dependent or potentially ciliary-independent roles of the JSRD genes in neurodevelopment.
Horizontal Gaze Palsy with Progressive Scoliosis (HGPPS)
HGPPS is a clinically and genetically homogeneous disorder in which hindbrain axons fail to cross the midline. Affected individuals are born with restricted horizontal gaze and and develop scoliosis within the first decade of life. HGPPS is an autosomal recessive trait and results from mutations in the ROBO3 gene (Jen et al. 2004). ROBO3 encodes a transmembrane receptor analogous to mouse Rig1/Robo3, with five Ig-like and three fibronectin-like extracellular motifs and three cytoplasmic signaling motifs. Indistinguishable phenotypes result from ROBO3 nonsense, frame-shift, splice-site, or missense mutations spread across the gene, supporting a complete loss of ROBO3 function. Although the disease gene was identified in affected members of consanguineous families harboring homozygous ROBO3 mutations, HGPPS is also present in individuals from nonconsanguineous families harboring compound heterozygous mutations (Chan et al. 2006).
Electrophysiological and neuroimaging studies in HGPPS support absence of decussating axons in the pons and medulla. Somatosensory and motor‐evoked-potential tests reveal ipsilateral (Jen et al. 2004; Haller et al. 2008) or predominantly ipsilateral (Amoiridis et al. 2006) rather than normal contralateral responses, reflecting uncrossed ascending dorsal column-medial lemniscal sensory pathways and descending corticospinal motor pathways. MRI reveals ventral flattening and hypoplasia of the hindbrain, and a butterfly-shaped medulla with a midline cleft (Jen et al. 2004). DTI confirms ipsilateral CST and sensory tracts (Haller et al. 2008), as well as failure of the SCP to decussate, absence of the major crossing fibers in the pons, and small cerebellar peduncles (Sicotte et al. 2006). Functional MRI reveals ipsilateral rather than the normal contralateral activation in the primary motor cortex following motor tasks (Haller et al. 2008). The cortex, corpus callosum, and exiting cranial nerves appear structurally normal (Jen et al. 2004; Bosley et al. 2005; Sicotte et al. 2006; Haller et al. 2008).
Robo3 is a divergent member of the Robo family of axon guidance molecules, and studies of the Robo3−/− (Rig-1−/−) mouse established that Robo3 is essential for midline crossing of hindbrain and spinal cord commissural (Sabatier et al. 2004) and precerebellar axons (Marillat et al. 2004). Robo3 is also necessary for midline crossing of precerebellar neurons (Marillat et al. 2004), and defects in neuronal migration may also contribute to the HGPPS phenotype. Robo3 alternative splicing produces two functionally antagonistic isoforms with distinct carboxy termini (Chen et al. 2008). Robo3.1 inhibits the responsiveness of commissural axons to Slit repellents and is present on commissural axons before and during midline crossing, whereas Robo3.2 is Slit-responsive and appears on the growth cone postcrossing to block re-crossing (Chen et al. 2008). HGPPS mutations reported to date alter nucleotides common to both isoforms.
Although the mechanism by which loss of ROBO3 leads to the HGPPS phenotype is not defined, the gaze palsy may result from errors in axon connectivity into and out of the abducens nucleus. The normal contralateral inputs onto the abducens nucleus from the pontine paramedian reticular formation and vestibular nuclei are predicted to be ipsilateral in HGPPS, and this would likely alter the firing patterns of motor and internuclear neurons. Axons of the abducens internuclear neurons would also fail to cross the midline via the medial longitudinal fasciculus to synapse on medial rectus motor neurons in the contralateral oculomotor nucleus, further perturbing horizontal gaze. Although the etiology of scoliosis is also speculative, HGPPS provides the first genetic evidence of a neurogenic cause for this disability. Finally, individuals with HGPPS perform normally on neuropsychological testing and have normal fine motor control without mirror movements (Amoiridis et al. 2006), suggesting that the pathologically ipsilateral corticospinal axons find their appropriate target, albeit on the wrong side.
HUMAN GENETIC DISORDERS OF CRANIAL NERVE GUIDANCE
Kallmann Syndrome
Individuals with Kallmann syndrome (KS) have congenital anosmia (lack of sense of smell) and hypogonadotropic hypogonadism (HH). In HH, the hypothalamus fails to release gonadotropin-releasing hormone (GnRH) that normally stimulates the pituitary gland to release sex hormones. Often, the lack of smell goes unnoticed and individuals with KS are not diagnosed until they fail to undergo secondary sexual development during their teenage years. It is proposed that errors in growth and guidance of olfactory axons can result in KS.
Both olfactory sensory neurons and GnRH neurons are born in the olfactory placode of the developing nose. Olfactory sensory axons then extend their growth cones through the cribriform plate into the central nervous system where they synapse with second-order mitral neurons within the olfactory bulb glomeruli. Mitral axons then extend in the olfactory tract to the piriform cortex. GnRH neurons migrate across the cribriform plate into the olfactory bulb anlage along a path that colocalizes with olfactory sensory axons (Schwanzel-Fukuda and Pfaff 1989; Wray et al. 1989), then migrate on to the hypothalamus, where they extend their axons to the median eminence, enabling neurosecretion into the hypophyseal portal circulation (Schwanzel-Fukuda and Pfaff 1989; Wray et al. 1989; Kim et al. 2008).
The only KS neuropathology report is of a 19-week male fetus with a family history of X-linked KS (Schwanzel-Fukuda et al. 1989). Although his olfactory axons passed through the cribriform plate, they ended prematurely in a tangle within the meninges, failing to make contact with the brain. Olfactory bulbs and tracts were absent, consistent with the observation that olfactory bulb development requires innervation from olfactory sensory neurons (Graziadei and Monti Graziadei 1986). GnRH expressing cells were not in their appropriate position in the hypothalamus, but instead were found in the nose, along the path of the olfactory axons, and within the tangle of axons in the meninges. Thus, at least the X-linked form of KS may result from defective olfactory axon guidance, with secondary failure in GnRH neuronal migration and olfactory bulb formation.
KS is genetically heterogeneous and can be inherited as an X-linked, autosomal dominant, and possibly autosomal recessive trait. Because affected individuals are often infertile without therapy, pedigrees tend to be small and two-thirds of cases are sporadic. Despite these challenges, six KS genes have been reported, accounting for approximately 30% of cases. These KS genes encode transmembrane receptors and ligands that may be important for growth cone guidance. Some KS proteins also interact with one other and with heparan sulfate proteoglycans to amplify downstream signaling pathways (Hu et al. 2003; LeCouter et al. 2003; Gonzalez-Martinez et al. 2004). Consistent with this, KS can be oligogenic, resulting from combinations of mutations in more than one KS gene (Dode et al. 2006; Pitteloud et al. 2007a; Canto et al. 2009).
X-linked KS is caused by loss-of-function mutations in KAL1 (Franco et al. 1991; Legouis et al. 1991), which is expressed in developing olfactory placode and olfactory bulb (Gonzalez-Martinez et al. 2004). Two-thirds of males harboring KAL1 mutations also have mirror movements and enlarged, aberrant ipsilateral CSTs (Quinton et al. 1996; Mayston et al. 1997; Krams et al. 1999; Quinton et al. 2001), supporting a role of KAL1 in guidance of CST as well as olfactory axons. KAL1 encodes the secreted glycoprotein anosmin-1 (Hardelin et al. 1999), which has cell adhesion, neurite outgrowth, and axon guidance and branch-promoting activities in vitro (Rugarli et al. 1996; Soussi-Yanicostas et al. 1996; Soussi-Yanicostas et al. 1998; Hardelin et al. 1999; Robertson et al. 2001; Soussi-Yanicostas et al. 2002; Gonzalez-Martinez et al. 2004). Direct studies of the role of KAL1 in axon guidance have been limited by the absence of Kal1 in the mouse genome.
KAL3 and KAL4 encode prokineticin-2 receptor and its ligand, PROKR2 and PROK2, respectively (Dode et al. 2006). PROK2 is expressed in the developing olfactory bulb (Ng et al. 2005), whereas the G-protein coupled receptor PROKR2 is expressed along the path of the olfactory axons and migrating GnRH neurons. PROKR2−/− and PROK2−/− mice have stalled olfactory sensory axons that fail to enter the CNS, arrested GnRH neuron migration, olfactory bulb hypoplasia, and reproductive system atrophy (Ng et al. 2005; Matsumoto et al. 2006; Pitteloud et al. 2007b). It is not yet known whether olfactory sensory neurons express PROKR2 on their growth cones and are attracted toward PROK2 in the olfactory bulb anlage (Pitteloud et al. 2007b).
KAL6 and KAL2 encode fibroblast growth factor receptor 1 and its ligand, FGFR1 and FGF8, respectively (Dode et al. 2003; Falardeau et al. 2008). Following conditional removal of Fgfr1 from mouse telencephalon and olfactory epithelium, the olfactory bulb fails to develop, but olfactory sensory axons successfully enter the forebrain (Hebert et al. 2003). Thus, these genetic forms of KS may not result from errors in olfactory sensory axon development. Notably, however, FGFR1 signaling promotes GnRH neurite outgrowth and may be necessary for GnRH axons to target the median eminence (Gill et al. 2004; Tsai et al. 2005; Gill and Tsai 2006).
Albinism
Individuals with oculocutaneous albinism (OCA) have absent melanin pigment in their eyes, hair, and skin, whereas males with ocular albinism (OA) lack eye pigment only. Individuals with either OCA or OA have increased contralateral and reduced ipsilateral projecting axons at the optic chiasm as well as hypopigmentation of the retinal pigment epithelium and iris, foveal hypoplasia, loss of binocular vision, reduced visual acuity, and nystagmus.
Melanin is synthesized within intracellular melanosomes, and in the eye is present in optic cup derived retinal pigment epithelial (RPE) cells and neural crest derived melanocytes of the iris. X-linked OA results from mutations in OA1, which encodes a G protein-coupled receptor on the melanosome membrane. Autosomal recessive OCA genes include TYR, encoding the enzyme tyrosinase that catalyzes rate-limiting steps in the melanin biosynthetic pathway; OCA2, encoding a protein regulating melanosome pH; TYRI1, encoding a tyrosinase-related catalase; and MATP, encoding a transporter mediating melanin synthesis.
During development, retinal ganglion cell (RGC) axons extend toward the optic disc, turn posterior, and exit the eye as the optic nerve (cranial nerve II). Within the middle cranial fossa, the left and right optic nerves join to form the optic chiasm where approximately 40% of the axons cross the midline. Both ipsilateral and contralateral axons then continue posterior to terminate in the lateral geniculate nucleus of the thalamus. There is little direct evidence in albinism for a primary defect in the guidance of axons at the chiasm (Colello and Jeffery 1991; Marcus et al. 1996; Jeffery and Erskine 2005), and instead there may be a defect in cell fate.
The retina contains two populations of sharply demarcated RGC; those positioned in the temporal retina extend axons that project ipsilateral, whereas those positioned nasally extend axons that decussate at the chiasm. In mature albino mammals, this line of demarcation is shifted toward the temporal periphery, corresponding to a decrease in ipsilateral projecting RGC axons (Guillery et al. 1995; Petros et al. 2008). In mouse, RPE melanin formation begins at E11 just before the onset of neuroblast division and proceeds in a graded fashion, and the amount of melanin in the RPE correlates with the percent of ipsilateral axons at the optic chiasm (Guillery et al. 1995; Ray et al. 2007; Petros et al. 2008). This has led to the hypothesis that pigment formation provides positional information to RGC neurons, committing them to ipsilateral or contralateral projecting axons (Ray et al. 2007). Albino mice have disorganized RGC neurons with perturbed proliferation and a reduced number of cells expressing Zic2, the zinc finger transcription factor that directs the uncrossed retinal projection (Rachel et al. 2002; Herrera et al. 2003; Williams et al. 2003; Tibber et al. 2006; Garcia-Frigola et al. 2008; Petros et al. 2008). Thus, although the precise role of melanin in RGC and chiasm development remains to be determined, the reduction in ipsilateral-projecting axons in albinism may result from a developmental shift in RGC specification and fate, rather than a primary defect in axon guidance.
Congenital Fibrosis of the Extraocular Muscles Type I
Congenital fibrosis of the extraocular muscles type 1 (CFEOM1) is a complex strabismus syndrome categorized as one of the congenital cranial dysinnervation disorders (CCDD) (Gutowski et al. 2003; Engle 2007). Affected individuals are born with bilateral blepharoptosis (drooping eyelids) and strabismus, and absence of fusion and binocular vision. The eyes look down at rest and cannot be elevated, whereas horizontal movement can range from absent to full. Affected individuals often have ocular synkinesis, including synergistic convergence, synergistic divergence, and Marcus Gunn jaw‐winking phenomenon (Fig. 1A).
Postmortem examination of an individual with CFEOM1 harboring the common KIF21A mutation (see below) revealed absence of the superior division of the oculomotor nerve and marked hypoplasia of the muscles this division innervates, the levator palpebrae superioris and superior rectus, that elevate the eyelid and eye, respectively. The oculomotor inferior division and abducens nerves were also small (Engle et al. 1997). Although the autopsy technique did not permit identification of aberrant innervation, subsequent MR imaging confirmed the autopsy findings and noted misinnervation of the lateral rectus muscle by an oculomotor nerve branch (Demer et al. 2005). Despite this strong clinical and radiological data supporting aberrant innervation in CFEOM1, however, it is not yet known if the primary defect is that of axon growth and guidance, pruning, or motor neuron survival.
CFEOM1 is inherited as an autosomal dominant trait and results from heterozygous mutations in KIF21A, which encodes a kinesin motor (Yamada et al. 2003). The pattern of KIF21A mutations suggests that CFEOM1 results from an alteration in, rather than haploinsufficiency of, KIF21A function. Eighty mutation-positive patients of multiple ethnicities reported to date harbor only 11 unique missense mutations, which are often de novo, and 75% harbor 2860C>T (R954W). These mutations alter only seven of the 1675 amino acids in KIF21A, of which five are located in the third coiled-coil domain of the KIF21A stalk and two in the motor domain (Yamada et al. 2003; Ali et al. 2004; Tiab et al. 2004; Lin et al. 2005; Shimizu et al. 2005; Yamada et al. 2005; Zhang et al. 2006; Chan et al. 2007; Lu et al. 2008; Flaherty et al. 2009; Rudolph et al. 2009).
Kif21a encodes an anterograde kinesin motor that is broadly expressed in rodent neuronal cell bodies, axons, and dendrites (Marszalek et al. 1999), and may interact with Big1 and Kank1 in vitro (Shen et al. 2008; Kakinuma and Kiyama 2009). Additional studies of wildtype and mutant KIF21A are necessary to determine the role of axon guidance in the etiology of CFEOM1.
Duane Retraction Syndrome
Duane retraction syndrome (DRS) is a CCDD affecting 1:1000 individuals. Affected individuals have restricted horizontal gaze greatest with attempted abduction (movement away from the midline), and ocular synkinesis resulting in globe retraction with attempted adduction (movement toward the midline) (Fig. 1B). Postmortem examinations of individuals with DRS found absence of abducens motor neurons and nerve, and aberrant innervation of the lateral rectus muscle by axons of the oculomotor nerve (Hotchkiss et al. 1980; Miller et al. 1982). Thus, when an affected individual attempts to adduct their eye, both the intended medial rectus and the pathologically innervated lateral rectus muscles contract, resulting in retraction of the eyeball into the orbit (Duane 1905). Cocontraction of the two muscles can be recorded by electromyography (Gunderson and Zeavin 1956; Huber 1984).
Genetic studies of rare families segregating autosomal dominant DRS led to the identification of CHN1 as a DRS gene (Miyake et al. 2008). Individuals harboring CHN1 mutations have a higher incidence of vertical movement abnormalities and bilateral eye involvement when compared to individuals with nonfamilial DRS (Chung et al. 2000; Demer et al. 2007). Consistent with this, MRI of individuals harboring CHN1 mutations can reveal hypoplasia of the oculomotor nerve and oculomotor-innervated muscles in addition to the expected abducens nerve hypoplasia and aberrant lateral rectus innervation (Demer et al. 2007). Together, these findings suggest that human CHN1 mutations alter the development of abducens and, to a lesser extent, oculomotor axons.
DRS CHN1 mutations identified to date are missense, and result in amino acid substitutions that alter α2-chimaerin, a Rac guanosine triphosphatase‐activating signaling protein containing a RacGAP domain, a C1 domain that binds to diacylglycerol, and an amino-terminal SH2 domain (Hall et al. 1993; Hall et al. 2001). α2-chimaerin is expressed widely in developing neurons of rodent (Hall et al. 1993; Hall et al. 2001) and human (Miyake et al. 2008). It serves as an effector for axon guidance, and mice with loss of α2-chimaerin have elevated RacGTP levels, disrupted ephrin/EphA4 signaling, and pathological midline re-crossing of corticospinal tract axons within the spinal cord (Brown et al. 2004; Beg et al. 2007; Iwasato et al. 2007; Shi et al. 2007; Wegmeyer et al. 2007). In contrast, human DRS CHN1 mutations are gain-of-function, resulting in hyperactive α2-chimaerin and lower RacGTP levels through several mechanisms including enhanced α2-chimaerin translation to the membrane (Miyake et al. 2008). Moreover, in ovo overexpression of mutant α2-chimaerin results in stalling, aberrant branching, and defasciculation of the oculomotor nerve (Miyake et al. 2008). The axon guidance molecules upstream and signaling pathway downstream of α2-chimaerin in the developing abducens and oculomotor axons are not yet known. Understanding why corticospinal and ocular axons are vulnerable to down- and up-regulation of this widely expressed signaling molecule may provide new insights into the regulation of axon guidance.
Pontine Tegmental Cap Dysplasia
Pontine tegmental cap dysplasia (PTCD) is a cerebellar, brain stem, and cranial nerve malformation syndrome (Maeoka et al. 1997; Ouanounou et al. 2005; Barth et al. 2007; Jissendi-Tchofo et al. 2009). The 12 affected children described to date have mild to severe developmental delay, ataxia, and a combination of restricted horizontal eye movements, ocular apraxia, facial weakness, deafness, and swallowing and feeding impairments. Neuroimaging reveals pontine hypoplasia with ventral flattening and dorsal protrusion of tissue into the fourth ventricle (“tegmental cap”). Cerebellar vermian hypoplasia and elongated and laterally misplaced SCP result in a modified molar-tooth sign. The middle and inferior cerebellar peduncles and cranial nerves VII and VIII are small. DTI reveals failure of the SCP, MCP, and axons of the pontine nuclei to decussate, and defines the tegmental cap as an ectopic dorsal transverse fiber bundle (Barth et al. 2007; Jissendi-Tchofo et al. 2009). Thus, PTCD represents an intriguing new human axon guidance phenotype that shares features with Joubert, HGPPS, and the CCDD syndromes. The reported children have neither a positive family history nor consanguineous parents, so it remains to be proved that PTCD is genetic. It is plausible, however, that it results from de novo dominant mutations or recessive mutations in an unidentified gene.
CONCLUDING REMARKS
Only a handful of human disorders have been purported to result from defects in axon guidance and, in most cases, much work remains to understand their molecular etiologies. It seems eminent, however, that advances in neuroimaging and electrophysiology will provide the necessary tools to accurately recognize new patterns of aberrant axon connectivity and permit ascertainment of phenotypically homogeneous patient cohorts for genetic study. Continued advances in genetic linkage analysis, association studies, and next-generation sequencing will then lead to identification of genetic variants among these cohorts that cause, or increase susceptibility to, defects in axon guidance. Additional clinical symptoms and signs resulting from defects in axon guidance may also become apparent. For example, it is intriguing to speculate whether synesthesia, in which a stimulus in one sensory modality triggers an automatic and consistent response in another modality, is a central nervous system parallel of synkinesis (Mattingley 2009).
The combination of these rapidly advancing fields may lead to the definition of more subtle guidance defects and the determination of their potential contribution to human disease, including neurodevelopmental and psychiatric disorders. Hints of advances to come include a recent genetic study of synesthesia (Asher et al. 2009), as well as the association of variants of AHI1 with autism and schizophrenia (Amann-Zalcenstein et al. 2006; Ingason et al. 2007; Alvarez Retuerto et al. 2008), of ROBO3 with autism (Anitha et al. 2008), of ROBO1 with dyslexia (Hannula-Jouppi et al. 2005), and of L1 with schizophrenia and major depression (Kurumaji et al. 2001; Laifenfeld et al. 2005). Finally, one can speculate on the contribution of variable axon guidance and connectivity to the normal spectrum of human cognition and behavior, and to the brain’s default network (Buckner et al. 2008).
ACKNOWLEDGMENTS
I thank members of my laboratory and many colleagues for ideas and suggestions relating to this article, Drs. Pratik Mukherjee and Elliott Sherr for their permission to reproduce figure 2, and National Institutes of Health (EY12498, EY13583, EY15298) and Howard Hughes Medical Institute for financial support.
Footnotes
Editors: Marc Tessier-Lavigne and Alex L. Kolodkin
Additional Perspectives on Neuronal Guidance available at www.cshperspectives.org
REFERENCES
- Ali M, Venkatesh C, Ragunath A, Kumar A 2004. Mutation analysis of the KIF21A gene in an Indian family with CFEOM1: Implication of CpG methylation for most frequent mutations. Ophthalmic Genet 25:247–255 [DOI] [PubMed] [Google Scholar]
- Alvarez Retuerto AI, Cantor RM, Gleeson JG, Ustaszewska A, Schackwitz WS, Pennacchio LA, Geschwind DH 2008. Association of common variants in the Joubert syndrome gene (AHI1) with autism. Hum Mol Genet 17:3887–3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann-Zalcenstein D, Avidan N, Kanyas K, Ebstein RP, Kohn Y, Hamdan A, Ben-Asher E, Karni O, Mujaheed M, Segman RH, et al. 2006. AHI1, a pivotal neurodevelopmental gene, and C6orf217 are associated with susceptibility to schizophrenia. Eur J Hum Genet 14:1111–1119 [DOI] [PubMed] [Google Scholar]
- Amoiridis G, Tzagournissakis M, Christodoulou P, Karampekios S, Latsoudis H, Panou T, Simos P, Plaitakis A 2006. Patients with horizontal gaze palsy and progressive scoliosis due to ROBO3 E319K mutation have both uncrossed and crossed central nervous system pathways and perform normally on neuropsychological testing. J Neurol Neurosurg Psychiatry 77:1047–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anitha A, Nakamura K, Yamada K, Suda S, Thanseem I, Tsujii M, Iwayama Y, Hattori E, Toyota T, Miyachi T, et al. 2008. Genetic analyses of roundabout (ROBO) axon guidance receptors in autism. Am J Med Genet B Neuropsychiatr Genet 147B:1019–1027 [DOI] [PubMed] [Google Scholar]
- Arts HH, Doherty D, van Beersum SE, Parisi MA, Letteboer SJ, Gorden NT, Peters TA, Marker T, Voesenek K, Kartono A, et al. 2007. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nat Genet 39:882–888 [DOI] [PubMed] [Google Scholar]
- Asher JE, Lamb JA, Brocklebank D, Cazier JB, Maestrini E, Addis L, Sen M, Baron-Cohen S, Monaco AP 2009. A whole-genome scan and fine-mapping linkage study of auditory-visual synesthesia reveals evidence of linkage to chromosomes 2q24, 5q33, 6p12, and 12p12. Am J Hum Genet 84:279–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baala L, Romano S, Khaddour R, Saunier S, Smith UM, Audollent S, Ozilou C, Faivre L, Laurent N, Foliguet B, et al. 2007. The Meckel-Gruber syndrome gene, MKS3, is mutated in Joubert syndrome. Am J Hum Genet 80:186–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badano JL, Mitsuma N, Beales PL, Katsanis N 2006. The ciliopathies: An emerging class of human genetic disorders. Annu Rev Genomics Hum Genet 7:125–148 [DOI] [PubMed] [Google Scholar]
- Barth PG, Majoie CB, Caan MW, Weterman MA, Kyllerman M, Smit LM, Kaplan RA, Haas RH, Baas F, Cobben JM, et al. 2007. Pontine tegmental cap dysplasia: A novel brain malformation with a defect in axonal guidance. Brain 130:2258–2266 [DOI] [PubMed] [Google Scholar]
- Beg AA, Sommer JE, Martin JH, Scheiffele P 2007. α2-chimaerin is an essential epha4 effector in the assembly of neuronal locomotor circuits. Neuron 55:768–778 [DOI] [PubMed] [Google Scholar]
- Bosley TM, Salih MA, Jen JC, Lin DD, Oystreck D, Abu-Amero KK, MacDonald DB, al Zayed Z, al Dhalaan H, Kansu T, et al. 2005. Neurologic features of horizontal gaze palsy and progressive scoliosis with mutations in ROBO3. Neurology 64:1196–1203 [DOI] [PubMed] [Google Scholar]
- Brown M, Jacobs T, Eickholt B, Ferrari G, Teo M, Monfries C, Qi RZ, Leung T, Lim L, Hall C 2004. α2-chimaerin, cyclin-dependent kinase 5/p35, and its target collapsin response mediator protein-2 are essential components in semaphorin 3A-induced growth-cone collapse. J Neurosci 24:8994–9004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL 2008. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38 [DOI] [PubMed] [Google Scholar]
- Buhusi M, Schlatter MC, Demyanenko GP, Thresher R, Maness PF 2008. L1 interaction with ankyrin regulates mediolateral topography in the retinocollicular projection. J Neurosci 28:177–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantagrel V, Silhavy JL, Bielas SL, Swistun D, Marsh SE, Bertrand JY, Audollent S, Attie-Bitach T, Holden KR, Dobyns WB, et al. 2008. Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am J Hum Genet 83:170–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto P, Munguia P, Soderlund D, Castro JJ, Mendez JP 2009. Genetic analysis in patients with Kallmann syndrome: Coexistence of mutations in prokineticin receptor 2 and KAL1. J Androl 30:41–45 [DOI] [PubMed] [Google Scholar]
- Castellani V, Chedotal A, Schachner M, Faivre-Sarrailh C, Rougon G 2000. Analysis of the L1-deficient mouse phenotype reveals cross-talk between Sema3A and L1 signaling pathways in axonal guidance. Neuron 27:237–249 [DOI] [PubMed] [Google Scholar]
- Castellani V, De Angelis E, Kenwrick S, Rougon G 2002. Cis and trans interactions of L1 with neuropilin-1 control axonal responses to semaphorin 3A. EMBO J 21:6348–6357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WM, Andrews C, Dragan L, Fredrick D, Armstrong L, Lyons C, Geraghty MT, Hunter DG, Yazdani A, Traboulsi EI, et al. 2007. Three novel mutations in KIF21A highlight the importance of the third coiled-coil stalk domain in the etiology of CFEOM1. BMC Gen 8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W-M, Traboulsi E, Arthur B, Friedman N, Andrews C, Engle E 2006. Horizontal gaze palsy with progressive scoliosis can result from compound heterozygous mutations in ROBO3. J Med Genet 43:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance PF, Cavalier L, Satran D, Pellegrino JE, Koenig M, Dobyns WB 1999. Clinical nosologic and genetic aspects of Joubert and related syndromes. J Child Neurol 14:660–666; discussion 669–672 [DOI] [PubMed] [Google Scholar]
- Chen Z, Gore BB, Long H, Ma L, Tessier-Lavigne M 2008. Alternative splicing of the Robo3 axon guidance receptor governs the midline switch from attraction to repulsion. Neuron 58:325–332 [DOI] [PubMed] [Google Scholar]
- Chow CW, Halliday JL, Anderson RM, Danks DM, Fortune DW 1985. Congenital absence of pyramids and its significance in genetic diseases. Acta Neuropathol 65:313–317 [DOI] [PubMed] [Google Scholar]
- Chung M, Stout JT, Borchert MS 2000. Clinical diversity of hereditary Duane’s retraction syndrome. Ophthalmology 107:500–503 [DOI] [PubMed] [Google Scholar]
- Cincotta M, Ziemann U 2008. Neurophysiology of unimanual motor control and mirror movements. Clin Neurophysiol 119:744–762 [DOI] [PubMed] [Google Scholar]
- Cohen NR, Taylor JS, Scott LB, Guillery RW, Soriano P, Furley AJ 1998. Errors in corticospinal axon guidance in mice lacking the neural cell adhesion molecule L1. Curr Biol 8:26–33 [DOI] [PubMed] [Google Scholar]
- Colello RJ, Jeffery G 1991. Evaluation of the influence of optic stalk melanin on the chiasmatic pathways in the developing rodent visual system. J Comp Neurol 305:304–312 [DOI] [PubMed] [Google Scholar]
- Dahme M, Bartsch U, Martini R, Anliker B, Schachner M, Mantei N 1997. Disruption of the mouse L1 gene leads to malformations of the nervous system. Nat Genet 17:346–349 [DOI] [PubMed] [Google Scholar]
- De Angelis E, Watkins A, Schafer M, Brummendorf T, Kenwrick S 2002. Disease-associated mutations in L1 CAM interfere with ligand interactions and cell-surface expression. Hum Mol Genet 11:1–12 [DOI] [PubMed] [Google Scholar]
- Delous M, Baala L, Salomon R, Laclef C, Vierkotten J, Tory K, Golzio C, Lacoste T, Besse L, Ozilou C, et al. 2007. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet 39:875–881 [DOI] [PubMed] [Google Scholar]
- Demer JL, Clark RA, Engle EC 2005. Magnetic resonance imaging evidence for widespread orbital dysinnervation in congenital fibrosis of extraocular muscles due to mutations in KIF21A. Invest Ophthalmol Vis Sci 46:530–539 [DOI] [PubMed] [Google Scholar]
- Demer JL, Clark RA, Lim KH, Engle EC 2007. Magnetic resonance imaging evidence for widespread orbital dysinnervation in dominant duane’s retraction syndrome linked to the DURS2 locus. Invest Ophthalmol Vis Sci 48:194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyanenko GP, Maness PF 2003. The L1 cell adhesion molecule is essential for topographic mapping of retinal axons. J Neurosci 23:530–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyanenko GP, Tsai AY, Maness PF 1999. Abnormalities in neuronal process extension, hippocampal development, and the ventricular system of L1 knockout mice. J Neurosci 19:4907–4920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dihne M, Bernreuther C, Sibbe M, Paulus W, Schachner M 2003. A new role for the cell adhesion molecule L1 in neural precursor cell proliferation, differentiation, and transmitter-specific subtype generation. J Neurosci 23:6638–6650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon-Salazar T, Silhavy JL, Marsh SE, Louie CM, Scott LC, Gururaj A, Al-Gazali L, Al-Tawari AA, Kayserili H, Sztriha L, et al. 2004. Mutations in the AHI1 gene, encoding jouberin, cause Joubert syndrome with cortical polymicrogyria. Am J Hum Genet 75:979–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dode C, Levilliers J, Dupont JM, De Paepe A, Le Du N, Soussi-Yanicostas N, Coimbra RS, Delmaghani S, Compain-Nouaille S, Baverel F, et al. 2003. Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat Genet 33:463–465 [DOI] [PubMed] [Google Scholar]
- Dode C, Teixeira L, Levilliers J, Fouveaut C, Bouchard P, Kottler ML, Lespinasse J, Lienhardt-Roussie A, Mathieu M, Moerman A, et al. 2006. Kallmann syndrome: Mutations in the genes encoding prokineticin-2 and prokineticin receptor-2. PLoS Genet 2:e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duane A 1905. Congenital deficiency of abduction, associated with impairment of adduction, retraction movements, contraction of the palpebral fissure and oblique movements of the eye. Arch Ophthalmol 34:133–159 [DOI] [PubMed] [Google Scholar]
- Engle EC 2007. Oculomotility disorders arising from disruptions in brainstem motor neuron development. Arch Neurol 64:633–637 [DOI] [PubMed] [Google Scholar]
- Engle EC, Goumnerov BC, McKeown CA, Schatz M, Johns DR, Porter JD, Beggs AH 1997. Oculomotor nerve and muscle abnormalities in congenital fibrosis of the extraocular muscles. Ann Neurol 41:314–325 [DOI] [PubMed] [Google Scholar]
- Falardeau J, Chung WC, Beenken A, Raivio T, Plummer L, Sidis Y, Jacobson-Dickman EE, Eliseenkova AV, Ma J, Dwyer A, et al. 2008. Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice. J Clin Invest 118:2822–2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferland RJ, Eyaid W, Collura RV, Tully LD, Hill RS, Al-Nouri D, Al-Rumayyan A, Topcu M, Gascon G, Bodell A, et al. 2004. Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat Genet 36:1008–1013 [DOI] [PubMed] [Google Scholar]
- Finckh U, Schroder J, Ressler B, Veske A, Gal A 2000. Spectrum and detection rate of L1CAM mutations in isolated and familial cases with clinically suspected L1-disease. Am J Med Genet 92:40–46 [DOI] [PubMed] [Google Scholar]
- Flaherty MP, Balachandran C, Jamieson R, Engle EC 2009. Congenital fibrosis of the extraocular muscles type 1, distinctive conjunctival changes and intrapapillary disc colobomata. Ophthalmic Genet 30:91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegauf M, Benzing T, Omran H 2007. When cilia go bad: Cilia defects and ciliopathies. Nat Rev Mol Cell Biol 8:880–893 [DOI] [PubMed] [Google Scholar]
- Franco B, Guioli S, Pragliola A, Incerti B, Bardoni B, Tonlorenzi R, Carrozzo R, Maestrini E, Pieretti M, Taillon-Miller P, et al. 1991. A gene deleted in Kallmann’s syndrome shares homology with neural cell adhesion and axonal path-finding molecules. Nature 353:529–536 [DOI] [PubMed] [Google Scholar]
- Friede RL, Boltshauser E 1978. Uncommon syndromes of cerebellar vermis aplasia. I: Joubert syndrome. Dev Med Child Neurol 20:758–763 [DOI] [PubMed] [Google Scholar]
- Garcia-Frigola C, Carreres MI, Vegar C, Mason C, Herrera E 2008. Zic2 promotes axonal divergence at the optic chiasm midline by EphB1-dependent and -independent mechanisms. Development 135:1833–1841 [DOI] [PubMed] [Google Scholar]
- Gerdes JM, Davis EE, Katsanis N 2009. The vertebrate primary cilium in development, homeostasis, and disease. Cell 137:32–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JC, Tsai PS 2006. Expression of a dominant negative FGF receptor in developing GNRH1 neurons disrupts axon outgrowth and targeting to the median eminence. Biol Reprod 74:463–472 [DOI] [PubMed] [Google Scholar]
- Gill JC, Moenter SM, Tsai PS 2004. Developmental regulation of gonadotropin-releasing hormone neurons by fibroblast growth factor signaling. Endocrinology 145:3830–3839 [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Keeler LC, Parisi MA, Marsh SE, Chance PF, Glass IA, Graham JM Jr, Maria BL, Barkovich AJ, Dobyns WB 2004. Molar tooth sign of the midbrain-hindbrain junction: Occurrence in multiple distinct syndromes. Am J Med Genet A 125A:125–134; discussion 117 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Martinez D, Kim SH, Hu Y, Guimond S, Schofield J, Winyard P, Vannelli GB, Turnbull J, Bouloux PM 2004. Anosmin-1 modulates fibroblast growth factor receptor 1 signaling in human gonadotropin-releasing hormone olfactory neuroblasts through a heparan sulfate-dependent mechanism. J Neurosci 24:10384–10392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorden NT, Arts HH, Parisi MA, Coene KL, Letteboer SJ, van Beersum SE, Mans DA, Hikida A, Eckert M, Knutzen D, et al. 2008. CC2D2A is mutated in Joubert syndrome and interacts with the ciliopathy-associated basal body protein CEP290. Am J Hum Genet 83:559–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf WD, Born DE, Shaw DW, Thomas JR, Holloway LW, Michaelis RC 2000. Diffusion-weighted magnetic resonance imaging in boys with neural cell adhesion molecule L1 mutations and congenital hydrocephalus. Ann Neurol 47:113–117 [PubMed] [Google Scholar]
- Graziadei PP, Monti Graziadei GA 1986. Principles of organization of the vertebrate olfactory glomerulus: An hypothesis. Neuroscience 19:1025–1035 [DOI] [PubMed] [Google Scholar]
- Guillery RW, Mason CA, Taylor JS 1995. Developmental determinants at the mammalian optic chiasm. J Neurosci 15:4727–4737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson T, Zeavin B 1956. Observations on the retraction syndrome of Duane. Arch Ophthalmol 55:576–580 [Google Scholar]
- Gutowski NJ, Bosley TM, Engle EC 2003. 110th ENMC International Workshop: The congenital cranial dysinnervation disorders (CCDDs). Naarden, The Netherlands, 25–27 October, 2002. Neuromuscul Disord 13:573–578 [DOI] [PubMed] [Google Scholar]
- Hall C, Michael GJ, Cann N, Ferrari G, Teo M, Jacobs T, Monfries C, Lim L 2001. α2-chimaerin, a Cdc42/Rac1 regulator, is selectively expressed in the rat embryonic nervous system and is involved in neuritogenesis in N1E-115 neuroblastoma cells. J Neurosci 21:5191–5202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C, Sin WC, Teo M, Michael GJ, Smith P, Dong JM, Lim HH, Manser E, Spurr NK, Jones TA, et al. 1993. α2-chimerin, an SH2-containing GTPase-activating protein for the ras-related protein p21rac derived by alternate splicing of the human n-chimerin gene, is selectively expressed in brain regions and testes. Mol Cell Biol 13:4986–4998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller S, Wetzel SG, Lutschg J 2008. Functional MRI, DTI and neurophysiology in horizontal gaze palsy with progressive scoliosis. Neuroradiology 50:453–459 [DOI] [PubMed] [Google Scholar]
- Halliday J, Chow CW, Wallace D, Danks DM 1986. X linked hydrocephalus: A survey of a 20 year period in Victoria, Australia. J Med Genet 23:23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula-Jouppi K, Kaminen-Ahola N, Taipale M, Eklund R, Nopola-Hemmi J, Kaariainen H, Kere J 2005. The axon guidance receptor gene ROBO1 is a candidate gene for developmental dyslexia. PLoS Genet 1:e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardelin JP, Julliard AK, Moniot B, Soussi-Yanicostas N, Verney C, Schwanzel-Fukuda M, Ayer-Le Lievre C, Petit C 1999. Anosmin-1 is a regionally restricted component of basement membranes and interstitial matrices during organogenesis: Implications for the developmental anomalies of X chromosome-linked Kallmann syndrome. Dev Dyn 215:26–44 [DOI] [PubMed] [Google Scholar]
- Hebert JM, Lin M, Partanen J, Rossant J, McConnell SK 2003. FGF signaling through FGFR1 is required for olfactory bulb morphogenesis. Development 130:1101–1111 [DOI] [PubMed] [Google Scholar]
- Herrera E, Brown L, Aruga J, Rachel RA, Dolen G, Mikoshiba K, Brown S, Mason CA 2003. Zic2 patterns binocular vision by specifying the uncrossed retinal projection. Cell 114:545–557 [DOI] [PubMed] [Google Scholar]
- Herron LR, Hill M, Davey F, Gunn-Moore FJ 2009. The intracellular interactions of the L1 family of cell adhesion molecules. Biochem J 419:519–531 [DOI] [PubMed] [Google Scholar]
- Hetts SW, Sherr EH, Chao S, Gobuty S, Barkovich AJ 2006. Anomalies of the corpus callosum: An MR analysis of the phenotypic spectrum of associated malformations. AJR Am J Roentgenol 187:1343–1348 [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J 2006. Topography of the human corpus callosum revisited–comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage 32:989–994 [DOI] [PubMed] [Google Scholar]
- Hortsch M 1996. The L1 family of neural cell adhesion molecules: Old proteins performing new tricks. Neuron 17:587–593 [DOI] [PubMed] [Google Scholar]
- Hotchkiss MG, Miller NR, Clark AW, Green WG 1980. Bilateral Duane’s retraction syndrome: A clinical-pathological case report. Arch Ophthalmol 98:870–874 [DOI] [PubMed] [Google Scholar]
- Hu Y, Tanriverdi F, MacColl GS, Bouloux PM 2003. Kallmann’s syndrome: Molecular pathogenesis. Int J Biochem Cell Biol 35:1157–1162 [DOI] [PubMed] [Google Scholar]
- Huber A 1984. Duane’s retraction syndrome; consideration on pathophysiology and etiology. In Strabismus II (ed.) Reinecke R., 345–361 Grune & Stratton, Orlando [Google Scholar]
- Ingason A, Sigmundsson T, Steinberg S, Sigurdsson E, Haraldsson M, Magnusdottir BB, Frigge ML, Kong A, Gulcher J, Thorsteinsdottir U, et al. 2007. Support for involvement of the AHI1 locus in schizophrenia. Eur J Hum Genet 15:988–991 [DOI] [PubMed] [Google Scholar]
- Iwasato T, Katoh H, Nishimaru H, Ishikawa Y, Inoue H, Saito YM, Ando R, Iwama M, Takahashi R, Negishi M, et al. 2007. Rac-GAP α-Chimerin regulates motor-circuit formation as a key mediator of EphrinB3/EphA4 forward signaling. Cell 130:742–753 [DOI] [PubMed] [Google Scholar]
- Jeffery G, Erskine L 2005. Variations in the architecture and development of the vertebrate optic chiasm. Prog Retin Eye Res 24:721–753 [DOI] [PubMed] [Google Scholar]
- Jen JC, Chan WM, Bosley TM, Wan J, Carr JR, Rub U, Shattuck D, Salamon G, Kudo LC, Ou J, et al. 2004. Mutations in a human ROBO gene disrupt hindbrain axon pathway crossing and morphogenesis. Science 304:1509–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jissendi-Tchofo P, Doherty D, McGillivray G, Hevner R, Shaw D, Ishak G, Leventer R, Barkovich AJ 2009. Pontine tegmental cap dysplasia: MR imaging and diffusion tensor imaging features of impaired axonal navigation. AJNR Am J Neuroradiol 30:113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosten EA, Gribnau AA 1989. Immunocytochemical localization of cell adhesion molecule L1 in developing rat pyramidal tract. Neurosci Lett 100:94–98 [DOI] [PubMed] [Google Scholar]
- Joubert M, Eisenring JJ, Andermann F 1968. Familial dysgenesis of the vermis: A syndrome of hyperventilation, abnormal eye movements and retardation. Neurology 18:302–303 [PubMed] [Google Scholar]
- Kakinuma N, Kiyama R 2009. A major mutation of KIF21A associated with congenital fibrosis of the extraocular muscles type 1 (CFEOM1) enhances translocation of Kank1 to the membrane. Biochem Biophys Res Commun 386:639–644 [DOI] [PubMed] [Google Scholar]
- Kamiguchi H, Lemmon V 1998. A neuronal form of the cell adhesion molecule L1 contains a tyrosine-based signal required for sorting to the axonal growth cone. J Neurosci 18:3749–3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiguchi H, Long KE, Pendergast M, Schaefer AW, Rapoport I, Kirchhausen T, Lemmon V 1998. The neural cell adhesion molecule L1 interacts with the AP-2 adaptor and is endocytosed via the clathrin-mediated pathway. J Neurosci 18:5311–5321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamnasaran D 2005. Agenesis of the corpus callosum: Lessons from humans and mice. Clin Invest Med 28:267–282 [PubMed] [Google Scholar]
- Kim SH, Hu Y, Cadman S, Bouloux P 2008. Diversity in fibroblast growth factor receptor 1 regulation: Learning from the investigation of Kallmann syndrome. J Neuroendocrinol 20:141–163 [DOI] [PubMed] [Google Scholar]
- Krams M, Quinton R, Ashburner J, Friston KJ, Frackowiak RS, Bouloux PM, Passingham RE 1999. Kallmann’s syndrome: Mirror movements associated with bilateral corticospinal tract hypertrophy. Neurology 52:816–822 [DOI] [PubMed] [Google Scholar]
- Kurumaji A, Nomoto H, Okano T, Toru M 2001. An association study between polymorphism of L1CAM gene and schizophrenia in a Japanese sample. Am J Med Genet 105:99–104 [PubMed] [Google Scholar]
- Laifenfeld D, Karry R, Klein E, Ben-Shachar D 2005. Alterations in cell adhesion molecule L1 and functionally related genes in major depression: A postmortem study. Biol Psychiatry 57:716–725 [DOI] [PubMed] [Google Scholar]
- LeCouter J, Lin R, Tejada M, Frantz G, Peale F, Hillan KJ, Ferrara N 2003. The endocrine-gland-derived VEGF homologue Bv8 promotes angiogenesis in the testis: Localization of Bv8 receptors to endothelial cells. Proc Natl Acad Sci 100:2685–2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Kim DI, Kim J, Kim DJ, Kim HD, Kim DS, Mori S 2005. Diffusion-tensor MR imaging and fiber tractography: A new method of describing aberrant fiber connections in developmental CNS anomalies. Radiographics 25:53–65; discussion 66–68 [DOI] [PubMed] [Google Scholar]
- Legouis R, Hardelin JP, Levilliers J, Claverie JM, Compain S, Wunderle V, Millasseau P, Le Paslier D, Cohen D, Caterina D, et al. 1991. The candidate gene for the X-linked Kallmann syndrome encodes a protein related to adhesion molecules. Cell 67:423–435 [DOI] [PubMed] [Google Scholar]
- Leinsinger GL, Heiss DT, Jassoy AG, Pfluger T, Hahn K, Danek A 1997. Persistent mirror movements: Functional MR imaging of the hand motor cortex. Radiology 203:545–552 [DOI] [PubMed] [Google Scholar]
- Lin LK, Chien YH, Wu JY, Wang AH, Chiang SC, Hwu WL 2005. KIF21A gene c.2860C>T mutation in congenital fibrosis of extraocular muscles type 1 and 3. Mol Vis 11:245–248 [PubMed] [Google Scholar]
- Lu S, Zhao C, Zhao K, Li N, Larsson C 2008. Novel and recurrent KIF21A mutations in congenital fibrosis of the extraocular muscles type 1 and 3. Arch Ophthalmol 126:388–394 [DOI] [PubMed] [Google Scholar]
- Maeoka Y, Yamamoto T, Ohtani K, Takeshita K 1997. Pontine hypoplasia in a child with sensorineural deafness. Brain Dev 19:436–439 [DOI] [PubMed] [Google Scholar]
- Maness PF, Schachner M 2007. Neural recognition molecules of the immunoglobulin superfamily: Signaling transducers of axon guidance and neuronal migration. Nat Neurosci 10:19–26 [DOI] [PubMed] [Google Scholar]
- Marcus RC, Wang LC, Mason CA 1996. Retinal axon divergence in the optic chiasm: Midline cells are unaffected by the albino mutation. Development 122:859–868 [DOI] [PubMed] [Google Scholar]
- Maria BL, Quisling RG, Rosainz LC, Yachnis AT, Gitten J, Dede D, Fennell E 1999. Molar tooth sign in Joubert syndrome: Clinical, radiologic, and pathologic significance. J Child Neurol 14:368–376 [DOI] [PubMed] [Google Scholar]
- Marillat V, Sabatier C, Failli V, Matsunaga E, Sotelo C, Tessier-Lavigne M, Chedotal A 2004. The slit receptor Rig-1/Robo3 controls midline crossing by hindbrain precerebellar neurons and axons. Neuron 43:69–79 [DOI] [PubMed] [Google Scholar]
- Marszalek JR, Weiner JA, Farlow SJ, Chun J, Goldstein LS 1999. Novel dendritic kinesin sorting identified by different process targeting of two related kinesins: KIF21A and KIF21B. J Cell Biol 145:469–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S, Yamazaki C, Masumoto KH, Nagano M, Naito M, Soga T, Hiyama H, Matsumoto M, Takasaki J, Kamohara M, et al. 2006. Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc Natl Acad Sci 103:4140–4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingley JB 2009. Attention, automaticity, and awareness in synesthesia. Ann N Y Acad Sci 1156:141–167 [DOI] [PubMed] [Google Scholar]
- Mayston MJ, Harrison LM, Quinton R, Stephens JA, Krams M, Bouloux PM 1997. Mirror movements in X-linked Kallmann’s syndrome. I. A neurophysiological study. Brain 120:1199–1216 [DOI] [PubMed] [Google Scholar]
- Millen KJ, Gleeson JG 2008. Cerebellar development and disease. Curr Opin Neurobiol 18:12–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NR, Kiel SM, Green WR, Clark AW 1982. Unilateral Duane’s retraction syndrome (type 1). Arch Ophthalmol 100:1468–1472 [DOI] [PubMed] [Google Scholar]
- Miyake N, Chilton J, Psatha M, Cheng L, Andrews C, Chan WM, Law K, Crosier M, Lindsay S, Cheung M, et al. 2008. Human CHN1 mutations hyperactivate α2-chimaerin and cause Duane’s retraction syndrome. Science 321:839–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KL, Li JD, Cheng MY, Leslie FM, Lee AG, Zhou QY 2005. Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science 308:1923–1927 [DOI] [PubMed] [Google Scholar]
- Noor A, Windpassinger C, Patel M, Stachowiak B, Mikhailov A, Azam M, Irfan M, Siddiqui ZK, Naeem F, Paterson AD, et al. 2008. CC2D2A, encoding a coiled-coil and C2 domain protein, causes autosomal-recessive mental retardation with retinitis pigmentosa. Am J Hum Genet 82:1011–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouanounou S, Saigal G, Birchansky S 2005. Mobius syndrome. AJNR Am J Neuroradiol 26:430–432 [PMC free article] [PubMed] [Google Scholar]
- Padgett KR, Maria BL, Yachnis AT, Blackband SJ 2002. Ex vivo high-resolution magnetic resonance imaging of the brain in Joubert’s syndrome. J Child Neurol 17:911–913 [PubMed] [Google Scholar]
- Parisi MA, Bennett CL, Eckert ML, Dobyns WB, Gleeson JG, Shaw DW, McDonald R, Eddy A, Chance PF, Glass IA 2004a. The NPHP1 gene deletion associated with juvenile nephronophthisis is present in a subset of individuals with Joubert syndrome. Am J Hum Genet 75:82–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi MA, Pinter JD, Glass IA, Field K, Maria BL, Chance PF, Mahurin RK, Cramer SC 2004b. Cerebral and cerebellar motor activation abnormalities in a subject with Joubert syndrome: Functional magnetic resonance imaging (MRI) study. J Child Neurol 19:214–218 [PubMed] [Google Scholar]
- Paul LK, Brown WS, Adolphs R, Tyszka JM, Richards LJ, Mukherjee P, Sherr EH 2007. Agenesis of the corpus callosum: Genetic, developmental and functional aspects of connectivity. Nat Rev Neurosci 8:287–299 [DOI] [PubMed] [Google Scholar]
- Petros TJ, Rebsam A, Mason CA 2008. Retinal axon growth at the optic chiasm: To cross or not to cross. Annu Rev Neurosci 31:295–315 [DOI] [PubMed] [Google Scholar]
- Pitteloud N, Quinton R, Pearce S, Raivio T, Acierno J, Dwyer A, Plummer L, Hughes V, Seminara S, Cheng YZ, et al. 2007a. Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J Clin Invest 117:457–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitteloud N, Zhang C, Pignatelli D, Li JD, Raivio T, Cole LW, Plummer L, Jacobson-Dickman EE, Mellon PL, Zhou QY, et al. 2007b. Loss-of-function mutation in the prokineticin 2 gene causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci 104:17447–17452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poretti A, Boltshauser E, Loenneker T, Valente EM, Brancati F, Il’yasov K, Huisman TA 2007. Diffusion tensor imaging in Joubert syndrome. AJNR Am J Neuroradiol 28:1929–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst M 1901. Uber den Bau des vollstanding balkenlosen GroBhirns. Arch Psychiatr 34:709–786 [Google Scholar]
- Quinton R, Duke VM, de Zoysa PA, Platts AD, Valentine A, Kendall B, Pickman S, Kirk JM, Besser GM, Jacobs HS, et al. 1996. The neuroradiology of Kallmann’s syndrome: A genotypic and phenotypic analysis. J Clin Endocrinol Metab 81:3010–3017 [DOI] [PubMed] [Google Scholar]
- Quinton R, Duke VM, Robertson A, Kirk JM, Matfin G, de Zoysa PA, Azcona C, MacColl GS, Jacobs HS, Conway GS, et al. 2001. Idiopathic gonadotrophin deficiency: Genetic questions addressed through phenotypic characterization. Clin Endocrinol 55:163–174 [DOI] [PubMed] [Google Scholar]
- Quisling RG, Barkovich AJ, Maria BL 1999. Magnetic resonance imaging features and classification of central nervous system malformations in Joubert syndrome. J Child Neurol 14:628–635; discussion 669–672 [DOI] [PubMed] [Google Scholar]
- Rachel RA, Dolen G, Hayes NL, Lu A, Erskine L, Nowakowski RS, Mason CA 2002. Spatiotemporal features of early neuronogenesis differ in wild-type and albino mouse retina. J Neurosci 22:4249–4263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray K, Chaki M, Sengupta M 2007. Tyrosinase and ocular diseases: Some novel thoughts on the molecular basis of oculocutaneous albinism type 1. Prog Retin Eye Res 26:323–358 [DOI] [PubMed] [Google Scholar]
- Robertson A, MacColl GS, Nash JA, Boehm MK, Perkins SJ, Bouloux PM 2001. Molecular modelling and experimental studies of mutation and cell-adhesion sites in the fibronectin type III and whey acidic protein domains of human anosmin-1. Biochem J 357:647–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph G, Nentwich M, Hellebrand H, Pollack K, Gordes R, Bau V, Kampik A, Meindl A 2009. KIF21A variant R954W in familial or sporadic cases of CFEOM1. Eur J Ophthalmol 19:667–674 [DOI] [PubMed] [Google Scholar]
- Rugarli EI, Ghezzi C, Valsecchi V, Ballabio A 1996. The Kallmann syndrome gene product expressed in COS cells is cleaved on the cell surface to yield a diffusible component. Hum Mol Genet 5:1109–1115 [DOI] [PubMed] [Google Scholar]
- Sabatier C, Plump AS, Le M, Brose K, Tamada A, Murakami F, Lee EY, Tessier-Lavigne M 2004. The divergent Robo family protein rig-1/Robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell 117:157–169 [DOI] [PubMed] [Google Scholar]
- Sayer JA, Otto EA, O’Toole JF, Nurnberg G, Kennedy MA, Becker C, Hennies HC, Helou J, Attanasio M, Fausett BV, et al. 2006. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet 38:674–681 [DOI] [PubMed] [Google Scholar]
- Schmid RS, Maness PF 2008. L1 and NCAM adhesion molecules as signaling coreceptors in neuronal migration and process outgrowth. Curr Opin Neurobiol 18:245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott GD, Wyke MA 1981. Congenital mirror movements. J Neurol Neurosurg Psychiatry 44:586–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M, Bick D, Pfaff DW 1989. Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Brain Res Mol Brain Res 6:311–326 [DOI] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M, Pfaff DW 1989. Origin of luteinizing hormone-releasing hormone neurons. Nature 338:161–164 [DOI] [PubMed] [Google Scholar]
- Shen X, Meza-Carmen V, Puxeddu E, Wang G, Moss J, Vaughan M 2008. Interaction of brefeldin A-inhibited guanine nucleotide-exchange protein (BIG) 1 and kinesin motor protein KIF21A. Proc Natl Acad Sci 105:18788–18793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Fu WY, Hung KW, Porchetta C, Hall C, Fu AK, Ip NY 2007. α2-chimaerin interacts with EphA4 and regulates EphA4-dependent growth cone collapse. Proc Natl Acad Sci 104:16347–16352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Okinaga A, Maruo T 2005. Recurrent mutation of the KIF21A gene in Japanese patients with congenital fibrosis of the extraocular muscles. Jpn J Ophthalmol 49:443–447 [DOI] [PubMed] [Google Scholar]
- Sicotte NL, Salamon G, Shattuck DW, Hageman N, Rub U, Salamon N, Drain AE, Demer JL, Engle EC, Alger JR, et al. 2006. Diffusion tensor MRI shows abnormal brainstem crossing fibers associated with ROBO3 mutations. Neurology 67:519–521 [DOI] [PubMed] [Google Scholar]
- Soussi-Yanicostas N, de Castro F, Julliard AK, Perfettini I, Chedotal A, Petit C 2002. Anosmin-1, defective in the X-linked form of Kallmann syndrome, promotes axonal branch formation from olfactory bulb output neurons. Cell 109:217–228 [DOI] [PubMed] [Google Scholar]
- Soussi-Yanicostas N, Faivre-Sarrailh C, Hardelin JP, Levilliers J, Rougon G, Petit C 1998. Anosmin-1 underlying the X chromosome-linked Kallmann syndrome is an adhesion molecule that can modulate neurite growth in a cell-type specific manner. J Cell Sci 111:2953–2965 [DOI] [PubMed] [Google Scholar]
- Soussi-Yanicostas N, Hardelin JP, Arroyo-Jimenez MM, Ardouin O, Legouis R, Levilliers J, Traincard F, Betton JM, Cabanie L, Petit C 1996. Initial characterization of anosmin-1, a putative extracellular matrix protein synthesized by definite neuronal cell populations in the central nervous system. J Cell Sci 109:1749–1757 [DOI] [PubMed] [Google Scholar]
- Spampinato MV, Kraas J, Maria BL, Walton ZJ, Rumboldt Z 2008. Absence of decussation of the superior cerebellar peduncles in patients with Joubert syndrome. Am J Med Genet A 146A:1389–1394 [DOI] [PubMed] [Google Scholar]
- Tiab L, d’Alleves Manzi V, Borruat FX, Munier F, Schorderet D 2004. Mutation analysis of KIF21A in congenital fibrosis of the extraocular muscles (CFEOM) patients. Ophthalmic Genet 25:241–246 [DOI] [PubMed] [Google Scholar]
- Tibber MS, Whitmore AV, Jeffery G 2006. Cell division and cleavage orientation in the developing retina are regulated by L-DOPA. J Comp Neurol 496:369–381 [DOI] [PubMed] [Google Scholar]
- Tovar-Moll F, Moll J, de Oliveira-Souza R, Bramati I, Andreiuolo PA, Lent R 2007. Neuroplasticity in human callosal dysgenesis: A diffusion tensor imaging study. Cereb Cortex 17:531–541 [DOI] [PubMed] [Google Scholar]
- Tsai PS, Moenter SM, Postigo HR, El Majdoubi M, Pak TR, Gill JC, Paruthiyil S, Werner S, Weiner RI 2005. Targeted expression of a dominant-negative fibroblast growth factor (FGF) receptor in gonadotropin-releasing hormone (GnRH) neurons reduces FGF responsiveness and the size of GnRH neuronal population. Mol Endocrinol 19:225–236 [DOI] [PubMed] [Google Scholar]
- Utsunomiya H, Yamashita S, Takano K, Okazaki M 2006. Arrangement of fiber tracts forming Probst bundle in complete callosal agenesis: Report of two cases with an evaluation by diffusion tensor tractography. Acta Radiol 47:1063–1066 [DOI] [PubMed] [Google Scholar]
- Vulliemoz S, Raineteau O, Jabaudon D 2005. Reaching beyond the midline: Why are human brains cross wired? Lancet Neurol 4:87–99 [DOI] [PubMed] [Google Scholar]
- Wahl M, Strominger Z, Jeremy RJ, Barkovich AJ, Wakahiro M, Sherr EH, Mukherjee P 2009. Variability of homotopic and heterotopic callosal connectivity in partial agenesis of the corpus callosum: A 3T diffusion tensor imaging and Q-ball tractography study. Am J Neuroradiol 30:282–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmeyer H, Egea J, Rabe N, Gezelius H, Filosa A, Enjin A, Varoqueaux F, Deininger K, Schnutgen F, Brose N, et al. 2007. EphA4-dependent axon guidance is mediated by the RacGAP α2-Chimaerin. Neuron 55:756–767 [DOI] [PubMed] [Google Scholar]
- Widjaja E, Blaser S, Raybaud C 2006. Diffusion tensor imaging of midline posterior fossa malformations. Pediatr Radiol 36:510–517 [DOI] [PubMed] [Google Scholar]
- Wiencken-Barger AE, Mavity-Hudson J, Bartsch U, Schachner M, Casagrande VA 2004. The role of L1 in axon pathfinding and fasciculation. Cereb Cortex 14:121–131 [DOI] [PubMed] [Google Scholar]
- Williams SE, Mann F, Erskine L, Sakurai T, Wei S, Rossi DJ, Gale NW, Holt CE, Mason CA, Henkemeyer M 2003. Ephrin-B2 and EphB1 mediate retinal axon divergence at the optic chiasm. Neuron 39:919–935 [DOI] [PubMed] [Google Scholar]
- Wray S, Grant P, Gainer H 1989. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci 86:8132–8136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachnis AT, Rorke LB 1999. Neuropathology of Joubert syndrome. J Child Neurol 14:655–659; discussion 669–672 [DOI] [PubMed] [Google Scholar]
- Yamada K, Andrews C, Chan WM, McKeown CA, Magli A, De Berardinis T, Loewenstein A, Lazar M, O’Keefe M, Letson R, et al. 2003. Heterozygous mutations of the kinesin KIF21A in congenital fibrosis of the extraocular muscles type 1 (CFEOM1). Nat Genet 35:318–321 [DOI] [PubMed] [Google Scholar]
- Yamada K, Hunter DG, Andrews C, Engle EC 2005. A novel KIF21A mutation in a patient with congenital fibrosis of the extraocular muscles and Marcus Gunn jaw-winking phenomenon. Arch Ophthalmol 123:1254–1259 [DOI] [PubMed] [Google Scholar]
- Yamasaki M, Thompson P, Lemmon V 1997. CRASH syndrome: Mutations in L1CAM correlate with severity of the disease. Neuropediatrics 28:175–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki MS, Abdel-Aleem A, Abdel-Salam G, Marsh SE, Silhavy JL, Barkovich AJ, Ross ME, Saleem SN, Dobyns WB, Gleeson JG 2008. The molar tooth sign: A new Joubert syndrome and related cerebellar disorders classification system tested in Egyptian families. Neurology 70:556–565 [DOI] [PubMed] [Google Scholar]
- Zhang XQ, Peng JH, Tang ZH, Xu CQ, Zhou X, Gong SX, Liu JY, Wang Q, Liu MG 2006. Mutation p.Arg954Trp of KIF21A causes congenital fibrosis of the extraocular muscles in a Chinese family. Yi chuan xue bao=Acta genetica Sinica 33:685–691 [DOI] [PubMed] [Google Scholar]